Engineered Hydrogels for Musculoskeletal Regeneration: Advanced Synthesis Strategies and Therapeutic Efficacy in Preclinical Models
Abstract
1. Introduction
2. Materials and Methods
3. Novel Design for Functionalized Composite Hydrogels
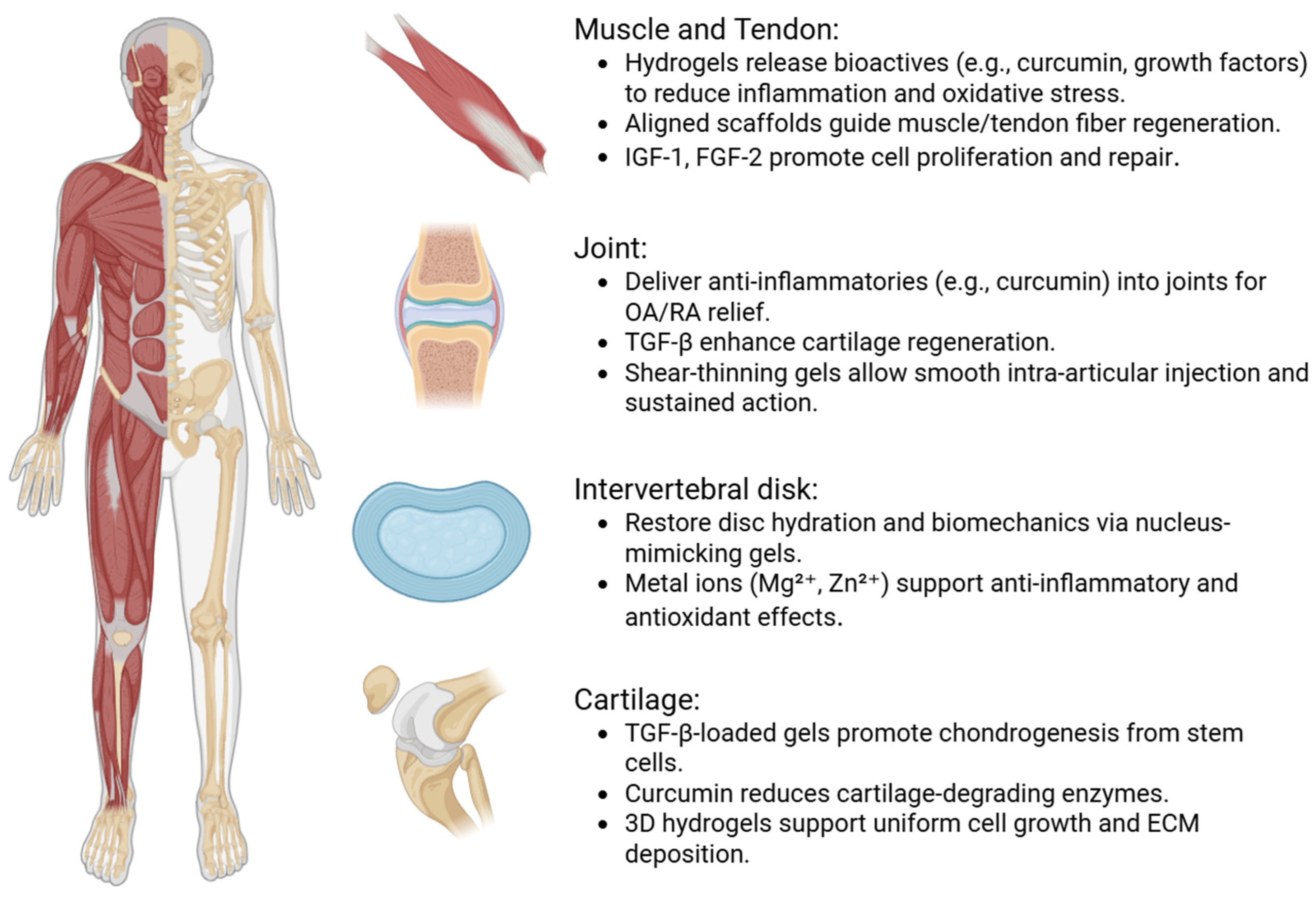
4. Therapeutic Outcomes in Musculoskeletal Models
5. Therapeutic Applications and Clinical Translation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sebbag, E.; Felten, R.; Sagez, F.; Sibilia, J.; Devilliers, H.; Arnaud, L. The World-Wide Burden of Musculoskeletal Diseases: A Systematic Analysis of the World Health Organization Burden of Diseases Database. Ann. Rheum. Dis. 2019, 78, 844–848. [Google Scholar] [CrossRef]
- Govaerts, R.; Tassignon, B.; Ghillebert, J.; Serrien, B.; De Bock, S.; Ampe, T.; El Makrini, I.; Vanderborght, B.; Meeusen, R.; De Pauw, K. Prevalence and Incidence of Work-Related Musculoskeletal Disorders in Secondary Industries of 21st Century Europe: A Systematic Review and Meta-Analysis. BMC Musculoskelet. Disord. 2021, 22, 751. [Google Scholar] [CrossRef] [PubMed]
- Grigore, A.; Coman, O.A.; Păunescu, H.; Costescu, M.; Fulga, I. Latest Insights into the In Vivo Studies in Murine Regarding the Role of TRP Channels in Wound Healing—A Review. Int. J. Mol. Sci. 2024, 25, 6753. [Google Scholar] [CrossRef] [PubMed]
- Diac, M.M.; Earar, K.; Damian, S.I.; Knieling, A.; Iov, T.; Shrimpton, S.; Castaneyra-Ruiz, M.; Wilkinson, C.; Bulgaru Iliescu, D. Facial Reconstruction: Anthropometric Studies Regarding the Morphology of the Nose for Romanian Adult Population I: Nose Width. Appl. Sci. 2020, 10, 6479. [Google Scholar] [CrossRef]
- Rusu, A. Compatibility Study of Four Binary Combinations of Active Ingredients for Dermal Film Forming Systems. FARMACIA 2020, 68, 800–811. [Google Scholar] [CrossRef]
- Rédai, E.-M.; Antonoaea, P.; Todoran, N.; Vlad, R.A.; Bîrsan, M.; Tătaru, A.; Ciurba, A. Development and Evaluation of Fluoxetine Fast Dissolving Films: An Alternative for Noncompliance in Pediatric Patients. Processes 2021, 9, 778. [Google Scholar] [CrossRef]
- Lungu, I.I.; Stefanache, A.; Crivoi, F.; Burec, A.-F.; Belei, D.; Cioanca, O.; Hancianu, M. Innovative Synthesis of Zinc and Selenium Complexes with Gallic Acid: Exploring Their Antioxidant Potential. Med.-Surg. J. 2024, 128, 177–188. [Google Scholar] [CrossRef]
- Lungu, I.I.; Cioanca, O.; Mircea, C.; Tuchilus, C.; Stefanache, A.; Huzum, R.; Hancianu, M. Insights into Catechin–Copper Complex Structure and Biologic Activity Modulation. Molecules 2024, 29, 4969. [Google Scholar] [CrossRef]
- Cheng, K.; Guo, Q.; He, Y.; Lu, Y.; Xie, R.; Li, C.; Wu, H. Artificial Intelligence in Sports Medicine: Could GPT-4 Make Human Doctors Obsolete? Ann. Biomed. Eng. 2023, 51, 1658–1662. [Google Scholar] [CrossRef]
- Mihai, C.; Chiriță, R.; Robu, V.; Untu, I. Predicting Suicide Risk among Male Offenders: The Role of Severe Personality Disorders. Rev. Cercet. Şi Interv. Soc. 2017, 57, 28–50. [Google Scholar]
- Costandache, G.I.; Munteanu, O.; Salaru, A.; Oroian, B.; Cozmin, M. An Overview of the Treatment of Eating Disorders in Adults and Adolescents: Pharmacology and Psychotherapy. Adv. Psychiatry Neurol. Psychiatr. Neurol. 2023, 32, 40–48. [Google Scholar] [CrossRef]
- Fischer, K.M.; Scott, T.E.; Browe, D.P.; McGaughey, T.A.; Wood, C.; Wolyniak, M.J.; Freeman, J.W. Hydrogels for Skeletal Muscle Regeneration. Regen. Eng. Transl. Med. 2021, 7, 353–361. [Google Scholar] [CrossRef]
- Zhang, F.-X.; Chien, M.-H.; Fan, Q.; Jiang, D. Advances in Bioadhesive Hydrogels for Musculoskeletal Tissue Application. Adv. Funct. Mater. 2024, 34, 2316540. [Google Scholar] [CrossRef]
- Balestri, W.; Morris, R.H.; Hunt, J.A.; Reinwald, Y. Current Advances on the Regeneration of Musculoskeletal Interfaces. Tissue Eng. Part B Rev. 2021, 27, 548–571. [Google Scholar] [CrossRef] [PubMed]
- Canciani, B.; Semeraro, F.; Herrera Millar, V.R.; Gervaso, F.; Polini, A.; Stanzione, A.; Peretti, G.M.; Di Giancamillo, A.; Mangiavini, L. In Vitro and In Vivo Biocompatibility Assessment of a Thermosensitive Injectable Chitosan-Based Hydrogel for Musculoskeletal Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 10446. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, S.; Li, E.N.; Fritch, M.R.; Alexander, P.G.; Lin, H.; Tuan, R.S. Tissue Engineering for Musculoskeletal Regeneration and Disease Modeling. In Organotypic Models in Drug Development; Schäfer-Korting, M., Stuchi Maria-Engler, S., Landsiedel, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 235–268. ISBN 978-3-030-70063-8. [Google Scholar]
- Călin, G.; Costescu, E.; Damir, D.L.; Mihai, C.; Grierosu, C. Pluridisciplinary Approaches in Global Postural Rehabilitation. Balneo PRM Res. J. 2024, 15, 711. [Google Scholar] [CrossRef]
- Costescu, E.; Ciuhodaru, T.; Calin, G.; Mihai, C.; Grierosu, C.; Damir, D.L. Non-Invasive Neuromodulatory Therapies Applied in Trigeminal Neuralgia. Balneo PRM Res. J. 2024, 15, 705. [Google Scholar] [CrossRef]
- Ma, Y.; Han, T.; Yang, Q.; Wang, J.; Feng, B.; Jia, Y.; Wei, Z.; Xu, F. Viscoelastic Cell Microenvironment: Hydrogel-Based Strategy for Recapitulating Dynamic ECM Mechanics. Adv. Funct. Mater. 2021, 31, 2100848. [Google Scholar] [CrossRef]
- Tremmel, D.M.; Sackett, S.D.; Feeney, A.K.; Mitchell, S.A.; Schaid, M.D.; Polyak, E.; Chlebeck, P.J.; Gupta, S.; Kimple, M.E.; Fernandez, L.A.; et al. A Human Pancreatic ECM Hydrogel Optimized for 3-D Modeling of the Islet Microenvironment. Sci. Rep. 2022, 12, 7188. [Google Scholar] [CrossRef]
- Pollot, B.E.; Rathbone, C.R.; Wenke, J.C.; Guda, T. Natural Polymeric Hydrogel Evaluation for Skeletal Muscle Tissue Engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 672–679. [Google Scholar] [CrossRef]
- Yang, P.; Li, C.; Lee, M.; Marzvanyan, A.; Zhao, Z.; Ting, K.; Soo, C.; Zheng, Z. Photopolymerizable Hydrogel-Encapsulated Fibromodulin-Reprogrammed Cells for Muscle Regeneration. Tissue Eng. Part A 2020, 26, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Bai, L.; Xu, M.; Dong, R.; Yin, Z.; Zhao, W.; Guo, B.; Hu, J. Magnetically Induced Anisotropic Conductive In Situ Hydrogel for Skeletal Muscle Regeneration by Promoting Cell Alignment and Myogenic Differentiation. Chem. Eng. J. 2024, 484, 149019. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Luo, S.; Chen, Z.; Zheng, X.; Kankala, R.K.; Chen, A.; Wang, S. 3D Bioprinting of Conductive Hydrogel for Enhanced Myogenic Differentiation. Regen. Biomater. 2021, 8, rbab035. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Chapter 2—Natural Polymers and the Hydrogels Prepared from Them. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. ISBN 978-0-12-816421-1. [Google Scholar]
- Gul, K.; Gan, R.-Y.; Sun, C.-X.; Jiao, G.; Wu, D.-T.; Li, H.-B.; Kenaan, A.; Corke, H.; Fang, Y.-P. Recent Advances in the Structure, Synthesis, and Applications of Natural Polymeric Hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 3817–3832. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hokmabad, V.R.; Del Bakhshayesh, A.R.; Asadi, N.; Salehi, R.; Nasrabadi, H.T. The Application of Hydrogels Based on Natural Polymers for Tissue Engineering. Curr. Med. Chem. 2020, 27, 2658–2680. [Google Scholar] [CrossRef]
- Resende, J.F.; Paulino, I.M.R.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Hydrogels Produced from Natural Polymers: A Review on Its Use and Employment in Water Treatment. Braz. J. Chem. Eng. 2023, 40, 23–38. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A. Chapter 6—The Physical and Chemical Properties of Hydrogels Based on Natural Polymers. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–172. ISBN 978-0-12-816421-1. [Google Scholar]
- Xu, Y.; Xu, C.; He, L.; Zhou, J.; Chen, T.; Ouyang, L.; Guo, X.; Qu, Y.; Luo, Z.; Duan, D. Stratified-Structural Hydrogel Incorporated with Magnesium-Ion-Modified Black Phosphorus Nanosheets for Promoting Neuro-Vascularized Bone Regeneration. Bioact. Mater. 2022, 16, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Xiong, H.; Yao, S.-Y.; Wang, S.; Li, S.; Chang, J.; Zhai, Z.; Guo, D.-S.; Fan, C.; Gao, C. Hypoxia and Matrix Metalloproteinase 13-Responsive Hydrogel Microspheres Alleviate Osteoarthritis Progression In Vivo. Small 2024, 20, 2308599. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liang, Y.; Zhang, J.; Bai, L.; Xu, M.; Han, Q.; Han, X.; Xiu, J.; Li, M.; Zhou, X.; et al. Synergistic Enhancement of Tendon-to-Bone Healing via Anti-Inflammatory and pro-Differentiation Effects Caused by Sustained Release of Mg2+/Curcumin from Injectable Self-Healing Hydrogels. Theranostics 2021, 11, 5911–5925. [Google Scholar] [CrossRef]
- Jiang, W.; Pan, Z.; Zhang, Z.; Yan, X.; Palasuberniam, P.; Xiao, J.; Shen, J.; Guo, D.; Zhang, Y.; Liu, H. An Injectable Composite Hydrogel Enhances Bone Regeneration by Rescuing Impaired Mitochondrial Biogenesis and Fusion of BMSCs under Inflammatory Conditions. ACS Appl. Polym. Mater. 2025, 7, 2297–2310. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, L.; Wei, D.; Liu, S.; Zhang, Z.; Lian, R.; Wang, L.; Chen, Y.; Jiang, J.; Xiao, Y.; et al. Injectable Biomimetic Hydrogel Guided Functional Bone Regeneration by Adapting Material Degradation to Tissue Healing. Adv. Funct. Mater. 2023, 33, 2213047. [Google Scholar] [CrossRef]
- Teng, C.; Tong, Z.; He, Q.; Zhu, H.; Wang, L.; Zhang, X.; Wei, W. Mesenchymal Stem Cells–Hydrogel Microspheres System for Bone Regeneration in Calvarial Defects. Gels 2022, 8, 275. [Google Scholar] [CrossRef]
- Lei, T.; Tong, Z.; Zhai, X.; Zhao, Y.; Zhu, H.; Wang, L.; Wen, Z.; Song, B. Chondroitin Sulfate Improves Mechanical Properties of Gelatin Hydrogel for Cartilage Regeneration in Rats. Adv. Biol. 2023, 7, 2300249. [Google Scholar] [CrossRef]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking Strategies for 3D Bioprinting of Polymeric Hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Peng, K.; Mitragotri, S. Covalently Crosslinked Hydrogels via Step-Growth Reactions: Crosslinking Chemistries, Polymers, and Clinical Impact. Adv. Mater. 2021, 33, 2006362. [Google Scholar] [CrossRef] [PubMed]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liu, C.; Liu, C.; Hu, F.; Li, X.; Wang, C.; Jian, X. Study on New Polymer Crosslinking Agents and Their Functional Hydrogels. Eur. Polym. J. 2020, 134, 109835. [Google Scholar] [CrossRef]
- Wu, S.; Gai, T.; Chen, J.; Chen, X.; Chen, W. Smart Responsive in Situ Hydrogel Systems Applied in Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2024, 12, 1389733. [Google Scholar] [CrossRef]
- Yang, J.; Xia, P.; Meng, F.; Li, X.; Xu, X. Bio-Functional Hydrogel Microspheres for Musculoskeletal Regeneration. Adv. Funct. Mater. 2024, 34, 2400257. [Google Scholar] [CrossRef]
- Clegg, J.R.; Adebowale, K.; Zhao, Z.; Mitragotri, S. Hydrogels in the Clinic: An Update. Bioeng. Transl. Med. 2024, 9, e10680. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.U.; Srinivasan, S.S.; Kumbar, S.G.; Moss, I.L. Hydrogel-Based Strategies for Intervertebral Disc Regeneration: Advances, Challenges and Clinical Prospects. Gels 2024, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Darai, A.; Pongkulapa, T.; Conley, B.; Yang, L.; Han, I.; Lee, K.-B. Injectable Bioorthogonal Hydrogel (BIOGEL) Accelerates Tissue Regeneration in Degenerated Intervertebral Discs. Bioact. Mater. 2023, 23, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, S.; Li, G.; Wang, C.; Guo, X.; Zhang, J.; Liu, J.; Xu, Y.; Wang, Y. Fabrication and Characterization of Phyllanthus Emblica Extract-Polyvinyl Alcohol/Carboxymethyl Cellulose Sodium Antioxidant Hydrogel and Its Application in Wound Healing. Pharmaceutics 2024, 16, 1531. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Zainul Armir, N.A.; Khairunnisa-Atiqah, M.K.; Wang, B. Electrovalent Effects of Sodium Carboxymethyl Cellulose and Hydroxyethyl Cellulose on Regeneration of Empty Fruit Bunch Cellulose to a Superabsorbent Hydrogel. Int. J. Biol. Macromol. 2024, 278, 134816. [Google Scholar] [CrossRef]
- Dienes, J.; Browne, S.; Farjun, B.; Amaral Passipieri, J.; Mintz, E.L.; Killian, G.; Healy, K.E.; Christ, G.J. Semisynthetic Hyaluronic Acid-Based Hydrogel Promotes Recovery of the Injured Tibialis Anterior Skeletal Muscle Form and Function. ACS Biomater. Sci. Eng. 2021, 7, 1587–1599. [Google Scholar] [CrossRef]
- Basurto, I.M.; Passipieri, J.A.; Gardner, G.M.; Smith, K.K.; Amacher, A.R.; Hansrisuk, A.I.; Christ, G.J.; Caliari, S.R. Photoreactive Hydrogel Stiffness Influences Volumetric Muscle Loss Repair. Tissue Eng. Part A 2022, 28, 312–329. [Google Scholar] [CrossRef]
- Chang, L.; Li, Y.; Li, M.; Liu, S.; Han, J.; Zhao, G.; Ji, C.; Lyu, Y.; Genin, G.M.; Bai, B.; et al. An Injectable, Biodegradable Magnetic Hydrogel System for Exogenous Promotion of Muscle Mass and Regeneration. Chem. Eng. J. 2021, 420, 130398. [Google Scholar] [CrossRef]
- Alheib, O.; da Silva, L.P.; da Silva Morais, A.; Mesquita, K.A.; Pirraco, R.P.; Reis, R.L.; Correlo, V.M. Injectable Laminin-Biofunctionalized Gellan Gum Hydrogels Loaded with Myoblasts for Skeletal Muscle Regeneration. Acta Biomater. 2022, 143, 282–294. [Google Scholar] [CrossRef]
- Shan, H.; Gao, X.; Zhang, M.; Huang, M.; Fang, X.; Chen, H.; Tian, B.; Wang, C.; Zhou, C.; Bai, J.; et al. Injectable ROS-Scavenging Hydrogel with MSCs Promoted the Regeneration of Damaged Skeletal Muscle. J. Tissue Eng. 2021, 12, 20417314211031378. [Google Scholar] [CrossRef]
- Prabhath, A.; Vernekar, V.N.; Esdaille, C.J.; Eisenberg, E.; Lebaschi, A.; Badon, M.; Seyedsalehi, A.; Dzidotor, G.; Tang, X.; Dyment, N.; et al. Pegylated Insulin-like Growth Factor-1 Biotherapeutic Delivery Promotes Rotator Cuff Regeneration in a Rat Model. J. Biomed. Mater. Res. A 2022, 110, 1356–1371. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Lu, L.; Hu, L.; Cheng, P.; Hu, Y.; Xie, X.; Dai, G.; Mi, B.; Liu, X.; Liu, G. Recent Advances in GelMA Hydrogel Transplantation for Musculoskeletal Disorders and Related Disease Treatment. Theranostics 2023, 13, 2015–2039. [Google Scholar] [CrossRef]
- Basurto, I.M.; Mora, M.T.; Gardner, G.M.; Christ, G.J.; Caliari, S.R. Aligned and Electrically Conductive 3D Collagen Scaffolds for Skeletal Muscle Tissue Engineering. Biomater. Sci. 2021, 9, 4040–4053. [Google Scholar] [CrossRef]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef]
- Ji, W.; Han, F.; Feng, X.; Shi, L.; Ma, H.; Lu, Y.; Tao, R. Cocktail-like Gradient Gelatin/Hyaluronic Acid Bioimplant for Enhancing Tendon-Bone Healing in Fatty-Infiltrated Rotator Cuff Injury Models. Int. J. Biol. Macromol. 2023, 244, 125421. [Google Scholar] [CrossRef]
- Paoli, A. Mesenchymal Stem Cell Exosomes for Skeletal Muscle Regeneration Following Trauma. Master’s Thesis, Saint Louis University, St. Louis, MO, USA, 2022. [Google Scholar]
- Fan, X.; Zhu, H.; Wang, J.; Dai, Z.; Zhang, S.; Huang, W.; Cai, R.; Qian, K. Water Transport-Modulated Highly Compressive Hydrogel for Total Biomimetic Sensing Intervertebral Disc. Small Methods 2025, 2500292. [Google Scholar] [CrossRef]
- Jia, H.; Lin, X.; Wang, D.; Wang, J.; Shang, Q.; He, X.; Wu, K.; Zhao, B.; Peng, P.; Wang, H.; et al. Injectable Hydrogel with Nucleus Pulposus-Matched Viscoelastic Property Prevents Intervertebral Disc Degeneration. J. Orthop. Transl. 2022, 33, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Du, D. Recent Advances of Hydrogel-Based Biomaterials for Intervertebral Disc Tissue Treatment: A Literature Review. J. Tissue Eng. Regen. Med. 2021, 15, 299–321. [Google Scholar] [CrossRef]
- Tang, G.; Zhou, B.; Li, F.; Wang, W.; Liu, Y.; Wang, X.; Liu, C.; Ye, X. Advances of Naturally Derived and Synthetic Hydrogels for Intervertebral Disk Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Rostamipoor, M.; Farsinnejad, A.; Razavi, R.; Varma, R.S.; Amiri, M. Classification and Medical Applications of Temperature-Sensitive Hydrogels as Promising Future Materials, with a Particular Emphasis on Chitosan-Based Hydrogels. Nanochem. Res. 2025, 10, 158–186. [Google Scholar]
- Pearson, J.J.; Temenoff, J.S. Growth Factor Immobilization Strategies for Musculoskeletal Disorders. Curr. Osteoporos. Rep. 2022, 20, 13–25. [Google Scholar] [CrossRef]
- Subbiah, R.; Ruehle, M.A.; Klosterhoff, B.S.; Lin, A.S.P.; Hettiaratchi, M.H.; Willett, N.J.; Bertassoni, L.E.; García, A.J.; Guldberg, R.E. Triple Growth Factor Delivery Promotes Functional Bone Regeneration Following Composite Musculoskeletal Trauma. Acta Biomater. 2021, 127, 180–192. [Google Scholar] [CrossRef]
- Wang, X.; He, W.; Huang, H.; Han, J.; Wang, R.; Li, H.; Long, Y.; Wang, G.; Han, X. Recent Advances in Hydrogel Technology in Delivering Mesenchymal Stem Cell for Osteoarthritis Therapy. Biomolecules 2024, 14, 858. [Google Scholar] [CrossRef]
- He, L.; Zhang, H.; Zhao, N.; Liao, L. A Novel Approach in Biomedical Engineering: The Use of Polyvinyl Alcohol Hydrogel Encapsulating Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for Enhanced Osteogenic Differentiation and Angiogenesis in Bone Regeneration. Int. J. Biol. Macromol. 2024, 270, 132116. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Y.; Xiang, Y.; Zhang, S.; Guo, X. Application of Smart Hydrogel Materials in Cartilage Injury Repair: A Systematic Review and Meta-Analysis. J. Biomater. Appl. 2024, 39, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, H.; Wu, C.; Shang, Y.; Wu, Q.; Wei, Q.; Zhang, Q.; Sun, Y.; Wang, Q. Tissue Fluid Triggered Enzyme Polymerization for Ultrafast Gelation and Cartilage Repair. Angew. Chem. 2021, 133, 20135–20140. [Google Scholar] [CrossRef]
- Cioanca, O.; Lungu, I.-I.; Mita-Baciu, I.; Robu, S.; Burlec, A.F.; Hancianu, M.; Crivoi, F. Extraction and Purification of Catechins from Tea Leaves: An Overview of Methods, Advantages, and Disadvantages. Separations 2024, 11, 171. [Google Scholar] [CrossRef]
- Grierosu, C.; Mihai, C.; Lungu, I.I.; Calin, G.; Stefanache, A.; Vasiliu, M.P.; Ivona, D. Stress-Induced Variability in Local Anesthesia Response: Exploring Implications for Dental Anesthetic Management. Rom. J. Oral. Rehabil. 2024, 16, 608–617. [Google Scholar] [CrossRef]
- Imere, A.; Ligorio, C.; O’Brien, M.; Wong, J.K.F.; Domingos, M.; Cartmell, S.H. Engineering a Cell-Hydrogel-Fibre Composite to Mimic the Structure and Function of the Tendon Synovial Sheath. Acta Biomater. 2021, 119, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yu, Q.; Duan, S.; Du, Y.; Shi, X.; Li, X.; Jiao, T.; Qin, Z.; He, X. Anti-Swelling, High-Strength, Anisotropic Conductive Hydrogel with Excellent Biocompatibility for Implantable Electronic Tendon. Adv. Funct. Mater. 2024, 34, 2309500. [Google Scholar] [CrossRef]
- Zhong, H.; Fang, Y.; Luo, M.; Wang, L.; Huang, J.; Dai, G.; Liu, K.; Wu, J.; Du, J. Deferoxamine-Loaded Injectable Chitosan-Grafted Chlorogenic Acid/Oxidized Hyaluronic Acid Hybrid Hydrogel with Antibacterial, Anti-Inflammatory, and Angiogenesis-Promoting Properties for Diabetic Wound Repair. ACS Appl. Mater. Interfaces 2024, 16, 28209–28221. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Dawson, J.I.; Oreffo, R.O.C.; Tabata, Y.; Kumar, D.; Aparicio, C.; Mutreja, I. Gelatin Methacryloyl Hydrogels for Musculoskeletal Tissue Regeneration. Bioengineering 2022, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.; Liu, W.; Madry, H.; Neisiany, R.E.; Cucchiarini, M. Functionalized Hydrogels as Smart Gene Delivery Systems to Treat Musculoskeletal Disorders. Adv. Colloid Interface Sci. 2024, 331, 103232. [Google Scholar] [CrossRef]
- Schreiner, M.M.; Raudner, M.; Szomolanyi, P.; Ohel, K.; Ben-Zur, L.; Juras, V.; Mlynarik, V.; Windhager, R.; Trattnig, S. Chondral and Osteochondral Femoral Cartilage Lesions Treated with GelrinC: Significant Improvement of Radiological Outcome over Time and Zonal Variation of the Repair Tissue Based on T2 Mapping at 24 Months. CARTILAGE 2021, 13, 604S–616S. [Google Scholar] [CrossRef]
- Øvrebø, Ø.; Perale, G.; Wojciechowski, J.P.; Echalier, C.; Jeffers, J.R.T.; Stevens, M.M.; Haugen, H.J.; Rossi, F. Design and Clinical Application of Injectable Hydrogels for Musculoskeletal Therapy. Bioeng. Transl. Med. 2022, 7, e10295. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.M.; Mithoefer, K.; Bhatia, S.; Cole, B.J. Enhanced Marrow-Stimulation Techniques. In Biologic Knee Reconstruction; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-1-003-52276-8. [Google Scholar]
- Huddleston, H.P.; Haunschild, E.D.; Wong, S.E.; Cole, B.J.; Yanke, A.B. Microfracture Augmentation Options for Cartilage Repair. In Cartilage Injury of the Knee: State-of-the-Art Treatment and Controversies; Krych, A.J., Biant, L.C., Gomoll, A.H., Espregueira-Mendes, J., Gobbi, A., Nakamura, N., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 205–217. ISBN 978-3-030-78051-7. [Google Scholar]
- Sciarretta, F.V.; Acosta, M. Orthobiologically Augmented Scaffolds in Cartilage Repair. In Regenerative Medicine in Sports and Orthopaedics: A New Approach; Gobbi, A., Nakamura, N., Lane, J.G., Dallo, I., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 387–398. ISBN 978-3-031-84693-9. [Google Scholar]


| Gel Composition | Crosslinking Mechanism | Functionalization | Mechanical Properties | Application | Reference |
|---|---|---|---|---|---|
| GelMA with Mg2+-modified black phosphorus nanosheets (GelMA-BP@Mg). | Chemical | Neurovascularization agents | Compressive modulus ~150 kPa | Bone regeneration | [31] |
| Hydrogel microspheres composed of methacrylate-modified sulfonated azocalix [4] arene (SAC4A-MA), methacrylated hyaluronic acid (HA-MA), and MMP-13-sensitive peptide crosslinkers; loaded with hydroxychloroquine via host–guest interactions. | Enzymatic | Hypoxia-responsive elements | Compressive modulus ~60 kPa | Osteoarthritis treatment | [32] |
| Self-healing injectable hydrogel (Cur&Mg-QCS/PF) composed of quaternized chitosan (QCS), poloxamer F127 (PF), loaded with Mg2+ and curcumin. | Chemical | Anti-inflammatory agents | Tensile strength ~800 kPa | Tendon-to-bone healing | [33] |
| Injectable hydrogel composed of methacrylated silk fibroin as the base material, mixed with platelet-rich plasma, and embedded with silk fibroin microspheres that contain the bioactive compound berberine. The gel is photocrosslinked in situ using ultraviolet light. | Physical/Chemical | Inflammatory-responsive agents | Compressive modulus ~120 kPa | Bone tissue engineering | [34] |
| Hybrid injectable biomimetic hydrogel synthesized by incorporating laponite (LP) and calcium phosphate cement (CPC) into gelatin via a one-step method. The resulting composite is referred to as LC hydrogel. | Adaptive degradation | Tissue healing synchronization | Compressive modulus ~100 kPa | Bone regeneration | [35] |
| Hydrogel microspheres were fabricated using light-induced crosslinking of GelMA via a microfluidic system to support adhesion and proliferation of bone marrow mesenchymal stem cells (BMSCs). | Physical | Cell encapsulation | Diameter 50–200 µm | Musculoskeletal regeneration | [36] |
| GelMA combined with oxidized chondroitin sulfate (OCS), where OCS provides aldehyde groups forming Schiff base bonds with GelMA to enhance mechanical strength and support cartilage regeneration. | Chemical | Cartilage regeneration | Compressive modulus ~80 kPa | Cartilage repair | [37] |
| Hydrogel | Experimental Model | Therapeutic Results | Reference |
|---|---|---|---|
| RGD-Heparin-MMP-degradable HyA | Rat TA (VML) | Functional recovery, neovascularization, myofiber ingrowth | [49] |
| Stiffness-tuned HyA (1.1–10.6 kPa) | Rat LD (VML) | Max force restoration (optimal at 3 kPa), reduced inflammation | [50] |
| Magnetic alginate + Fe3O4 nanoparticles | Mouse TA (VML) | Improved muscle force, volume, CSA via magnetic stimulation | [51] |
| PEG hydrogel + laminin peptide | Mouse muscle injury | Pro-regenerative macrophage polarization, improved fiber organization | [52] |
| ROS-scavenging gelatin-PEG hydrogel | Mouse VML | Increased Pax7, MyoD, reduced oxidative stress, enhanced regeneration | [53] |
| PEG hydrogel with IGF-1 + HGF | Rat VML | Greater force recovery, enhanced fiber size, angiogenesis | [54] |
| GelMA hydrogel + MSCs + PDGF | Mouse muscle defect | MSC engraftment, angiogenesis, increased functional recovery | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calin, G.; Costescu, M.; Nour, M.; Ciuhodaru, T.; Denisa, B.-M.; Duceac, L.D.; Mihai, C.; Munteanu, M.F.; Trifunschi, S.; Oancea, A.; et al. Engineered Hydrogels for Musculoskeletal Regeneration: Advanced Synthesis Strategies and Therapeutic Efficacy in Preclinical Models. Polymers 2025, 17, 2094. https://doi.org/10.3390/polym17152094
Calin G, Costescu M, Nour M, Ciuhodaru T, Denisa B-M, Duceac LD, Mihai C, Munteanu MF, Trifunschi S, Oancea A, et al. Engineered Hydrogels for Musculoskeletal Regeneration: Advanced Synthesis Strategies and Therapeutic Efficacy in Preclinical Models. Polymers. 2025; 17(15):2094. https://doi.org/10.3390/polym17152094
Chicago/Turabian StyleCalin, Gabriela, Mihnea Costescu, Marcela Nour (Cârlig), Tudor Ciuhodaru, Batîr-Marin Denisa, Letitia Doina Duceac, Cozmin Mihai, Melania Florina Munteanu, Svetlana Trifunschi, Alexandru Oancea, and et al. 2025. "Engineered Hydrogels for Musculoskeletal Regeneration: Advanced Synthesis Strategies and Therapeutic Efficacy in Preclinical Models" Polymers 17, no. 15: 2094. https://doi.org/10.3390/polym17152094
APA StyleCalin, G., Costescu, M., Nour, M., Ciuhodaru, T., Denisa, B.-M., Duceac, L. D., Mihai, C., Munteanu, M. F., Trifunschi, S., Oancea, A., & Damir, D. L. (2025). Engineered Hydrogels for Musculoskeletal Regeneration: Advanced Synthesis Strategies and Therapeutic Efficacy in Preclinical Models. Polymers, 17(15), 2094. https://doi.org/10.3390/polym17152094




.jpg)



