Evaluation of Various Thiourea Derivatives as Reducing Agents in Two-Component Methacrylate-Based Materials
Abstract
1. Introduction
2. Experimental Section
2.1. Chemical Compounds
2.2. Evaluation of Unfilled Materials
2.2.1. Preparation of the Formulations
2.2.2. Polymerization Processes Followed by Optical Pyrometry
2.2.3. Flexural Strength and Flexural Modulus
2.3. Evaluation of Filled Materials
2.3.1. Formulation of Self-Cure (SC) Composites
2.3.2. Flexural Strength and Flexural Modulus
2.3.3. Determination of the Double-Bond Conversion by NIR Spectrometry
2.3.4. Working Time
2.4. Evaluation of the Chemical Mechanisms of Thiourea-Based Redox Systems
2.4.1. UV-Vis Experiment
2.4.2. Electron Spin Resonance
2.4.3. Cyclic Voltammetry
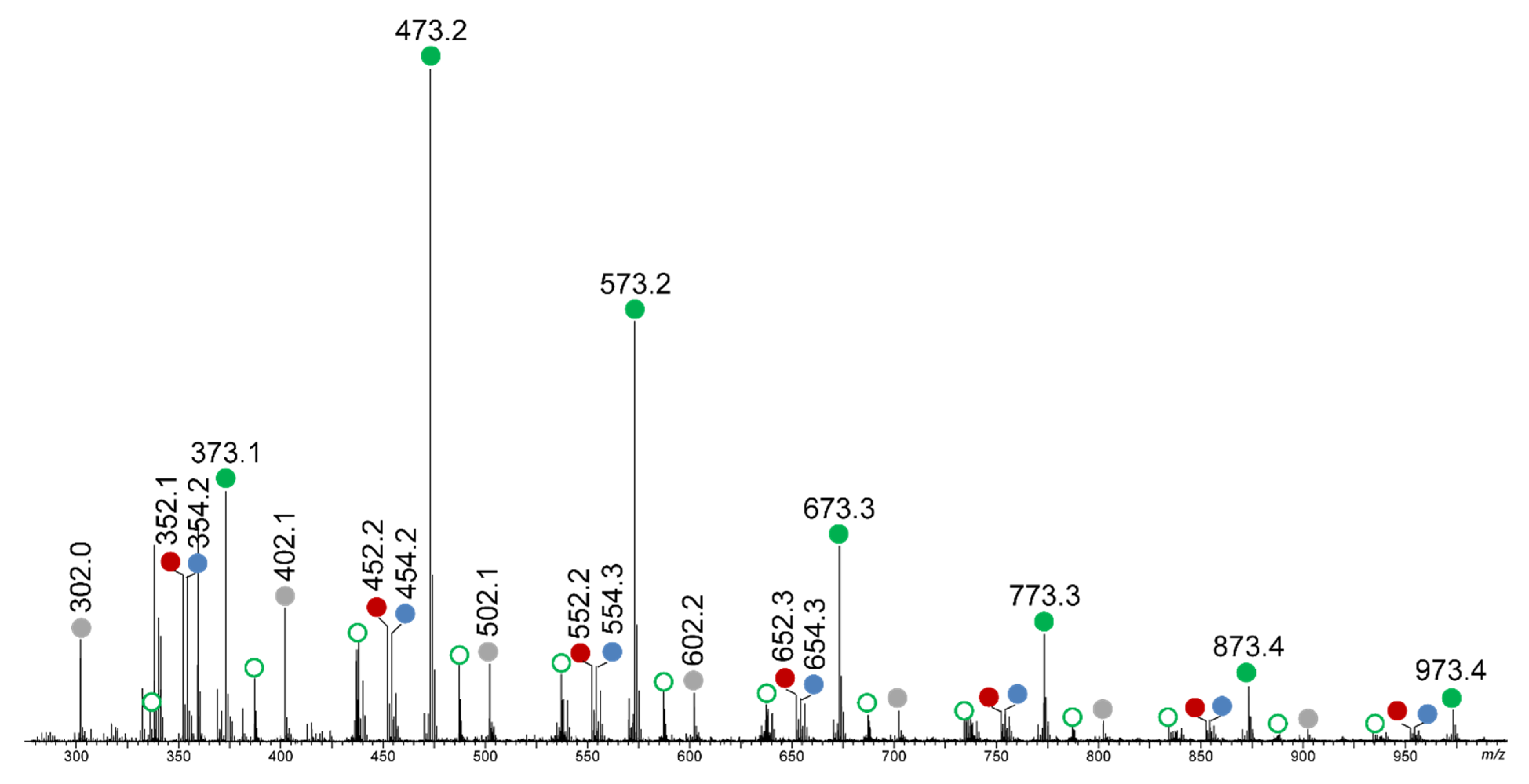
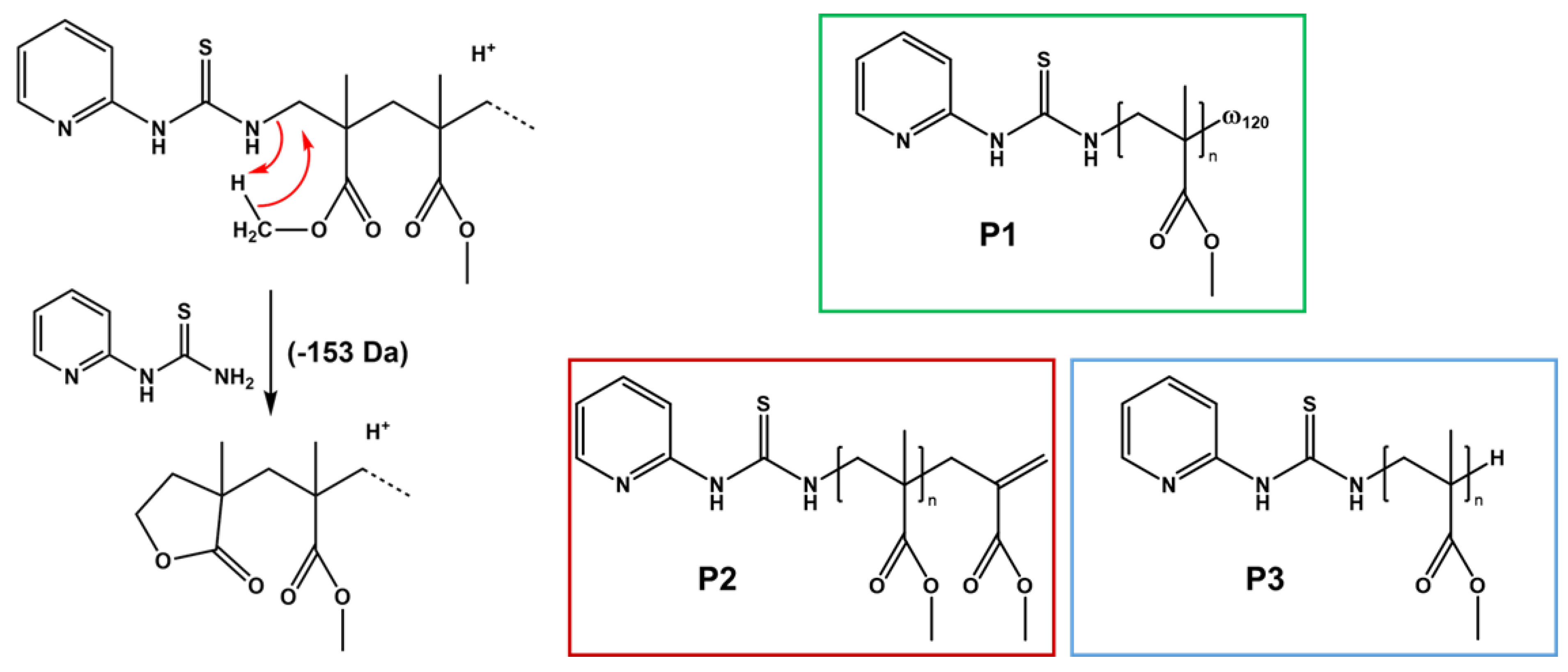
2.4.4. Mass Spectrometry Analysis of Initiating Fragment
2.4.5. Molecular Modelling
3. Results and Discussion
3.1. Evaluation of CHP//TUs1-6 + Cu(acac)2 Redox Initiator Systems in Filler-Free Two-Component Materials via Pyrometry
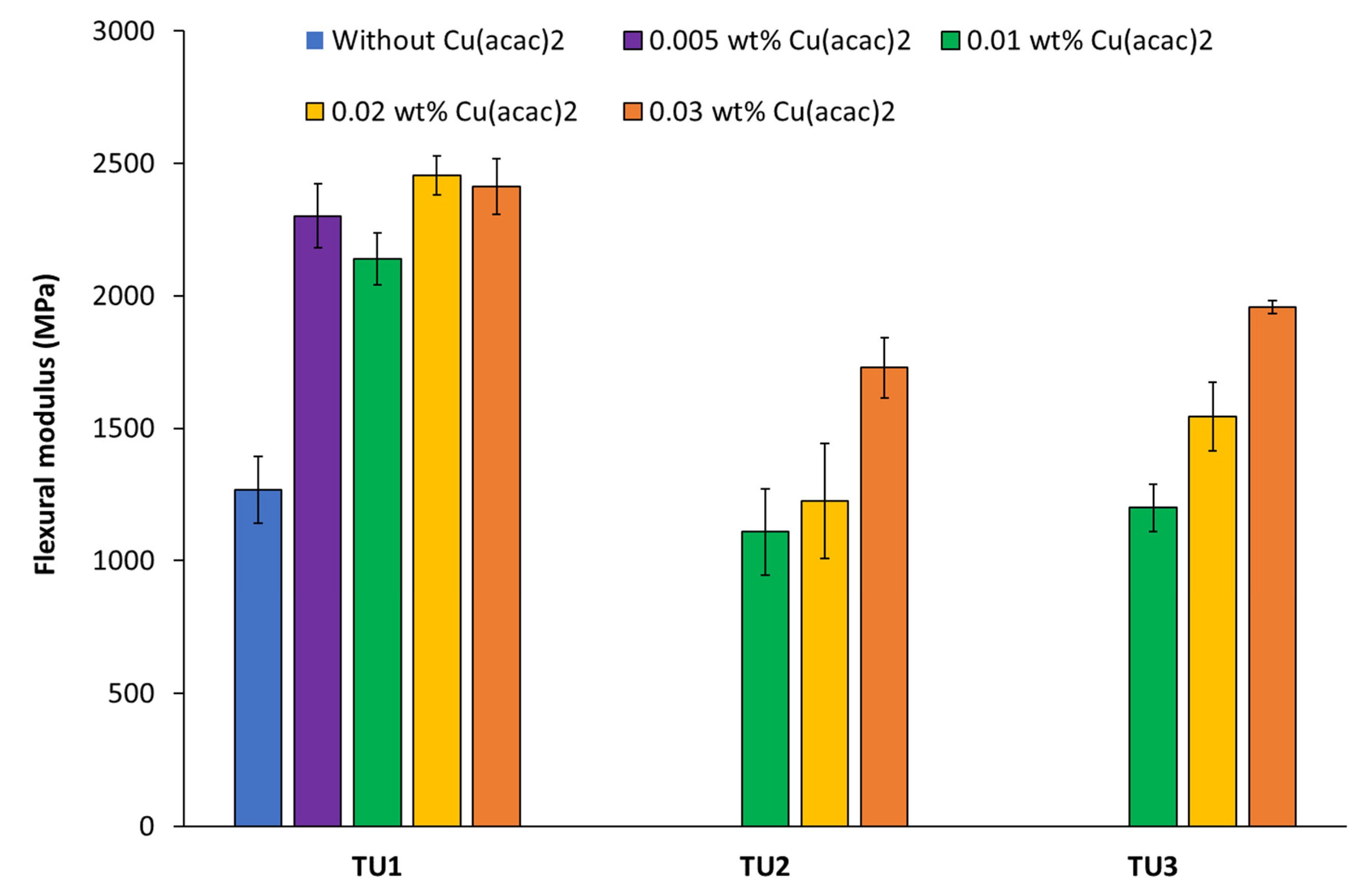
3.2. Determination of the Flexural Strength/Modulus of Filler-Free Two-Component Materials Based on CHP//TUs1-6 + Cu(acac)2 Redox Initiator Systems
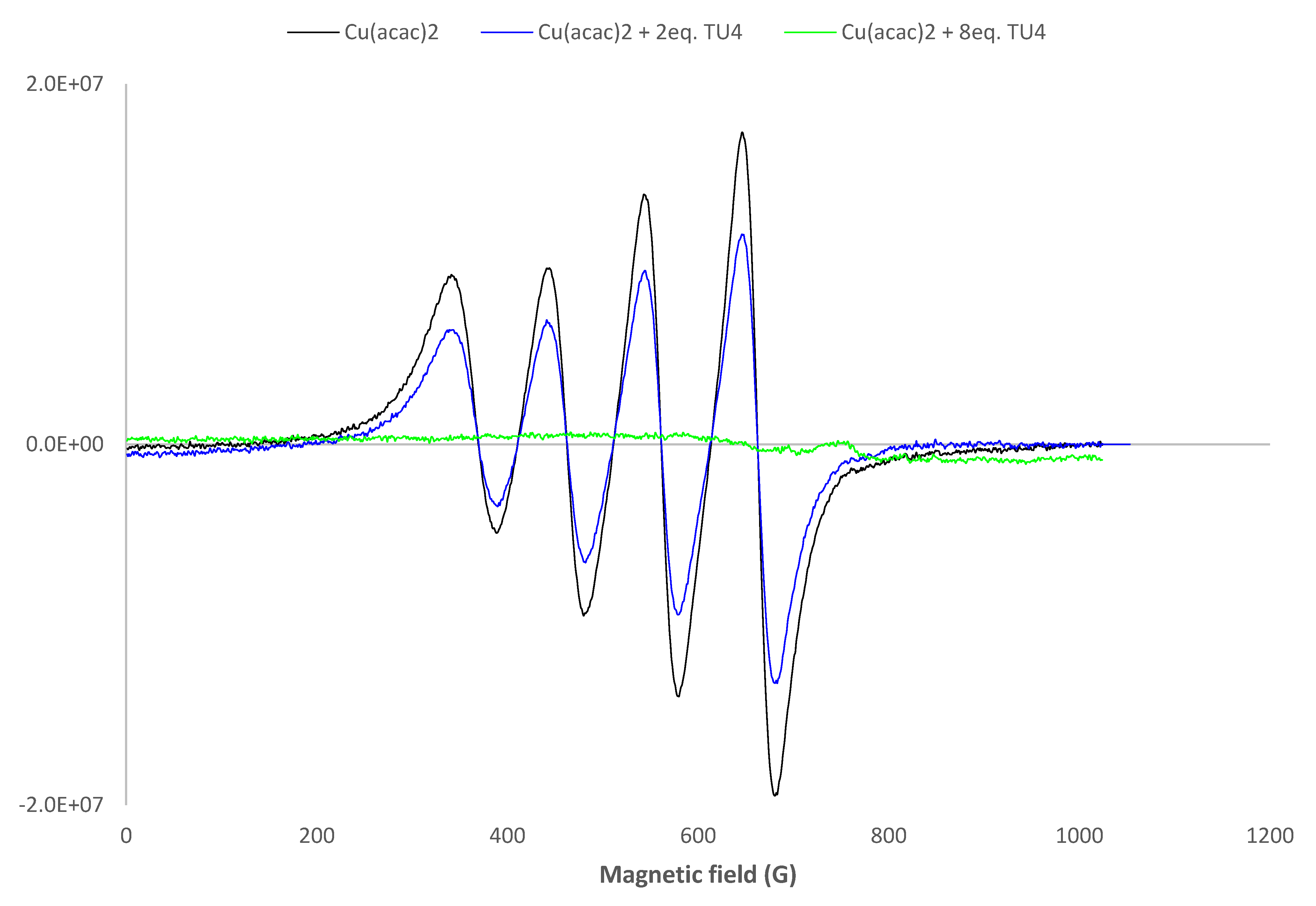
3.3. Investigation of the Chemical Mechanisms: Cyclic Voltammetry (CV); UV Analysis; ESR Experiments and Mass Spectrometry (MS) Analysis of Initiating Fragments
3.4. Evaluation of Two-Component Flowable Composites Based on CHP//TU1, TU4, or TU6 + Cu(acac)2 Redox Initiator Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moszner, N.; Hirt, T. New polymer-chemical developments in clinical dental polymer materials: Enamel–dentin adhesives and restorative composites. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4369–4402. [Google Scholar] [CrossRef]
- Palin, W.M.; Ferracane, J.L. Handbook of Oral Biomaterials; Jenny Stanford Publishing: New Delhi, India, 2014; pp. 213–254. [Google Scholar]
- Tanoue, N.; Koishi, Y.; Atsuta, M.; Matsumura, H. Properties of dual-curable luting composites polymerized with single and dual curing modes. J. Oral Rehabil. 2003, 30, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Duymus, Z.Y.; Yanikoglu, N.D.; Alkurt, M. Evaluation of the flexural strength of dual-cure composite resin cements. J. Biomed. Mater. Res. Part B 2013, 101B, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Stansbury, J.W.; Burke, F.J.T. Self-adhesive resin cements—chemistry, properties and clinical considerations. J. Oral Rehabil. 2011, 38, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, H.; Wang, Y. Different depth-related polymerization kinetics of dual-cure, bulk-fill composites. Dent. Mater. 2019, 35, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Vandewalker, J.P.; Casey, J.A.; Lincoln, T.A.; Vandewalle, K.S. Properties of dual-cure, bulk-fill composite resin restorative materials. Gen. Dent. 2016, 64, 68–73. [Google Scholar] [PubMed]
- Maletin, A.; Kneževic, M.J.; Koprivica, D.D.; Veljovic, T.; Puškar, T.; Milekic, B.; Ristic, I. Dental Resin-Based Luting Materials—Review. Plymers 2023, 15, 4156. [Google Scholar] [CrossRef] [PubMed]
- Heboyan, A.; Vardanyan, A.; Karobari, M.I.; Marya, A.; Avagyan, T.; Tebyaniyan, H.; Mustafa, M.; Rokaya, D.; Avetisyan, A. Dental Luting Cements: An Updated Comprehensive Review. Molecules 2023, 28, 1619. [Google Scholar] [CrossRef] [PubMed]
- Achilias, D.S.; Sideridou, I.D. Kinetics of the Benzoyl Peroxide/Amine Initiated Free-Radical Polymerization of Dental Dimethacrylate Monomers: Experimental Studies and Mathematical Modeling for TEGDMA and Bis-EMA. Macromolecules 2004, 37, 4254–4265. [Google Scholar] [CrossRef]
- Sideridou, I.D.; Achilias, D.S.; Karava, O. Reactivity of Benzoyl Peroxide/Amine System as an Initiator for the Free Radical Polymerization of Dental and Orthopaedic Dimethacrylate Monomers: Effect of the Amine and Monomer Chemical Structure. Macromolecules 2006, 39, 2072–2080. [Google Scholar] [CrossRef]
- Zoller, A.; Gigmes, D.; Guillaneuf, Y. Simulation of radical polymerization of methyl methacrylate at room temperature using a tertiary amine/BPO initiating system. Polym. Chem. 2015, 6, 5719–5727. [Google Scholar] [CrossRef]
- Moszner, N.; Köhler, T.; Schädlich, J.; Angermann, J. Dental Materials Based on Redox Systems with Low-Odour Cumene Hydroperoxide Derivatives. U.S. Patent US11357709B2, 14 June 2022. [Google Scholar]
- Yuji, I.; Yoshinori, K.; Kawashima, T. Polymerization Initiator Composition Controlling Polymerization at Interface and Curable Composition Containing Same. European Patent EP0480785B1, 27 December 1996. [Google Scholar]
- Hecht, R.; Ludsteck, M. Initiator System for Acid Dental Formulations. U.S. Patent US6953535B2, 11 October 2005. [Google Scholar]
- Falsafi, A.; Kalgutkar, R.S.; Oxman, J.D. Dental Compositions and Methods with Arylsulfinate Salts. U.S. Patent US7465758B2, 16 December 2008. [Google Scholar]
- Kawashima, M.; Omura, I. Polymerizable Composition. European Patent EP0408357B1, 1 June 1994. [Google Scholar]
- Guggenberger, R.A.; Hecht, R.; Ludsteck, M.; Raia, G.; Stippschild-Boxler, A. Two-Component Self-Adhesive Dental Composition, Process of Production and Use Thereof. U.S. Patent US10932994B2, 2 March 2021. [Google Scholar]
- Thetiot, E.; Ohl, C.; Vidal, L.; Catel, Y.; Lamparth, I.; Fässler, P.; Lalevée, J. Reactivity of dihydropyridines as reducing agents in redox initiating systems. Eur. Polym. J. 2024, 209, 112905. [Google Scholar] [CrossRef]
- Misra, G.S.; Bajpai, U.D.N.; Trekoval, J. Role of thiourea in redox polymerization. JMS Rev. Macromol. Chem. Phys. 1984, C24, 335–353. [Google Scholar] [CrossRef]
- Hennen, H.; Utterodt, A. Two-Component Initiator System (Amine-Free) with Very Good Storage Stability and Particular Suitability for Acid Systems. European Patent EP1754465B1, 5 October 2011. [Google Scholar]
- Burtscher, P.; Gianasmidis, A.; Moszner, N. Photopolymerizable and Dual-Curing Dental Materials Based on Thiourea Derivatives. U.S. Patent US10195121B2, 5 February 2019. [Google Scholar]
- Falsafi, A.; Mitra, S.B. Composition Containing a Polymerizable Reducing Agent, Kit, and Method. U.S. Patent US7541393B2, 2 June 2009. [Google Scholar]
- Lamparth, I.; Fässler, P.; Schnur, T.; Thetiot, E.; Lalevée, J.; Catel, Y. Polymerizable thioureas as innovative reducing agents for self-cured and dual-cured dental materials. Dent. Mater. 2022, 38, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Catel, Y.; Angermann, J.; Grob, B.; Fässler, P.; Lamparth, I.; Schnur, T. Acylthiourea oligomers as promising reducing agents for dimethacrylate-based two-component dental materials. Dent. Mater. 2023, 39, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevee, J. Redox two-component initiated free radical and cationic polymerizations: Concepts, reactions and applications. Prog. Polym. Sci. 2019, 94, 33–56. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Gigmes, D.; Nechab, M.; Morlet-Savary, F.; Dietlin, C.; Gree, S.; Fouassier, J.P.; Lalevee, J. Metal Acetylacetonate−Bidentate Ligand Interaction (MABLI) (Photo)activated Polymerization: Toward High Performance AmineFree, Peroxide-Free Redox Radical (Photo)initiating Systems. Polym. Chem. 2018, 9, 1371–1378. [Google Scholar] [CrossRef]
- Wang, D.; Garra, P.; Szillat, F.; Fouassier, J.P.; Lalevee, J. Silane Based Redox Initiating Systems: Toward a Safer Amine-Free, Peroxide-Free, and Metal-Free Approach. Macromolecules 2019, 52, 3351–3358. [Google Scholar] [CrossRef]
- Garra, P.; Dumur, F.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevee, J. A New Highly Efficient Amine-Free and Peroxide-Free Redox System for Free Radical Polymerization under Air with Possible Light Activation. Macromolecules 2016, 49, 6296–6309. [Google Scholar] [CrossRef]
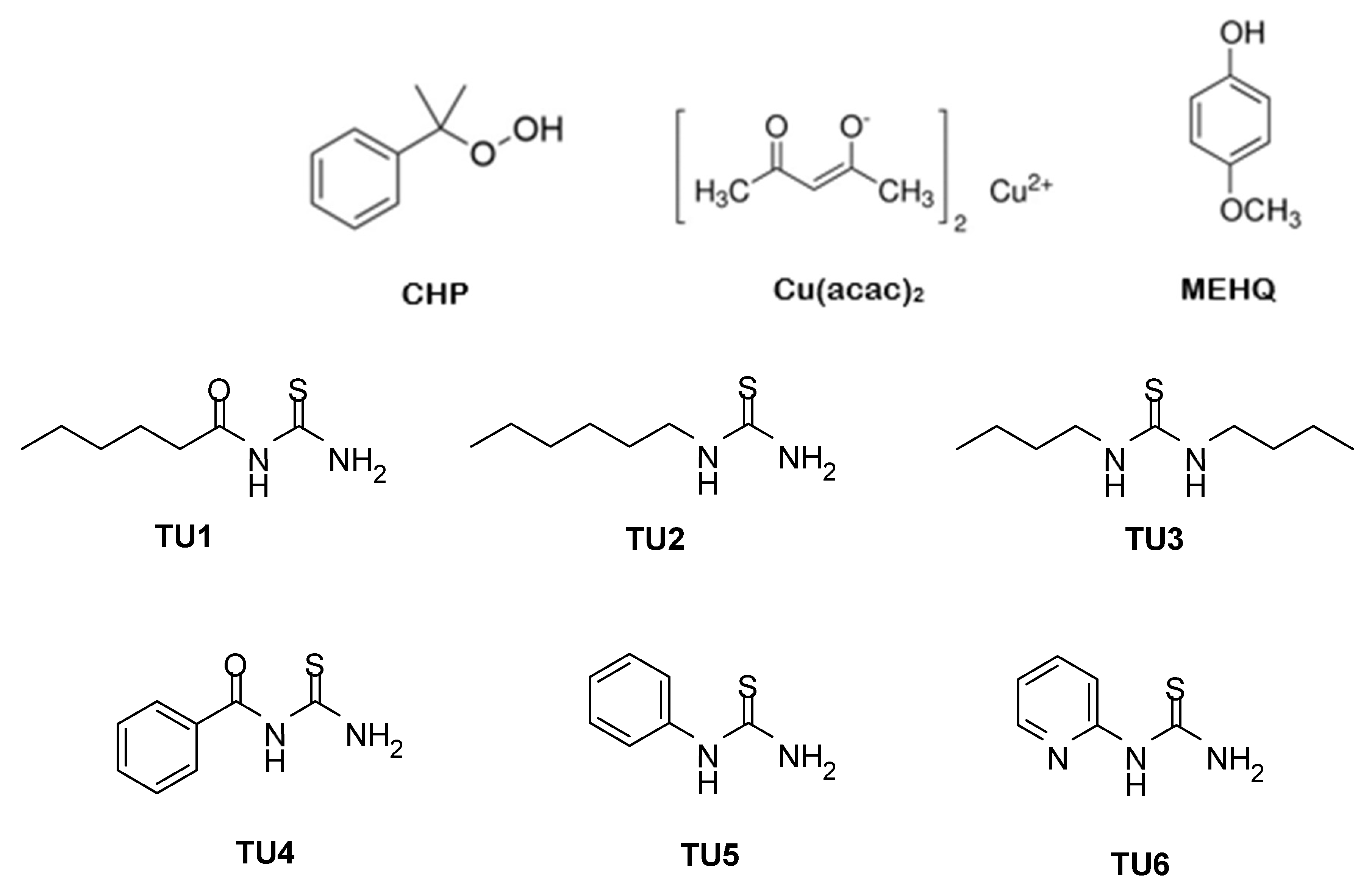
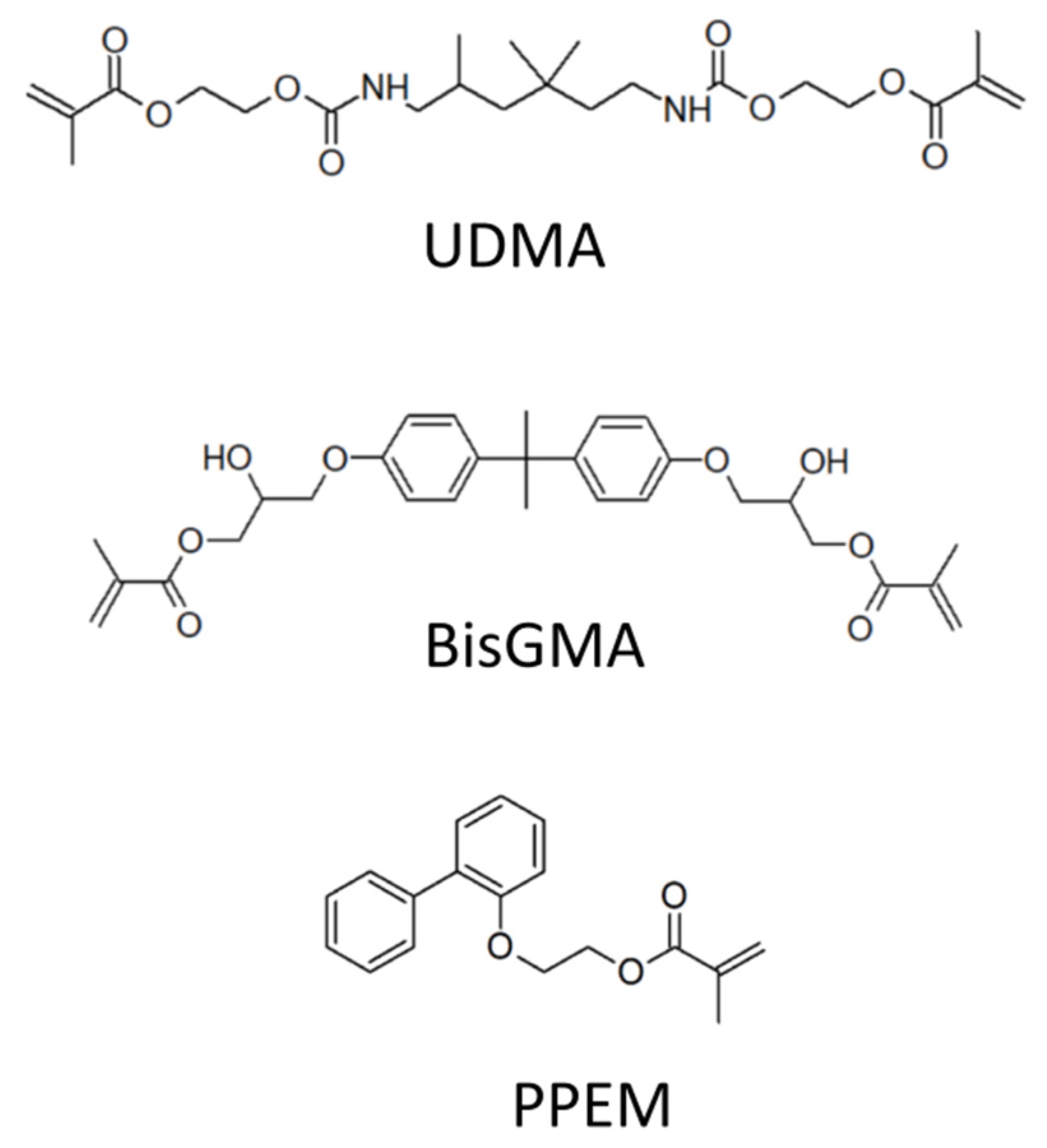

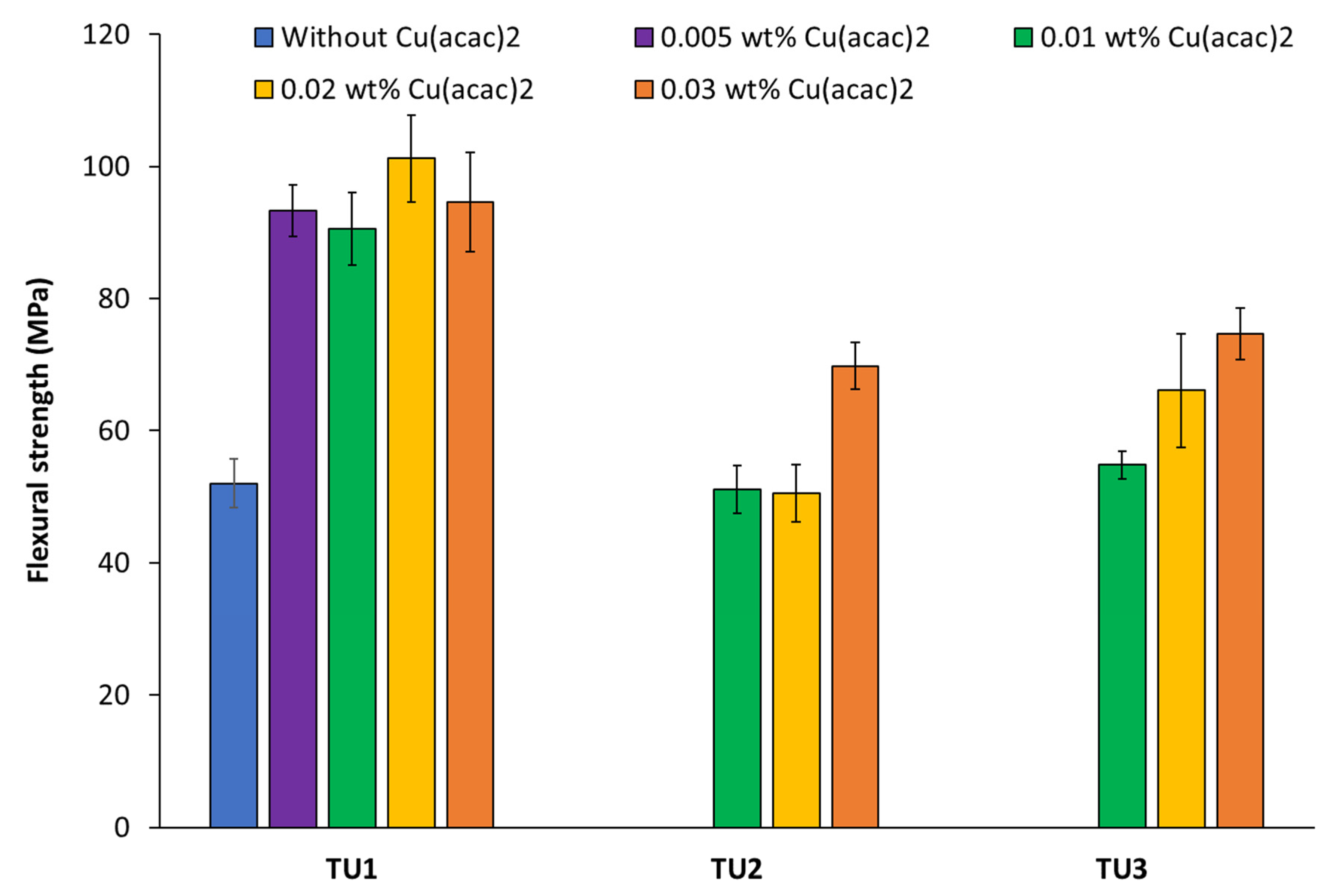
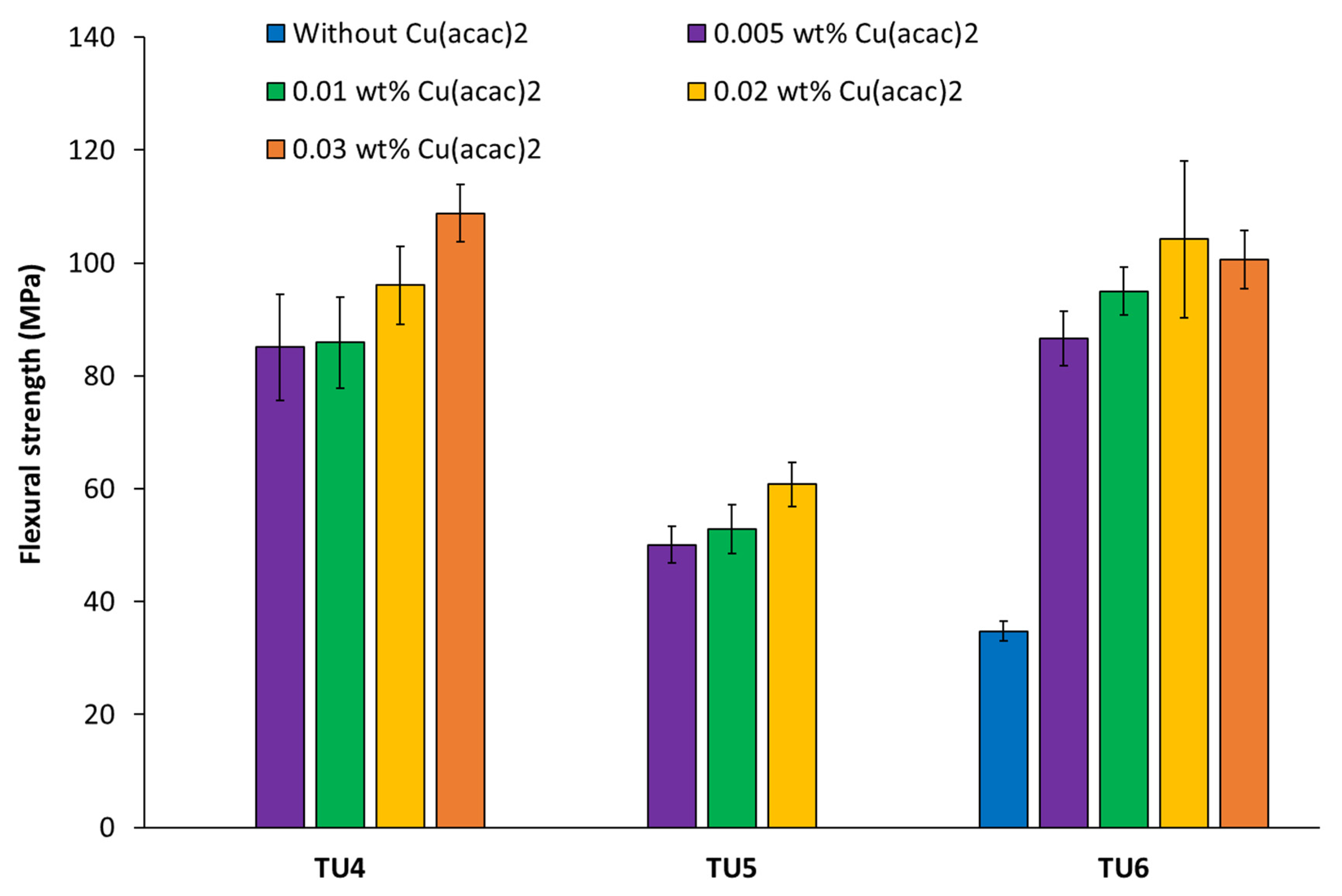





| Oxidizing Agent/Reducing Agent | Cu(acac)2 (wt% in the Second Component) | Flexural Strength (MPa) | Flexural Modulus (MPa) |
|---|---|---|---|
| CHP//TU1 | 0.01 | 90.5 ± 5.5 | 2065 ± 154 |
| CHP//TU2 | 0.01 | 51.1 ± 3.6 | 1108 ± 163 |
| CHP//TU3 | 0.01 | 54.8 ± 2.1 | 1200 ± 89 |
| CHP//TU4 | 0.01 | 85.9 ± 8.1 | 2216 ± 342 |
| CHP//TU5 | 0.01 | 52.9 ± 4.3 | 1080 ± 99 |
| CHP//TU6 | 0.01 | 95.0 ± 4.2 | 2437 ± 88 |
| Systems | Eox (V) | Ered (V) |
|---|---|---|
| TU1 | 1.15 | −0.55 |
| TU2 | 0.85 | −0.35 |
| TU3 | 0.78 | −0.33 |
| TU4 | 1.15 | −0.78 |
| TU5 | 0.79 | −0.35 |
| TU6 | 0.80 | −0.20 |
| Thiourea | TU1 | TU2 | TU3 | TU4 | TU5 | TU6 |
|---|---|---|---|---|---|---|
| [TU] (mol/L) | 0.0192 | 0.0207 | 0.0177 | 0.0185 | 0.0219 | 0.0218 |
| Color |  | |||||
| Position of the H-Abstraction | BDE N-H (kcal/mol) | Spin Density (N) | Spin Density (S) |
|---|---|---|---|
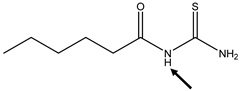 | 81.1 | 0.02 | 0.71 |
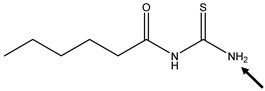 | 93.5 | 0.00 | 0.86 |
 | 96.3 | 0.5 | 0.34 |
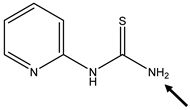 | 104.9 | 0.39 | 0.7 |
| Oxidizing Agent/Reducing Agent | Cu(acac)2 (wt% in the Monomer Mixture) | FS (MPa) | FM (MPa) | Working Time (s) | DBC (%) |
|---|---|---|---|---|---|
| CHP//TU1 | 0.005 | 113.4 ± 9.9 | 6321 ± 237 | 242 ± 6 | 79 ± 1 |
| CHP//TU1 | 0.01 | 107.9 ± 4.7 | 6241 ± 263 | 132 ± 1 | 77 ± 1 |
| CHP//TU1 | 0.02 | 107.4 ± 9.9 | 6419 ± 246 | 88 ± 3 | 77 ± 2 |
| CHP//TU4 | 0.01 | 122.0 ± 10.5 | 7132 ± 213 | 440 ± 31 | 76 ± 1 |
| CHP//TU4 | 0.02 | 117.8 ± 8.5 | 7212 ± 127 | 280 ± 14 | 77 ± 1 |
| CHP//TU4 | 0.03 | 114.6 ± 8.8 | 6856 ± 103 | 236 ± 29 | 78 ± 2 |
| CHP//TU4 | 0.05 | 117.4 ± 8.4 | 7041 ± 251 | 193 ± 3 | 78 ± 1 |
| CHP//TU6 | 0.005 | 120.9 ± 8.2 | 6219 ± 221 | 169 ± 8 | 81 ± 1 |
| CHP//TU6 | 0.01 | 104.6 ± 11.0 | 6710 ± 317 | 115 ± 3 | 79 ± 3 |
| CHP//TU6 | 0.02 | 115.7 ± 6.9 | 6788 ± 355 | 81 ± 2 | 80 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohl, C.; Thetiot, E.; Charles, L.; Catel, Y.; Fässler, P.; Lalevée, J. Evaluation of Various Thiourea Derivatives as Reducing Agents in Two-Component Methacrylate-Based Materials. Polymers 2025, 17, 2017. https://doi.org/10.3390/polym17152017
Ohl C, Thetiot E, Charles L, Catel Y, Fässler P, Lalevée J. Evaluation of Various Thiourea Derivatives as Reducing Agents in Two-Component Methacrylate-Based Materials. Polymers. 2025; 17(15):2017. https://doi.org/10.3390/polym17152017
Chicago/Turabian StyleOhl, Coralie, Estelle Thetiot, Laurence Charles, Yohann Catel, Pascal Fässler, and Jacques Lalevée. 2025. "Evaluation of Various Thiourea Derivatives as Reducing Agents in Two-Component Methacrylate-Based Materials" Polymers 17, no. 15: 2017. https://doi.org/10.3390/polym17152017
APA StyleOhl, C., Thetiot, E., Charles, L., Catel, Y., Fässler, P., & Lalevée, J. (2025). Evaluation of Various Thiourea Derivatives as Reducing Agents in Two-Component Methacrylate-Based Materials. Polymers, 17(15), 2017. https://doi.org/10.3390/polym17152017









