Synthesis and Properties of Degradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Derived from Waste Fish Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Cultivation Conditions
2.2. Waste Fish Oil (WFO)
2.3. The Parameters of the Bacterial Growth Process
2.4. Polymer Properties
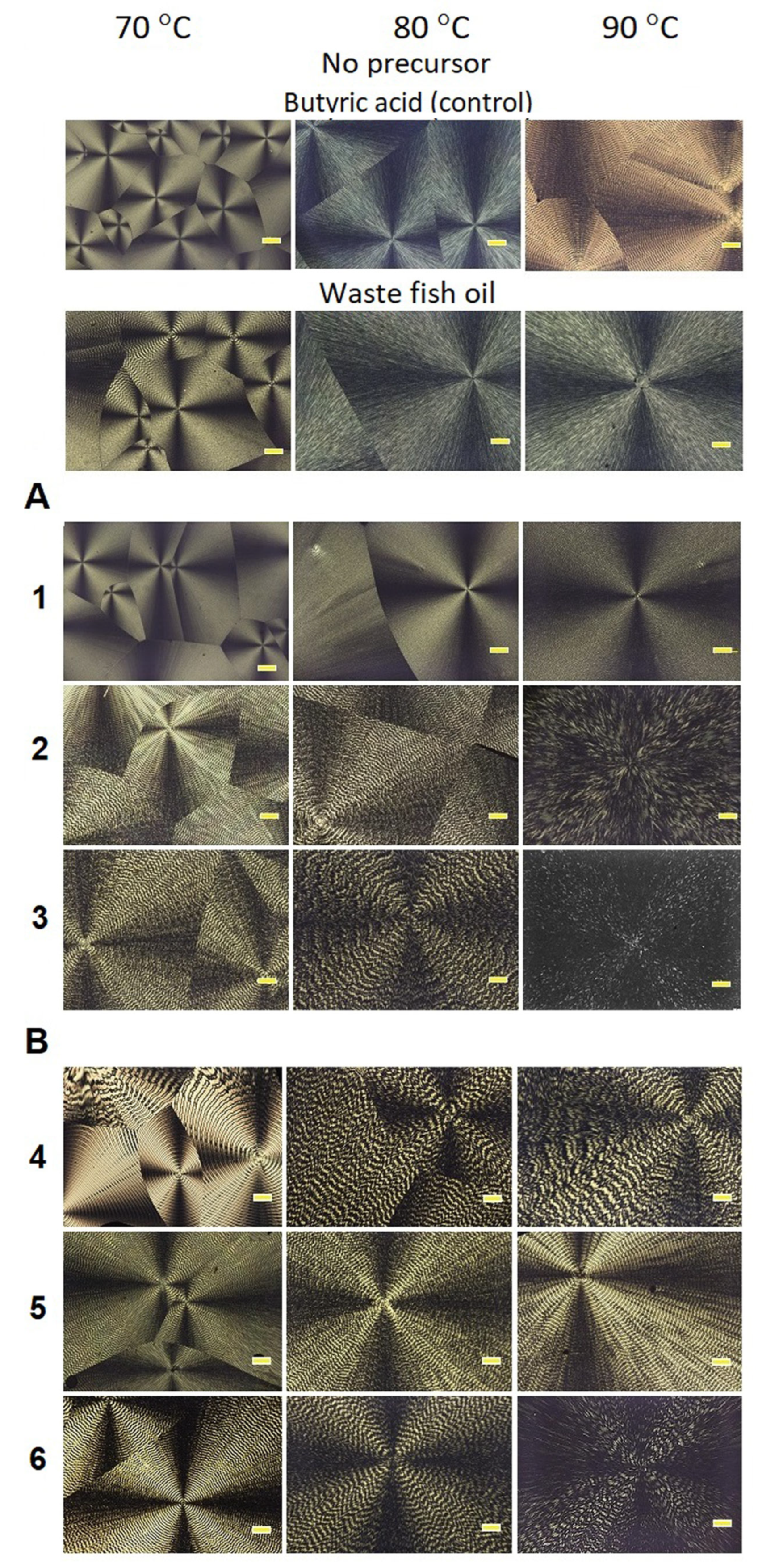
2.5. Study of Spherulite Morphology
2.6. Statistics
3. Results
3.1. Synthesis of P(3HB-co-3HV) Using Waste Fish Oil as the Main Carbon Substrate
3.2. Physicochemical Properties of P(3HB-co-3HV) with Different Ratios of 3HB to 3HV Monomers Synthesized on WFO or Butyric Acid
3.3. Thermal Behavior and Exothermic Crystallization of P(3HB-co-3HV) Copolymers with Different Monomer Ratios Synthesized by C. necator B-10646 on WFO or Butyric Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, G.Q. Plastics completely synthesized by bacteria: Polyhydroxyalkanoates. In Plastics from Bacteria: Natural Functions and Applications; Chen, G.Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–37. [Google Scholar] [CrossRef]
- Sudesh, K. Practical Guide to Microbial Polyhydroxyalkanoates; Smitthes: London, UK, 2010; 158p. [Google Scholar]
- Volova, T.G.; Shishatskaya, E.I.; Sinskey, A.J. Degradable Polymers: Production, Properties, Applications; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2013; 380p. [Google Scholar]
- Mitra, R.; Xu, T.; Chen, G.Q.; Xiang, H.; Han, J. An updated overview on the regulatory circuits of polyhydroxyalkanoates synthesis. Microb. Biotechnol. 2022, 15, 1446–1470. [Google Scholar] [CrossRef]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.Q. Grand challenges for industrializing polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. A new wave of industrialization of PHA biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Adak, S.; Kayalvizhi, R.; Bishai, M.; Jacob, S.; Kundu, D. Advancements in microbial production of polyhydroxyalkanoates (PHA) from wastes for sustainable active food packaging: An eclectic review. Biocatal. Agric. Biotechnol. 2024, 60, 103288. [Google Scholar] [CrossRef]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.M.; Wnek, G.E. Poly(hydroxyalkanoates): Emerging biopolymers in Biomedical Fields and Packaging Industries for a Circular Economy. Biomed. Mater. Devices 2025, 3, 19–44. [Google Scholar] [CrossRef]
- Bolla, M.L.; Pettinato, M.; Ferrari, P.F.; Fabiano, B.; Perego, P. Polyhydroxyalkanoates production from laboratory to industrial scale: A review. Int. J. Biol. Macromol. 2025, 310, 143255. [Google Scholar] [CrossRef] [PubMed]
- Cromwick, A.M.; Foglia, T.; Lenz, R.W. The microbial production of poly(hydroxyalkanoates) from tallow. Appl. Microbiol. Biotechnol. 1996, 46, 464–469. [Google Scholar] [CrossRef]
- Lemoigne, M. Études sur l’autolyse microbienne origine de l’acide β-oxybutyrique formé par autolyse. Ann. Inst. Pasteur 1927, 41, 148. [Google Scholar]
- Macrae, R.M.; Wilkinson, J.F. The influence of cultural conditions on poly-β-hydroxybutyrate synthesis in Bacillus megaterium. Proc. R. Phys. Soc. Edinb. 1958, 27, 73–78. [Google Scholar]
- Senior, P.J.; Dawes, E.A. The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem. J. 1973, 134, 225–238. [Google Scholar] [CrossRef]
- King, P.P. Biotechnology, an industrial view. J. Chem. Technol. Biotechnol. 1982, 32, 2–8. [Google Scholar] [CrossRef]
- Howells, E.R. Opportunities in biotechnology for the chemical industry. Chem. Ind. 1982, 7, 508–511. [Google Scholar]
- Bergmann, A.; Owen, A. Dielectric relaxation spectroscopy of poly[(R)-3-hydroxybutyrate] (PHB) during crystallization. Polym. Int. 2004, 53, 863–868. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Zhang, Z.; Quinn, E.C.; Olmedo-Martínez, J.L.; Caputo, M.R.; Franklin, K.A.; Müller, A.J.; Chen, E.Y.X. Toughening brittle Bio-P3HB with synthetic P3HB of engineered stereomicrostructures. Angew. Chem. Int. Ed. 2023, 62, e202311264. [Google Scholar] [CrossRef] [PubMed]
- Wallen, L.L.; Rohwedder, W.K. Poly-.beta.-hydroxyalkanoate from activated sludge. Environ. Sci. Technol. 1974, 8, 576–579. [Google Scholar] [CrossRef]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Let. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Spyros, A.; Kimmich, R.; Briese, B.H.; Jendrossek, D. 1H NMR Imaging Study of Enzymatic Degradation in Poly(3-hydroxybutyrate) and Poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Evidence for Preferential Degradation of the Amorphous Phase by PHB Depolymerase B from Pseudomonas lemoignei. Macromolecules 1977, 30, 8218–8225. [Google Scholar] [CrossRef]
- Kim, D.Y.; Rhee, Y.H. Bacterial Poly(3-hydroxyalkanoates) Bearing Carbon−Carbon Triple Bonds. Macromolecules 1998, 31, 4760–4763. [Google Scholar] [CrossRef]
- Pazur, R.J.; Hocking, P.J.; Raymond, S.; Marchessault, R.H. Crystal structure of syndiotactic poly(β-hydroxybutyrate) from X-ray fiber and powder diffraction analyses and molecular modeling. Macromolecules 1998, 31, 6585–6592. [Google Scholar] [CrossRef]
- Nagata, M.; Machida, T.; Sakai, W.; Tsutsumi, N. Synthesis, characterization, and enzymatic degradation studies on novel network aliphatic polyesters. Macromolecules 1998, 31, 6450–6454. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly(3-hydroxy)butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Avella, M.; Rota, G.L.; Martuscelli, E.; Raimo, M.; Sadocco, P.; Elegir, G.; Riva, R. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and wheat straw fibre composites: Thermal, mechanical properties and biodegradation behaviour. J. Mater. Sci. 2000, 35, 829–836. [Google Scholar] [CrossRef]
- Bluhm, T.L.; Hamer, G.K.; Marchessault, R.H.; Fyfe, C.A.; Veregin, R.P. Isodimorphism in bacterial poly(β-hydroxybutyrate-co-β-hydroxyvalerate). Macromolecules 1986, 19, 2871–2876. [Google Scholar] [CrossRef]
- Scandola, M.; Ceccorulli, G.; Pizzoli, M.; Gazzano, M. Study of the crystal phase and crystallization rate of bacterial poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Macromolecules 1992, 25, 1405–1410. [Google Scholar] [CrossRef]
- Kunioka, M.; Tamaki, A.; Doi, Y. Crystalline and thermal properties of bacterial copolyesters: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1989, 22, 694–697. [Google Scholar] [CrossRef]
- Bloembergen, S.; Holden, D.A.; Hamer, G.K.; Bluhm, T.L.; Marchessault, R.H. Studies of composition and crystallinity of bacterial poly(β-hydroxybutyrate-co-β-hydroxyvalerate). Macromolecules 1986, 19, 2865–2871. [Google Scholar] [CrossRef]
- Poirier, Y.; Nawrath, C.; Somerville, C. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Biotechnology 1995, 13, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Langford, A.; Chan, C.M.; Pratt, S.; Garvey, C.J.; Laycock, B. The morphology of crystallisation of PHBV/PHBV copolymer blends. Eur. Polym. J. 2019, 112, 104–119. [Google Scholar] [CrossRef]
- Mai, J.; Garvey, C.J.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and characterisation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) multi-block copolymers comprising blocks of differing 3-hydroxyvalerate contents. Chem. Eng. J. 2023, 475, 146175. [Google Scholar] [CrossRef]
- Mai, J.; Pratt, S.; Laycock, B.; Chan, C.M. Synthesis and Characterisation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-b-poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Multi-Block Copolymers Produced Using Diisocyanate Chemistry. Polymers 2023, 15, 3257. [Google Scholar] [CrossRef]
- Mai, J.; Chan, C.M.; Laycock, B.; Pratt, S. Understanding the reaction of hydroxy-terminated poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) random copolymers with a Monoisocyanate. Macromolecules 2023, 56, 2328–2338. [Google Scholar] [CrossRef]
- Mai, J.; Kockler, K.; Parisi, E.; Chan, C.M.; Pratt, S.; Laycock, B. Synthesis and physical properties of polyhydroxyalkanoate (PHA)-based block copolymers: A review. Int. J. Biol. Macromol. 2024, 263, 130204. [Google Scholar] [CrossRef]
- Luzier, W.D. Materials derived from biomass/biodegradable materials. Proc. Natl. Acad. Sci. USA 1992, 89, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Lawless, S.; Laycock, B.; Lant, P.; Pratt, S. Solvent-based synthesis, structural elucidation and thermal characterisation of free radical grafted PHBV. Polym. Degrad. Stab. 2024, 229, 110976. [Google Scholar] [CrossRef]
- Doi, Y. Microbial polyester. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 1365. [Google Scholar] [CrossRef]
- Doi, Y.; Segawa, A.; Kawaguchi, Y.; Kunioka, M. Cyclic nature of poly(3-hydroxyalkanoate) metabolism in Alcaligenes eutrophus. FEMS Microbiol. Lett. 1990, 67, 165–169. [Google Scholar] [CrossRef]
- Akiyama, M.; Taima, Y.; Doi, Y. Production of poly (3-hydroxyalkanoates) by a bacterium of the genus Alcaligenes utilizing long-chain fatty acids. Appl. Microbiol. Biotechnol. 1992, 37, 698–701. [Google Scholar] [CrossRef]
- Doi, Y.; Kitamura, S.; Abe, H. Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1995, 28, 4822–4828. [Google Scholar] [CrossRef]
- Slater, S.; Houmiel, K.L.; Tran, M.; Mitsky, T.A.; Taylor, N.B.; Padgette, S.R.; Gruys, K.J. Multiple β-ketothiolases mediate poly(β-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J. Bacteriol. 1998, 180, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Green, P.R.; Kemper, J.; Schechtman, L.; Guo, L.; Satkowski, M.; Fiedler, S.; Steinbüchel, A.; Rehm, B.H. Formation of short chain length/medium chain length polyhydroxyalkanoate copolymers by fatty acid β-oxidation inhibited Ralstonia eutropha. Biomacromolecules 2002, 3, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Tamaki, A.; Kunioka, M.; Soga, K. Production of copolyesters of 3-hydroxybutyrate and 3-hydroxyvalerate by Alcaligenes eutrophus from butyric and pentanoic acids. Appl. Microbiol. Biotechnol. 1988, 28, 330–334. [Google Scholar] [CrossRef]
- Kobayashi, G.; Tanaka, K.; Itoh, H.; Tsuge, T.; Sonomoto, K.; Ishizaki, A. Fermentative production of P(3HB-co-3HV) from propionic acid by Alcaligenes eutrophus in fed-batch culture with pH-stat continuous substrate feeding method. Biotechnol. Let. 2000, 22, 1067–1069. [Google Scholar] [CrossRef]
- Squio, C.R.; Marangoni, C.; De Vecchi, C.S.; Aragão, G.M.F. Phosphate feeding strategy during production phase improves poly(3-hydroxybutyrate-co-3-hydroxyvalerate) storage by Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2003, 61, 257–260. [Google Scholar] [CrossRef]
- Choi, G.G.; Kim, M.W.; Kim, J.Y.; Rhee, Y.H. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with high molar fractions of 3-hydroxyvalerate by a threonine-overproducing mutant of Alcaligenes sp. SH-69. Biotechnol. Let. 2003, 25, 665–670. [Google Scholar] [CrossRef]
- Marangoni, C.; Furigo, A.; Falcão de Aragão, G.M. Oleic acid improves poly(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Ralstonia eutropha in inverted sugar and propionic acid. Biotechnol. Let. 2000, 22, 1635–1638. [Google Scholar] [CrossRef]
- Tanaka, K.; Ishizaki, A.; Kanamaru, T.; Kawano, T. Production of poly(D-3-hydroxybutyrate) from CO2, H2, and O2 by high cell density autotrophic cultivation of Alcaligenes eutrophus. Biotechnol. Bioeng. 1995, 45, 268–275. [Google Scholar] [CrossRef]
- Ishizaki, A.; Tanaka, K.; Taga, N. Microbial production of poly-D-3-hydroxybutyrate from CO2. Appl. Microbiol. Biotechnol. 2001, 57, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Kalacheva, G.; Altukhova, O.V. Autotrophic synthesis of polyhydroxyalkanoates by the bacteria Ralstonia eutropha in the presence of carbon monoxide. Appl. Microbiol. Biotechnol. 2002, 58, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Zhila, N.O.; Shishatskaya, E.I.; Sukovatyi, A.G. Fiziko-Himicheskie Svoistva Polyhidroxyalkanoatov Razlichnoi Structury. Usloviya Polucheniya i Producenty (Physicochemical Properties of Polyhydroxyalkanoates of Different Structure. Biosynthesis Conditions and Producers). RF Patent (Database) 2012620288, 15 March 2012. (In Russian). [Google Scholar]
- Huang, T.Y.; Duan, K.J.; Huang, S.Y.; Chen, C.W. Production of polyhydroxyalkanoates from inexpensive extruded rice bran and starch by Haloferax mediterranei. J. Ind. Microbiol. Biotechnol. 2006, 33, 701–706. [Google Scholar] [CrossRef]
- Laycock, B.; Arcos-Hernandez, M.V.; Langford, A.; Pratt, S.; Werker, A.; Halley, P.J.; Lant, P.A. Crystallisation and fractionation of selected polyhydroxyalkanoates produced from mixed cultures. New Biotechnol. 2014, 31, 345–356. [Google Scholar] [CrossRef]
- Jo, S.Y.; Lim, S.H.; Lee, J.Y.; Son, J.; Choi, J.I.; Park, S.J. Microbial production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate), from lab to the shelf: A review. Int. J. Biol. Macromol. 2024, 274, 133157. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Haldar, D.; Sharma, C.; Chopra, J.; Chakrabortty, S.; Dilip, K.J. Innovations in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and nanocomposites for sustainable food packaging via biochemical biorefinery platforms: A comprehensive review. Int. J. Biol. Macromol. 2024, 283, 137574. [Google Scholar] [CrossRef] [PubMed]
- Pu, N.; Wang, M.R.; Li, Y.; Li, Z.J. Metabolic engineering of Salinivibrio sp. TGB10 for PHBV biosynthesis with a high 3-hydroxyvalerate fraction from starch and propionate. Int. J. Biol. Macromol. 2025, 308, 142359. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving biological production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) co-polymer: A critical review. Rev. Environ. Sci. Biotechnol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Talan, A.; Kaur, R.; Tyagi, R.D.; Drogui, P. Bioresource technology reports bioconversion of oily waste to polyhydroxyalkanoates: Sustainable technology with circular bioeconomy approach and multidimensional impacts. Bioresour. Technol. Rep. 2020, 11, 100496. [Google Scholar] [CrossRef]
- Tan, J.; Jia, S.; Ramakrishna, S. Accelerating plastic circularity: A critical assessment of the pathways and processes to circular plastics. Processes 2023, 11, 1457. [Google Scholar] [CrossRef]
- Soni, R.; Debbarma, P.; Suyal, D.C.; Goel, R. Advanced Strategies for Biodegradation of Plastic Polymers; Springer: Cham, Switzerland, 2024; 419p. [Google Scholar] [CrossRef]
- Thuoc, V.D.; My, D.N.; Loan, T.T.; Sudesh, K. Utilization of waste fish oil and glycerol as carbon sources for polyhydroxyalkanoate production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019, 141, 885–892. [Google Scholar] [CrossRef]
- Argiz, L.; Gonzalez-Cabaleiro, R.; Correa-Galeote, D.; del Rio, A.V.; Mosquera-Corral, A. Open-culture biotechnological process for triacylglycerides and polyhydroxyalkanoates recovery from industrial waste fish oil under saline conditions. Sep. Purif. Technol. 2021, 270, 118805. [Google Scholar] [CrossRef]
- Sangkharak, K.; Paichid, N.; Yunu, T.; Klomklao, S.; Prasertsan, P. Utilisation of tuna condensate waste from the canning industry as a novel substrate for polyhydroxyalkanoate production. Biomass Convers. Biorefinery 2021, 11, 2053–2064. [Google Scholar] [CrossRef]
- Correa-Galeote, D.; Argiz, L.; Val del Rio, A.; Mosquera-Corral, A.; Juarez-Jimenez, B.; Gonzalez-Lopez, J.; Rodelas, B. Dynamics of PHA-accumulating bacterial communities fed with lipid-rich liquid effluents from fish-canning industries. Polymers 2022, 14, 1396. [Google Scholar] [CrossRef]
- Thuoc, V.D.; Anh, V.T. Bioconversion of crude fish Oil into poly-3-hydroxybutyrate by Ralstonia sp. M91. Appl. Biochem. Microbiol. 2021, 57, 219–225. [Google Scholar] [CrossRef]
- Loan, T.T.; Trang, D.T.Q.; Huy, P.Q.; Ninh, P.X.; Thuoc, V.D. A fermentation process for the production of poly(3-hydroxybutyrate) using waste cooking oil or waste fish oil as inexpensive carbon substrate. Biotechnol. Rep. 2022, 33, e00700. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Shishatskaya, E.I.; Volova, T.G. Synthesis and properties of polyhydroxyalkanoates on waste fish oil from the production of canned sprats. Processes 2023, 11, 2113. [Google Scholar] [CrossRef]
- Zhila, N.O.; Kiselev, E.G.; Volkov, V.V.; Mezenova, O.Y.; Sapozhnikova, K.Y.; Shishatskaya, E.I.; Volova, T.G. Properties of Degradable Polyhydroxyalkanoates Synthesized from New Waste Fish Oils (WFOs). Int. J. Mol. Sci. 2023, 24, 14919. [Google Scholar] [CrossRef]
- Volova, T.; Zhila, N.; Sapozhnikova, K.; Menshikova, O.; Kiselev, E.; Sukovatyi, A.; Volkov, V.; Peterson, I.; Ipatova, N.; Shishatskaya, E. From Waste to Biopolymer: Synthesis of P(3HB-co-4HB) from Renewable Fish Oil. J. Ren. Mater. 2025, 13, 413. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I. Shtamm bakterii Cupriavidus eutrophus VKPM B-10646—Producent Polyhidroxyalkanoatov i Sposob ich Poluchenia (Bacterial Strain Cupriavidus eutrophus VKPM B-10646—Producer of Polyhydroxyalkanoates and Method for Their Production). RF Patent 2439143, 10 January 2012. (In Russian). [Google Scholar]
- Bazhenov, E.; Baydalinova, L.; Volkov, V.; Grimm, T. Experiments in obtaining low molecular weight peptides from various types of fish by-products. AIP Conf. Proc. 2022, 2636, 020012. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, A.I.; Arasimovich, V.V.; Smirnova-Ikonnikova, M.I.; Yarosh, N.P.; Lukovnikova, G.A. Metody Biokhimicheskogo Issledovaniya Rastenii (Methods of Biochemical Plant Research); Kolos: Leningrad, Russia, 1972. (In Russian) [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Canad. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.O.; Sapozhnikova, K.Y.; Berezovskaya, A.V.; Kiselev, E.G.; Shishatskaya, E.I.; Vasiliev, A.D.; Thomas, S.; Volova, T.G. Biosynthesis and Properties of Sulfur-Containing Polyhydroxyalkanoates (PHAs) Produced by Wild-Type Strain Cupriavidus necator B-10646. Polymers 2023, 15, 1005. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Volova, T.; Demidenko, A.; Kiselev, E.; Baranovskiy, S.; Shishatskaya, E.; Zhila, N. Polyhydroxyalkanoate synthesis based on glycerol and implementation of the process under conditions of pilot production. Appl. Microbiol. Biotechnol. 2019, 103, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of degradable polyhydroxyalkanoates with different monomer compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, N.O.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasilies, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef]
- Zinn, M.; Weilenmann, H.U.; Hany, R.; Schmid, M.; Egli, T.H. Tailored synthesis of poly([R]-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/HV) in Ralstonia eutropha DSM 428. Acta Biotechnol. 2003, 23, 309–316. [Google Scholar] [CrossRef]
- Grousseau, E.; Blanchet, E.; Déléris, S.; Albuquerque, M.G.; Paul, E.; Uribelarrea, J.L. Phosphorus limitation strategy to increase propionic acid flux towards 3-hydroxyvaleric acid monomers in Cupriavidus necator. Bioresour. Technol. 2014, 153, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Ferre-Guell, A.; Winterburn, J. Increased production of polyhydroxyalkanoates with controllable composition and consistent material properties by fed-batch fermentation. Biochem. Eng. J. 2019, 141, 35–42. [Google Scholar] [CrossRef]
- Kiselev, E.G.; Demidenko, A.V.; Zhila, N.O.; Volkov, V.V.; Volova, T.G. Waste Fish Oil is a Promising Substrate for Productive Synthesis of Degradable Polyhydroxyalkanoates. J. Polym. Environ. 2025, 33, 1022–1034. [Google Scholar] [CrossRef]
- Pramanik, N.; Das, R.; Rath, T.; Kundu, P.P. Microbial degradation of linseed oil-based elastomer and subsequent accumulation of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer. Appl. Biochem. Biotechnol. 2014, 174, 1613–1630. [Google Scholar] [CrossRef] [PubMed]
- Alfano, S.; Pagnanelli, F.; Martinelli, A. Rapid Estimation of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Composition Using ATR-FTIR. Polymers 2023, 15, 4127. [Google Scholar] [CrossRef]
- Gorodzha, S.N.; Muslimov, A.R.; Syromotina, D.S.; Timin, A.S.; Tcvetkov, N.Y.; Lepik, K.V.; Petrova, A.V.; Surmeneva, M.A.; Gorin, D.A.; Sukhorukov, G.B.; et al. A comparison study between electrospun polycaprolactone and piezoelectric poly(3-hydroxybutyrate-co-3-hydroxyvalerate) scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2017, 160, 48–59. [Google Scholar] [CrossRef]
- Izumi, C.M.; Temperini, M.L. FT-Raman investigation of biodegradable polymers: Poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Vib. Spectrosc. 2010, 54, 127–132. [Google Scholar] [CrossRef]
- Volova, T.G.; Zhila, N.O.; Shishatskaya, E.I.; Mironov, P.V.; Vasil’ev, A.D.; Sukovatyi, A.G.; Sinskey, A.J. The physicochemical properties of polyhydroxyalkanoates with different chemical structures. Polym. Sci. Ser. A 2013, 55, 427–437. [Google Scholar] [CrossRef]
- Volova, T.; Sapozhnikova, K.; Zhila, N. Cupriavidus necator B-10646 growth and polyhydroxyalkanoates production on different plant oils. Int. J. Biol. Macromol. 2020, 164, 121–130. [Google Scholar] [CrossRef]
- Eesaee, M.; Ghassemi, P.; Nguyen, D.D.; Thomas, S.; Elkoun, S.; Nguyen-Tri, P. Morphology and crystallization behaviour of polyhydroxyalkanoates-based blends and composites: A review. Biochem. Eng. J. 2022, 187, 108588. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Xu, P.; Yang, W.; Chen, M.; Dong, W.; Ma, P. Crystallization of microbial polyhydroxyalkanoates: A review. Int. J. Biol. Macromol. 2022, 209, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.H.; Kummerlöwe, C.; Kammer, H.W. Crystallization and melting behavior of poly(3-hydroxybutyrate)-based blends. Macromol. Chem. Phys. 2004, 205, 664–675. [Google Scholar] [CrossRef]
- Volova, T.G.; Zhila, N.O.; Kiselev, E.G.; Sukovatyi, A.G.; Lukyanenko, A.V.; Shishatskaya, E.I. Biodegradable polyhydroxyalkanoates with a different set of valerate monomers: Chemical structure and physicochemical properties. Int. J. Mol. Sci. 2023, 24, 14082. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, B. Macromolecular Physics: Crystal Melting, 1st ed.; Academic Press: Cambridge, MA, USA, 1980; 363p. [Google Scholar]
- Gazzano, M.; Focarete, M.L.; Riekel, C.; Ripamonti, A.; Scandola, M. Structural Investigation of Poly(3-hydroxybutyrate) Spherulites by Microfocus X-Ray Diffraction. Macromol. Chem. Phys. 2001, 202, 1405–1409. [Google Scholar] [CrossRef]
- Hobbs, J.K.; Binger, D.R.; Keller, A.; Barham, P.J. Spiralling optical morphologies in spherulites of poly(hydroxybutyrate). J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1575–1583. [Google Scholar] [CrossRef]
- Peng, S.; An, Y.; Chen, C.; Fei, B.; Zhuang, Y.; Dong, L. Isothermal crystallization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Eur. Polym. J. 2003, 39, 1475–1480. [Google Scholar] [CrossRef]
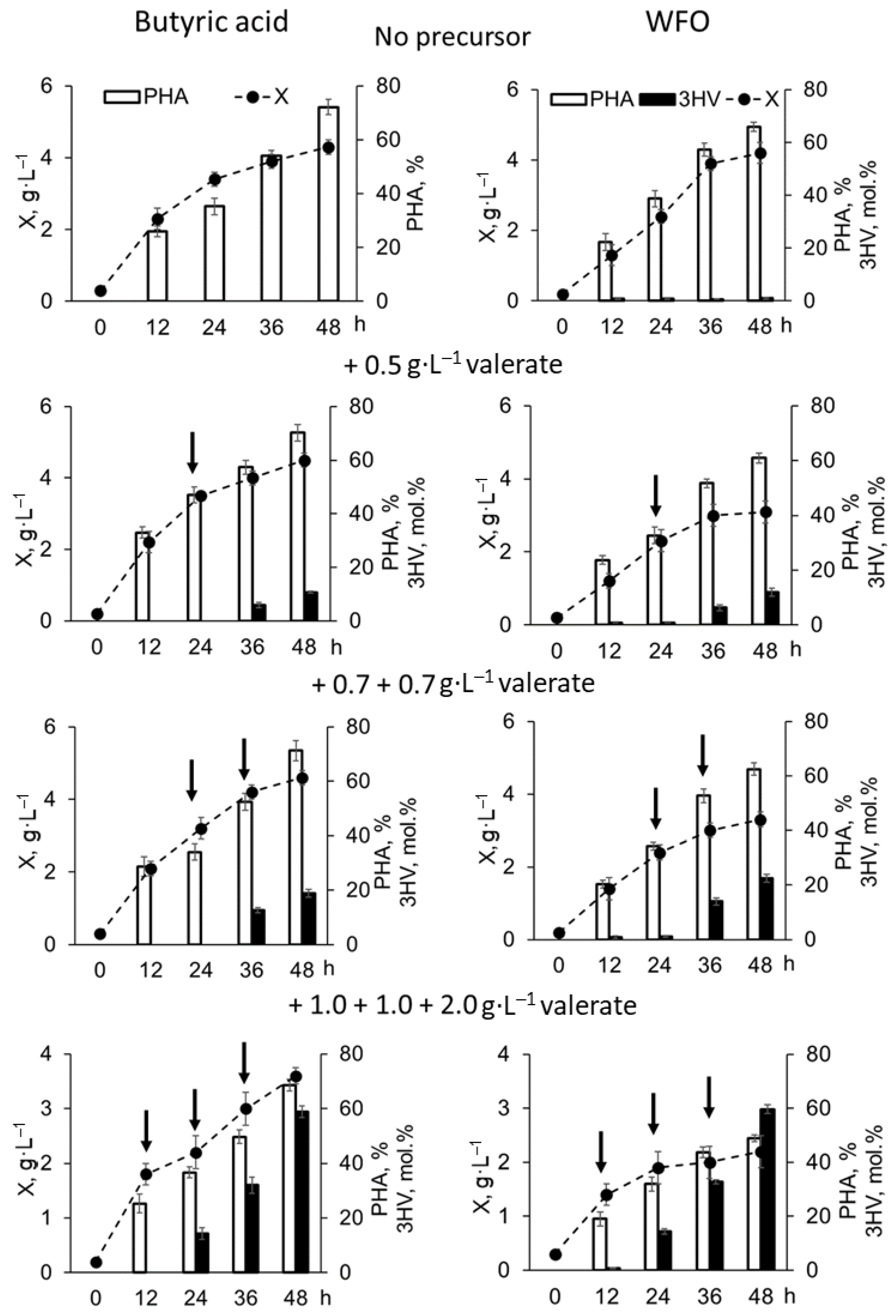
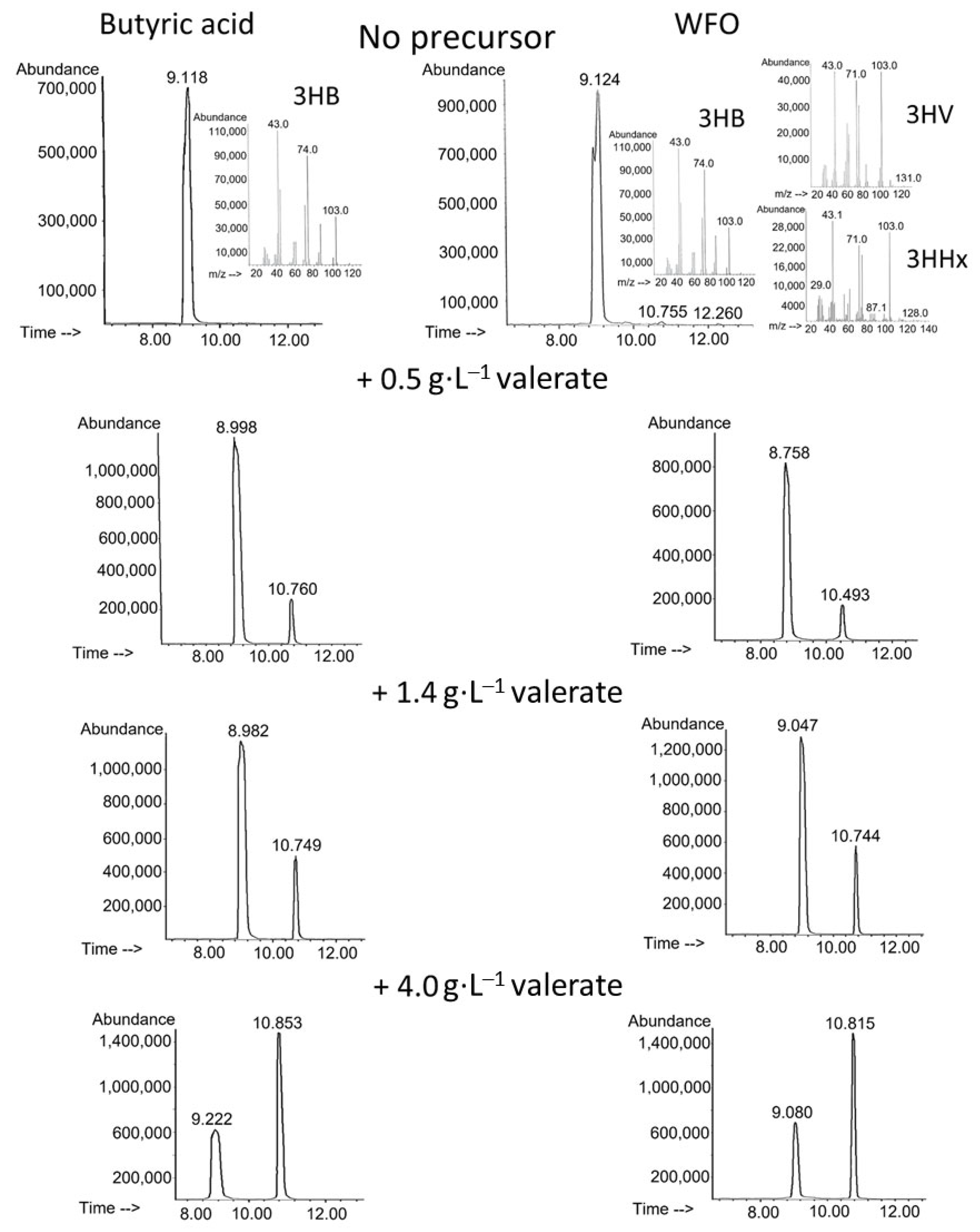
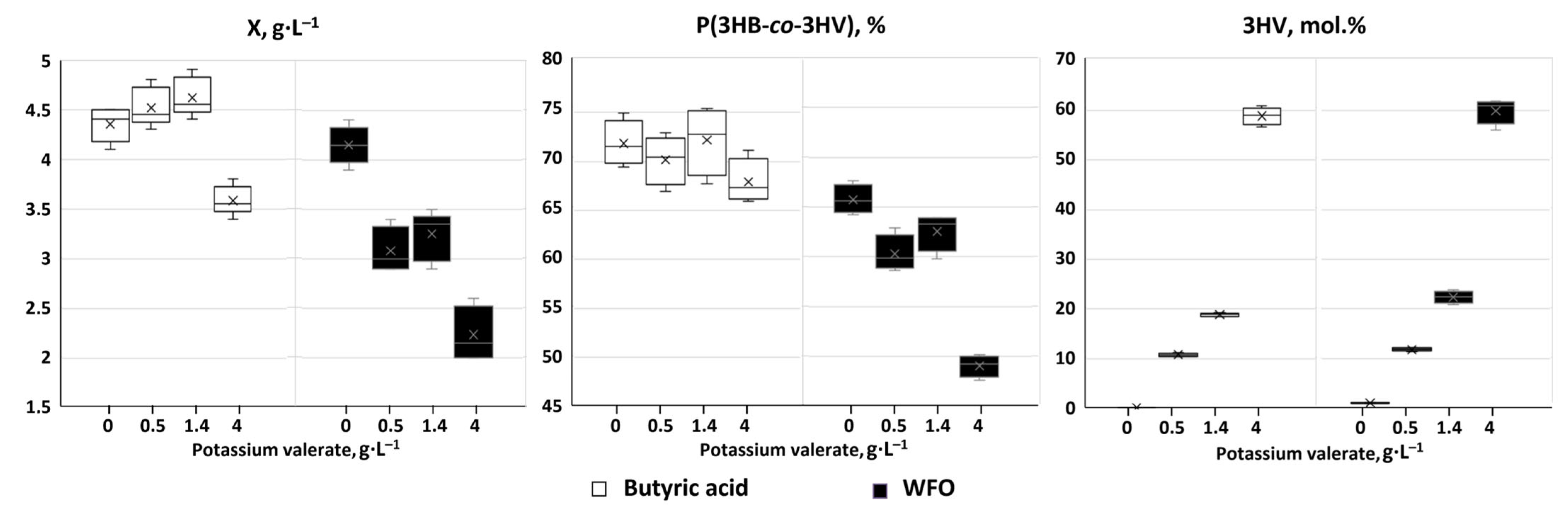

| Number of Potassium Valerate Additions, ⅀ Concentration (g·L−1) | X (g·L−1) | P(3HB-co-3HV) | 3HV (mol.%) | Productivity (g·L−1·h−1) | ||
|---|---|---|---|---|---|---|
| (g·L−1) | (%) | Biomass, PX | P(3HB-co-3HV), PP(3HB/3HV) | |||
| Butyric acid (control) | ||||||
| No precursor | 4.3 ± 0.2 | 3.1 ± 0.2 | 72 ± 3 | - | 0.090 ± 0.004 | 0.065 ± 0.004 |
| 1 addition (0.5 g·L−1), total 0.5 g·L−1 | 4.5 ± 0.2 | 3.2 ± 0.2 | 70 ± 3 | 10.7 ± 0.4 | 0.094 ± 0.004 | 0.066 ± 0.004 |
| 2 additions (0.7 + 0.7 g·L−1), total 1.4 g·L−1 | 4.6 ± 0.2 | 3.3 ± 0.3 | 71 ± 4 | 18.9 ± 0.4 | 0.096 ± 0.004 | 0.069 ± 0.006 |
| 3 additions (1.0 + 1.0 + 2.0 g·L−1), total 4.0 g·L−1 | 3.6 ± 0.1 | 2.4 ± 0.2 | 67 ± 3 | 58.8 ± 2.1 | 0.074 ± 0.002 | 0.050 ± 0.004 |
| Waste fish oil | ||||||
| No precursor | 4.2 ± 0.3 | 2.7 ± 0.2 | 66 ± 3 | 1.1 ± 0.1 | 0.087 ± 0.005 | 0.057 ± 0.005 |
| 1 addition (0.5 g·L−1), total 0.5 g·L−1 | 3.1 ± 0.3 | 1.9 ± 0.1 | 61 ± 2 | 11.9 ± 0.4 | 0.065 ± 0.006 | 0.039 ± 0.003 |
| 2 additions (0.7 + 0.7 g·L−1), total 1.4 g·L−1 | 3.3 ± 0.3 | 2.0 ± 0.2 | 63 ± 2 | 22.2 ± 1.5 | 0.068 ± 0.007 | 0.042 ± 0.004 |
| 3 additions (1.0 + 1.0 + 2.0 g·L−1), total 4.0 g·L−1 | 2.2 ± 0.3 | 1.1 ± 0.2 | 49 ± 2 | 59.7 ± 3.1 | 0.047 ± 0.005 | 0.023 ± 0.003 |
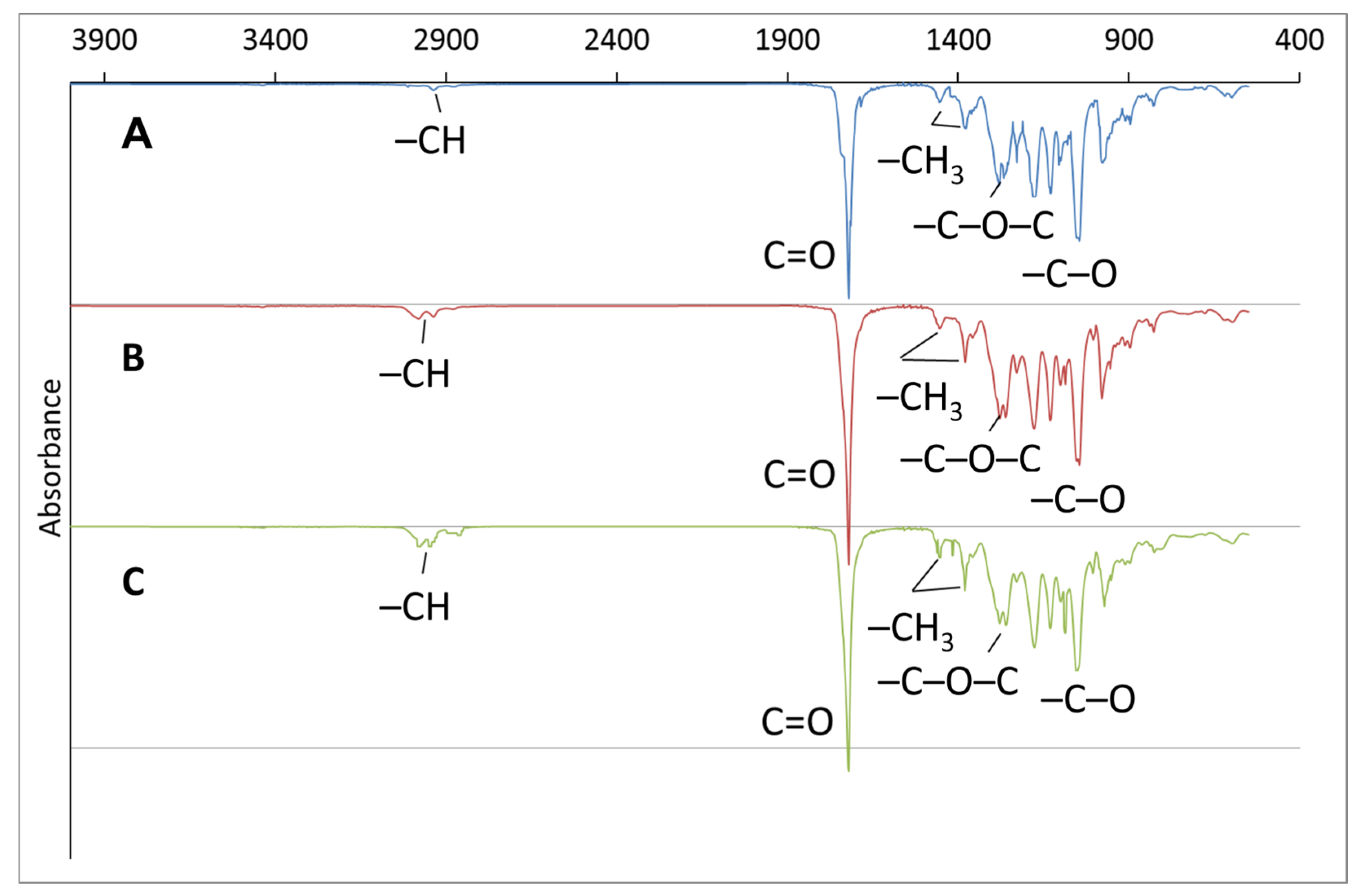
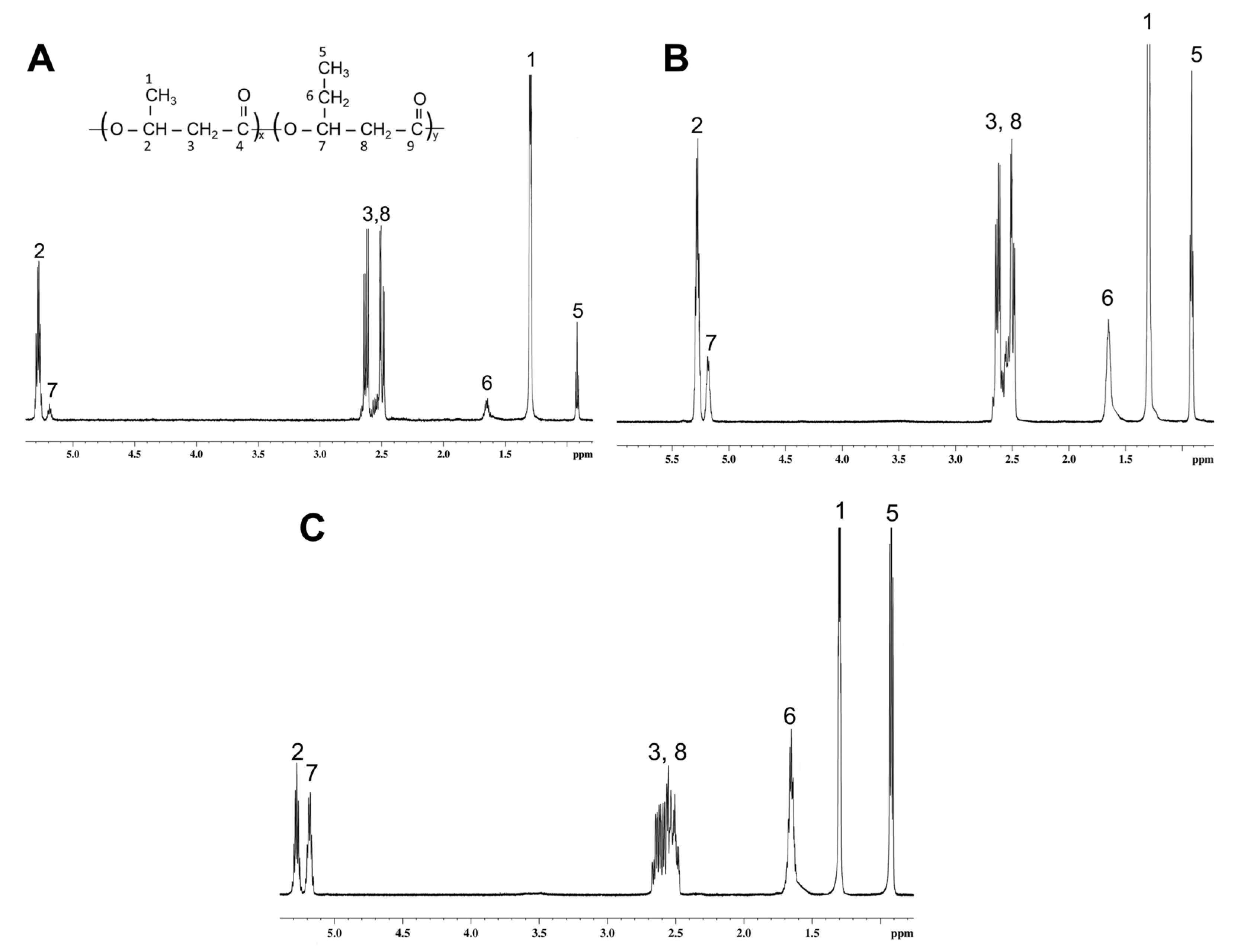
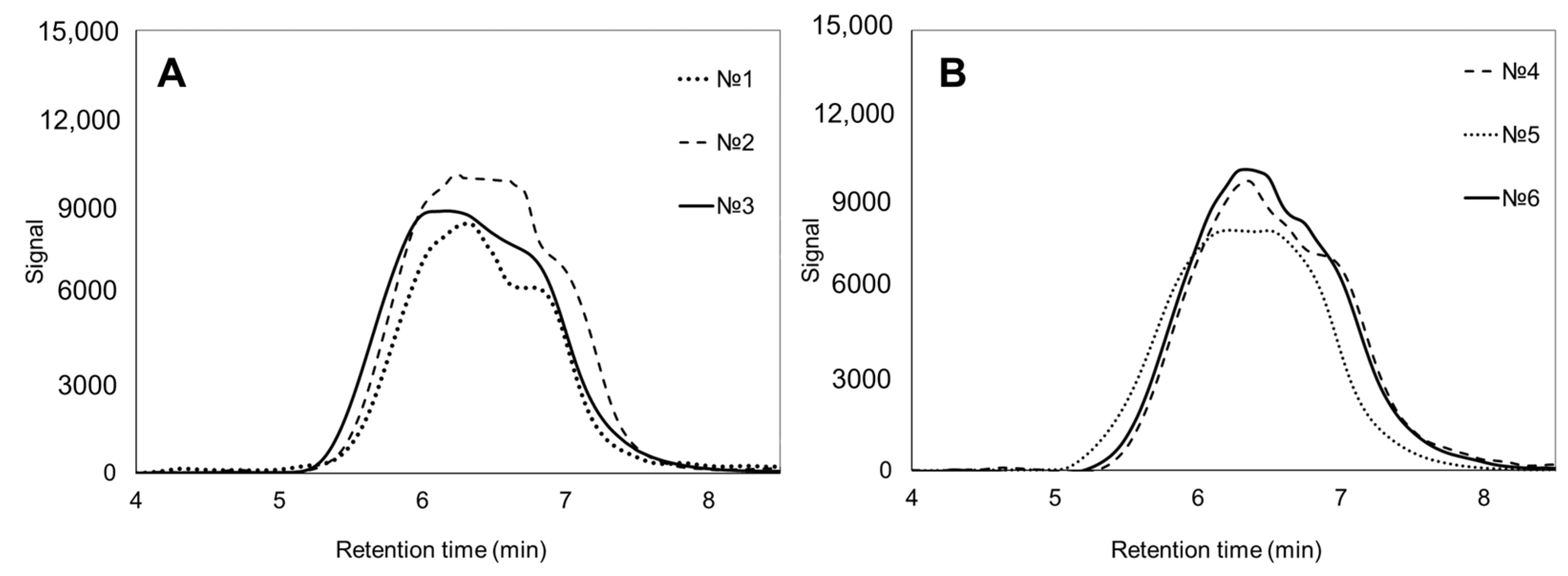
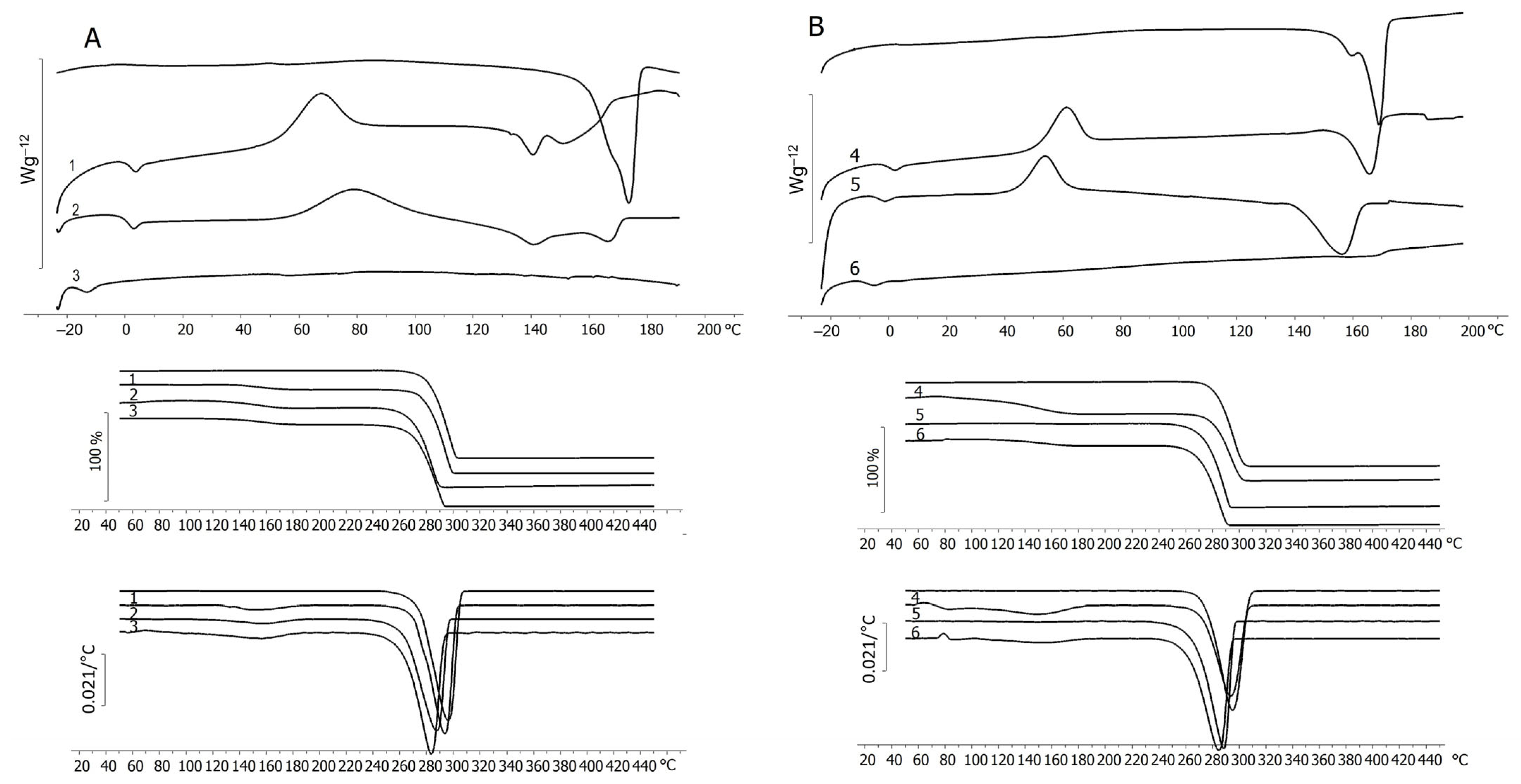
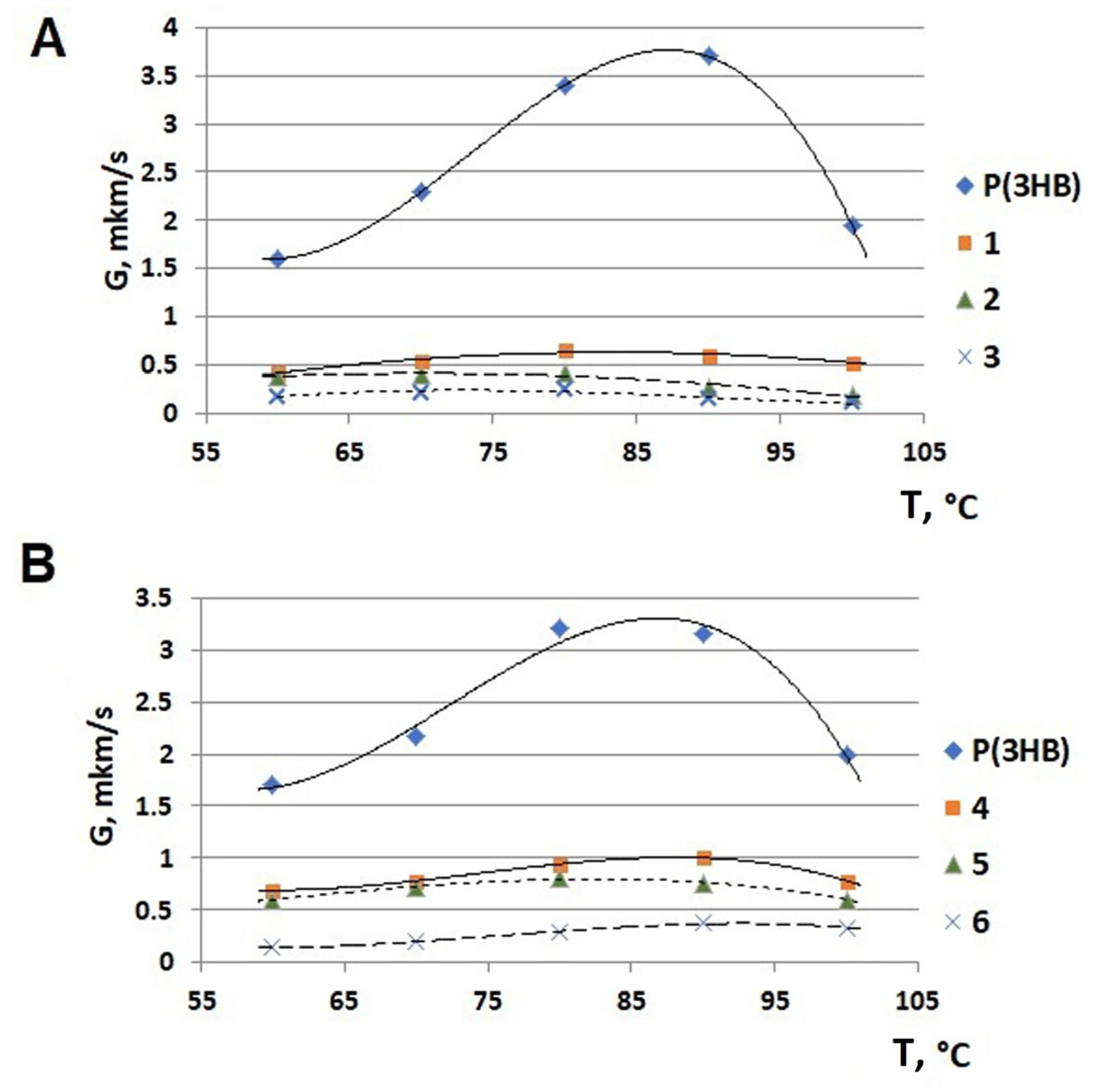
| No. | Potassium Valerate (g·L−1) | PHA Composition, mol.% | Mn, kDa | Mw, kDa | Đ | Cx, % | Tmelt, °C | Tcryst, °C | Tg, °C | Tdegr, °C | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3HB | 3HV | 3HHx | ||||||||||
| Without precursors | ||||||||||||
| Butyric acid | 100 | 0 | 0 | 190 ± 6 | 418 ± 8 | 2.2 | 62.0 ± 2.0 | 173.2 ± 0.2 | 70.6 ± 2.2 | – 1 | 281.9 ± 2.3 | |
| WFO | 98.6 ± 0.2 | 1.0 ± 0.1 | 0.4 ± 0.0 | 219 ± 8 | 528 ± 7 | 2.4 | 45.1 ± 1.0 | 168.7 ± 0.1 | 63.6 ± 1.1 | 4.0 ± 0.5 | 282.7 ± 2.2 | |
| Butyric acid | ||||||||||||
| 1 | 0.5 | 89.3 ± 0.5 | 10.7 ± 0.4 | 0 | 194 ± 16 | 458 ± 6 | 2.4 | 50.4 ± 2.2 | 140.7 ± 0.3 151.5 ± 0.2 | 67.2 ± 1.6 | 0.4 ± 0.1 | 142.4 ± 1.5 280.4 ± 3.1 |
| 2 | 1.4 | 81.1 ± 0.4 | 18.9 ± 0.4 | 0 | 165 ± 9 | 430 ± 5 | 2.6 | 30.2 ± 2.7 | 141.1 ± 0.2 166.4 ± 0.3 | 78.5 ± 2.1 | −1.1 ± 0.2 | 119.6 ± 1.3 268.3 ± 2.5 |
| 3 | 4.0 | 41.2 ± 1.7 | 58.8 ± 2.1 | 0 | 193 ± 10 | 520 ± 9 | 2.7 | – | – | – | −16.6 ± 0.4 | 128.1 ± 1.3 271.8 ± 2.1 |
| WFO | ||||||||||||
| 4 | 0.5 | 88.1 ± 0.7 | 11.9 ± 0.4 | 0 | 130 ± 5 | 390 ± 1 | 3.0 | 32.2 ± 1.2 | 165.8 ± 0.2 | 61.2 ± 2.9 | −1.4 ± 60.1 | 280.7 ± 2.1 |
| 5 | 1.4 | 77.8 ± 1.7 | 22.2 ± 1.5 | 0 | 206 ± 2 | 573 ± 9 | 2.6 | 36.5 ± 3.5 | 156.0 ± 0.3 | 53.6 ± 2.3 | −3.9 ± 60.2 | 275.2 ± 2.8 |
| 6 | 4.0 | 40.3 ± 2.2 | 59.7 ± 3.1 | 0 | 142 ± 10 | 417 ± 8 | 2.9 | 3.5 ± 0.9 | 163.9 ± 0.1 | – | −9.0 ± 0.1 | 270.4 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volova, T.G.; Kiselev, E.G.; Sukovatyi, A.G.; Zhila, N.O.; Sapozhnikova, K.Y.; Ipatova, N.D.; Shishatskii, P.O. Synthesis and Properties of Degradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Derived from Waste Fish Oil. Polymers 2025, 17, 2171. https://doi.org/10.3390/polym17162171
Volova TG, Kiselev EG, Sukovatyi AG, Zhila NO, Sapozhnikova KY, Ipatova ND, Shishatskii PO. Synthesis and Properties of Degradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Derived from Waste Fish Oil. Polymers. 2025; 17(16):2171. https://doi.org/10.3390/polym17162171
Chicago/Turabian StyleVolova, Tatiana G., Evgeniy G. Kiselev, Alexey G. Sukovatyi, Natalia O. Zhila, Kristina Yu. Sapozhnikova, Natalia D. Ipatova, and Peter O. Shishatskii. 2025. "Synthesis and Properties of Degradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Derived from Waste Fish Oil" Polymers 17, no. 16: 2171. https://doi.org/10.3390/polym17162171
APA StyleVolova, T. G., Kiselev, E. G., Sukovatyi, A. G., Zhila, N. O., Sapozhnikova, K. Y., Ipatova, N. D., & Shishatskii, P. O. (2025). Synthesis and Properties of Degradable Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Derived from Waste Fish Oil. Polymers, 17(16), 2171. https://doi.org/10.3390/polym17162171









