Controllable Structure and Fluorescence Enhancement of ACQ Dye Nanoparticles Based on the FNP Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of CyINPs by FNP
2.4. Synthesis of CyINPs by Thermodynamic-Driven Method
2.5. Encapsulation Efficiency of CyINPs
2.6. Cell Culture
2.7. Cytotoxicity of CyINPs
2.7.1. Dark Cytotoxicity of CyINPs
2.7.2. Cytotoxicity of Photothermal Effect Caused by CyINPs
2.8. Statistical Analysis
3. Results and Discussion
3.1. Selection of Counterion Molecules
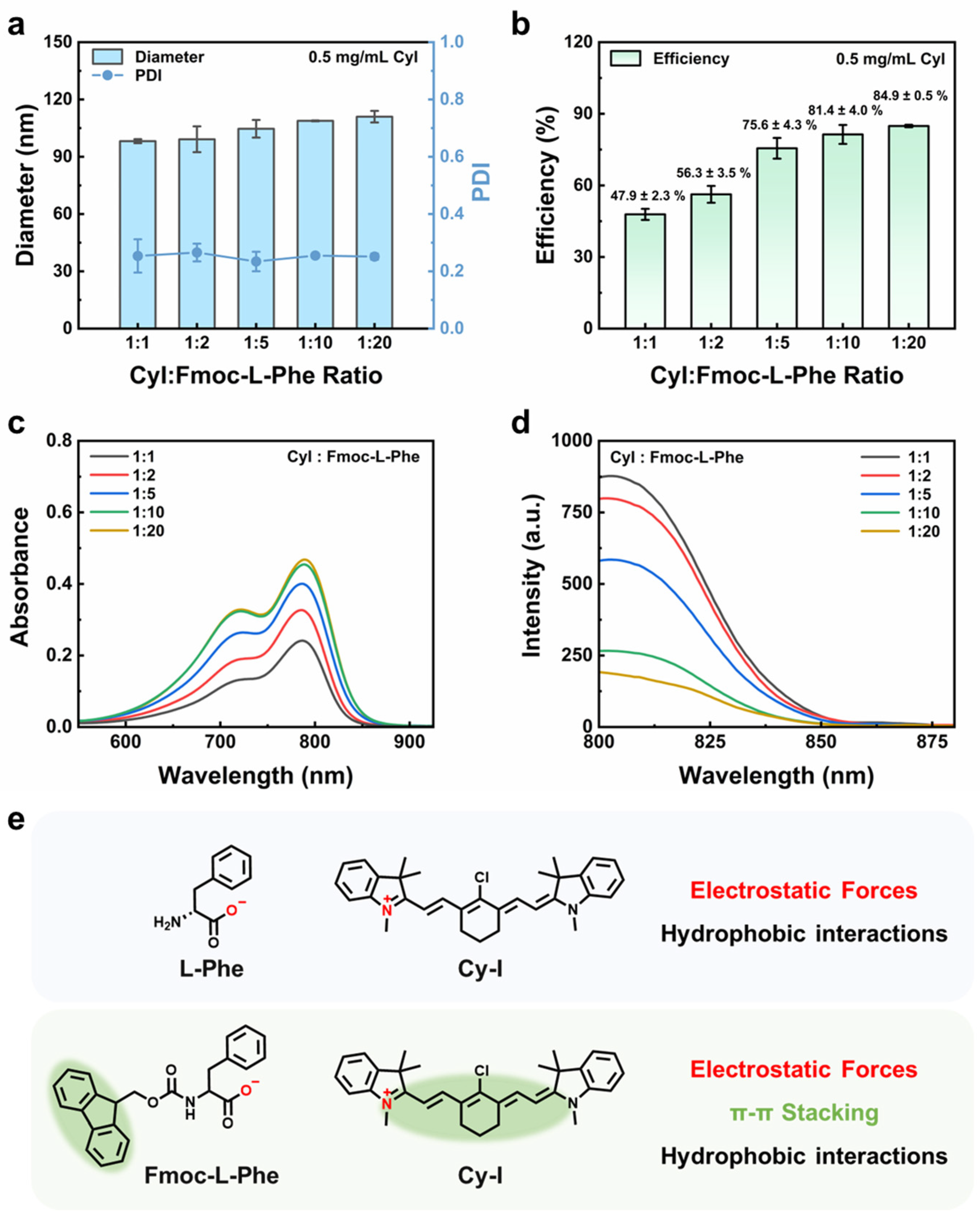
3.2. Effect of Fmoc-L-Phe Anionic Ligand Ratio on Fluorescence Enhancement
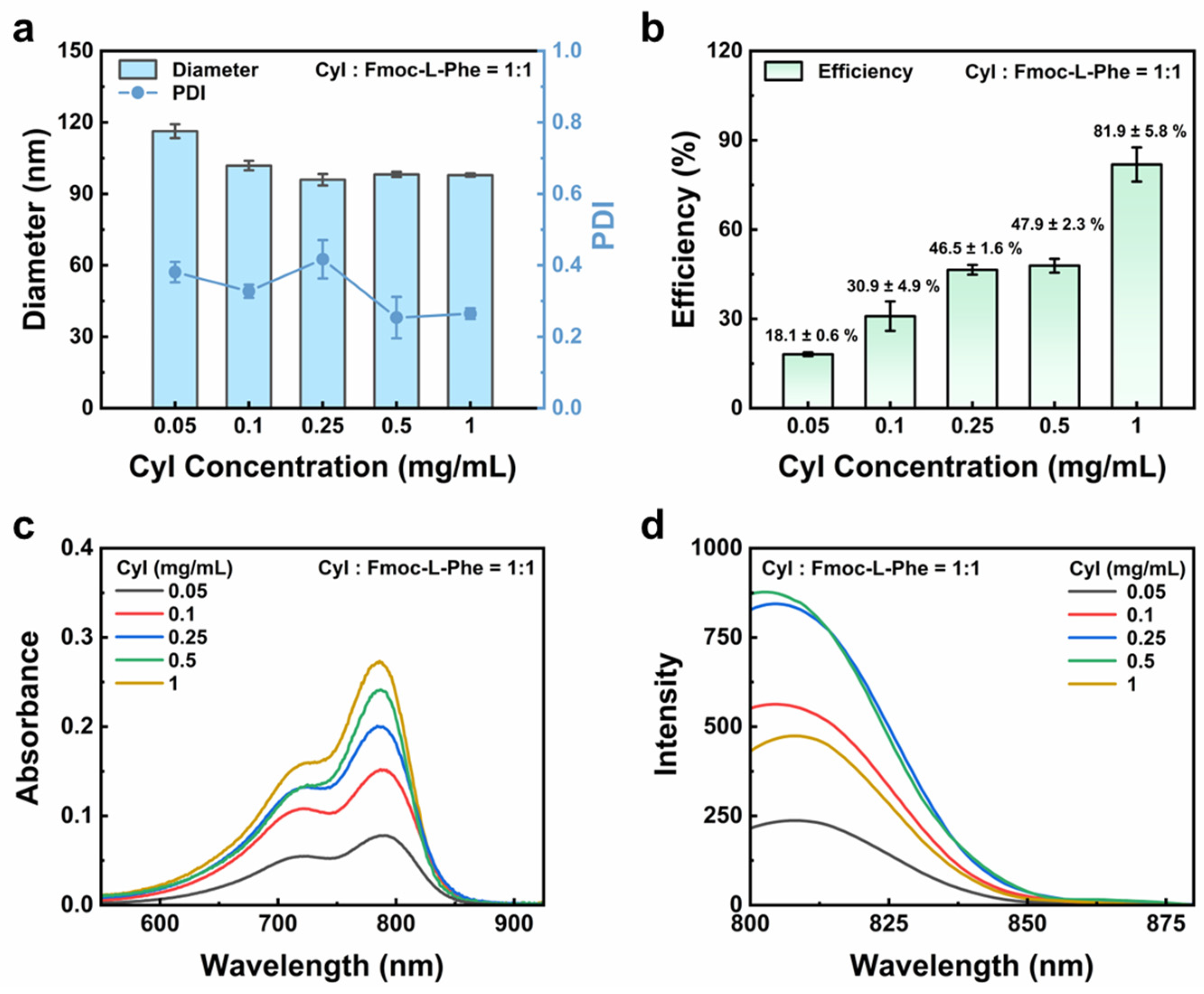
3.3. Screening of CyI Concentrations
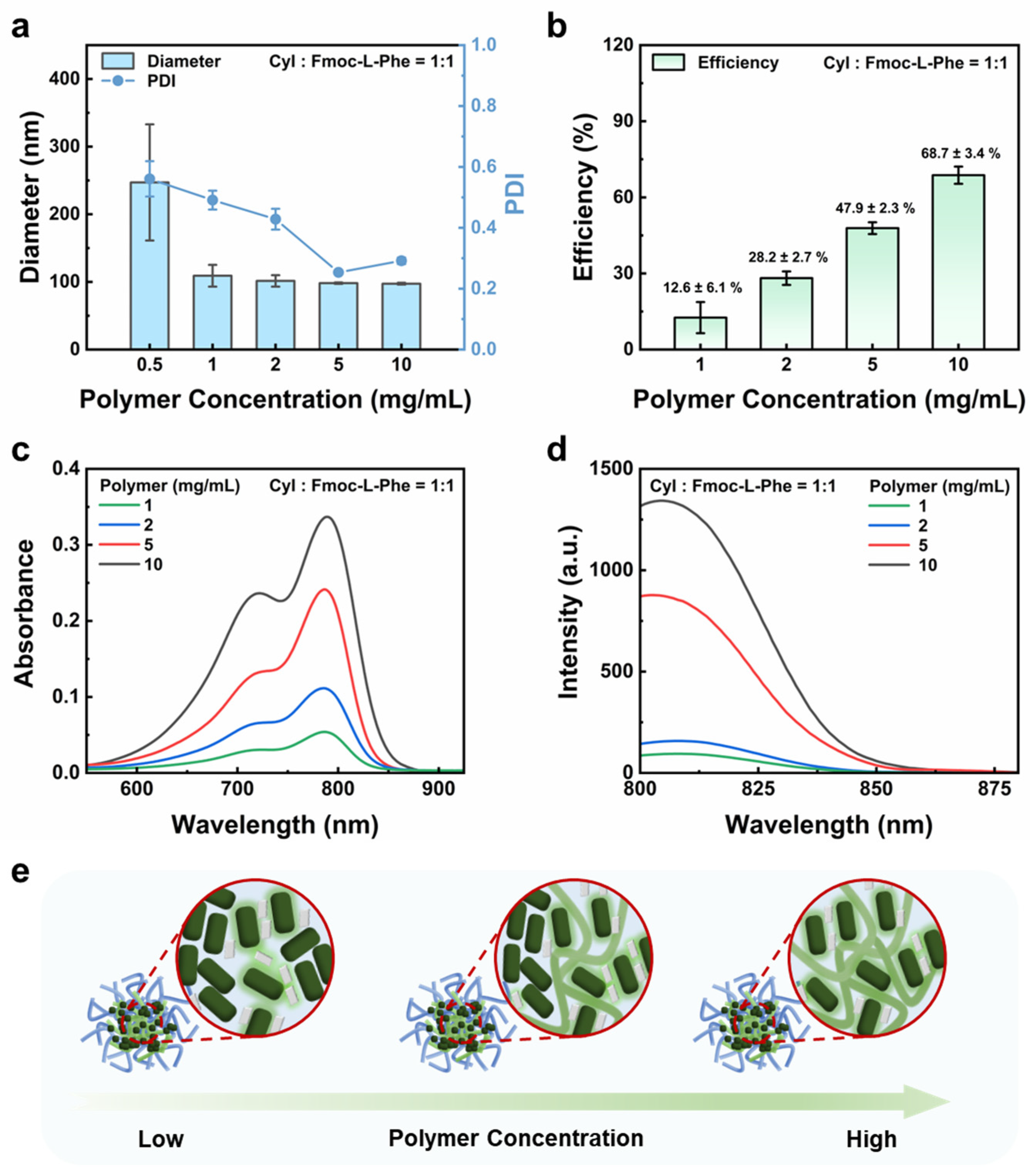
3.4. Impact of Polymer Concentration on CyI Dispersion
3.5. Particle Performance of Optimized CyINPs
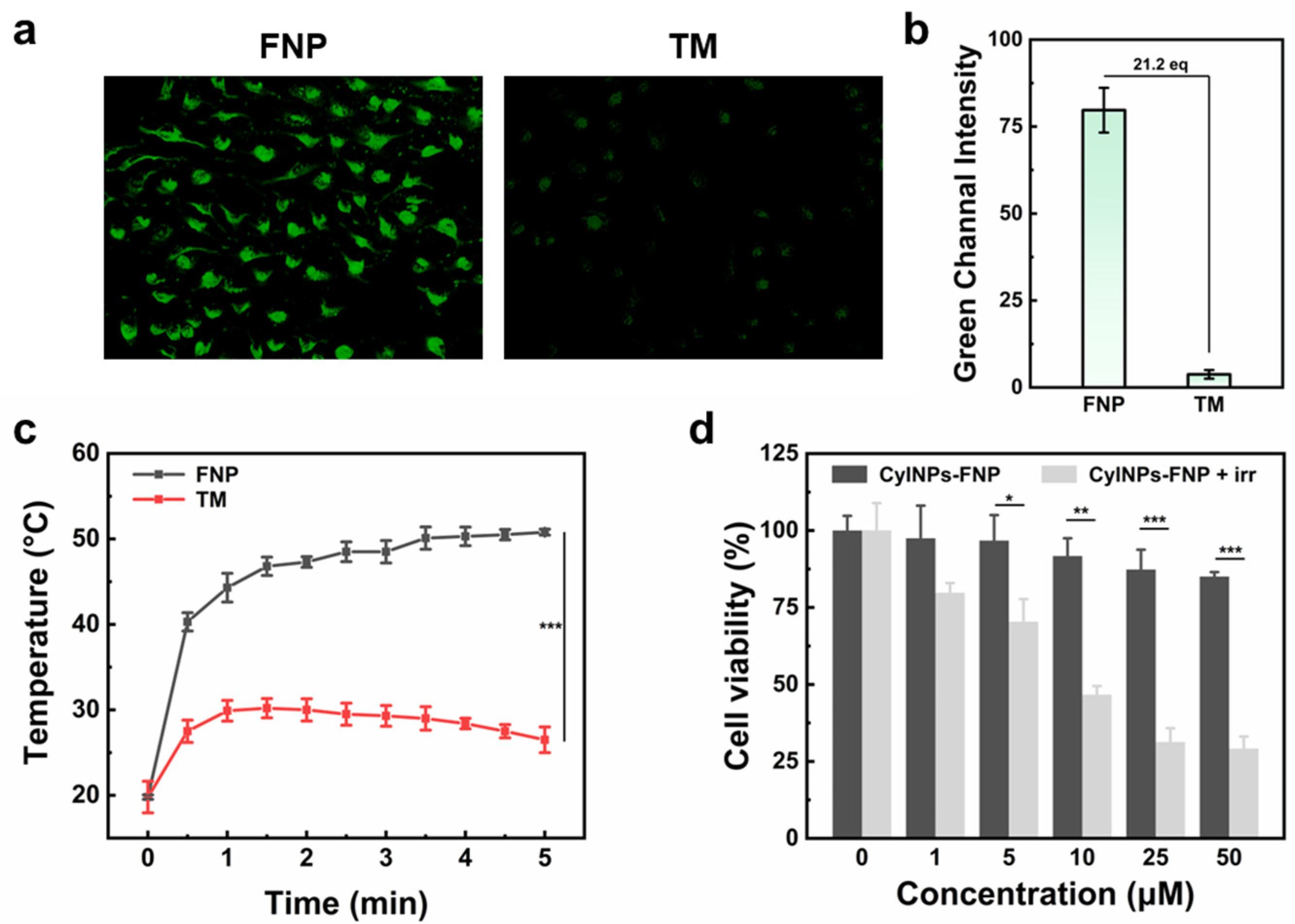
3.6. Cell Cytotoxicity and Photothermal Effect of CyINPs-FNP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uthaman, S.; Huh, K.M.; Park, I.K. Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomater. Res. 2018, 22, 22. [Google Scholar] [CrossRef]
- Hristova-Panusheva, K.; Xenodochidis, C.; Georgieva, M.; Krasteva, N. Nanoparticle-mediated drug delivery systems for precision targeting in oncology. Pharmaceuticals 2024, 17, 677. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, G.; You, X.; Wang, X. Cuproptosis nanomedicine: Clinical challenges and opportunities for anti-tumor therapy. Chem. Eng. J. 2024, 495, 153373. [Google Scholar] [CrossRef]
- Luo, W.; Zhan, T. The new era of pancreatic cancer treatment: Application of nanotechnology breaking through bottlenecks. Cancer Lett. 2024, 594, 216979. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliver. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Luo, Q.; Li, Z.; Li, X.; Gan, H.; Gu, Z.; Gong, Q.; Luo, K. Stimuli-activatable nanomedicine meets cancer theranostics. Theranostics 2023, 13, 5386–5417. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Chen, X.; Dobrovolskaia, M.A.; Lammers, T. Cancer nanomedicine. Nat. Rev. Cancer 2022, 22, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Gawne, P.J.; Ferreira, M.; Papaluca, M.; Grimm, J.; Decuzzi, P. New opportunities and old challenges in the clinical translation of nanotheranostics. Nat. Rev. Mater. 2023, 8, 783–798. [Google Scholar] [CrossRef]
- Miranda, D.; Wan, C.; Kilian, H.I.; Mabrouk, M.T.; Zhou, Y.; Jin, H.; Lovell, J.F. Indocyanine green binds to DOTAP liposomes for enhanced optical properties and tumor photoablation. Biomater. Sci. 2019, 7, 3158–3164. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, P.; Tofighi, S.; Sharma, R.; Ensley, T.R.; Jang, S.-H.; Hagan, D.J.; Van Stryland, E.W.; Jen, A.K.-Y. Zwitterionic cyanine–cyanine salt: Structure and optical properties. J. Phys. Chem. C 2016, 120, 15378–15384. [Google Scholar] [CrossRef]
- She, Z.-P.; Wang, W.-X.; Mao, G.-J.; Jiang, W.-L.; Wang, Z.-Q.; Li, Y.; Li, C.-Y. A near-infrared fluorescent probe for accurately diagnosing cancer by sequential detection of cysteine and H+. Chem. Commun. 2021, 57, 4811–4814. [Google Scholar] [CrossRef]
- Yoon, S.A.; Kim, W.; Sharma, A.; Verwilst, P.; Won, M.; Lee, M.H. A fluorescent Cy7-mercaptopyridine for the selective detection of glutathione over homocysteine and cysteine. Sensors 2018, 18, 2897. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Yue, X.; Dai, Z. Cyanine conjugates in cancer theranostics. Bioact. Mater. 2021, 6, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yu, X.; Jiang, M.; Zhu, L.; Zhang, Y.; Yang, W.; Xi, W.; Li, G.; Qian, J. Excretable IR-820 for in vivo NIR-II fluorescence cerebrovascular imaging and photothermal therapy of subcutaneous tumor. Theranostics 2019, 9, 5706–5719. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Z.; Wu, Z.; He, Y.; Shan, C.; Chai, P.; Ma, C.; Tian, M.; Teng, J.; Jin, D.; et al. Mitochondrial dynamics quantitatively revealed by STED nanoscopy with an enhanced squaraine variant probe. Nat. Commun. 2020, 11, 3699. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, J.C.; Dempsey, G.T.; Sun, E.; Zhuang, X. Phosphine quenching of cyanine dyes as a versatile tool for fluorescence microscopy. J. Am. Chem. Soc. 2013, 135, 1197–1200. [Google Scholar] [CrossRef]
- Chakraborty, S.; Liao, I.C.; Adler, A.; Leong, K.W. Electrohydrodynamics: A facile technique to fabricate drug delivery systems. Adv. Drug Deliv. Rev. 2009, 61, 1043–1054. [Google Scholar] [CrossRef]
- Yoon, H.-J.; Lee, H.-S.; Lim, J.-Y.; Park, J.-H. Liposomal indocyanine green for enhanced photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 5683–5691. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, W. Stability enhancement of fluorophores for lighting up practical application in bioimaging. Chem. Soc. Rev. 2015, 44, 4179–4184. [Google Scholar] [CrossRef]
- Ou, Y.-F.; Xiang, H.-Y.; Yang, X.; Wang, R.-X.; Huan, S.-Y.; Yuan, L.; Ren, T.-B.; Zhang, X.-B. Constructing stable and wavelength-extended heptamethine cyanines via donor ectopic substitution for NIR-IIa/b bioimaging. Angew. Chem. Int. Ed. 2025, 64, e202423978. [Google Scholar] [CrossRef]
- Lee, S.; Jung, J.S.; Jo, G.; Yang, D.H.; Koh, Y.S.; Hyun, H. Near-infrared fluorescent sorbitol probe for targeted photothermal cancer therapy. Cancers 2019, 11, 1286. [Google Scholar] [CrossRef]
- Cheng, D.; Peng, J.; Lv, Y.; Su, D.; Liu, D.; Chen, M.; Yuan, L.; Zhang, X. De novo design of chemical stability near-infrared molecular probes for high-fidelity hepatotoxicity evaluation in vivo. J. Am. Chem. Soc. 2019, 141, 6352–6361. [Google Scholar] [CrossRef]
- James, N.S.; Chen, Y.; Joshi, P.; Ohulchanskyy, T.Y.; Ethirajan, M.; Henary, M.; Strekowsk, L.; Pandey, R.K. Evaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors:part-1. Theranostics 2013, 3, 692–702. [Google Scholar] [CrossRef]
- Liu, Y.; Kathan, K.; Saad, W.; Prud’homme, R.K. Ostwald ripening of β-carotene nanoparticles. Phys. Rev. Lett. 2007, 98, 036102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Prud’homme, R.K. Thermodynamic limits on drug loading in nanoparticle cores. J. Pharm. Sci. 2008, 97, 4904–4914. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Guo, Z.; Liu, Y.; Shi, P.; Tian, H.; Zhu, W.-H. A sequence-activated AND logic dual-channel fluorescent probe for tracking programmable drug release. Chem. Sci. 2018, 9, 6176–6182. [Google Scholar] [CrossRef]
- ISO 13321:1996 E; Particle Size Analysis—Photon Correlation Spectroscopy. International Organization for Standardization (ISO): Geneva, Switzerland, 1996.
- ISO 22412:2008; Particle Size Analysis—Dynamic Light Scattering (DLS). International Organization for Standardization (ISO): Geneva, Switzerland, 2008.
- Liu, Y.; Cheng, C.; Liu, Y.; Prud’homme, R.K.; Fox, R.O. Mixing in a multi-inlet vortex mixer (MIVM) for flash nano-precipitation. Chem. Eng. Sci. 2008, 63, 2829–2842. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Liu, Y.; Gu, K.; Tan, J.; Shi, P.; Yang, D.; Guo, Z.; Zhu, W.; Guo, X.; et al. Morphology tuning of aggregation-induced emission probes by flash nanoprecipitation: Shape and size effects on in vivo imaging. ACS Appl. Mater. Interfaces 2018, 10, 25186–25193. [Google Scholar] [CrossRef]
- Wu, Y.; Yuan, C.; Jia, X.; Zheng, Z.; Yang, X.; Yang, J.; Kayitmazer, B.A.; Ahmad, A.; Ramzan, N.; Xu, Y. Efficient ROS activation by highly stabilized aqueous ICG encapsulated upconversion nanoparticles for tumor cell imaging and therapeutics. Chem. Eng. J. 2023, 452, 139343. [Google Scholar] [CrossRef]
- Johnson, B.K.; Prud’homme, R.K. Chemical processing and micromixing in confined impinging jets. AIChE J. 2003, 49, 2264–2282. [Google Scholar] [CrossRef]
- Saad, W.S.; Prud’homme, R.K. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today 2016, 11, 212–227. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, X. Simulations on time scales and conversion of fast competing reactions in rapid mixing. Chem. Eng. J. 2018, 336, 741–747. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, Y.; Guo, Z.; Xu, Y. Controllable Structure and Fluorescence Enhancement of ACQ Dye Nanoparticles Based on the FNP Process. Polymers 2025, 17, 2152. https://doi.org/10.3390/polym17152152
Wu Y, Zhang Y, Guo Z, Xu Y. Controllable Structure and Fluorescence Enhancement of ACQ Dye Nanoparticles Based on the FNP Process. Polymers. 2025; 17(15):2152. https://doi.org/10.3390/polym17152152
Chicago/Turabian StyleWu, Yue, Yutao Zhang, Zhiqian Guo, and Yisheng Xu. 2025. "Controllable Structure and Fluorescence Enhancement of ACQ Dye Nanoparticles Based on the FNP Process" Polymers 17, no. 15: 2152. https://doi.org/10.3390/polym17152152
APA StyleWu, Y., Zhang, Y., Guo, Z., & Xu, Y. (2025). Controllable Structure and Fluorescence Enhancement of ACQ Dye Nanoparticles Based on the FNP Process. Polymers, 17(15), 2152. https://doi.org/10.3390/polym17152152





