Two Novel Low-Bandgap Copolymers Based on Indacenodithiophene/Indacenodithienothiophene and Benzothiadiazole Dicarboxylic Imide: Structural Design and DFT/TD-DFT Investigation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Polymers
2.2.1. Synthesis of PIDTBDI
2.2.2. Synthesis of PIDTTBDI
2.3. Computational Details
3. Results and Discussion
3.1. Polymer Synthesis
3.2. Thermal Properties and Powder X-Ray Diffraction Studies
3.3. Optical Properties
3.4. Density Functional Theory (DFT) Analysis
3.5. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noman, M.; Khan, Z.; Jan, S.T. A comprehensive review on the advancements and challenges in perovskite solar cell technology. RSC Adv. 2024, 14, 5058. [Google Scholar] [CrossRef]
- Ajayan, J.; Nirmal, D.; Mohankumar, P.; Saravanan, M.; Jagadesh, M.; Arivazhagan, L. A review of photovoltaic performance of organic/inorganic solar cells for future renewable and sustainable energy technologies. Superlattices Microstruct. 2020, 143, 106549. [Google Scholar] [CrossRef]
- Shahsavari, A.; Akbari, M. Potential of solar energy in developing countries for reducing energy-related Emissions. Renew. Sustain. Energy Rev. 2018, 90, 275–291. [Google Scholar] [CrossRef]
- Donaghy, T.Q.; Healy, N.; Jiang, C.Y.; Battle, C.P. Fossil fuel racism in the United States: How phasing out coal, oil, and gas can protect communities. Energy Res. Soc. Sci. 2023, 100, 103104. [Google Scholar] [CrossRef]
- Gurney, R.S.; Lidzey, D.G.; Wang, T. A review of non-fullerene polymer solar cells: From device physics to morphology Control. Rep. Prog. Phys. 2019, 82, 036601. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Shuai, Z.; Wei, Z. A-π-D-π-A Electron-Donating Small Molecules for Solution-Processed Organic Solar Cells: A Review. Macromol. Rapid Commun. 2017, 38, 1700470. [Google Scholar] [CrossRef]
- Hao, D.; Qi, L.; Tairab, A.M.; Ahmed, A.; Azam, A.; Luo, D.; Pan, Y.; Zhang, Z.; Yan, J. Solar energy harvesting technologies for PV self-powered applications: A comprehensive review. Renew. Energy 2022, 188, 678–697. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, F.R.; Qi, F.; Heumüller, T.; Distler, A.; Egelhaaf, H.-J.; Li, N.; Chow, P.C.Y.; Brabec, C.J.; Jen, A.K.-Y.; et al. Renewed Prospects for Organic Photovoltaics. Chem. Rev. 2022, 122, 14180–14274. [Google Scholar] [CrossRef]
- Pankow, R.M.; Thompson, B.C. The development of conjugated polymers as the cornerstone of organic electronics. Polymer 2020, 207, 122874. [Google Scholar] [CrossRef]
- Ding, L.; Yu, Z.-D.; Wang, X.-Y.; Yao, Z.-F.; Lu, Y.; Yang, C.-Y.; Wang, J.-Y.; Pei, J. Polymer Semiconductors: Synthesis, Processing, and Applications. Chem. Rev. 2023, 123, 7421–7497. [Google Scholar] [CrossRef]
- Al-Azzawi, A.G.S.; Aziz, S.B.; Dannoun, E.M.A.; Iraqi, A.; Nofal, M.M.; Murad, A.R.; Hussein, A.M.A. Mini Review on the Development of Conjugated Polymers: Steps towards the Commercialization of Organic Solar Cells. Polymers 2023, 15, 164. [Google Scholar] [CrossRef]
- Li, Z.; Chueh, C.-C.; Jen, A.K.-Y. Recent advances in molecular design of functional conjugated polymers for high-performance polymer solar cells. Prog. Polym. Sci. 2019, 99, 101175. [Google Scholar] [CrossRef]
- Machín, A.; Márquez, F. Advancements in Photovoltaic Cell Materials: Silicon, Organic, and Perovskite Solar Cells. Materials 2024, 17, 1165. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, R.; Li, Y.; Li, Y. Applications of organic solar cells in wearable electronics. Wearable Electron. 2024, 1, 26–40. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Wan, X.; Chen, Y. Recent progress in flexible organic solar cells. Escience 2023, 3, 100085. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Cui, C.; Li, Y. Flexible and Semitransparent Organic Solar Cells. Adv. Energy Mater. 2017, 8, 1701791. [Google Scholar] [CrossRef]
- Sun, L.; Fukuda, K.; Someya, T. Recent progress in solution-processed flexible organic photovoltaics. npj Flex. Electron. 2022, 6, 89. [Google Scholar] [CrossRef]
- Søndergaard, R.; Hösel, M.; Angmo, D.; Larsen-Olsen, T.T.; Krebs, F.C. Roll-to-roll fabrication of polymer solar cells. Mater. Today 2012, 15, 36–49. [Google Scholar] [CrossRef]
- Krebs, F.C. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Głowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef]
- Søndergaard, R.; Hösel, M.; Krebs, F.C. Roll-to-Roll Fabrication of Large Area Functional Organic Materials. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 16–34. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1993, 270, 1789–1791. [Google Scholar] [CrossRef]
- Wang, G.; Melkonyan, F.S.; Facchetti, A.; Marks, T.J. All-Polymer Solar Cells: Recent Progress, Challenges, and Prospects. Angew. Chem. Int. Ed. 2019, 58, 4129–4142. [Google Scholar] [CrossRef]
- Duan, C.; Ding, L. The new era for organic solar cells: Non-fullerene small molecular acceptors. Sci. Bull. 2020, 65, 1231–1233. [Google Scholar] [CrossRef]
- Tong, Y.; Xiao, Z.; Du, X.; Zuo, C.; Li, Y.; Lv, M.; Yuan, Y.; Yi, C.; Hao, F.; Hua, Y. Progress of the key materials for organic solar cells. Sci. China Chem. 2020, 63, 758–765. [Google Scholar] [CrossRef]
- Hou, L.; Hou, J.; Chen, H.-Y.; Zhang, S.; Jiang, Y.; Chen, T.L.; Yang, Y. Bandgap and Molecular Level Control of the Low-Bandgap Polymers Based on 3,6-Dithiophen-2-yl-2,5-dihydropyrrolo [3,4-c]pyrrole-1,4-dione toward Highly Efficient Polymer Solar Cells. Macromolecules 2009, 42, 6564–6571. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% Efficiency Organic Photovoltaic Cells Enabled by a Chlorinated Acceptor with Increased Open-Circuit Voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef]
- Kang, I.; Yun, H.-J.; Chung, D.S.; Kwon, S.-K.; Kim, Y.-H. Record High Hole Mobility in Polymer Semiconductors via Side-Chain Engineering. J. Am. Chem. Soc. 2013, 135, 14896–14899. [Google Scholar] [CrossRef]
- Geng, Y.; Tang, A.; Tajima, K.; Zeng, Q.; Zhou, E. Conjugated materials containing dithieno[3,2-b:2’,3’-d]pyrrole and its derivatives for organic and hybrid solar cell applications. J. Mater. Chem. A 2019, 7, 64. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Q.; You, W. Molecular engineering of conjugated polymers for solar cells: An updated report. Adv. Mater. 2017, 29, 1601391. [Google Scholar] [CrossRef]
- Yao, H.; Ye, L.; Zhang, H.; Li, S.; Zhang, S.; Hou, J. Molecular Design of Benzodithiophene-Based Organic Photovoltaic Materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef]
- Deshmukh, K.D.; Matsidik, R.; Prasad, S.K.K.; Connal, L.A.; Liu, A.C.Y.; Gann, E.; Thomsen, L.; Hodgkiss, J.M.; Sommer, M.; McNeill, C.R. Tuning the Molecular Weight of the Electron Accepting Polymer in All-Polymer Solar Cells: Impact on Morphology and Charge Generation. Adv. Funct. Mater. 2018, 28, 1707185. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Hi, H.; Abdullah, S.N.; Brza, M.A.; Abdulwahid, R.T. Influence of Fluorine Substitution on the Optical, Thermal, Electrochemical and Structural Properties of Carbazole-Benzothiadiazole Dicarboxylic Imide Alternate Copolymers. Polymers 2020, 12, 2910. [Google Scholar] [CrossRef]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef]
- Al-Azzawi, A.G.S.; Iraqi, A.; Aziz, S.B.; Zhang, Y.; Murad, A.R.; Hadi, J.M.; Lidzey, D.G. Synthesis, Optical and Electrochemical Properties of Naphthothiadiazole-Based Donor-Acceptor Polymers and Their Photovoltaic Applications. Int. J. Electrochem. Sci. 2021, 16, 21125. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-Junction Organic Photovoltaic Cell with 19% Efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.-C.; Wang, Z.; Ma, W.; Wang, J.; Yin, Z.; Tang, C.; Cai, D.; Zheng, Q. Indacenodithiophene-based wide bandgap copolymers for high performance single-junction and tandem polymer solar cells. Nano Energy 2017, 33, 313–324. [Google Scholar] [CrossRef]
- Gao, W.; Liu, T.; Hao, M.; Wu, K.; Zhang, C.; Sun, Y.; Yang, C. Dithieno [3,2-b:2’,3’-d]pyridin-5(4H)-one based D–A type copolymers with wide bandgaps of up to 2.05 eV to achieve solar cell efficiencies of up to 7.33%. Chem. Sci. 2016, 7, 6167–6175. [Google Scholar] [CrossRef]
- Negash, A.; Genene, Z.; Eachambadi, R.T.; Verstappen, P.; Brande, N.V.D.; Kesters, J.; D'HAen, J.; Wang, E.; Vandewal, K.; Maes, W.; et al. Ladder-type high gap conjugated polymers based on indacenodithieno[3,2-b]thiophene and bithiazole for organic photovoltaics. Org. Electron. 2019, 74, 211–217. [Google Scholar] [CrossRef]
- Khelifi, W.; Luscombe, C.K. Recent developments in indacenodithiophene and indacenodithienothiophene-based donor-acceptor conjugated polymers: From design to device performance in organic electronics. Prog. Polym. Sci. 2024, 151, 101804. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Pan, Z.; Zhang, B.; Yang, X.; Gu, J.; Chen, Y. Indacenodithiophene: A promising building block for high performance polymer solar cells. J. Mater. Chem. A 2017, 5, 10798–10814. [Google Scholar] [CrossRef]
- Liang, C.; Wang, H. Indacenodithiophene-based D-A conjugated polymers for application in polymer solar cells. Org. Electron. 2017, 50, 443–457. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, C.Y.; Fan, Y.L.; Hung, L.I.; Chen, C.P.; Ting, C. Low-bandgap conjugated polymer for high efficient photovoltaic Applications. Chem. Commun. 2010, 46, 6503–6505. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Yip, H.-L.; Chen, K.-S.; Zeigler, D.F.; Sun, Y.; Jen, A.K.-Y. Indacenodithiophene and Quinoxaline-Based Conjugated Polymers for Highly Efficient Polymer Solar Cells. Chem. Mater. 2011, 23, 2289–2291. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Tan, J.; Zhang, S.; Huo, L.; Hu, W.; Li, Y.; Hou, J. Influence of D/A Ratio on Photovoltaic Performance of a Highly Efficient Polymer Solar Cell System. Adv. Mater. 2012, 24, 6536–6541. [Google Scholar] [CrossRef]
- Zhang, X.; Bronstein, H.; Kronemeijer, A.J.; Smith, J.; Kim, Y.; Kline, R.J.; Richter, L.J.; Anthopoulos, T.D.; Sirringhaus, H.; Song, K.; et al. Molecular origin of high field-effect mobility in an indacenodithiophene–benzothiadiazole copolymer. Nat. Commun. 2013, 4, 2238. [Google Scholar] [CrossRef]
- Venkateshvaran, D.; Nikolka, M.; Sadhanala, A.; Lemaur, V.; Zelazny, M.; Kepa, M.; Hurhangee, M.; Kronemeijer, A.J.; Pecunia, V.; Nasrallah, I.; et al. Approaching disorder-free transport in high-mobility conjugated polymers. Nature 2014, 515, 384–388. [Google Scholar] [CrossRef]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular optimisation enables over 13% efficiency in organic solar cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef]
- Yan, C.; Wu, Y.; Wang, J.; Li, R.; Cheng, P.; Bai, H.; Zhan, Z.; Ma, W.; Zhan, X. Enhancing performance of non-fullerene organic solar cells via side chain engineering of fused-ring electron acceptors. Dye. Pigment. 2017, 139, 627–634. [Google Scholar] [CrossRef]
- Jiang, Z.-Q.; Wang, T.-T.; Wu, F.-P.; Lin, J.-D.; Liao, L.-S. Recent advances in electron acceptors with ladder-type backbone for organic solar cells. J. Mater. Chem. A 2018, 6, 17256–17287. [Google Scholar] [CrossRef]
- Yi, H.; Al-Faifi, S.; Iraqi, A.; Watters, D.C.; Kingsley, J.; Lidzey, D.G. Carbazole and thienyl benzo [1,2,5]thiadiazole based polymers with improved open circuit voltages and processability for application in solar cells. J. Mater. Chem. 2011, 21, 13649. [Google Scholar] [CrossRef]
- Zhang, Y.; Chien, S.-C.; Chen, K.-S.; Yip, H.-L.; Sun, Y.; Davies, J.A.; Chen, F.-C.; Jen, A.K.-Y. Increased open circuit voltage in fluorinated benzothiadiazole-based alternating conjugated polymers. Chem. Commun. 2011, 47, 11026–11028. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Z.; Wei, W.; Jiang, Z. Fluorinated Benzothiadiazole-Based Conjugated Polymers for High-Performance Polymer Solar Cells without Any Processing Additives or Post-treatments. J. Am. Chem. Soc. 2013, 135, 17060–17068. [Google Scholar] [CrossRef]
- Wang, L.; Cai, D.; Zheng, Q.; Tang, C.; Chen, S.-C.; Yin, Z. Low Band Gap Polymers Incorporating a Dicarboxylic Imide-Derived Acceptor Moiety for Efficient Polymer Solar Cells. ACS Macro Lett. 2013, 2, 605–608. [Google Scholar] [CrossRef]
- Pron, M. Leclerc, Imide/amide based π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1815–1831. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Abdulwahid, R.T.; Hussen, S.A. Optical, Electrochemical, Thermal, and Structural Properties of Synthesized Fluorene/Dibenzosilole-Benzothiadiazole Dicarboxylic Imide Alternating Organic Copolymers for Photovoltaic Applications. Coatings 2020, 10, 1147. [Google Scholar] [CrossRef]
- Li, H.; Sun, S.; Mhaisalkar, S.; Zin, M.T.; Lam, Y.M.; Grimsdale, A.C. A high voltage solar cell using a donor–acceptor conjugated polymer based on pyrrolo [3,4-f]-2,1,3-benzothiadiazole-5,7-dione. J. Mater. Chem. A 2014, 2, 17925–17933. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Ashraf, R.S.; Treat, N.D.; Schroeder, B.C.; Donaghey, J.E.; White, A.J.P.; Stingelin, N.; McCulloch, I. 2,1,3-Benzothiadiazole-5,6-Dicarboxylic Imide–A Versatile Building Block for Additive- and Annealing-Free Processing of Organic Solar Cells with Efficiencies Exceeding 8%. Adv. Mater. 2015, 27, 948–953. [Google Scholar] [CrossRef]
- Yu, J.; Ornelas, J.L.; Tang, Y.; Uddin, M.A.; Guo, H.; Yu, S.; Wang, Y.; Woo, H.Y.; Zhang, S.; Xing, G.; et al. 2,1,3-Benzothiadiazole-5,6-dicarboxylic imide-Based Polymer Semiconductors for Organic Thin-Film Transistors and Polymer Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 42167–42178. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Almeataq, M.S.; Abdullah, S.N.; Brza, M.A. Characteristics of Low Band Gap Copolymers Containing Anthracene-Benzothiadiazole Dicarboxylic Imide: Synthesis, Optical, Electrochemical, Thermal and Structural Studies. Polymers 2020, 13, 62. [Google Scholar] [CrossRef]
- Xu, Y.; Chueh, C.; Yip, H.; Ding, F.; Li, Y.; Li, C.; Li, X.; Chen, W.; Jen, A.K. Improved Charge Transport and Absorption Coefficient in Indacenodithieno [3,2-b]thiophene-based Ladder-Type Polymer Leading to Highly Efficient Polymer Solar Cells. Adv. Mater. 2012, 24, 6356–6361. [Google Scholar] [CrossRef]
- Sun, Y.; Chien, S.-C.; Yip, H.-L.; Zhang, Y.; Chen, K.-S.; Zeigler, D.F.; Chen, F.-C.; Lin, B.; Jen, A.K.-Y. High-mobility low-bandgap conjugated copolymers based on indacenodithiophene and thiadiazolo [3,4-c]pyridine units for thin film transistor and photovoltaic applications. J. Mater. Chem. 2011, 21, 13247–13255. [Google Scholar] [CrossRef]
- Al-Azzawi, A.G.; Aziz, S.B.; Iraqi, A.; Murad, A.R.; Abdulwahid, R.T.; Alshehri, S.M.; Ahamad, T. Impact of ethynylene linkers on the optical and electrochemical properties of benzothiadiazole based alternate conjugated polymers. Arab. J. Chem. 2021, 14, 103320. [Google Scholar] [CrossRef]
- Alqurashy, B.A.; Cartwright, L.; Iraqi, A.; Zhang, Y.; Lidzey, D.G. Pyrene–benzothiadiazole-based copolymers for application in photovoltaic devices. Polym. Adv. Technol. 2016, 28, 193–200. [Google Scholar] [CrossRef]
- Frisch, G.W.T.M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16 Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Chemcraf-Graphical Software for Visualization of Quantum Chemistry Computations. Version 1.8, Build 489. 2016. Available online: https://www.chemcraftprog.com (accessed on 12 February 2024).
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results Obtained with the Correlation-Energy Density Functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Petersilka, M.; Gossmann, U.; Gross, E. Excitation energies from time-dependent density-functional theory. Phys. Rev. Lett. 1996, 76, 1212. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sanchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W.J. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Murad, A.R.; Iraqi, A.; Aziz, S.B.; Abdullah, S.N.; Brza, M.A.; Saeed, S.R.; Abdulwahid, R.T. Fabrication of Alternating Copolymers Based on Cyclopentadithiophene-Benzothiadiazole Dicarboxylic Imide with Reduced Optical Band Gap: Synthesis, Optical, Electrochemical, Thermal, and Structural Properties. Polymers 2021, 13, 63. [Google Scholar] [CrossRef]
- Alqurashy, B.A.; Iraqi, A.; Zhang, Y.; Lidzey, D.G. Pyrene-benzo [1,2,5]thiadiazole based conjugated polymers for application in BHJ solar cells. J. Saudi Chem. Soc. 2020, 24, 484–491. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Akl, A.S.; Elhadi, M. Estimation of crystallite size, lattice parameter, internal strain and crystal impurification of nanocrystalline Al3Ni20Bx alloy by Williamson-Hall method. J. Ovonic Res. 2020, 16, 323–335. [Google Scholar]
- Irfan, H.; Racik, K.M.; Anand, S. Microstructural evaluation of CoAl2 O 4 nanoparticles by Williamson–Hall and size–strain plot methods. J. Asian Ceram. Soc. 2018, 6, 54–62. [Google Scholar] [CrossRef]
- Madivalappa, S.; Basavaraj, R.; Chethan, P.; Aarti, D.; Jisha, P. Insights and perspectives on PVDF/MgO NCs films: Structural and optical properties for optoelectronic device applications. Results Chem. 2024, 11, 101764. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductors; Plenum: New York, NY, USA, 1974; pp. 159–220. [Google Scholar]
- Chen, K.-S.; Zhang, Y.; Yip, H.-L.; Sun, Y.; Davies, J.A.; Ting, C.; Chen, C.-P.; Jen, A.K.-Y. Highly efficient indacenodithiophene-based polymeric solar cells in conventional and inverted device configurations. Org. Electron. 2011, 12, 794–801. [Google Scholar] [CrossRef]
- Pan, X.; Bjuggren, J.M.; Jevric, M.; Tan, W.L.; McNeill, C.R.; Andersson, M.R. Achieving High-Efficiency Organic Photovoltaics from a New Completely Amorphous Donor Polymer. Chem. Mater. 2022, 34, 5103–5115. [Google Scholar] [CrossRef]
- Liu, S.; Yi, S.; Qing, P.; Li, W.; Gu, B.; He, Z.; Zhang, B. Molecular engineering enhances the charge carriers transport in wide band-gap polymer donors based polymer solar cells. Molecules 2020, 24, 4101. [Google Scholar] [CrossRef]
- Chochos, C.L.; Leclerc, N.; Gasparini, N.; Zimmerman, N.; Tatsi, E.; Katsouras, A.; Moschovas, D.; Serpetzoglou, E.; Konidakis, I.; Fall, S.; et al. The role of chemical structure in indacenodithienothiophene-alt-benzothiadiazole copolymers for high performance organic solar cells with improved photo-stability through minimization of burn-in loss. J. Mater. Chem. A 2017, 5, 25064–25076. [Google Scholar] [CrossRef]
- An, L.; Tong, J.; Huang, Y.; Liang, Z.; Li, J.; Yang, C.; Wang, X. Elevated Photovoltaic Performance in Medium Bandgap Copolymers Composed of Indacenodi-thieno [3 2-b] thiophene and Benzothiadiazole Subunits by modulating the π-bridge. Polymers 2020, 12, 368. [Google Scholar] [CrossRef]
- Intemann, J.J.; Yao, K.; Li, Y.X.; Yip, H.L.; Xu, Y.X.; Liang, P.W.; Chueh, C.C.; Ding, F.Z.; Yang, X.; Li, X. Highly efficient inverted organic solar cells through material and interfacial engineering of indacenodithieno [3,2-b]thiophene-based polymers and devices. Adv. Funct. Mater. 2014, 24, 1465–1473. [Google Scholar] [CrossRef]
- Chochos, C.L.; Katsouras, A.; Gasparini, N.; Koulogiannis, C.; Ameri, T.; Brabec, C.J.; Avgeropoulos, A. Rational Design of High-Performance Wide-Bandgap (≈2 eV) Polymer Semiconductors as Electron Donors in Organic Photovoltaics Exhibiting High Open Circuit Voltages (≈1 V). Macromol. Rapid Commun. 2017, 38, 1600614. [Google Scholar] [CrossRef]
- Alsoghier, H.M.; Selim, M.A.; Salman, H.M.; Rageh, H.M.; Santos, M.A.; Ibrahim, S.A.; Dongol, M.; Soga, T.; Abuelwafa, A.A. NMR spectroscopic, linear and non-linear optical properties of 1,3-benzothiazol-2-yl-(phenylhydrazono) acetonitrile (BTPA) azo dye. J. Mole Struct. 2018, 1179, 315–324. [Google Scholar]








| Polymer | Mn (Da) a | Mw (Da) a | PDI | Td (°C) b | λmax (nm) | Eg opt (eV) c | |
|---|---|---|---|---|---|---|---|
| Solution | Film | ||||||
| PIDTBDI | 4334 | 10673 | 2.46 | 270 | 580 | 625 | 1.58 |
| PIDTTBDI | 6236 | 7149 | 1.14 | 390 | 605 | 657 | 1.57 |
| Compound | λmax (nm) | f | Assignment | Hole | Particle |
|---|---|---|---|---|---|
| PIDTBDI | 543 | 0.373 | H-1→L |  | 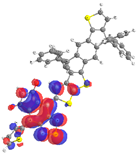 |
| PIDTTBDI-a | 589 | 0.388 | H-1→L |  |  |
| PIDTTBDI-b | 597 | 0.447 | H-1→L |  |  |
| Compound | HOMO (eV) | LUMO (eV) | Energy Gap (eV) |
|---|---|---|---|
| PIDTBDI | −5.480 | −3.470 | 2.010 |
| PIDTTBDI-a | −5.435 | −3.460 | 1.975 |
| PIDTTBDI-b | −5.431 | −3.501 | 1.930 |
| Polymer | HOMO (eV) a | LUMO (eV) a | Eg elec (eV) b |
|---|---|---|---|
| PIDTBDI | −5.30 | −3.61 | 1.69 |
| PIDTTBDI | −5.28 | −3.59 | 1.69 |
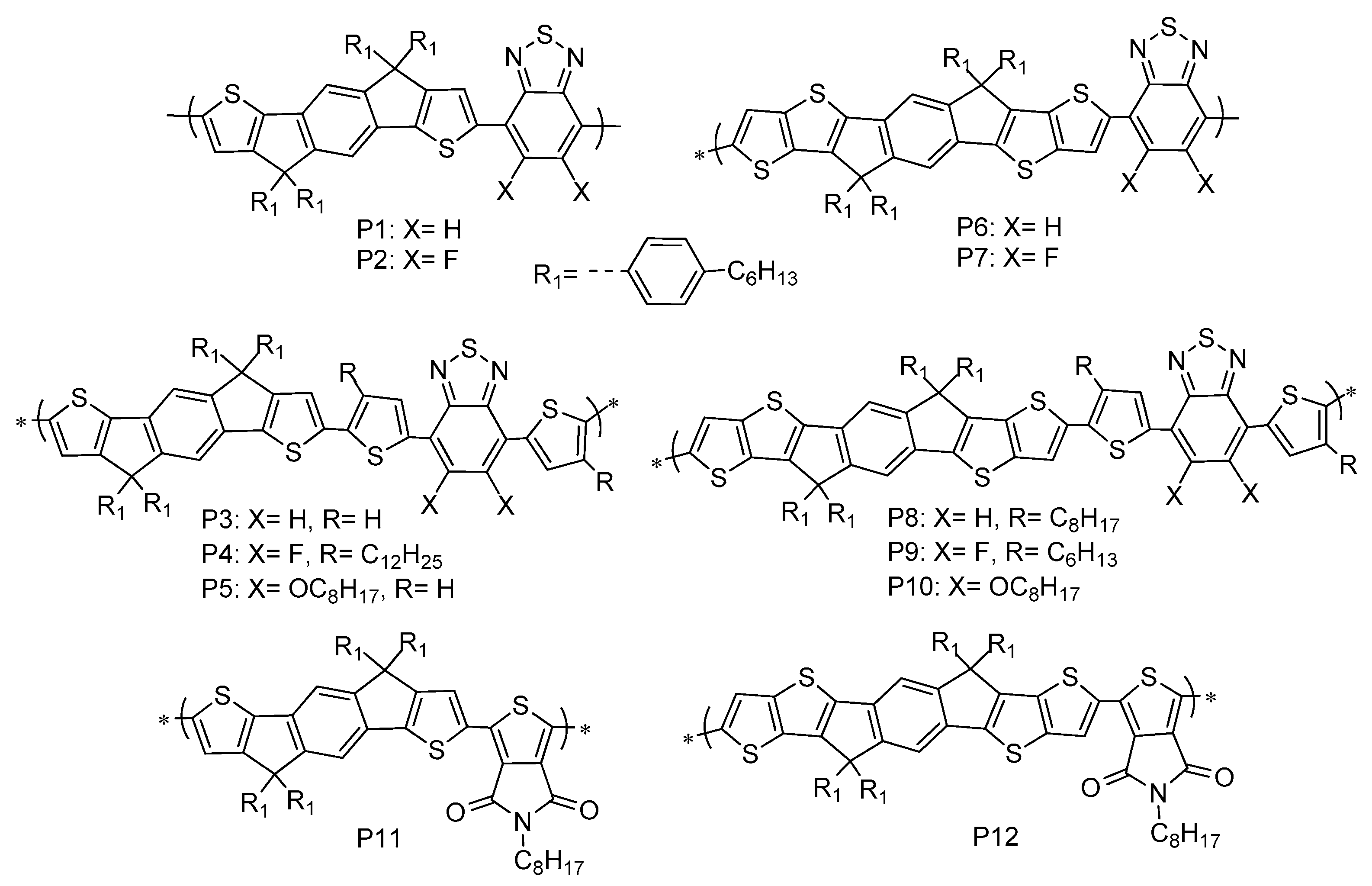
| Polymer | Eg opt (eV) b | HOMO (eV) c | LUMO (eV) c | Eg elec (eV) d | Td (°C) | PCE (%) | Ref |
|---|---|---|---|---|---|---|---|
| PIDTBDI | 1.58 | −5.30 | −3.61 | 1.69 | 270 | - | This work |
| PIDTTBDI | 1.57 | −5.28 | −3.59 | 1.69 | 390 | - | |
| P1 | 1.75 | −5.23 | −3.52 | 1.71 | - | 6.30 | [81] |
| P2 | 1.78 | −5.46 | −3.56 | 1.90 | 5.97 | [62] | |
| P3 | 1.74 | −5.19 | −3.49 | 1.70 | - | 4.40 | [81] |
| P4 | 1.80 | −5.71 | −3.70 | 2.01 | - | 12.7 | [82] |
| P5 | 1.89 | −5.29 | −3.53 | 1.76 | 335 | 6.05 | [83] |
| P6 | 1.75 | −5.75 | −3.64 | 2.11 | - | 6.20 | [84] |
| P7 | 1.78 | −5.82 | −3.71 | 2.11 | - | 6.10 | [84] |
| P8 | 1.79 | −5.28 | −3.47 | 1.81 | - | 2.05 | [85] |
| P9 | 1.75 | −5.30 | −3.40 | 1.90 | - | 4.40 | [86] |
| P10 | 1.91 | −5.25 | −3.64 | 1.62 | 362 | 6.12 | [83] |
| P11 | 1.96 | −5.50 | −3.54 | 1.96 | - | 3.68 | [87] |
| P12 | 1.96 | −5.45 | −3.49 | 1.96 | - | 5.85 | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqurashy, B.A.; Murad, A.R.; Alsaedi, W.H.; Altayeb, B.M.; Elroby, S.A.; Jedidi, A. Two Novel Low-Bandgap Copolymers Based on Indacenodithiophene/Indacenodithienothiophene and Benzothiadiazole Dicarboxylic Imide: Structural Design and DFT/TD-DFT Investigation. Polymers 2025, 17, 2050. https://doi.org/10.3390/polym17152050
Alqurashy BA, Murad AR, Alsaedi WH, Altayeb BM, Elroby SA, Jedidi A. Two Novel Low-Bandgap Copolymers Based on Indacenodithiophene/Indacenodithienothiophene and Benzothiadiazole Dicarboxylic Imide: Structural Design and DFT/TD-DFT Investigation. Polymers. 2025; 17(15):2050. https://doi.org/10.3390/polym17152050
Chicago/Turabian StyleAlqurashy, Bakhet A., Ary R. Murad, Wael H. Alsaedi, Bader M. Altayeb, Shaaban A. Elroby, and Abdesslem Jedidi. 2025. "Two Novel Low-Bandgap Copolymers Based on Indacenodithiophene/Indacenodithienothiophene and Benzothiadiazole Dicarboxylic Imide: Structural Design and DFT/TD-DFT Investigation" Polymers 17, no. 15: 2050. https://doi.org/10.3390/polym17152050
APA StyleAlqurashy, B. A., Murad, A. R., Alsaedi, W. H., Altayeb, B. M., Elroby, S. A., & Jedidi, A. (2025). Two Novel Low-Bandgap Copolymers Based on Indacenodithiophene/Indacenodithienothiophene and Benzothiadiazole Dicarboxylic Imide: Structural Design and DFT/TD-DFT Investigation. Polymers, 17(15), 2050. https://doi.org/10.3390/polym17152050








