Multifunctional, Biocompatible Hybrid Surface Coatings Combining Antibacterial, Hydrophobic and Fluorescent Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisation
2.3. Synthesis Methods
2.3.1. Synthesis of Silicon Quantum Dots (Si QDs)
2.3.2. Prehydrolysis of the Fluorosilane Surface Agent
2.3.3. Synthesis of Ag Nanoparticles by the Turkevich Method
2.3.4. Synthesis of the Blank Hybrid Coating by the Sol-Gel Technique Using the Spray Method
2.3.5. Synthesis of the Multifunctional Hybrid Surface Coating via the Step-by-Step Method
2.3.6. Physical and Mechanical Properties of Multifunctional Hybrid Surface Coatings
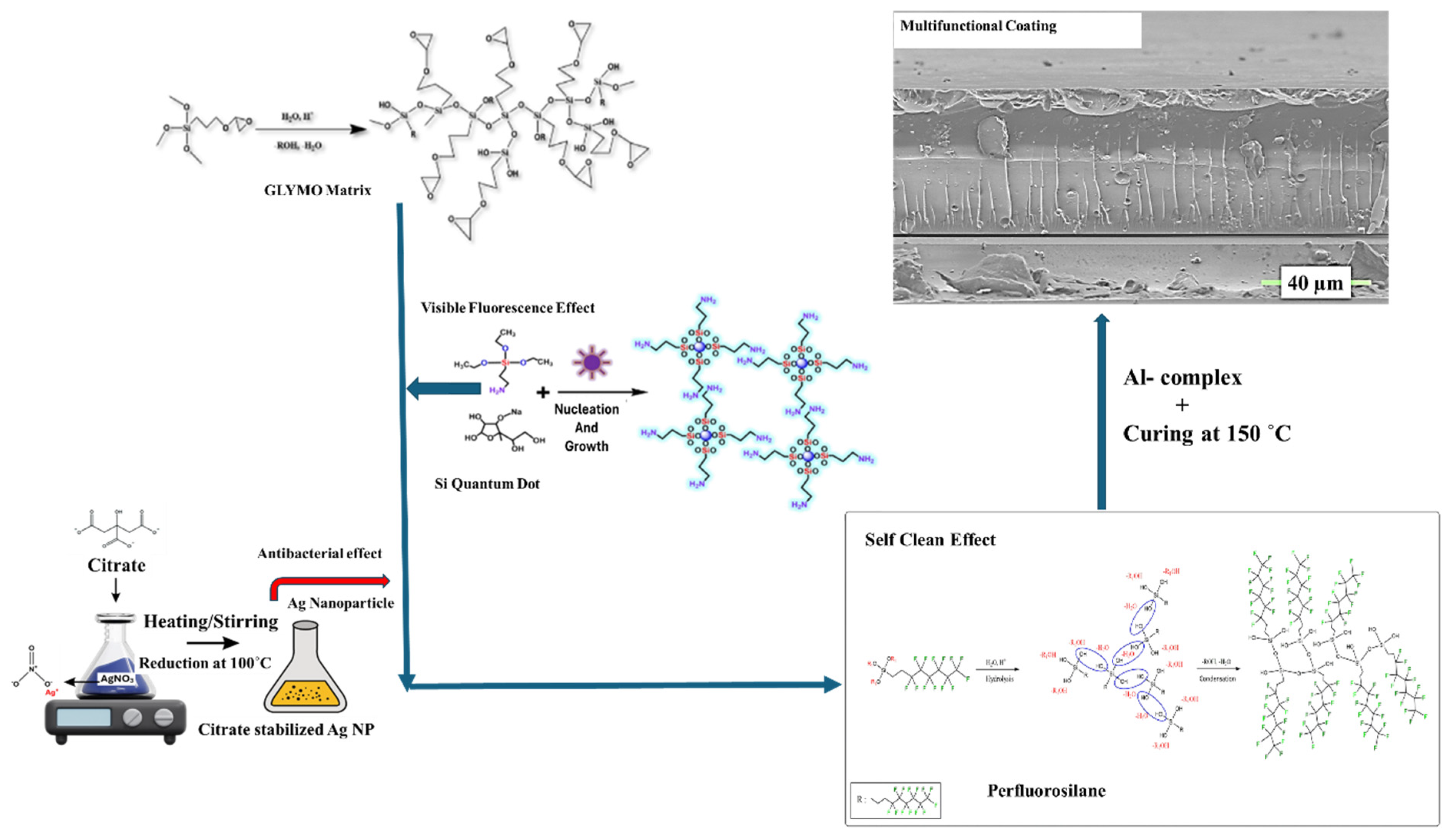
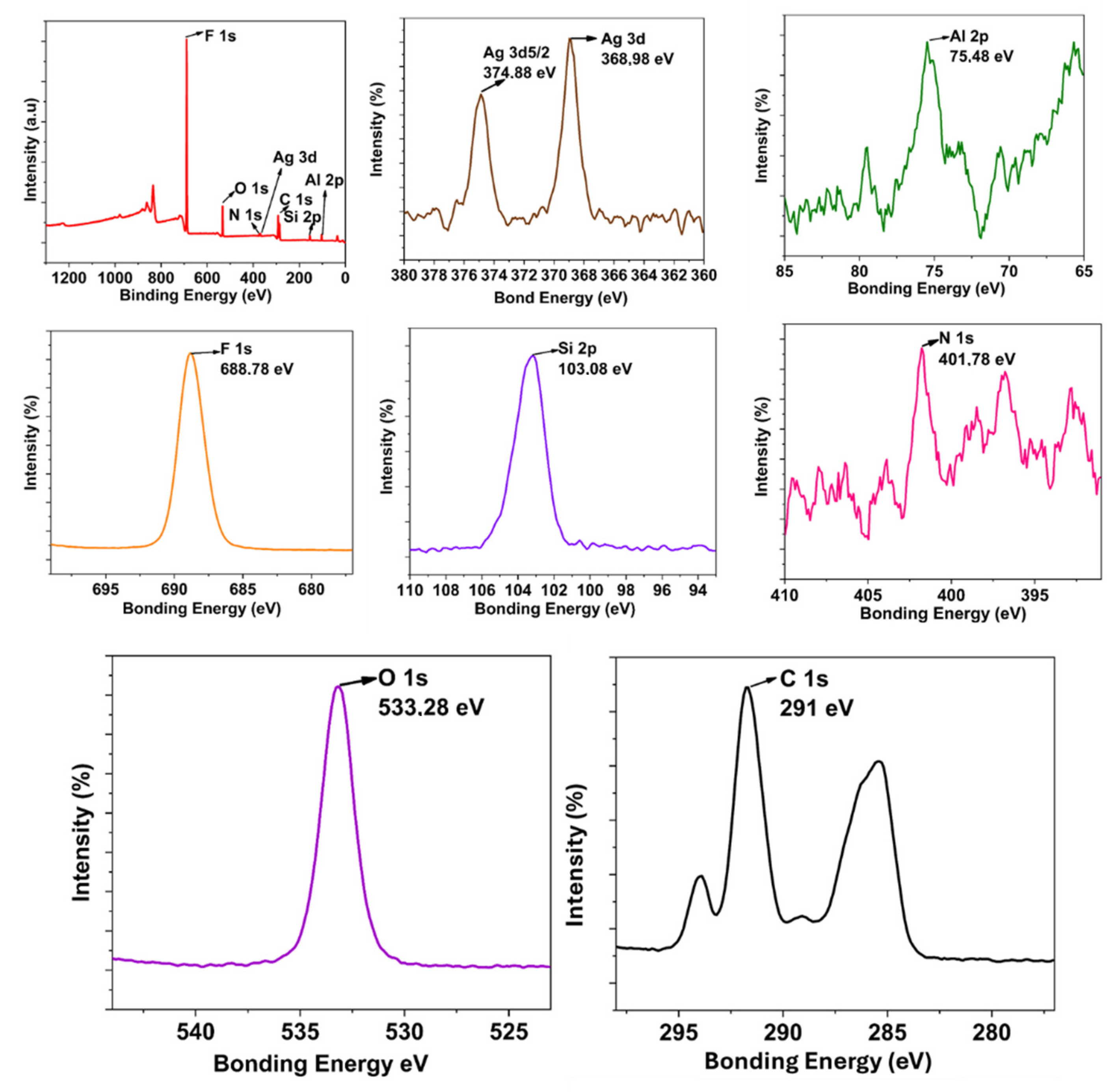
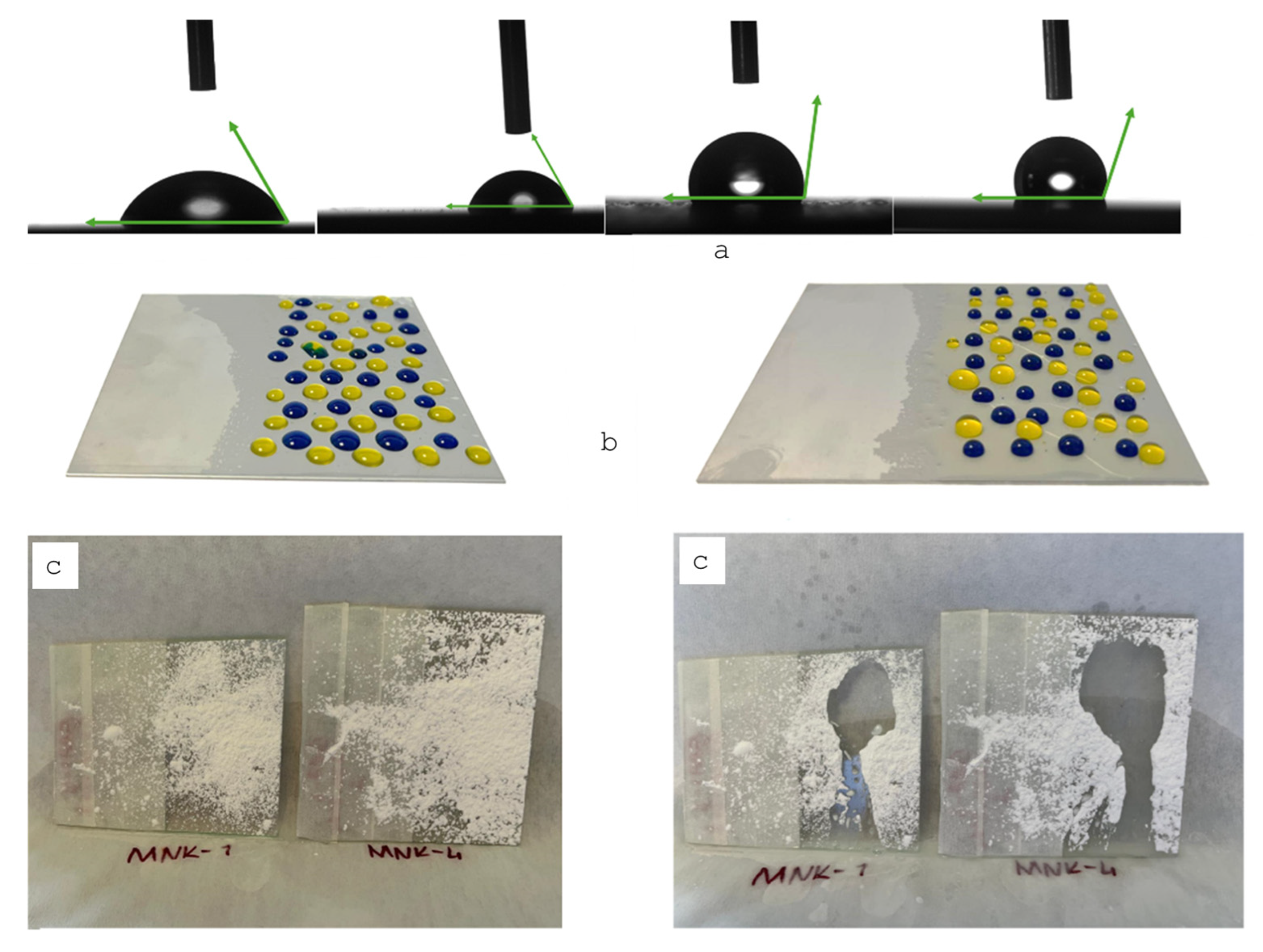
3. Results and Discussion
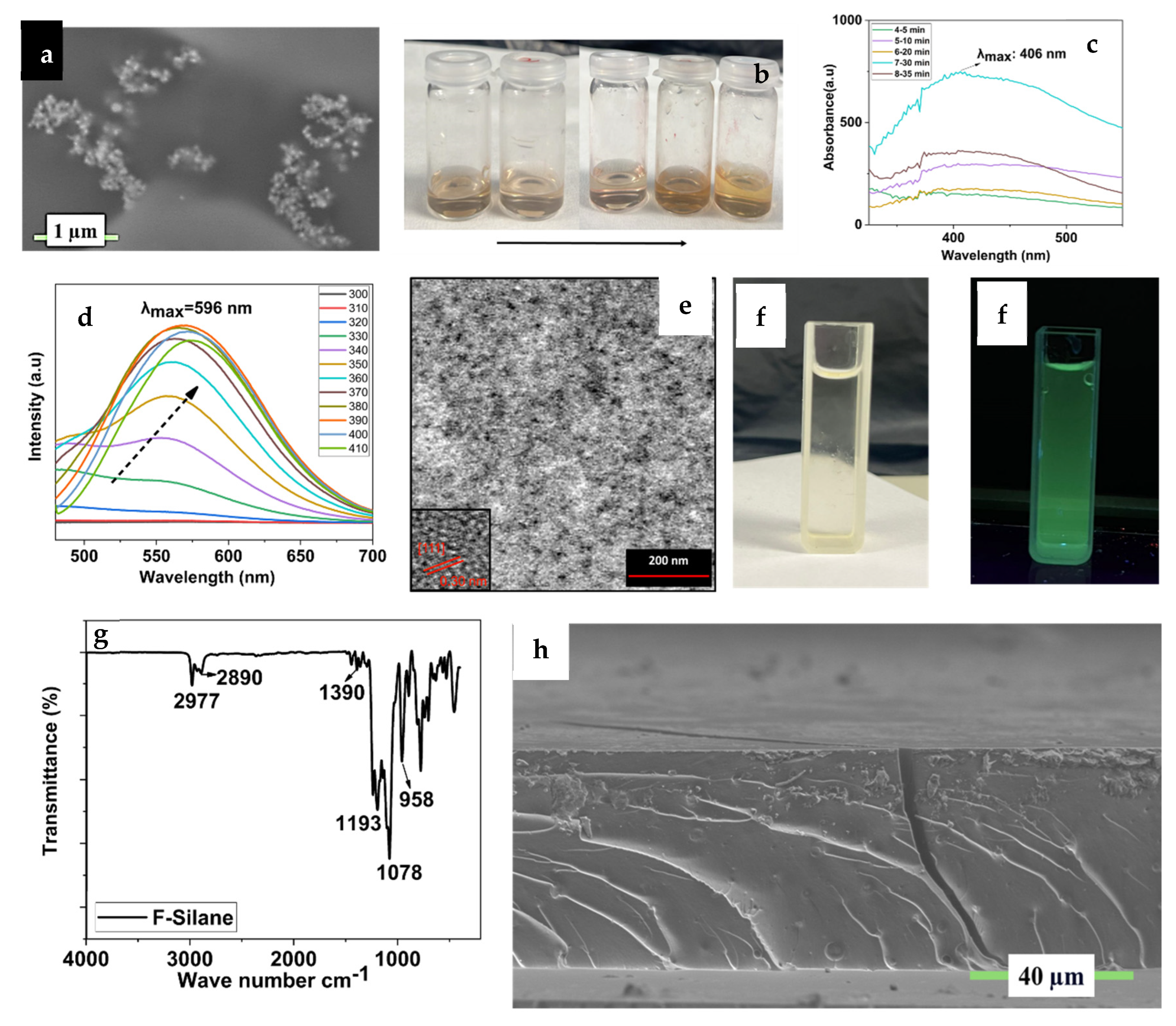
3.1. Synthesis Ag NP, Si QD, and Perfluorosilane Precursors and Blank Hybrid Coating
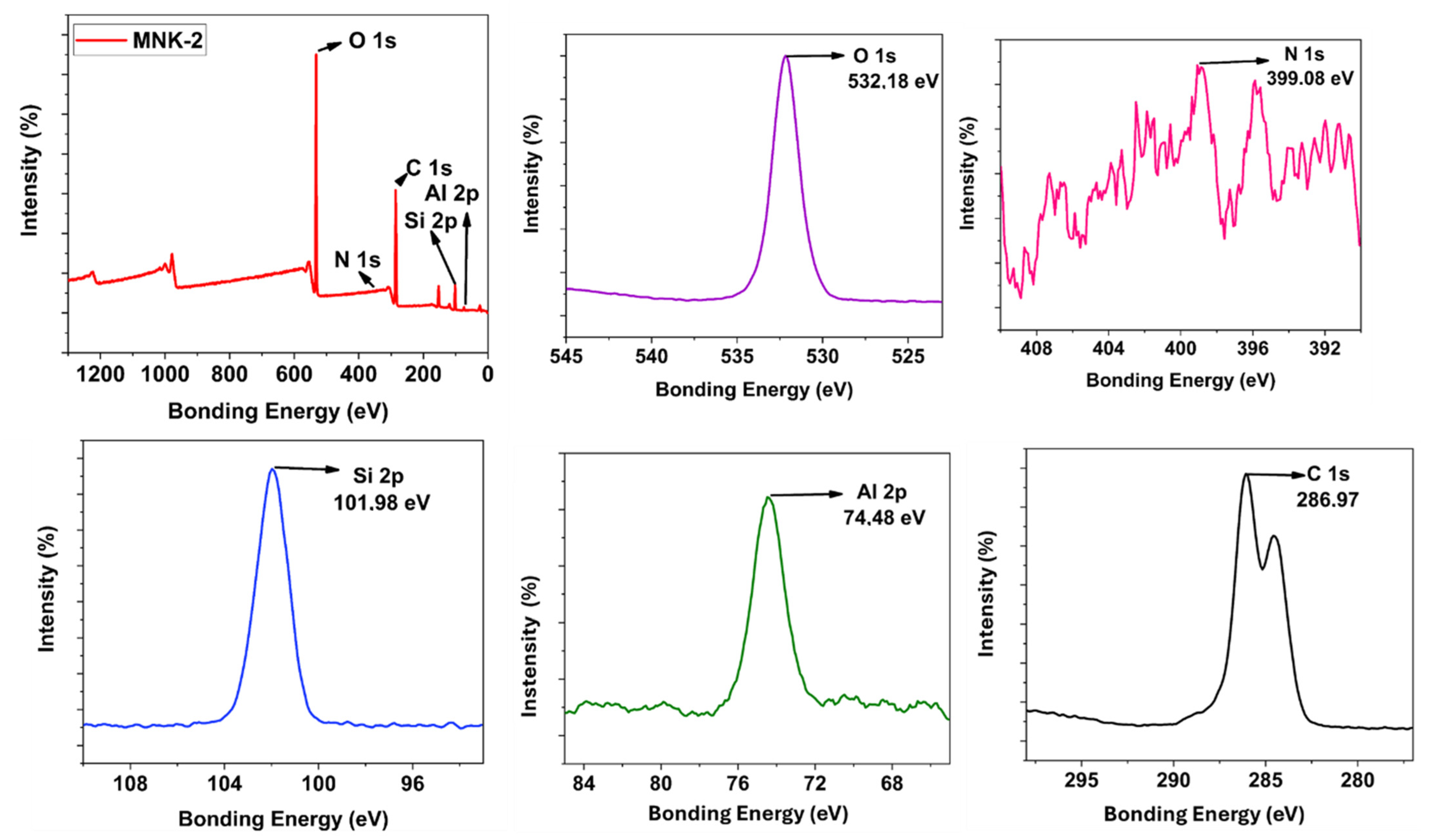
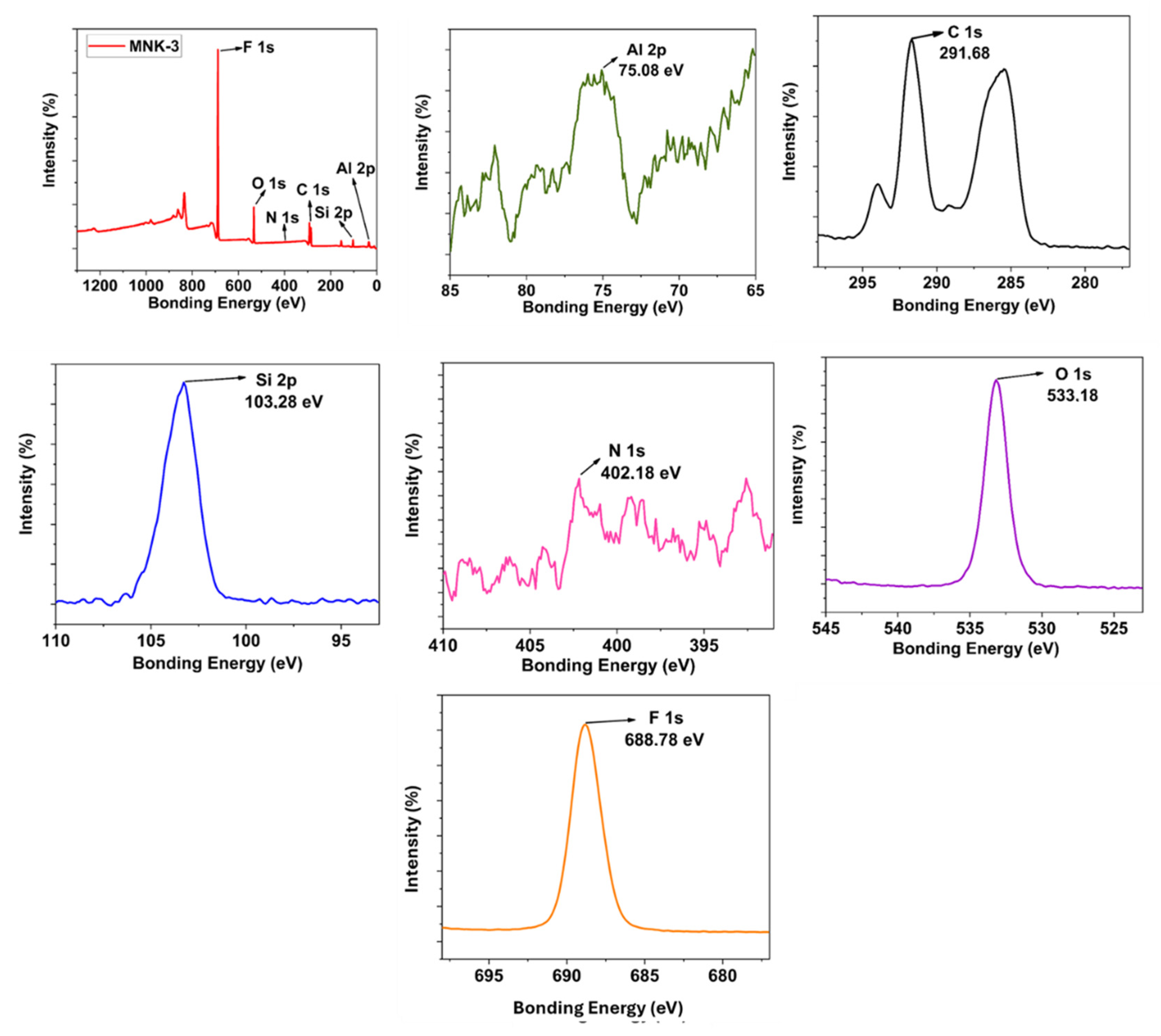
3.2. Synthesis and Step-by-Step Investigation of the Multifunctional Hybrid Coating
3.3. Material Applications of the Multifunctional Hybrid Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahay, R.; Reddy, V.J.; Ramakrishna, S. Synthesis and applications of multifunctional composite nanomaterials. Int. J. Mech. Mater. Eng. 2014, 9, 25. [Google Scholar] [CrossRef]
- Arslan, O.; Arpac, E.; Sayılkan, H. Siliconcarbide embedded hybrid nanocomposites as abrasion resistant coating. J. Inorg. Organomet. Polym. Mater. 2007, 20, 284–292. [Google Scholar] [CrossRef]
- Schmidt, H.; Wolter, H. Organically modified ceramics and their applications. J. Non-Cryst. Solids 1990, 121, 428–435. [Google Scholar] [CrossRef]
- Ferreira, A.D.B.; Nóvoa, P.R.; Marques, A.T. Multifunctional Material Systems: A state-of-the-art review. Compos. Struct. 2016, 151, 3–35. [Google Scholar] [CrossRef]
- Salonitis, K.; Pandremenos, J.; Paralikas, J.; Chryssolouris, G. Multifunctional materials: Engineering applications and processing challenges. Int. J. Adv. Manuf. Technol. 2010, 49, 803–826. [Google Scholar] [CrossRef]
- Schottner, G. Hybrid Sol-Gel-Derived Polymers: Applications of Multifunctional Materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Lu, D.; Yao, Z.; Jiao, L.; Waheed, M.; Sun, Z.; Zhang, L. Separation mechanism, selectivity enhancement strategies and advanced materials for mono-/multivalent ion-selective nanofiltration membrane. Adv. Membr. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Arslan, O.; Abalı, Y. Controlled modulation of 1D ZnO nano/micro structures: Evaluation of the various effects on the photocatalytic activity. J. Phys. Chem. Solids 2017, 108, 88–97. [Google Scholar] [CrossRef]
- Costa, C.M.; Cardoso, V.F.; Martins, P.; Correia, D.M.; Gonçalves, R.; Costa, P.; Correia, V.; Ribeiro, C.; Fernandes, M.M.; Martins, P.M.; et al. Smart and Multifunctional Materials Based on Electroactive Poly(vinylidene fluoride): Recent Advances and Opportunities in Sensors, Actuators, Energy, Environmental, and Biomedical Applications. Chem. Rev. 2023, 123, 11392–11487. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, X.; Qiao, Z.; Kottilil, D.; Shi, D.; Fan, W.; Yuan, Y.D.; Yu, X.; Babusenan, A.; Zhang, M.; et al. Tunable Nonlinear Optical Properties Based on Metal–Organic Framework Single Crystals. Adv. Opt. Mater. 2024, 12, 2302405. [Google Scholar] [CrossRef]
- Chen, X.; Leishman, M.; Bagnall, D.; Nasiri, N. Nanostructured gas sensors: From air quality and environmental monitoring to healthcare and medical applications. Nanomaterials 2021, 11, 1927. [Google Scholar] [CrossRef]
- Sadeque, M.S.B.; Chowdhury, H.K.; Rafique, M.; Durmuş, M.A.; Ahmed, M.K.; Hasan, M.M.; Erbas, A.; Sarpkaya, İ.; Incia, F.; Ordu, M. Hydrogel-integrated optical fiber sensors and their applications: A comprehensive review. J. Mater. Chem. C 2023, 11, 9383. [Google Scholar] [CrossRef]
- Ippili, S.; Jung, J.; Thomas, A.M.; Vuong, V.; Lee, J.; Sha, M.S.; Sadasivuni, K.K.; Jella, V.; Yoon, S. An Overview of Polymer Composite Films for Antibacterial Display Coatings and Sensor Applications. Polymers 2022, 15, 3791. [Google Scholar] [CrossRef]
- Zarkov, A. Sol–Gel Technology Applied to Materials Science: Synthesis, Characterization and Applications. Materials 2024, 17, 462. [Google Scholar] [CrossRef] [PubMed]
- Lendlein, A.; Trask, R.S. Multifunctional materials: Concepts, function-structure relationships, knowledge-based design, translational materials research. Multifunct. Mater. 2018, 1, 010201. [Google Scholar] [CrossRef]
- ASTM D3359-23; Standard Test Methods for Rating Adhesion by Tape Test. ASTM: West Conshohocken, PA, USA, 2023.
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Montjoy, D.G.; Bahng, J.H.; Eskafi, A.; Hou, H.; Kotov, N.A. Omnidispersible hedgehog particles with multilayer coatings for multiplexed biosensing. J. Am. Chem. Soc. 2018, 140, 7835–7845. [Google Scholar] [CrossRef]
- Marcelo, G.A.; Lodeiro, C.; Capelo, J.L.; Lorenzo, J.; Oliveira, E. Magnetic, fluorescent and hybrid nanoparticles: From synthesis to application in biosystems. J. Am. Chem. Soc. 2020, 106, 110104. [Google Scholar] [CrossRef]
- Al Ragib, A.; Al Amin, A.; Alanazi, Y.M.; Kormoker, T.; Uddin, M.; Siddique, A.B.; Barai, H.R. Multifunctional carbon dots in nanomaterial surface modification: A descriptive review. Carbon Res. 2023, 2, 37. [Google Scholar] [CrossRef]
- Gorup, L.F.; Longo, E.; Leite, E.R.; Camargo, E.R. Moderating effect of ammonia on particle growth and stability of quasi-monodisperse silver nanoparticles synthesized by the Turkevich method. J. Colloid Interface Sci. 2011, 360, 355–358. [Google Scholar] [CrossRef]
- Arslan, O.; Uyar, T. Multifunctional electrospun polymeric nanofibrous mats for catalytic reduction, photocatalysis and sensing. Nanoscale 2017, 9, 9606–9614. [Google Scholar] [CrossRef]
- Arslan, O.; Aytaç, Z.; Uyar, T. Fluorescent Si QD Decoration onto Flexible Polymeric Electrospun Nanofibrous Mat for Colorimetric Sensing of TNT. J. Mater. Chem. C 2017, 5, 1816–1825. [Google Scholar] [CrossRef]
- Rananavare, A.P.; Jain, R.; Lee, J. Recent developments in superhydrophobic textiles: A status review. J. Coat. Technol. Res. 2025, 22, 549–580. [Google Scholar] [CrossRef]
- Qi, H.; Zhang, C.; Guo, H.; Zheng, W.; Yang, J.; Zhou, X.; Zhang, L. Bioinspired Multifunctional Protein Coating for Antifogging, Self-Cleaning, and Antimicrobial Properties. ACS Appl. Mater. Interfaces 2019, 11, 24504–24511. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilzadeh, P.; Sadeghi, M.T.; Bahramian, A.; Fakhroueian, Z.; Zarbakhsh, A. Superamphiphobic Surfaces Prepared by Coating Multifunctional Nanofluids. ACS Appl. Mater. Interfaces 2016, 8, 32011–32020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, W.; Li, Z.; Yang, G.; Kuang, C.; Xu, S.; Li, X.; Qing, Y.; Wu, Y. Multifunctional Durable Superhydrophobic Coating Construct by Rosin Acid with Oil–Water Separation and Antimicrobial Property. ACS Appl. Polym. Mater. 2025, 7, 7871–7883. [Google Scholar] [CrossRef]
- Arslan, O.; Aytac, Z.; Uyar, T. Superhydrophobic, hybrid, electrospun cellulose acetate nanofibrous mats for oil/water separation by tailored surface modification. ACS Appl. Mater. Interfaces 2016, 8, 19747–19754. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, G.; Cui, J.; Wu, N.; Zhao, B.; Xu, J.; Zhao, N. Superior Hard but Quickly Reversible Si–O–Si Network Enables Scalable Fabrication of Transparent, Self-Healing, Robust, Programmable Multifunctional Nanocomposite Coatings. J. Am. Chem. Soc. 2022, 144, 436–445. [Google Scholar] [CrossRef]
- Du, M.; Peng, M.; Mai, B.; Hu, F.; Zhang, X.; Chen, Y.; Wang, C. A multifunctional hybrid inorganic-organic coating fabricated on magnesium alloy surface with antiplatelet adhesion and antibacterial activities. Surf. Coat. Technol. 2020, 384, 125336. [Google Scholar] [CrossRef]
- Améndola, I.F.; Velasco, M.I.; Acosta, R.H.; Illia, G.J.S.; Contreras, C.B. Smart hybrid copolymer-coated silica nanosystems with dual responsiveness as a carrier for positive charged molecules. Eur. Polym. J. 2024, 204, 112717. [Google Scholar] [CrossRef]
- Alessi, B.; Macias-Montero, M.; Maddi, C.; Maguire, P.; Svrcek, V.; Mariotti, D. Bridging energy bands to the crystalline and amorphous states of Si QDs. Faraday Discuss. 2020, 222, 390–404. [Google Scholar] [CrossRef]
- Wallart, X.; de Villeneuve, C.H.; Allongue, P. Truly Quantitative XPS Characterization of Organic Monolayers on Silicon: Study of Alkyl and Alkoxy Monolayers on H−Si(111). J. Am. Chem. Soc. 2005, 127, 7871–7878. [Google Scholar] [CrossRef]
- Golvari, P.; Alkameh, K.; Kuebler, S.M. Si–H Surface Groups Inhibit Methacrylic Polymerization: Thermal Hydrosilylation of Allyl Methacrylate with Silicon Nanoparticles. Langmuir 2022, 38, 8366–8373. [Google Scholar] [CrossRef]
- Du, G.; Li, G.; Qiu, S.; Liu, L.; Zheng, Y.; Liu, X. Low-Cost Water Soluble Silicon Quantum Dots and Biocompatible Fluorescent Composite Films. Part. Part. Syst. Charact. 2021, 38, 2100173. [Google Scholar] [CrossRef]
- Macan, J.; Ivankovic, H.; Ivankovic, M.; Mencer, H.J. Synthesis and characterization of organic–inorganic hybrids based on epoxy resin and 3-glycidyloxypropyltrimethoxysilane. J. Appl. Polym. Sci. 2004, 92, 498–505. [Google Scholar] [CrossRef]
- Parola, S.; Julián-López, B.; Carlos, L.D.; Sanchez, C. Optical Properties of Hybrid Organic-Inorganic Materials and their Applications. Adv. Funct. Mater. 2016, 26, 6506–6544. [Google Scholar] [CrossRef]
- Nguyen, T.-D. Portraits of colloidal hybrid nanostructures: Controlled synthesis and potential applications. Colloids Surf. B Biointerfaces 2013, 103, 326–344. [Google Scholar] [CrossRef]
- Tian, Z.; Li, S.; Chen, Y.; Li, L.; An, Z.; Zhang, Y.; Tong, A.; Zhang, H.; Liu, Z.; An, B. Self-Healing Coating with a Controllable Release of Corrosion Inhibitors by Using Multifunctional Zinc Oxide Quantum Dots as Valves. ACS Appl. Mater. Interfaces 2022, 14, 47188–47197. [Google Scholar] [CrossRef]
- Patra, R.; Vijay, B.; Raju, K.S.; Gobi, K.; Subasri, R. Dual functional coatings: Comparative study of corrosion indicators for corrosion reporting and self-healing on MgAZ31 alloy. J. Alloys Compd. 2025, 1029, 180755. [Google Scholar] [CrossRef]
- Chen, Y.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Y.; Silberschmidt, V.V.; Wilson, P.; Chen, Z. Mechanically Robust Transparent Anti-Icing Coatings: Roles of Dispersion Status of Titanate Nanotubes. Adv. Mater. Interfaces 2018, 5, 1800773. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.; Gui, H.; Du, D.; Du, Y.; Song, X.-M.; Liang, F. Superhydrophobic Coating Derived from the Spontaneous Orientation of Janus Particles. ACS Appl. Mater. Interfaces 2021, 13, 25392–25399. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Zhou, Y.; Zhou, Y. Multifunctional Antibacterial Materials for the Control of Hazardous Microbes and Chemicals: A Review. ACS EST Water 2021, 1, 479–497. [Google Scholar] [CrossRef]
- Visagamani, A.M.; Shanthi, D.; Muthukrishnaraj, A.; Venkatadri, B.; Ahamed, J.I.; Kaviyarasu, K. Innovative Preparation of Cellulose-Mediated Silver Nanoparticles for Multipurpose Applications: Experiment and Molecular Docking Studies. ACS Omega 2023, 8, 38860–38870. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Mitra, S.; Yang, T.; Jew, A.D.; Badireddy, A.R.; Lowry, G.V.; Brown, G.E., Jr. Effect of Chloride on the Dissolution Rate of Silver Nanoparticles and Toxicity to E. coli. Environ. Sci. Technol. 2013, 47, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Milburn, L.A.; Robidillo, C.J.T.; Dalangin, R.; Shen, Y.; Veinot, J.G.C. A Complementary Silicon Quantum Dot-Enzyme Platform for the Selective Detection of Nitroaromatic Compounds: Explosives versus Nerve Agents, Leanne. ACS Appl. Nano Mater. 2022, 5, 11984–11990. [Google Scholar] [CrossRef]
- Bose, S.; Ganayee, M.A.; Mondal, B.; Baidya, A.; Chennu, S.; Mohanty, J.S.; Pradeep, T. Synthesis of Silicon Nanoparticles from Rice Husk and their Use as Sustainable Fluorophores for White Light Emission. ACS Sustain. Chem. Eng. 2018, 6, 6203–6210. [Google Scholar] [CrossRef]
- Sreejith, S.; Ajayan, J.; Radhika, J.M.; Reddy, N.V.U.; Manikandan, M.; Mathew, J.K. Recent Progress in Silicon Quantum Dots Sensors: A Review. Silicon 2024, 16, 6313–6335. [Google Scholar] [CrossRef]
- Khan, M.S.H.; Rahaman, M.T.; Pranta, A.D.; Hasan, M.K. Eco-friendly organic nanomaterials for multifunctional textiles: Sources, applications, recent advances and future prospects towards sustainability. Int. J. Environ. Sci. Technol. 2025, 22, 7353–7410. [Google Scholar] [CrossRef]
- Akhtar, M.; Shahzadi, S.; Arshad, M.; Akhtar, T. Muhammad Ramzan Saeed Ashraf Janjua Metal oxide-polymer hybrid composites: A comprehensive review on synthesis and multifunctional applications. RSC Adv. 2025, 15, 18173–18208. [Google Scholar] [CrossRef]
- Zong, W.; Gao, B.; Qu, B.; Han, S.; Zhang, X. Advances in bioinspired hydrophobic materials: From surface engineering to multifunctional applications. Mater. Sci. Eng. B 2025, 322, 118564. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asan, G.; Arslan, O. Multifunctional, Biocompatible Hybrid Surface Coatings Combining Antibacterial, Hydrophobic and Fluorescent Applications. Polymers 2025, 17, 2139. https://doi.org/10.3390/polym17152139
Asan G, Arslan O. Multifunctional, Biocompatible Hybrid Surface Coatings Combining Antibacterial, Hydrophobic and Fluorescent Applications. Polymers. 2025; 17(15):2139. https://doi.org/10.3390/polym17152139
Chicago/Turabian StyleAsan, Gökçe, and Osman Arslan. 2025. "Multifunctional, Biocompatible Hybrid Surface Coatings Combining Antibacterial, Hydrophobic and Fluorescent Applications" Polymers 17, no. 15: 2139. https://doi.org/10.3390/polym17152139
APA StyleAsan, G., & Arslan, O. (2025). Multifunctional, Biocompatible Hybrid Surface Coatings Combining Antibacterial, Hydrophobic and Fluorescent Applications. Polymers, 17(15), 2139. https://doi.org/10.3390/polym17152139







