Carboxymethyl Polysaccharides/Montmorillonite Biocomposite Films and Their Sorption Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Sodium Montmorillonite
2.3. Preparation of Polysaccharide-Based Films
2.4. Methods
2.4.1. Solubility in Water
2.4.2. Swelling in Water
2.4.3. Moisture Absorption
2.4.4. DMTA
2.4.5. XRD
2.4.6. FTIR
2.4.7. TEM
2.4.8. Mechanical Properties
3. Results and Discussion
3.1. Solubility in Water
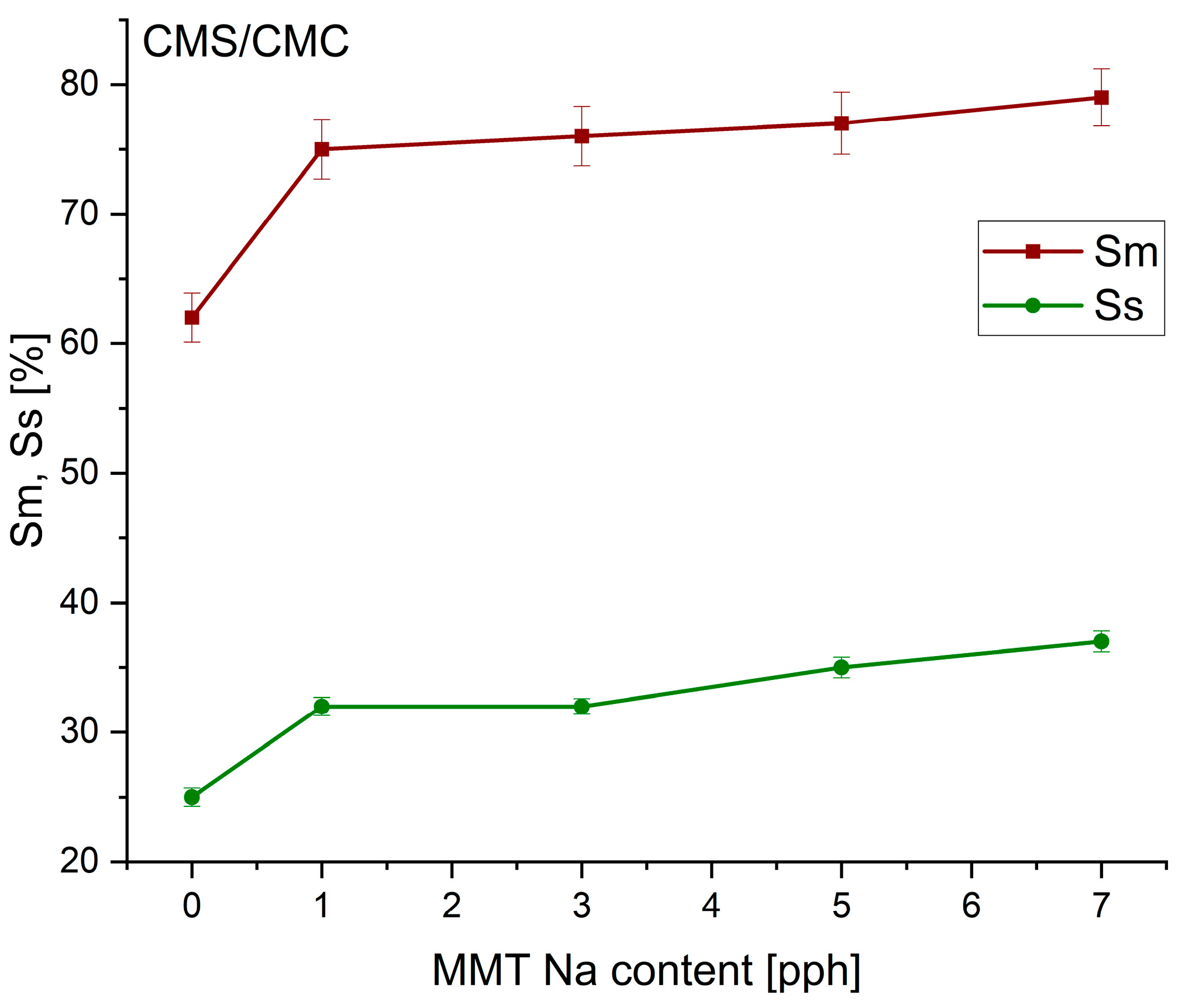
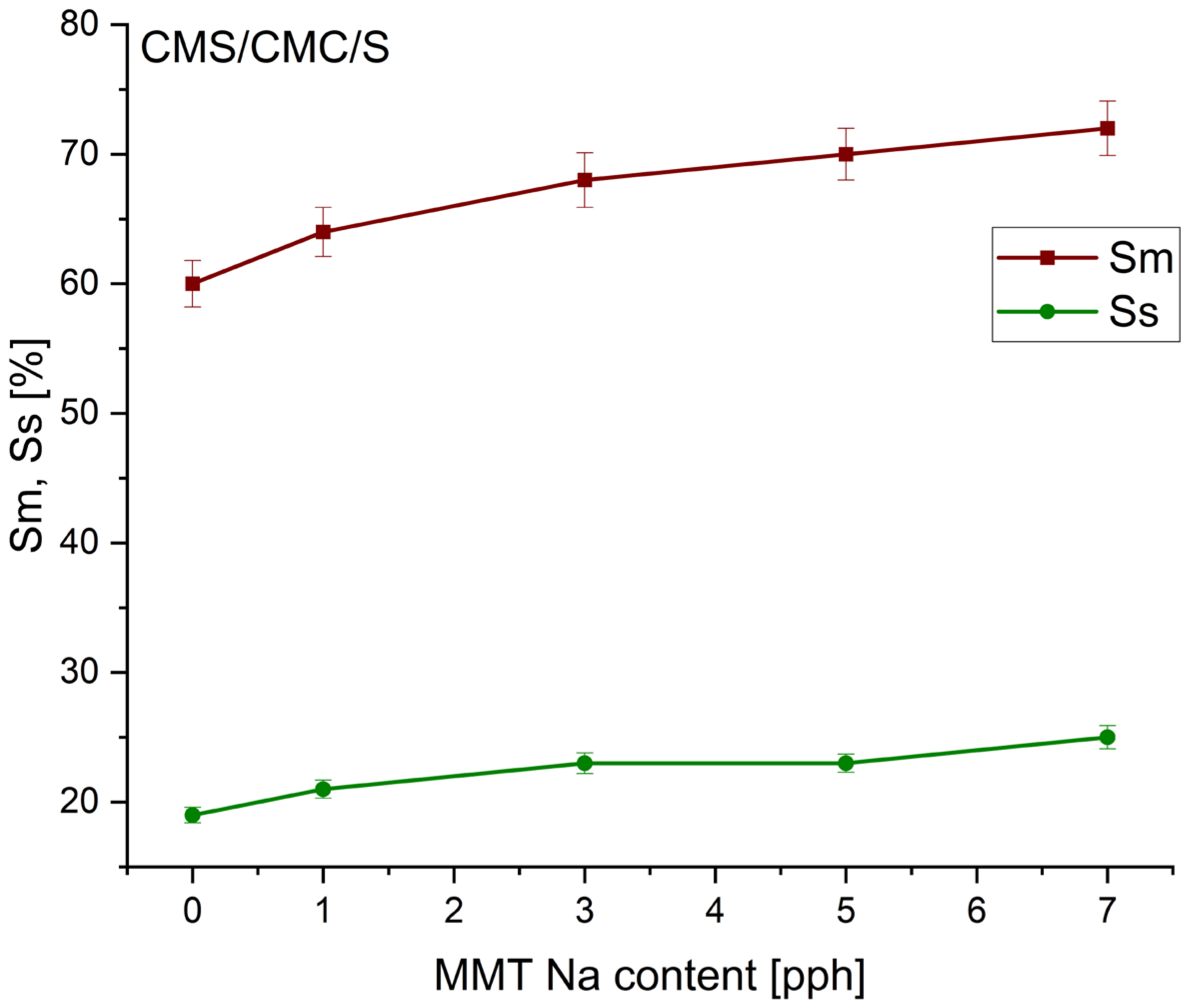
3.2. The Swelling Effect
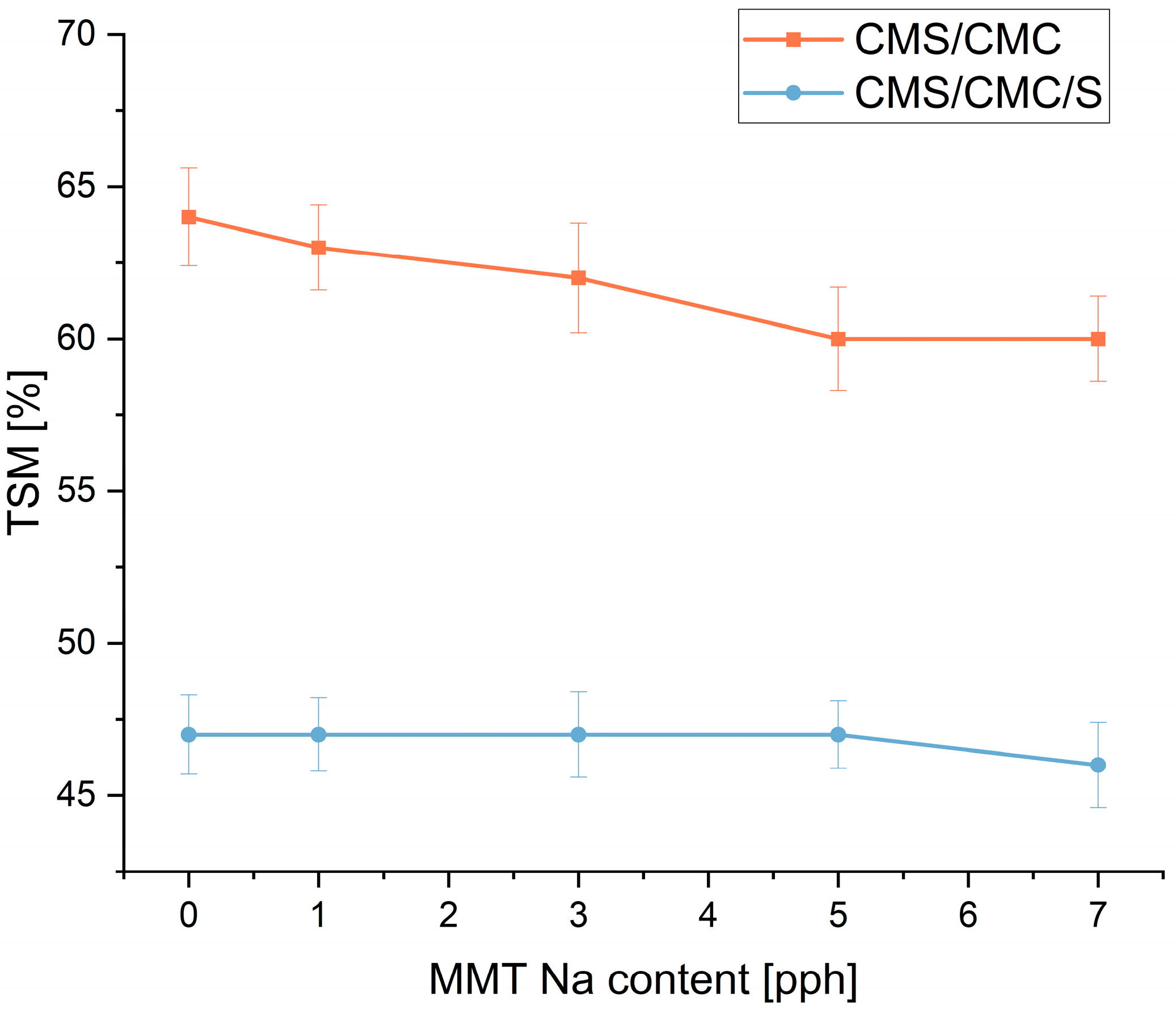
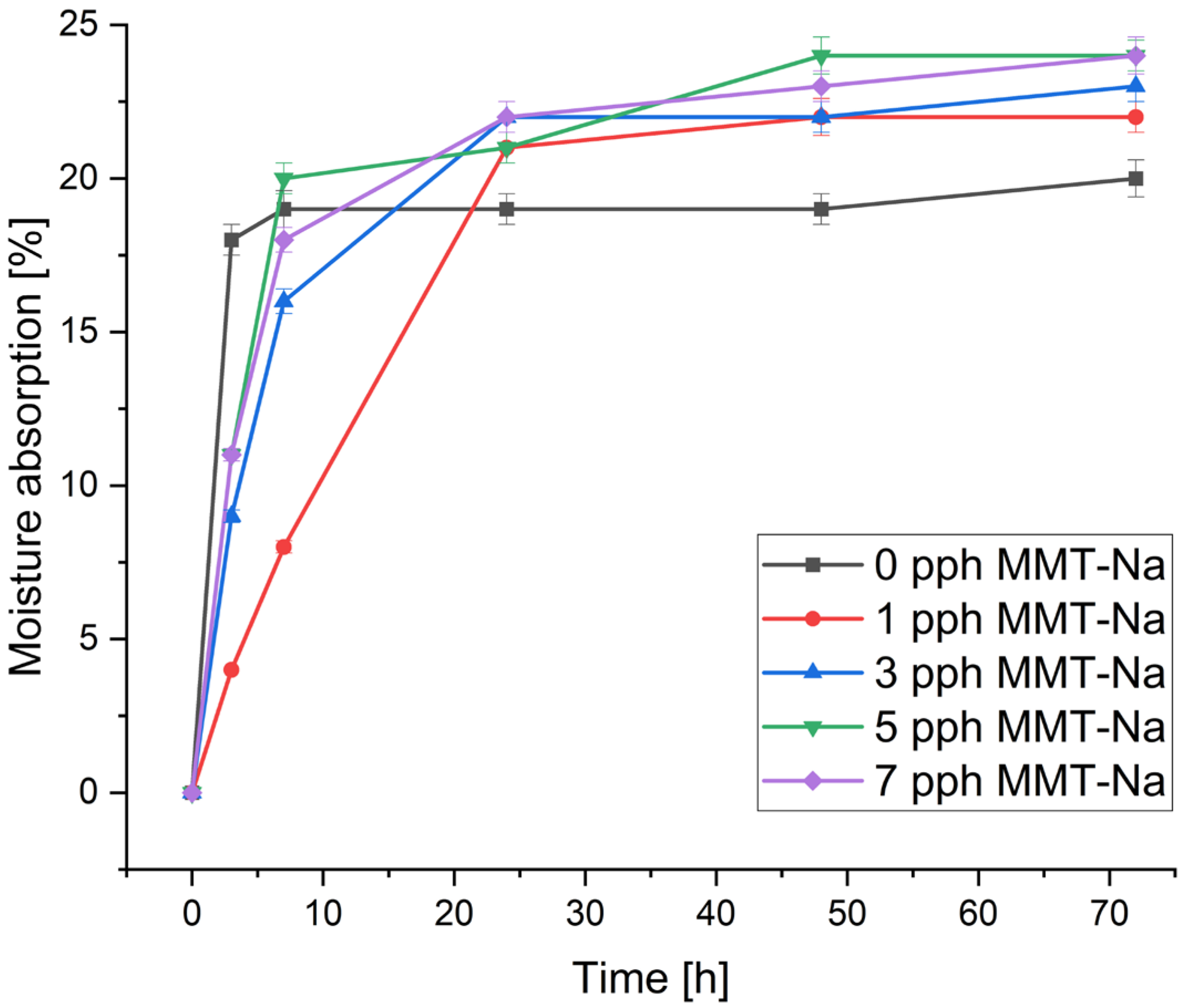
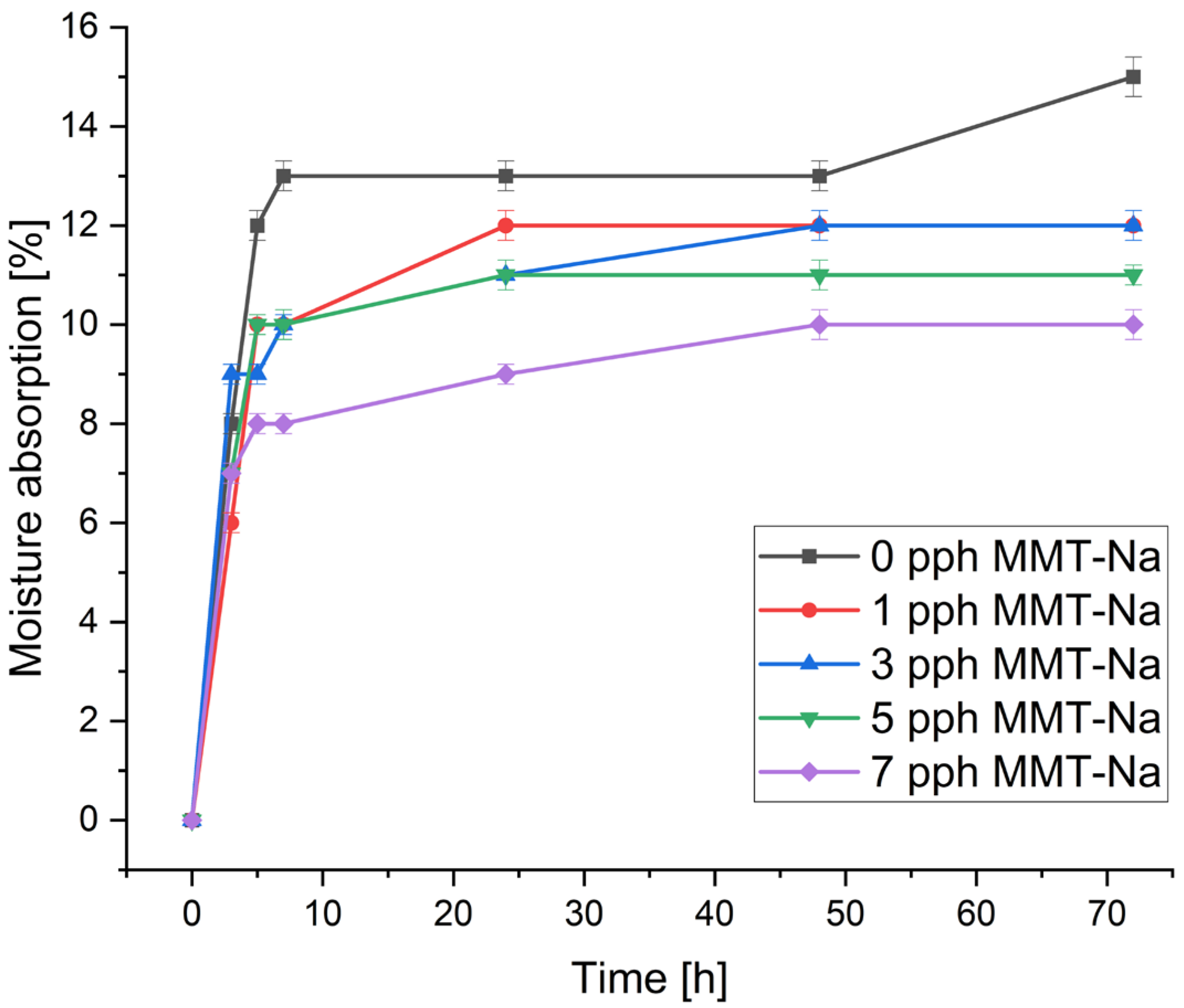
3.3. Moisture Absorption
3.4. DMTA
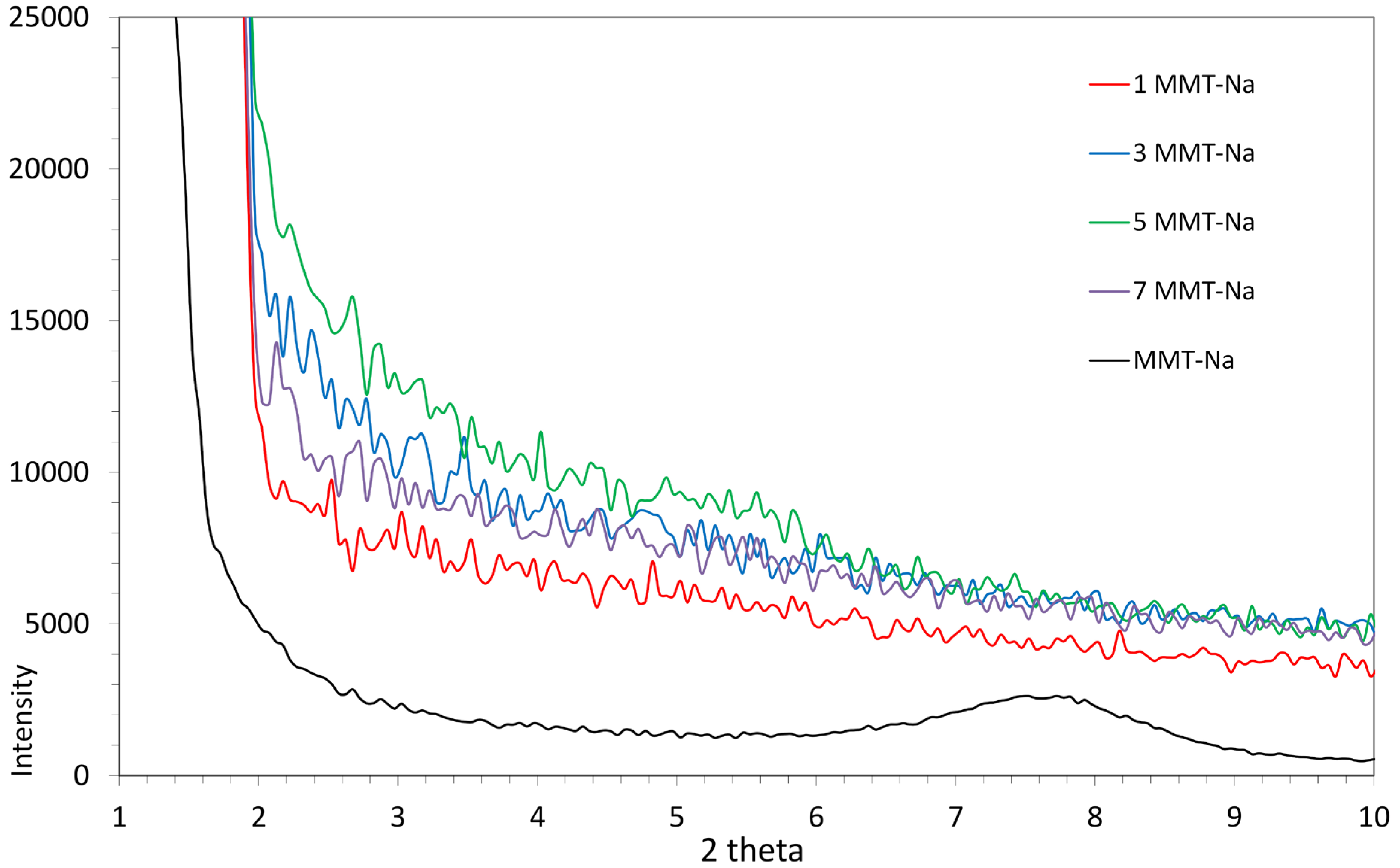
3.5. Measurement of X-Ray Diffraction Analysis (XRD)
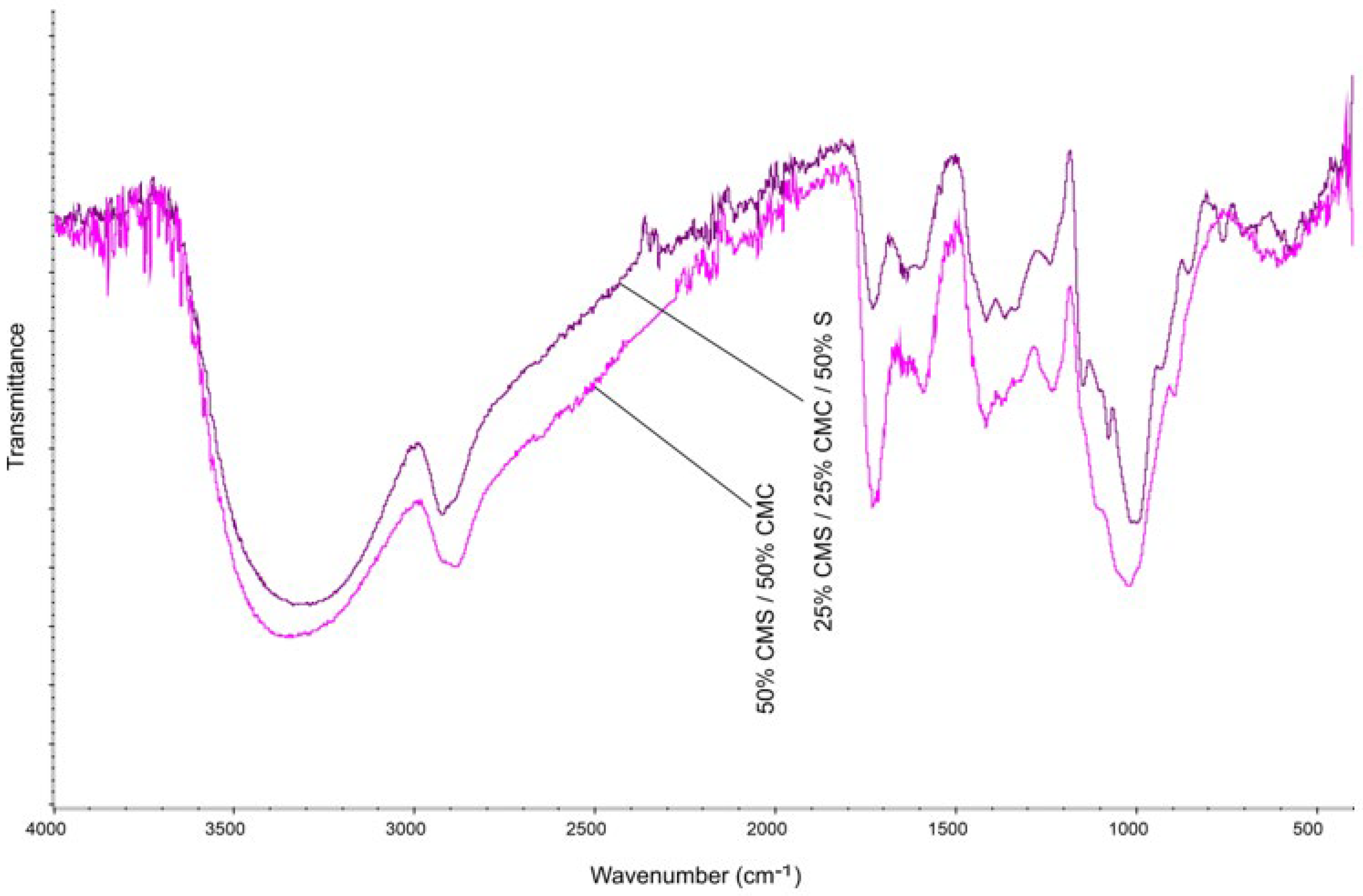
3.6. Fourier Transform Infrared Spectroscopy FTIR
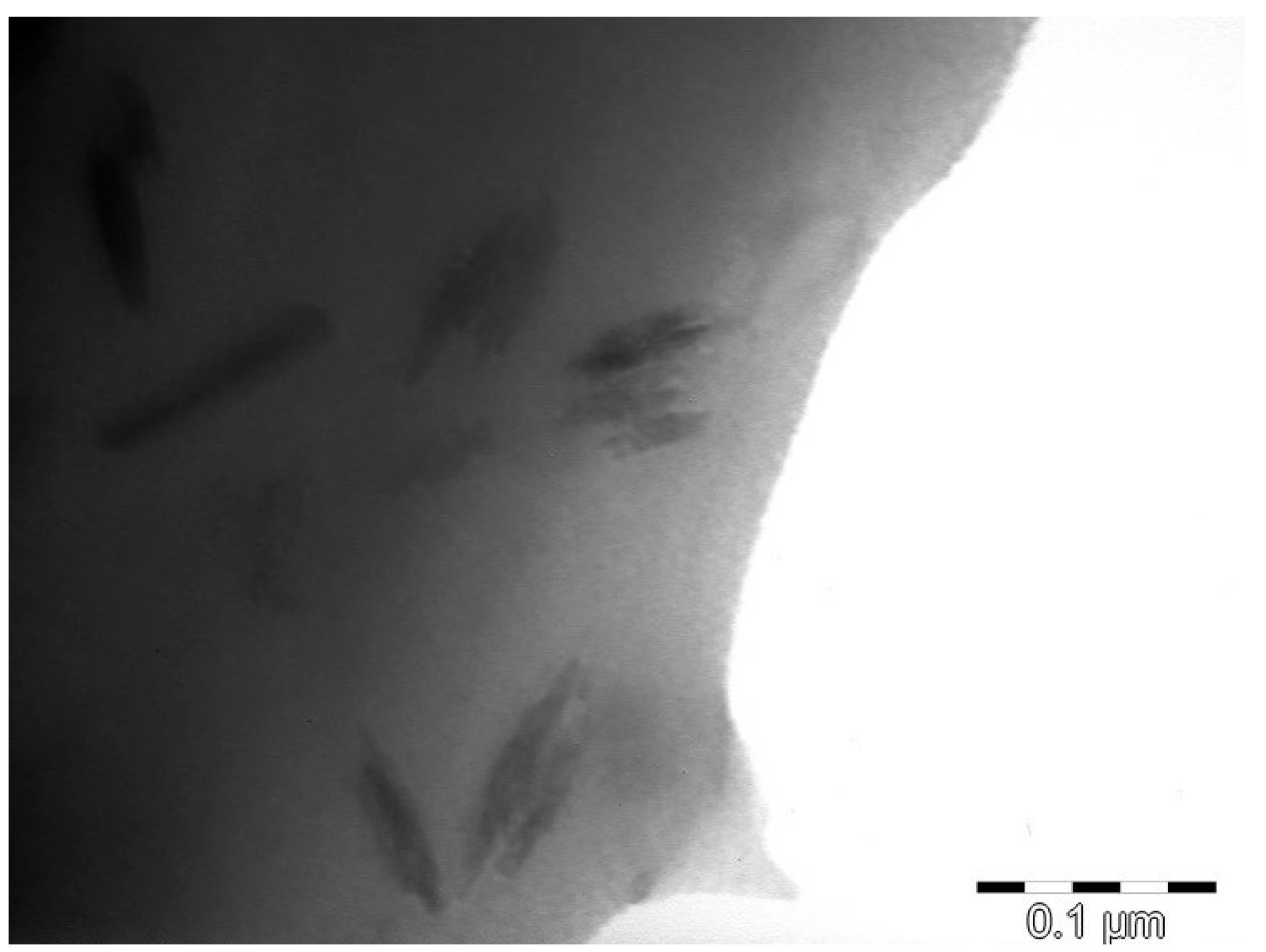
3.7. Transmission Electron Microscope Analysis (TEM)
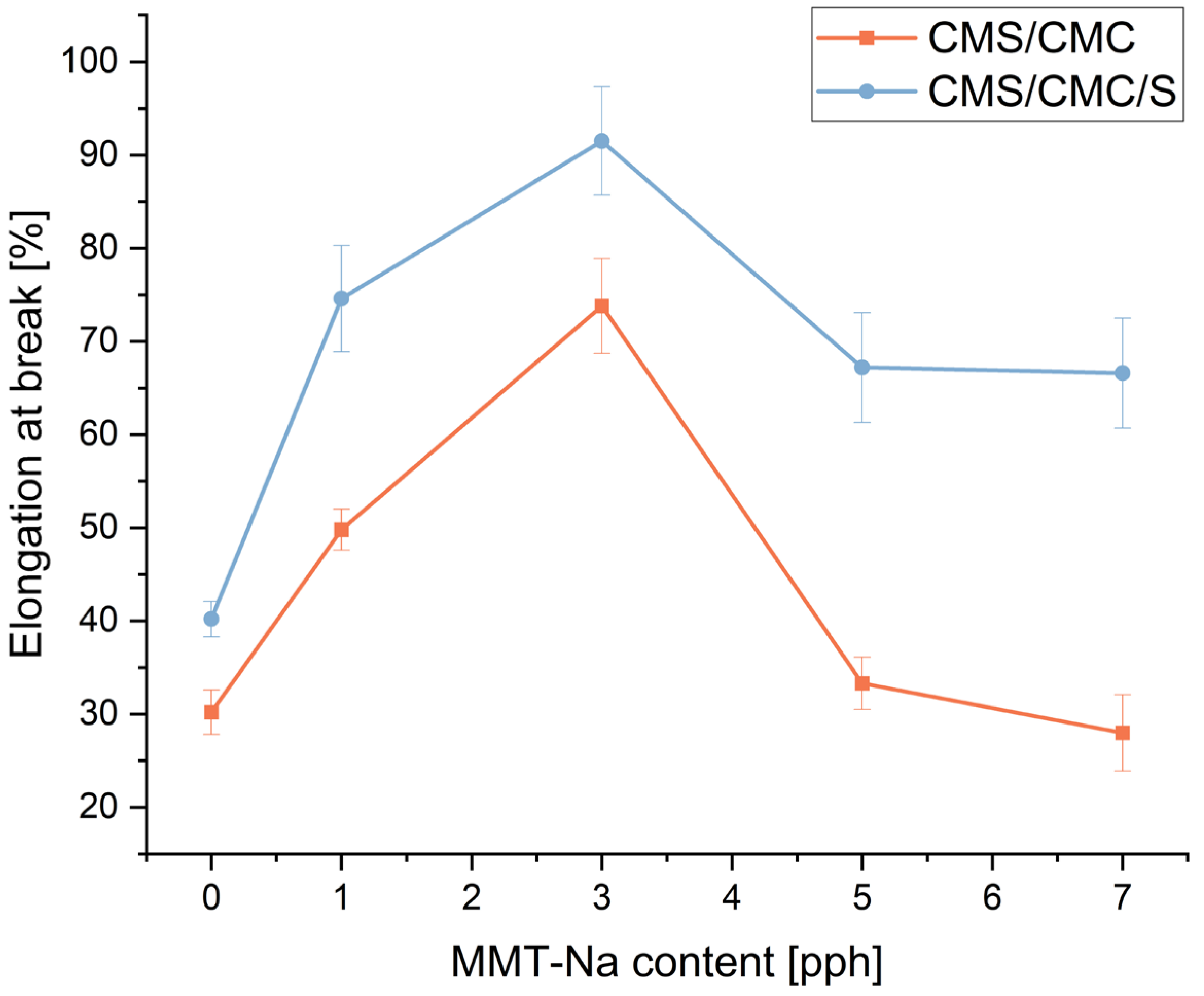
3.8. Mechanical Properties
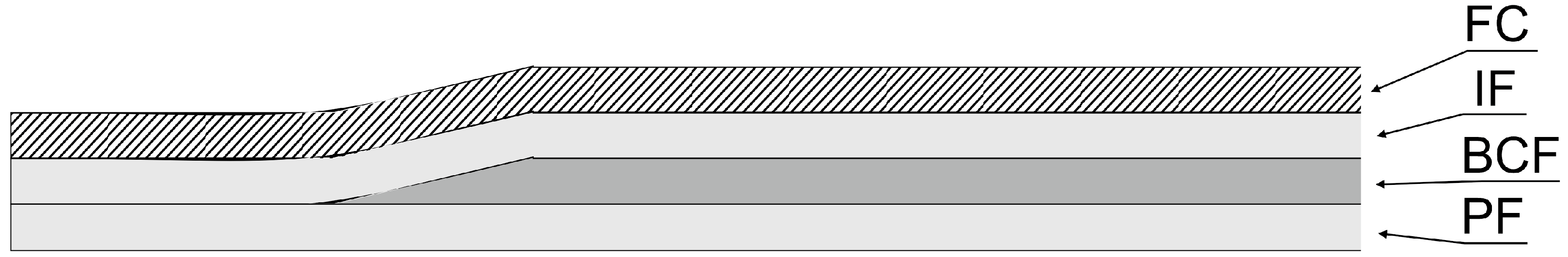
3.9. Wound Dressing Model Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rhim, J.-W.; Gennadios, A.; Weller, C.L.; Cezeirat, C.; Hanna, M.A. Soy Protein Isolate–Dialdehyde Starch Films. Ind. Crops Prod. 1998, 8, 195–203. [Google Scholar] [CrossRef]
- Riar, C.S. Studies on Influence of Chemical Modification, Plasticizer and Starch Concentration on Some Characteristics of Biodegradable Film. Mater. Sci. Forum 2016, 842, 129–156. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Fan, L.; Ma, S. A Review of Wheat Starch Analyses: Methods, Techniques, Structure and Function. Int. J. Biol. Macromol. 2022, 203, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Amaraweera, S.M.; Gunathilake, C.; Gunawardene, O.H.P.; Fernando, N.M.L.; Wanninayaka, D.B.; Dassanayake, R.S.; Rajapaksha, S.M.; Manamperi, A.; Fernando, C.A.N.; Kulatunga, A.K.; et al. Development of Starch-Based Materials Using Current Modification Techniques and Their Applications: A Review. Molecules 2021, 26, 6880. [Google Scholar] [CrossRef]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch Modification for Non-Food, Industrial Applications: Market Intelligence and Critical Review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef] [PubMed]
- Admase, A.T.; Mersha, D.A.; Kebede, A.Y. Cassava Starch-Based Hot Melt Adhesive for Textile Industries. Sci. Rep. 2024, 14, 20927. [Google Scholar] [CrossRef]
- Molavi, H.; Behfar, S.; Ali Shariati, M.; Kaviani, M.; Atarod, S. A review on biodegradable starch based film. J. Microbiol. Biotechnol. Food Sci. 2015, 4, 456–461. [Google Scholar] [CrossRef]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based Films and Food Coatings: An Overview. Starch-Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Chang, H.; Korma, S.A.; Chen, J.; Yang, T. Preparation, Properties, and Characterization of Dual-Modified Tapioca Starch and Its Composite Film. Ind. Crops Prod. 2024, 222, 119840. [Google Scholar] [CrossRef]
- Spychaj, T.; Wilpiszewska, K.; Zdanowicz, M. Medium and High Substituted Carboxymethyl Starch: Synthesis, Characterization and Application. Starch-Stärke 2013, 65, 22–33. [Google Scholar] [CrossRef]
- Silva, D.A.; de Paula, R.C.M.; Feitosa, J.P.A.; de Brito, A.C.F.; Maciel, J.S.; Paula, H.C.B. Carboxymethylation of Cashew Tree Exudate Polysaccharide. Carbohydr. Polym. 2004, 58, 163–171. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Zdanowicz, M. The Effect of Citric Acid on Physicochemical Properties of Hydrophilic Carboxymethyl Starch-Based Films. J. Polym. Environ. 2019, 27, 1379–1387. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the Barrier and Mechanical Properties of Corn Starch-Based Edible Films: Effect of Citric Acid and Carboxymethyl Cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Menzel, C.; Olsson, E.; Plivelic, T.S.; Andersson, R.; Johansson, C.; Kuktaite, R.; Järnström, L.; Koch, K. Molecular Structure of Citric Acid Cross-Linked Starch Films. Carbohydr. Polym. 2013, 96, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.A.; Rahman, N.A.; Talip, N.; Yacob, N. Citric Acid Cross-Linking of Carboxymethyl Sago Starch Based Hydrogel for Controlled Release Application. Macromol. Symp. 2018, 382, 1800086. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Johansson, C. Mechanical and Barrier Properties of Starch-Based Films Plasticized with Two- or Three Component Deep Eutectic Solvents. Carbohydr. Polym. 2016, 151, 103–112. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Chen, Y.; Huang, C.; Zhong, N.; Hu, Y. Preparation and Characterization of Ternary Polysaccharide Hydrogels Based on Carboxymethyl Cellulose, Carboxymethyl Chitosan, and Carboxymethyl β-Cyclodextrin. Int. J. Biol. Macromol. 2024, 271, 132604. [Google Scholar] [CrossRef]
- Morais, M.A.P.; Silva, M.; Barros, M.; Halley, P.; Almeida, Y.; Vinhas, G. Impact of Citric Acid on Guar Gum Carboxymethylcellulose Crosslinked Blend Films. J. Appl. Polym. Sci. 2024, 141, e56162. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Singh, A. Base Triggered Release of Insecticide from Bentonite Reinforced Citric Acid Crosslinked Carboxymethyl Cellulose Hydrogel Composites. Carbohydr. Polym. 2017, 156, 303–311. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical Properties of Starch–CMC–Nanoclay Biodegradable Films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Rahmani, E.; Shamsabadipour, A.; Samadi, A.; Esmaeili, J.; Arshad, R.; Rahdar, A.; Tavangarian, F.; Pandey, S. Novel Carboxymethyl Cellulose Based Nanocomposite: A Promising Biomaterial for Biomedical Applications. Process Biochem. 2023, 130, 211–226. [Google Scholar] [CrossRef]
- Tudoroiu, E.-E.; Dinu-Pîrvu, C.-E.; Albu Kaya, M.G.; Popa, L.; Anuța, V.; Prisada, R.M.; Ghica, M.V. An Overview of Cellulose Derivatives-Based Dressings for Wound-Healing Management. Pharmaceuticals 2021, 14, 1215. [Google Scholar] [CrossRef]
- Ali, M.; Ullah, S.; Ullah, S.; Shakeel, M.; Afsar, T.; Husain, F.M.; Amor, H.; Razak, S. Innovative Biopolymers Composite Based Thin Film for Wound Healing Applications. Sci. Rep. 2024, 14, 27415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- González-Seligra, P.; Guz, L.; Ochoa-Yepes, O.; Goyanes, S.; Famá, L. Influence of Extrusion Process Conditions on Starch Film Morphology. LWT 2017, 84, 520–528. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of Probiotic Bacteria in Carboxymethyl Cellulose-Based Edible Film and Assessment of Quality Parameters. LWT 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Kim, S.; Cho, D.-H.; Kweon, D.-K.; Jang, E.-H.; Hong, J.-Y.; Lim, S.-T. Improvement of Mechanical Properties of Orodispersible Hyaluronic Acid Film by Carboxymethyl Cellulose Addition. Food Sci. Biotechnol. 2020, 29, 1233–1239. [Google Scholar] [CrossRef]
- Kong, P.; Rosnan, S.M.; Enomae, T. Carboxymethyl Cellulose–Chitosan Edible Films for Food Packaging: A Review of Recent Advances. Carbohydr. Polym. 2024, 346, 122612. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; He, L.; Liu, Y. Improving Nisin Production by Encapsulated Lactococcus Lactis with Starch/Carboxymethyl Cellulose Edible Films. Carbohydr. Polym. 2021, 251, 117062. [Google Scholar] [CrossRef]
- Cagnin, C.; Simões, B.M.; Yamashita, F.; de Carvalho, G.M.; Grossmann, M.V.E. PH Sensitive Phosphate Crosslinked Films of Starch-carboxymethyl Cellulose. Polym. Eng. Sci. 2021, 61, 388–396. [Google Scholar] [CrossRef]
- Ounkaew, A.; Kasemsiri, P.; Jetsrisuparb, K.; Uyama, H.; Hsu, Y.-I.; Boonmars, T.; Artchayasawat, A.; Knijnenburg, J.T.N.; Chindaprasirt, P. Synthesis of Nanocomposite Hydrogel Based Carboxymethyl Starch/Polyvinyl Alcohol/Nanosilver for Biomedical Materials. Carbohydr. Polym. 2020, 248, 116767. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Markowska-Szczupak, A.; Spychaj, T. Carboxymethyl Starch/Medium Chain Fatty Acid Compositions: Rheological Changes During Storage and Selected Film Properties. Starch-Stärke 2020, 72, 1900240. [Google Scholar] [CrossRef]
- Tavares, K.M.; de Campos, A.; Mitsuyuki, M.C.; Luchesi, B.R.; Marconcini, J.M. Corn and Cassava Starch with Carboxymethyl Cellulose Films and Its Mechanical and Hydrophobic Properties. Carbohydr. Polym. 2019, 223, 115055. [Google Scholar] [CrossRef] [PubMed]
- Laoutid, F.; Gaudon, P.; Taulemesse, J.-M.; Lopez Cuesta, J.M.; Velasco, J.I.; Piechaczyk, A. Study of Hydromagnesite and Magnesium Hydroxide Based Fire Retardant Systems for Ethylene–Vinyl Acetate Containing Organo-Modified Montmorillonite. Polym. Degrad. Stab. 2006, 91, 3074–3082. [Google Scholar] [CrossRef]
- Malko, M.; Antosik, A.K.; Wróblewska, A.; Czech, Z.; Wilpiszewska, K.; Miądlicki, P.; Michalkiewicz, B. Montmorillonite as the Catalyst in Oxidation of Limonene with Hydrogen Peroxide and in Isomerization of Limonene. Pol. J. Chem. Technol. 2017, 19, 50–58. [Google Scholar] [CrossRef]
- Antosik, A.K.; Wilpiszewska, K.; Czech, Z. Carboxymethylated Polysaccharide-Based Films as Carriers for Acrylic Pressure-Sensitive Adhesives. Int. J. Adhes. Adhes. 2017, 73, 75–79. [Google Scholar] [CrossRef]
- Antosik, A.K.; Miądlicki, P.; Wilpiszewska, K.; Markowska-Szczupak, A.; Koren, Z.C.; Wróblewska, A. Polysaccharide Films Modified by Compounds of Natural Origin and Silver Having Potential Medical Applications. Cellulose 2021, 28, 7257–7271. [Google Scholar] [CrossRef]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable Starch/Clay Nanocomposite Films for Food Packaging Applications. Food Chem. 2005, 93, 467–474. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric Acid Cross-Linking of Starch Films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- ISO 527-3:2018; Plastics—Determination of Tensile Properties, Part 3: Test Conditions for Films and Sheets. International Organization for Standardization: Geneva, Switzerland, 2018.
- Roy, N.; Saha, N.; Kitano, T.; Saha, P. Biodegradation of PVP–CMC Hydrogel Film: A Useful Food Packaging Material. Carbohydr. Polym. 2012, 89, 346–353. [Google Scholar] [CrossRef]
- Antosik, A.K.; Wilpiszewska, K. Natural Composites Based on Polysaccharide Derivatives: Preparation and Physicochemical Properties. Chem. Pap. 2018, 72, 3215–3218. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Antosik, A.K.; Spychaj, T. Novel Hydrophilic Carboxymethyl Starch/Montmorillonite Nanocomposite Films. Carbohydr. Polym. 2015, 128, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, M.; Spychaj, T. Viscosity Changes of Aqueous Carboxymethyl Starch by Partial Crosslinking and Montmorillonite Addition. J. Appl. Polym. Sci. 2014, 131, 40793. [Google Scholar] [CrossRef]
- Wilpiszewska, K.; Zdanowicz, M.; Spychaj, T. Carboxymethyl Starch/Montmorillonite Aqueous Dispersions: The Effect of Components and Mixing Method on Rheoviscometric Characteristics. Adv. Polym. Technol. 2013, 32, 1–6. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Utilization of Carboxymethyl Cellulose from Durian Rind Agricultural Waste to Improve Physical Properties and Stability of Rice Starch-Based Film. J. Polym. Environ. 2019, 27, 286–298. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Rokosa, M.; Pieczykolan, M.; Antosik, A.K.; Skórczewska, K. Biocomposites Based on Wheat Flour with Urea-Based Eutectic Plasticizer and Spent Coffee Grounds: Preparation, Physicochemical Characterization, and Study of Their Influence on Plant Growth. Materials 2024, 17, 1212. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Sałasińska, K. Characterization of Thermoplastic Starch Plasticized with Ternary Urea-Polyols Deep Eutectic Solvent with Two Selected Fillers: Microcrystalline Cellulose and Montmorillonite. Polymers 2023, 15, 972. [Google Scholar] [CrossRef]
- Wilpiszewska, K. Hydrophilic Films Based on Starch and Carboxymethyl Starch. Pol. J. Chem. Technol. 2019, 21, 26–30. [Google Scholar] [CrossRef]
- Yanli, W.; Wenyuan, G.; Xia, L. Carboxymethyl Chinese Yam Starch: Synthesis, Characterization, and Influence of Reaction Parameters. Carbohydr. Res. 2009, 344, 1764–1769. [Google Scholar] [CrossRef]
- Jiang, Q.; Gao, W.; Li, X.; Liu, Z.; Huang, L.; Xiao, P. Synthesis and Properties of Carboxymethyl Pueraria Thomsonii Benth. Starch. Starch/Staerke 2011, 63, 692–699. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Johansson, C. Impact of Additives on Mechanical and Barrier Properties of Starch-based Films Plasticized with Deep Eutectic Solvents. Starch-Stärke 2017, 69, 1700030. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antosik, A.K.; Bartkowiak, M.; Zdanowicz, M.; Wilpiszewska, K. Carboxymethyl Polysaccharides/Montmorillonite Biocomposite Films and Their Sorption Properties. Polymers 2025, 17, 2130. https://doi.org/10.3390/polym17152130
Antosik AK, Bartkowiak M, Zdanowicz M, Wilpiszewska K. Carboxymethyl Polysaccharides/Montmorillonite Biocomposite Films and Their Sorption Properties. Polymers. 2025; 17(15):2130. https://doi.org/10.3390/polym17152130
Chicago/Turabian StyleAntosik, Adrian Krzysztof, Marcin Bartkowiak, Magdalena Zdanowicz, and Katarzyna Wilpiszewska. 2025. "Carboxymethyl Polysaccharides/Montmorillonite Biocomposite Films and Their Sorption Properties" Polymers 17, no. 15: 2130. https://doi.org/10.3390/polym17152130
APA StyleAntosik, A. K., Bartkowiak, M., Zdanowicz, M., & Wilpiszewska, K. (2025). Carboxymethyl Polysaccharides/Montmorillonite Biocomposite Films and Their Sorption Properties. Polymers, 17(15), 2130. https://doi.org/10.3390/polym17152130





