Effect of Ozone on Nonwoven Polylactide/Natural Rubber Fibers
Abstract
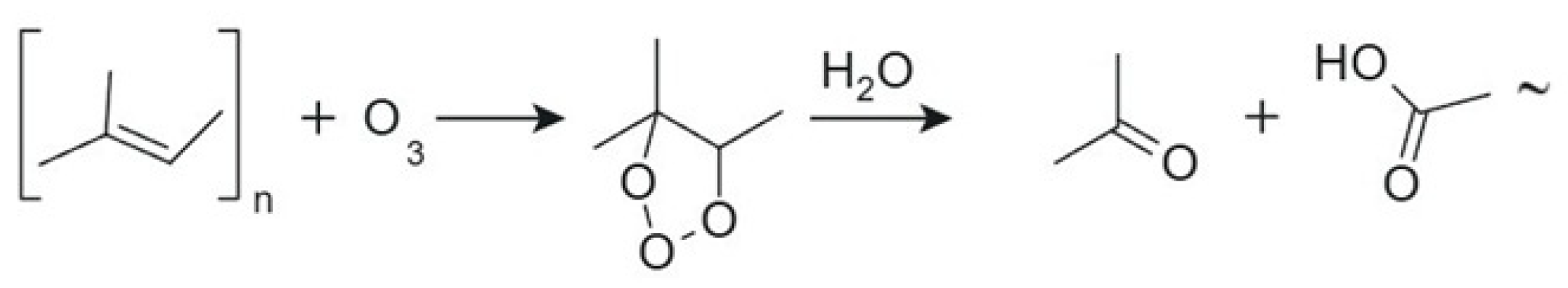
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Differential Scanning Calorimetry (DSC)
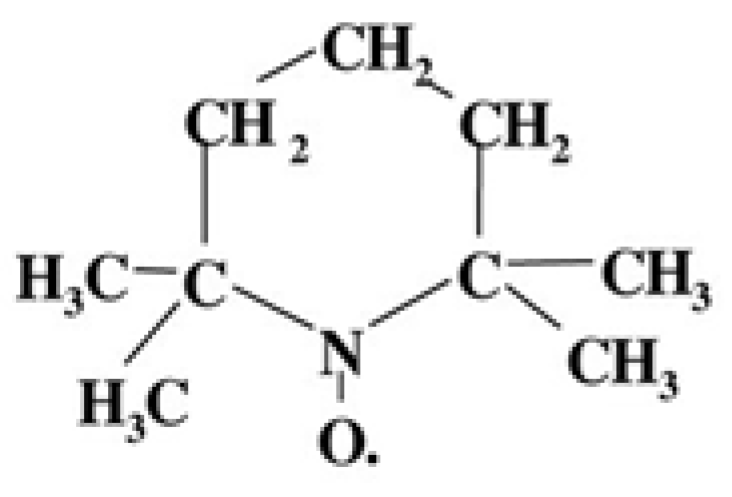
2.3. Electron Paramagnetic Resonance
2.4. Morphology of the Sample
2.5. FTIR Spectroscopy
2.6. Ozone Aging
2.7. X-Ray Diffraction
2.8. Melt Flow Rate Measurement
2.9. Statistical Processing
3. Results and Discussion
3.1. DSC Analysis
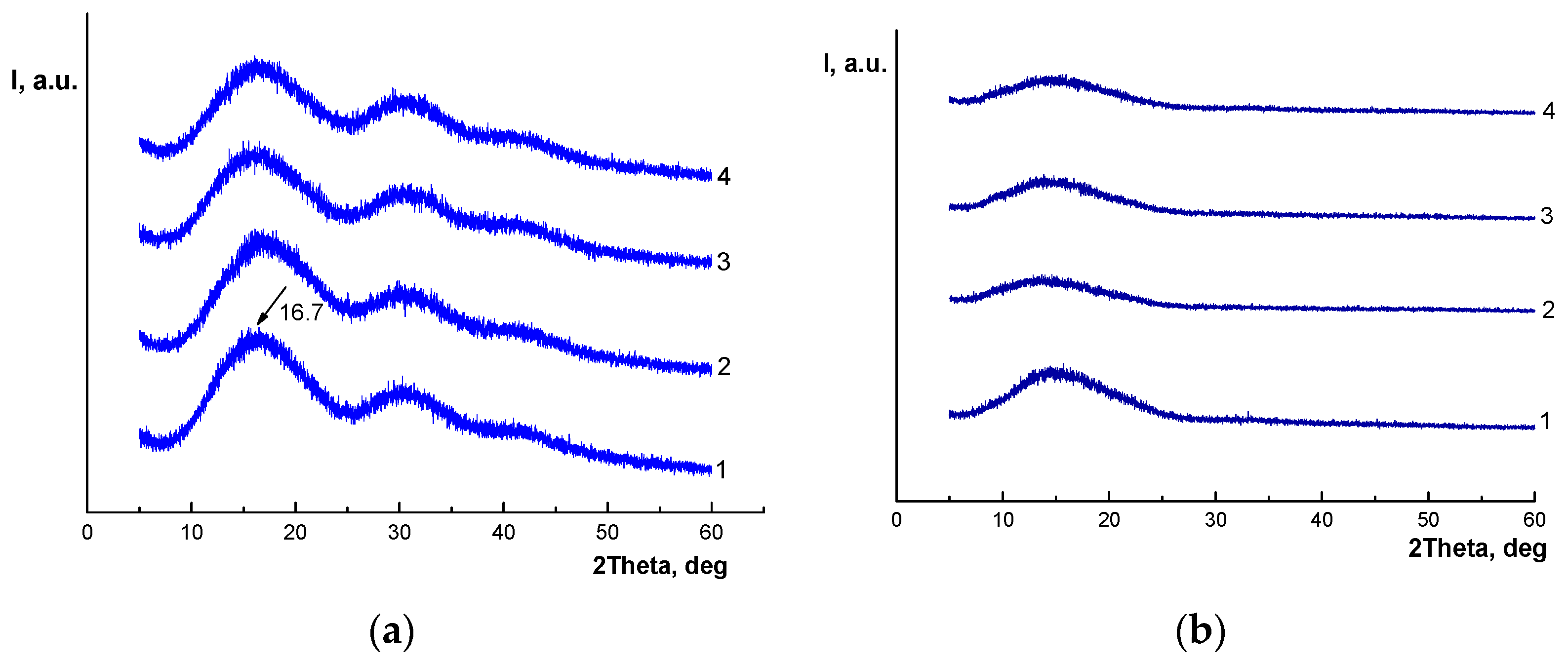
3.2. X-Ray Diffraction
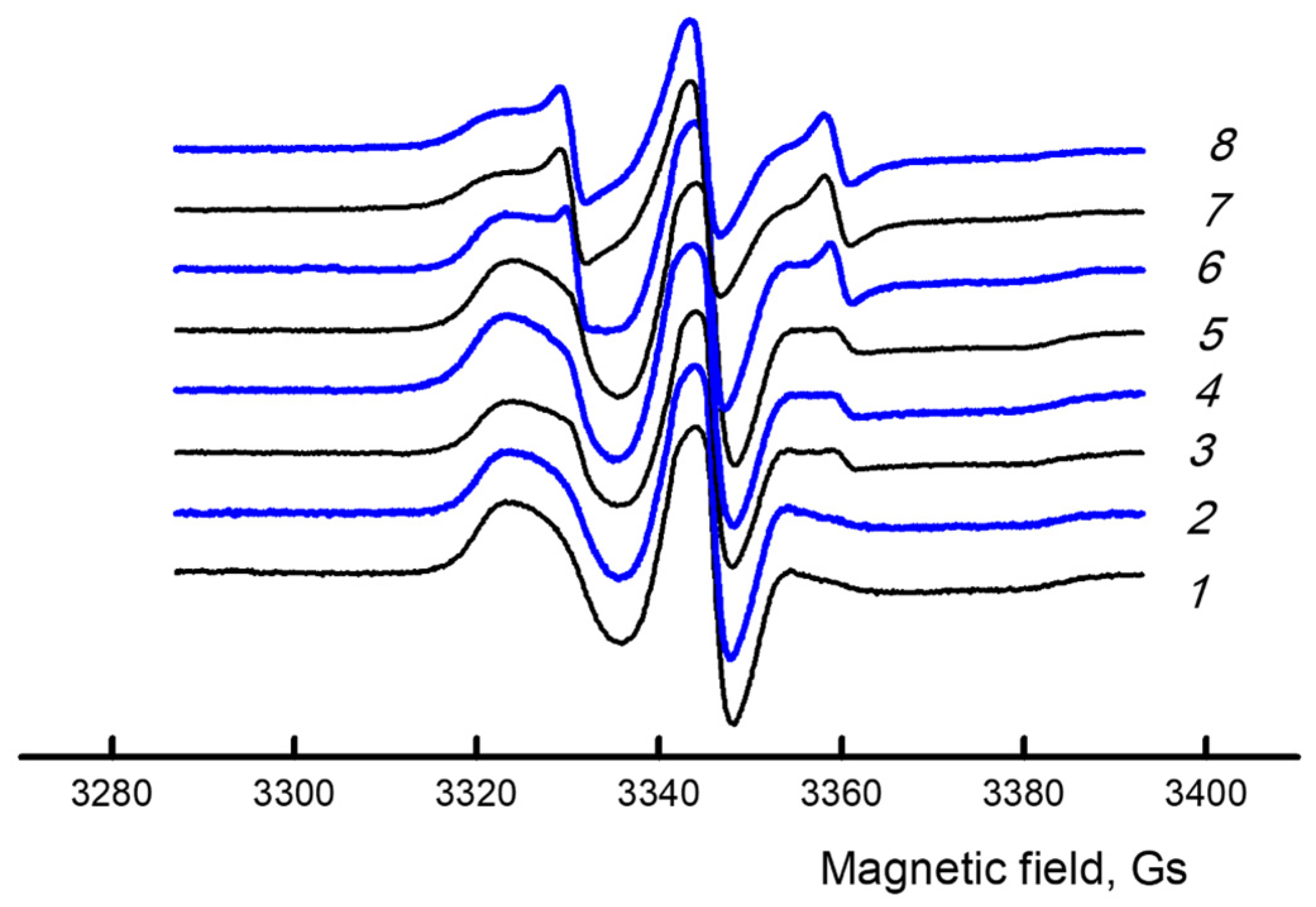
3.3. EPR (Macromolecular Mobility Study)
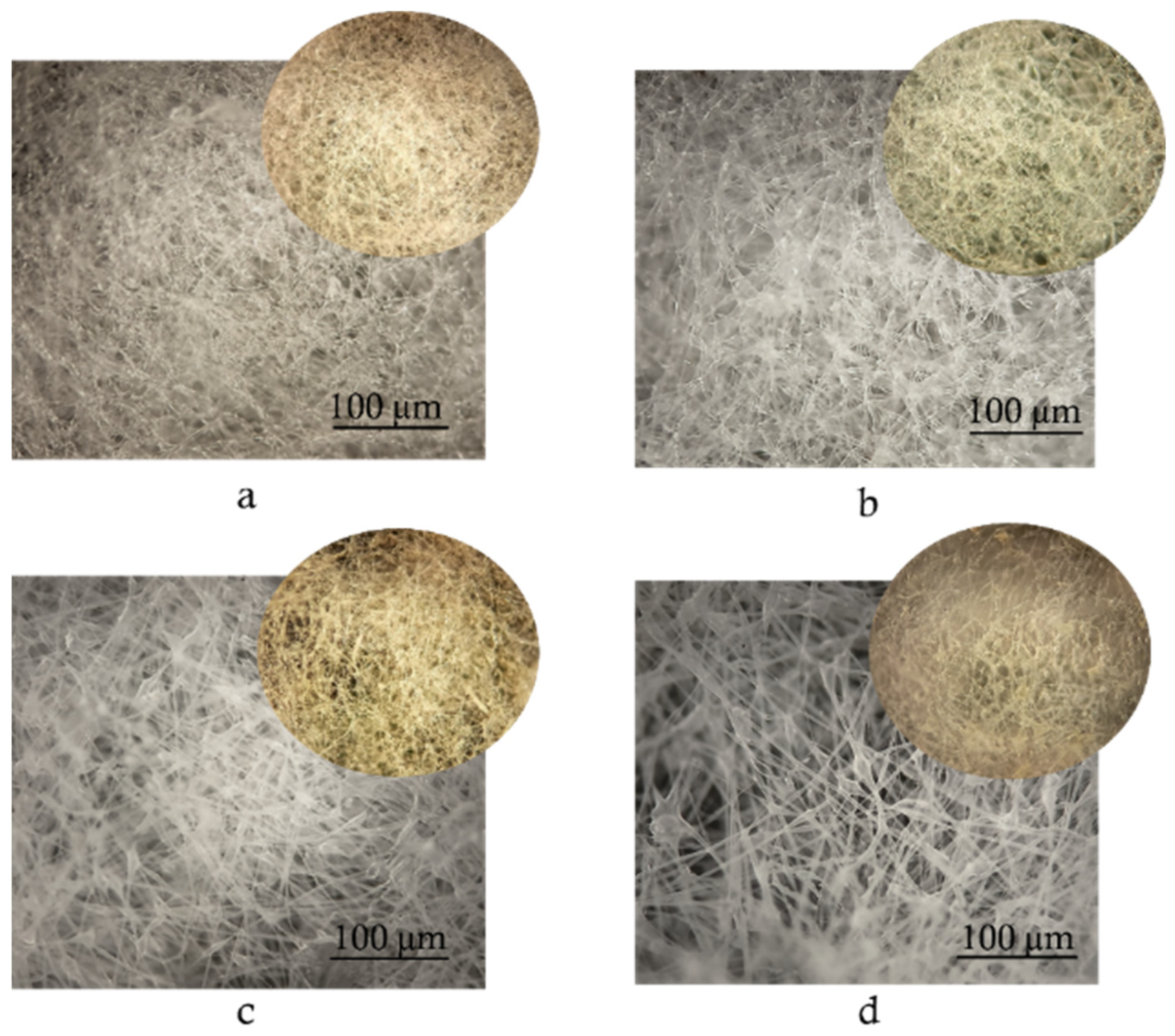
3.4. Morphology of Samples
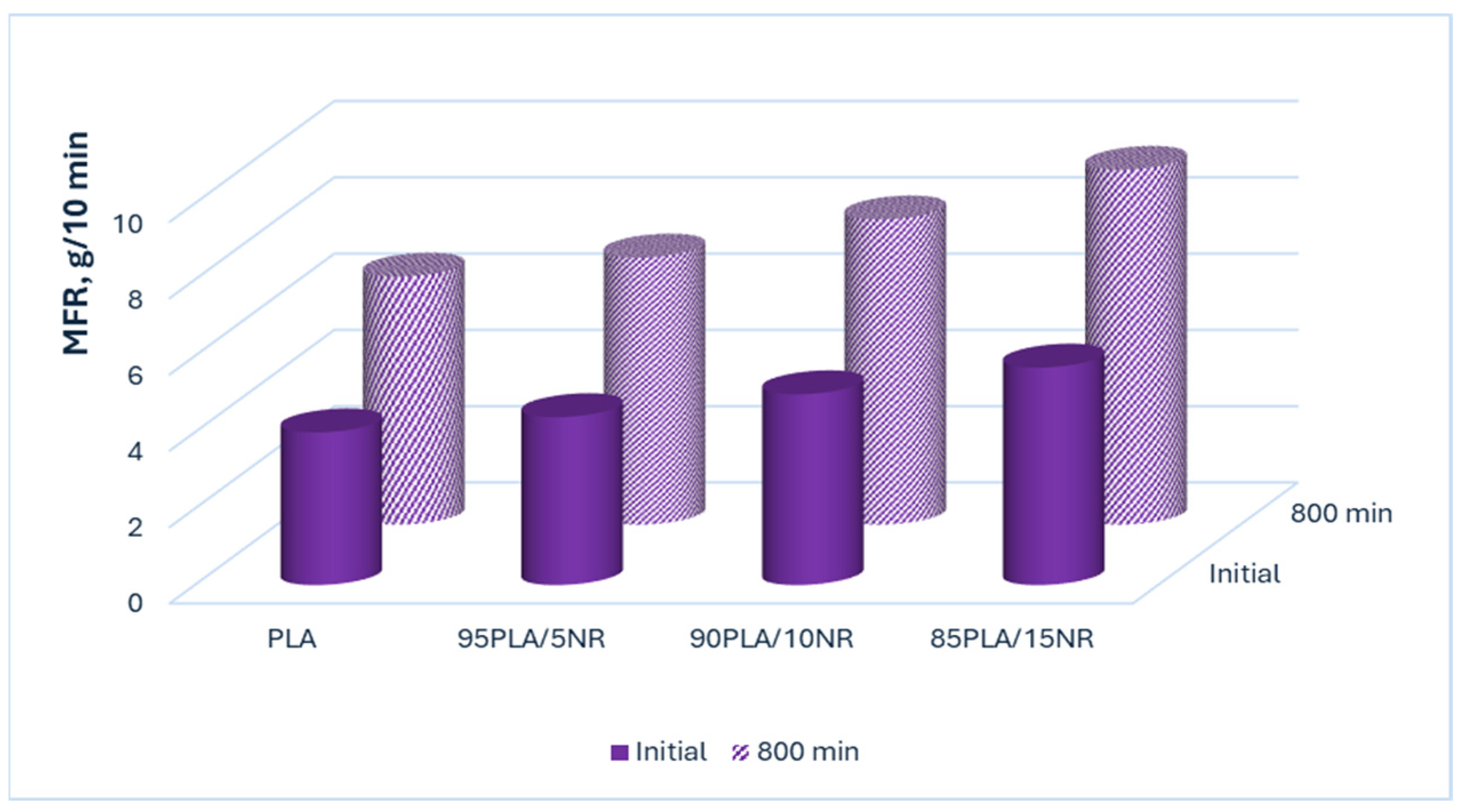
3.5. Melt Flow Rate
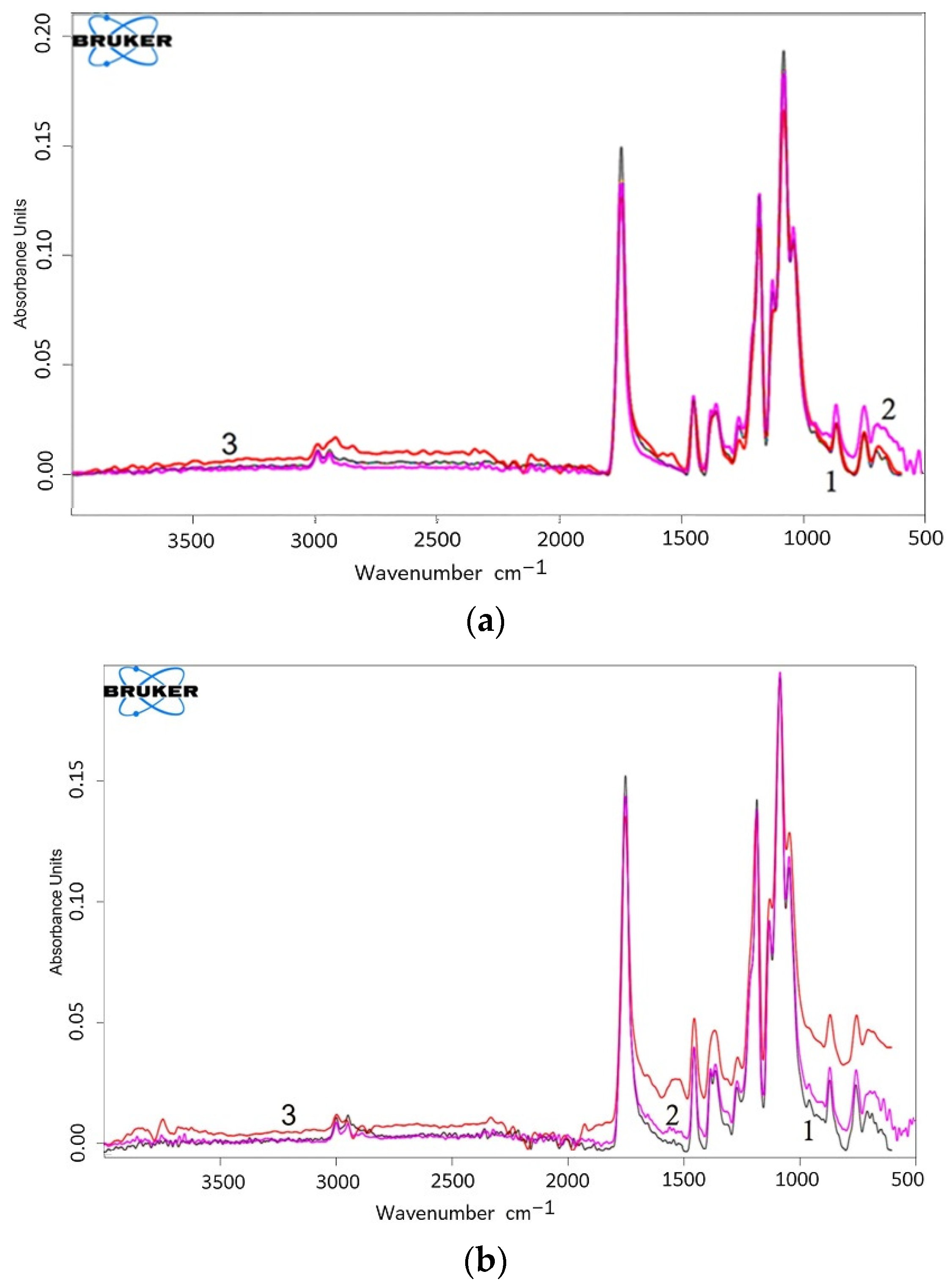
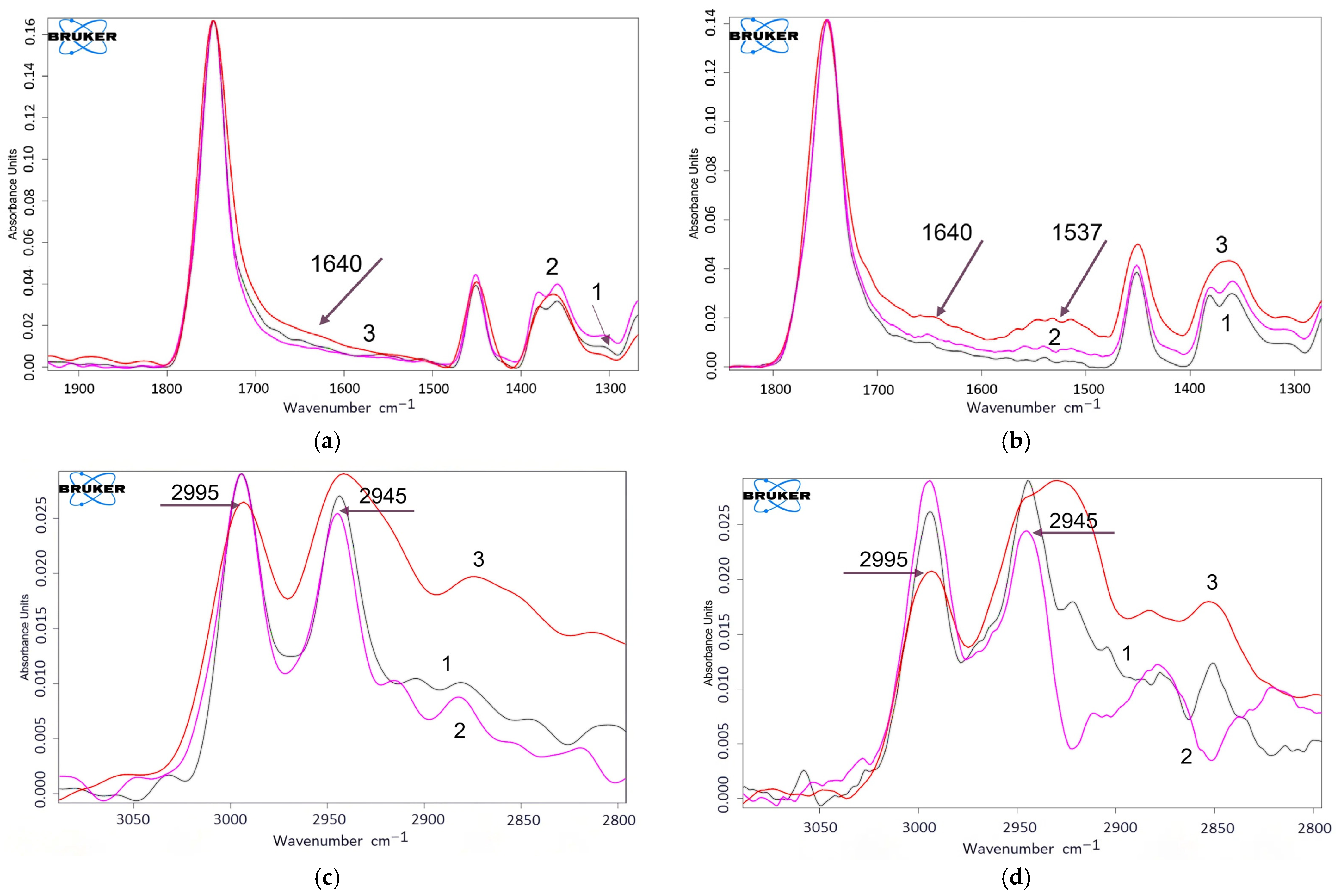
3.6. FTIR-Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLA | Polylactide |
| NR | Natural Rubber |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier Transform Infrared Radiation |
| MFR | Melt Flow Rate |
| XRD | X-ray Diffraction |
| ATR | Attenuated total reflection |
References
- Kim, J.; Kang, S.; Seong, I.; Jeon, J.W.; Lee, D.; Kim, J.-H.; Kwon, D.-J. Advancing CFRP durability: Interfacial and weathering performance of epoxy and acrylic matrices. Compos. B Eng. 2025, 297, 112315. [Google Scholar] [CrossRef]
- Podzorova, M.; Tertyshnaya, Y.; Khramkova, A.; Ivanitskikh, A. Effect of UV-Irradiation and Ozone Exposure on Thermal and Mechanical Properties of PLA/LDPE. Films Modern Trends in Manufacturing Technologies and Equipment. Mater. Res. Proc. 2022, 21, 76–80. [Google Scholar] [CrossRef]
- Zhao, W.; He, J.; Yu, P.; Jiang, X.; Zhang, L. Recent progress in the rubber antioxidants: A review. Polym. Degrad. Stab. 2023, 207, 110223. [Google Scholar] [CrossRef]
- Rose, K.; Tenberge, K.B.; Steinbüchel, A. Identification and characterization of genes from Streptomyces sp. strain K30 responsible for clear zone formation on natural rubber latex and poly(cis-1,4-isoprene) rubber degradation. Biomacromolecules 2005, 6, 180–188. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Podzorova, M.V.; Khramkova, A.V.; Ovchinnikov, V.A.; Krivandin, A.V. Structural Rearrangements of Polylactide/Natural Rubber Composites during Hydro- and Biotic Degradation. Polymers 2023, 15, 1930. [Google Scholar] [CrossRef]
- Podzorova, M.V.; Tertyshnaya, Y.V. Kinetic patterns for thermal oxidation of binary and ternary blends based on polylactide and polyethylene. Russ. Chem. Bull. 2021, 70, 1791–1797. [Google Scholar] [CrossRef]
- Cataldo, F. On the Ozone Protection of Polymers Having Non-Conjugated Unsaturation. Polym. Degrad. Stab. 2001, 72, 287–296. [Google Scholar] [CrossRef]
- Kruza, M.; Lewis, A.C.; Morrison, G.C.; Carslaw, N. Impact of Surface Ozone Interactions on Indoor Air Chemistry: A Modeling Study. Indoor Air 2017, 27, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Eng, A.H.; Kodama, S.; Nagata, K.; Kawasaki, H. Reaction of Moist Ozone with Natural Rubber. J. Rubb. Res. 1998, 1, 133–145. [Google Scholar]
- Treib, C.; Loos, K.; Johlitz, M.; Lion, A. Ozone ageing: Experimental methods on pristine and protected natural rubber. Contin. Mech. Thermodyn. 2022, 34, 1563–1577. [Google Scholar] [CrossRef]
- Cataldo, F. Protection Mechanism of Rubbers from Ozone Attack. Ozone Sci. Eng. J. Int. Ozone Assoc. 2019, 41, 358–368. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Xia, H.; Zhang, Q.; Ying, T.; Hu, T. Removal and reduction of selected organic micro-pollutants in effluent sewage by the ozone-based oxidation processes. Chem. Eng. J. 2015, 269, 245–254. [Google Scholar] [CrossRef]
- Huang, C.; Shi, Y.; El-Din, M.G.; Liu, Y. Optimization of ozonation combined with integrated fixed-film activated sludge (IFAS) in the treatment of oil sands process-affected water (OSPW). Int. Biodeterior. Biodegr. 2016, 112, 31–41. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Luo, X.; Wang, R.; Li, Y.; Shao, H.; Chen, Z. Effect of deoxynivalenol detoxification by ozone treatment in wheat grains. Food Control 2016, 66, 137–144. [Google Scholar] [CrossRef]
- Zeng, Z.; Zou, H.; Li, X.; Arowo, M.; Sun, B.; Chen, J.; Chu, G.; Shao, L. Degradation of phenol by ozone in the presence of Fenton reagent in a rotating packed bed. Chem. Eng. J. 2013, 229, 404–411. [Google Scholar] [CrossRef]
- Kuleznev, V.N.; Shershnev, V.A. Chemistry and Physics of Polymers, 3rd ed.; Lan: St. Petersburg, Russia, 2014; p. 368. [Google Scholar]
- Kamaruddin, S.; Muhr, A.H. Investigation of Ozone Cracking on Natural Rubber. J. Rubber Res. 2018, 21, 73–93. [Google Scholar] [CrossRef]
- Zheng, T.; Zheng, X.; Zhan, S.; Zhou, J.; Liao, S. Study on the ozone aging mechanism of Natural Rubber. Polym. Degrad. Stab. 2021, 186, 109514. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Nowaczyk, J.; Kadac, K. Effect of ozone exposure on thermal and structural properties of polylactide based composites. Polym. Test. 2016, 56, 299–307. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Nowaczyk, J.; Kadac, K. Effect of compatibilizig agent on the properties of polylactide and polylactide based composite during ozone exposure. Polym. Test. 2017, 60, 283. [Google Scholar] [CrossRef]
- Abdullah, M.F.; Andriyan, A.; Muhamad, F.; Ang, B.C. Effect of core-to-shell flowrate ratio on morphology, crystallinity, mechanical properties and wettability of poly(lactic acid) fibers prepared via modified coaxial electrospinning. Polymer 2021, 237, 124378. [Google Scholar] [CrossRef]
- Karpova, S.G.; Tertyshnaya, Y.V.; Podzorova, M.V.; Popov, A.A. Effect of Exposure in Aqueous Medium at Elevated Temperature on the Structure of Nonwoven Materials Based on Polylactide and Natural Rubber. Polym. Sci. Ser. A 2021, 62, 515–525. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H. Thermal and thermo-oxidative degradation kinetics and characteristics of poly (lactic acid) and its composites. Waste Manag. 2019, 87, 335–344. [Google Scholar] [CrossRef]
- Dana, H.R.; Ebrahimi, F. Synthesis, properties, and applications of polylactic acid-based polymers. Polym. Engin. Sci. 2023, 63, 22–43. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Lobanov, A.V.; Morokov, E.S.; Buzanov, G.A.; Abushakhmanova, Z.R. Polylactide—Meso-Substituted Arylporphyrin Composites: Structure, Properties and Antibacterial Activity. Polymers 2023, 15, 1027. [Google Scholar] [CrossRef]
- Shekhar, N.; Mondal, A. Synthesis, properties, environmental degradation, processing, and applications of Polylactic Acid (PLA): An overview. Polym. Bull. 2024, 81, 11421–11457. [Google Scholar] [CrossRef]
- Steinbüchel, L.A. Biodegradation of Natural and Synthetic Rubbers; Wiley-VCH Verlag GmbH & Co. KGaA.: Münster, Germany, 2005. [Google Scholar]
- Povernov, P.A.; Shibryaeva, L.S.; Lyusova, L.R.; Kotova, S.V.; Zykova, A.K. The Influence of Mixing Conditions on the Morphology of Poly-3-Hydroxybutyrate and Nitrile-Butadiene Rubber Polymer Compositions. Polymer Sci. Ser. D 2022, 15, 628–632. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Podzorova, M.V.; Karpova, S.G.; Krivandin, A.V. Structural Features of Polylactide and Natural Rubber Films Produced by Solution Casting. J. Phys. Chem. B 2024, 18, 592–598. [Google Scholar] [CrossRef]
- Xu, C.; Yuan, D.; Fu, L.; Chen, Y. Physical blend of PLA/NR with co-continuous phase structure: Preparation, rheology property, mechanical properties and morphology. Polym. Test. 2014, 37, 94–101. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Pavićević, A.; Luo, J.; Popović-Bijelić, A.; Mojović, M. Maleimido-proxyl as an EPR spin label for the evaluation of conformational changes of albumin. Eur. Biophys. J. 2017, 46, 773–787. [Google Scholar] [CrossRef]
- ISO 9073-2:1995; Textiles—Test Methods for Nonwovens. ISO—International Organization for Standardization: Geneva, Switzerland, 1995.
- GOST 11645-2021 (ISO 1133-1:2011); Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 1: Standard Method. Interstate Council for Standardization, Metrology and Certification (ISC): Moscow, Russia, 2021.
- Kuleznev, V.N. Polymer Blends; Chemistry: Moscow, Russia, 1980; pp. 125–172. [Google Scholar]
- Di Vona, M.L. Annealing of Polymer Membranes. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Jayanth, N.; Jaswanthraj, K.; Sandeep, S.; Mallaya, N.H.; Siddharth, S.R. Effect of heat treatment on mechanical properties of 3D printed PLA. J. Mech. Behav. Biomed. Mater. 2021, 123, 104764. [Google Scholar] [CrossRef]
- Hart, K.R.; Dunn, R.M.; Wetzel, E.D. Increased fracture toughness of additively manufactured semi-crystalline thermoplastics via thermal annealing. Polymer 2020, 211, 123091. [Google Scholar] [CrossRef]
- Gao, P.; Alanazi, S.; Masato, D. Crystallization of Polylactic Acid with Organic Nucleating Agents under Quiescent Conditions. Polymers 2024, 16, 320. [Google Scholar] [CrossRef]
- Zhang, J.; Tashiro, K.; Tsuji, H.; Domb, A.J. Disorder-to-order phase transition and multiple melting behavior of poly(l-lactide) investigated by simultaneous measurements of WAXD and DSC. Macromolecules 2008, 41, 1352–1357. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Cho, Y. Estimation of Synthetic Rubber Lifespan Based on Ozone Accelerated Aging Tests. Polymers 2025, 17, 819. [Google Scholar] [CrossRef] [PubMed]
- Podzorova, M.V.; Tertyshnaya, Y.V. Dynamics of the degradation of polylactide-natural rubber films under the influence of UV-irradiation. J. Phys. Chem. B 2024, 18, 419–424. [Google Scholar] [CrossRef]
- Ol’khov, A.A.; Kucherenko, E.L.; Zhul’kina, A.L.; Iordanskii, A.L.; Shibryaeva, L.S.; Tertyshnaya, Y.V.; Kovaleva, A.N. Resistance to thermal oxidation of ethylene propylene rubber and polyhydroxybutyrate blends. Int. Polym. Sci. Technol. 2017, 44, 11–14. [Google Scholar] [CrossRef]
- Margolin, A.L.; Vorontsov, N.V.; Monakhova, T.V.; Popov, A.A. Thermal oxidation of blends of polypropylene and polyamide 6/66. Effect of inhibition. Polym. Degrad. Stab. 2024, 220, 110627. [Google Scholar] [CrossRef]
- Vorontsov, N.V.; Popov, A.A.; Margolin, A.L. The effect of thermal oxidation on biodegradation of isotactic polypropylene, polyamide 6/66-4 and their mixtures. AIP Conf. Proc. 2019, 2167, 020388. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Skorokhodova, A.N.; Anpilova, A.Y.; Olkhov, A.A. Promotive Effect of Non-Woven Polylactide/Natural Rubber Composites on Growth and Biochemical Constituents of Purple Basil (Ocimum basilicum L.). J. Compos. Sci. 2024, 8, 102. [Google Scholar] [CrossRef]
- Molinaro, S.; Romero, M.C.; Boaro, M.; Sensidoni, A.; Lagazio, C.; Morris, M.; Kerry, J. Effect of nanoclay-type and PLA optical purity on the characteristics of PLA-based nanocomposite films. J. Food Eng. 2013, 117, 113–123. [Google Scholar] [CrossRef]
- Wang, X.; Pan, H.; Yang, K.; Zhang, P. Cracking, structural, and mechanical property changes of SIBR and related elastomers during the ozone aging process. Polym. Degrad. Stab. 2022, 195, 109774. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, N.; Sachdeva, A. Factors affecting the ageing of polymer composite: A state of art. Polym. Degrad. Stab. 2024, 221, 110670. [Google Scholar] [CrossRef]
- Zaidi, L.; Kaci, M.; Bruzaud, S.; Bourmaud, A.; Grohens, Y. Effect of natural weather on the structure and properties of polylactide/Cloisite 30B nanocomposites. Polym. Degrad. Stab. 2010, 95, 1751. [Google Scholar] [CrossRef]
- West, C.; McTaggart, R.; Letcher, T.; Raynie, D.; Roy, R. Effects of gamma irradiation upon the mechanical and chemical properties of 3D-printed samples of polylactic acid. J. Manuf. Sci. Eng. 2019, 141, 041002-1. [Google Scholar] [CrossRef]
- Krikorian, V.; Pochan, D.J. Crystallization behavior of poly(L-lactic acid) nanocomposites: Nucleation and growth probed by infrared spectroscopy. Macromolecules 2005, 38, 6520. [Google Scholar] [CrossRef]
- Rodrigues, F.H.A.; Santos, E.F.; Feitosa, J.P.A.; Ricardo, N.M.P.S.; Heatley, F. Ozonation of unstretched natural rubber film from Hevea brasiliensis studied by ozone consumption and 13C NMR. Polym. Int. 2004, 53, 733–739. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.; Karpova, S.; Moskovskiy, M.; Dorokhov, A. Electrospun Polylactide/Natural Rubber Fibers: Effect Natural Rubber Content on Fiber Morphology and Properties. Polymers 2021, 13, 2232. [Google Scholar] [CrossRef]
- Bitinis, N.; Verdejo, R.; Maya, E.M.; Espuche, E.; Cassagnau, P.; Lopez-Manchado, M.A. Physicochemical properties of organoclay filled polylactic acid/natural rubber blend bionanocomposites. Compos. Sci. Technol. 2012, 72, 305–313. [Google Scholar] [CrossRef]

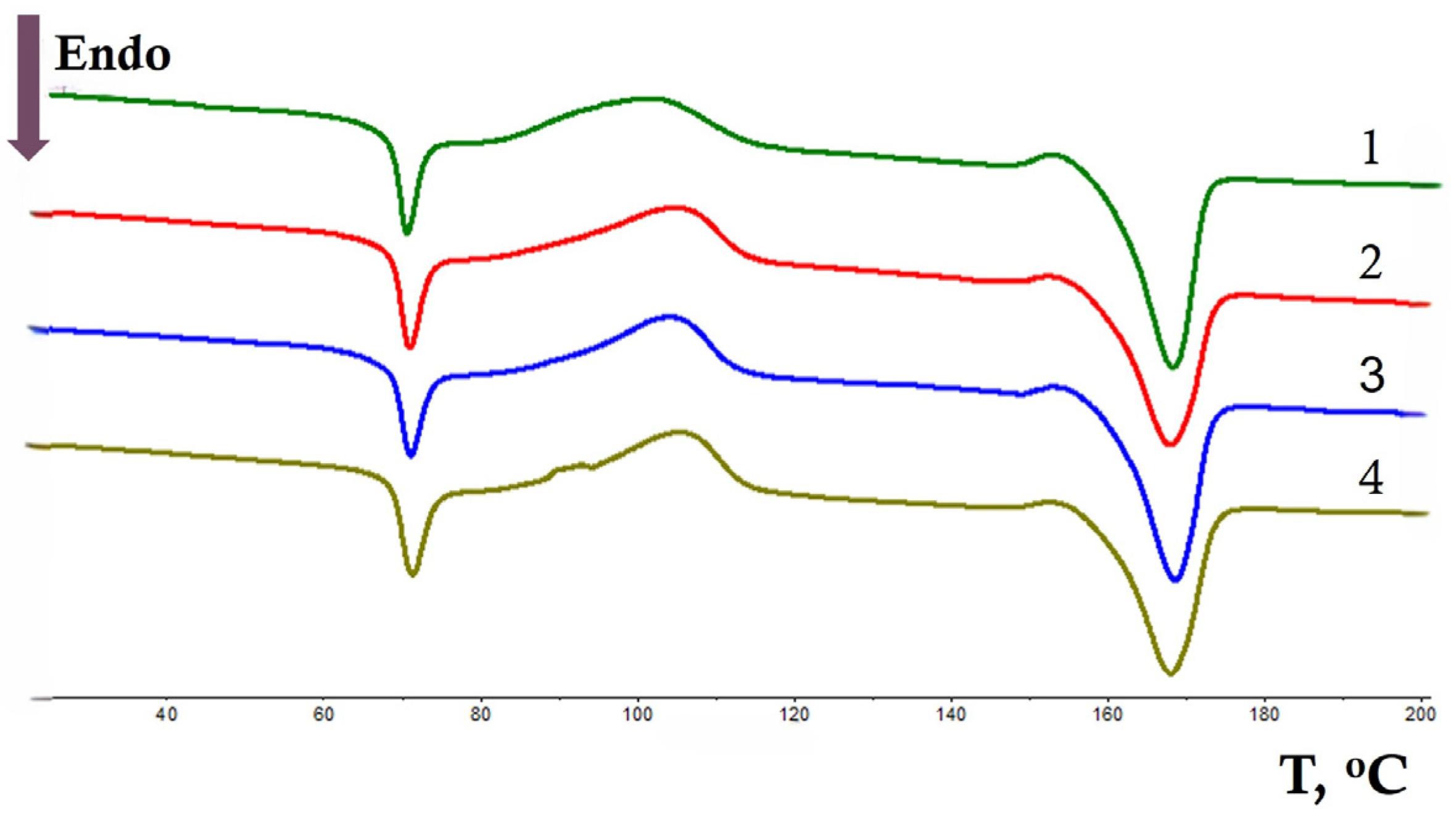
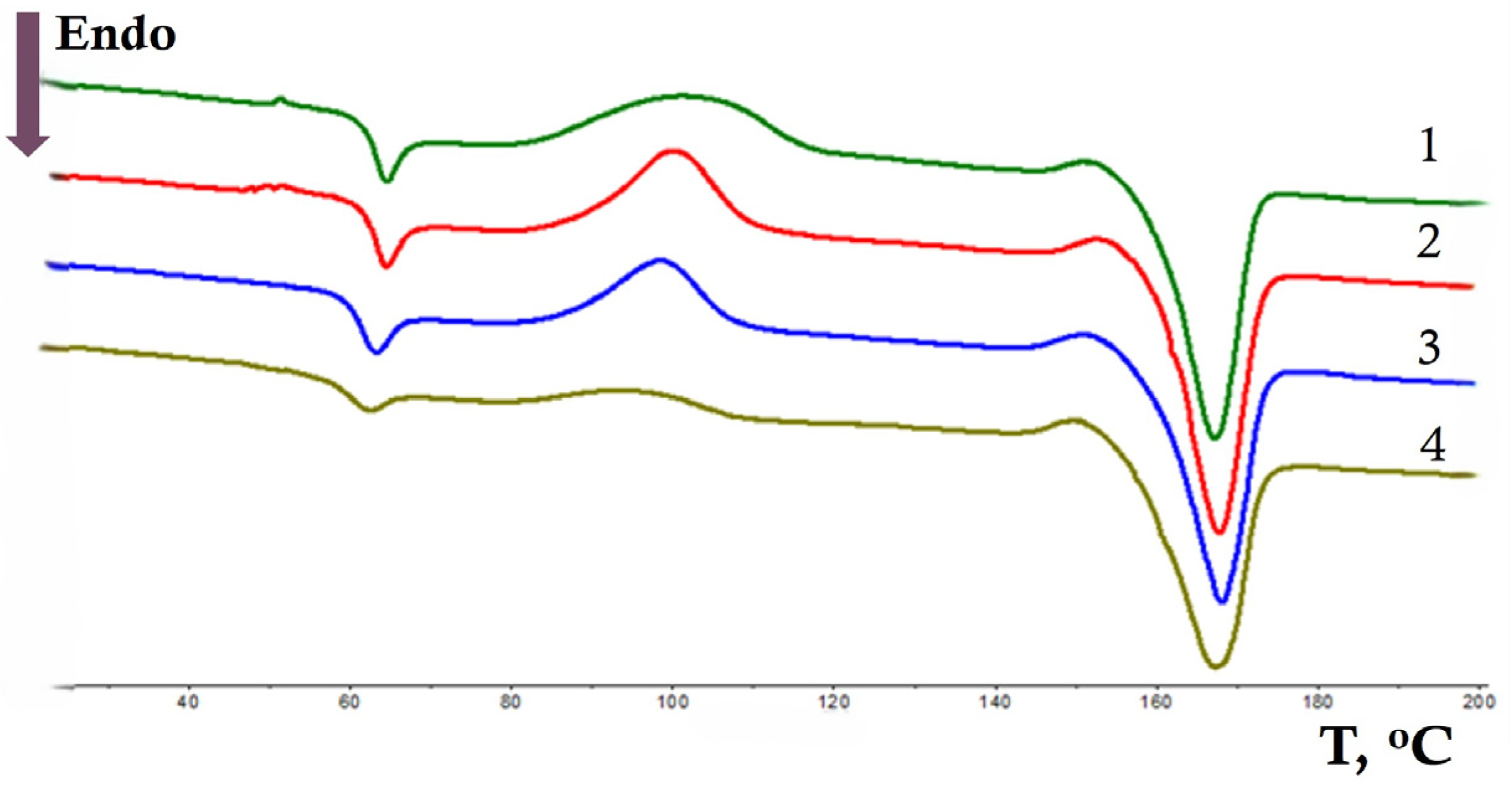

| NR, wt.% | Tg, °C (Δ ± 0.3 °C) | Tcc, °C (Δ ± 0.3 °C) | Tm, °C (Δ ± 0.3 °C) | χc, % (Δ ± 1%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min | 400 min | 800 min | 0 | 400 | 800 | 0 | 400 | 800 | 0 | 400 | 800 | |
| 0 | 66 | 64 | 61 | 101 | 102 | 103 | 167 | 167 | 167 | 16 | 17 | 20 |
| 5 | 67 | 64 | 61 | 104 | 102 | 100 | 168 | 167 | 167 | 11 | 15 | 23 |
| 10 | 68 | 62 | 59 | 104 | 101 | 98 | 168 | 167 | 167 | 13 | 18 | 25 |
| 15 | 68 | 63 | 59 | 105 | 100 | 94 | 168 | 167 | 167 | 12 | 20 | 30 |
| NR, wt.% | Rotational Correlation Time, τc × 10−10, s−1 | |||
|---|---|---|---|---|
| 0 min | 200 min | 400 min | 800 min | |
| 0 | 80.2 ± 0.3 | 65.0 ± 0.5 | 46.6 ± 0.7 | 34.3 ± 0.5 |
| 5 | 42.4 ± 0.6 | 34.1 ± 0.7 | 28.7 ± 0.5 | 14.1 ± 0.6 |
| 10 | 31.3 ± 0.3 | 22.0 ± 0.4 | 15.5 ± 0.6 | 8.2 ± 0.4 |
| 15 | 20.5 ± 0.4 | 13.5 ± 0.5 | 10.2 ± 0.4 | 4.5 ± 0.2 |
| NR Content, wt.% | dav, µm | Bulk Density g/cm3 |
|---|---|---|
| 0 | 5.7–7.4/5.6–7.3 | 0.160/0.157 |
| 5 | 6.4–8.5/6.2–8.4 | 0.172/0.167 |
| 10 | 6.7–9.3/6.4–9.2 | 0.178/0.172 |
| 15 | 6.7–9.9/6.3–9.7 | 0.182/0.174 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tertyshnaya, Y.V.; Karpova, S.G.; Podzorova, M.V. Effect of Ozone on Nonwoven Polylactide/Natural Rubber Fibers. Polymers 2025, 17, 2102. https://doi.org/10.3390/polym17152102
Tertyshnaya YV, Karpova SG, Podzorova MV. Effect of Ozone on Nonwoven Polylactide/Natural Rubber Fibers. Polymers. 2025; 17(15):2102. https://doi.org/10.3390/polym17152102
Chicago/Turabian StyleTertyshnaya, Yulia V., Svetlana G. Karpova, and Maria V. Podzorova. 2025. "Effect of Ozone on Nonwoven Polylactide/Natural Rubber Fibers" Polymers 17, no. 15: 2102. https://doi.org/10.3390/polym17152102
APA StyleTertyshnaya, Y. V., Karpova, S. G., & Podzorova, M. V. (2025). Effect of Ozone on Nonwoven Polylactide/Natural Rubber Fibers. Polymers, 17(15), 2102. https://doi.org/10.3390/polym17152102








