Thermoresponsive Behavior, Degradation, and Bioactivity of Nanohydroxyapatite on Graphene Oxide Nanoscroll-Enhanced Poly(N-isopropylacrylamide)-Based Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. Oxidative Degradation
2.2. Thermal Behavior, Aqueous Stability, and Phase Transition
2.3. Chemical Analysis
2.4. Morphological and Elemental Analysis
2.5. Antioxidant Behavior
2.6. Statistical Analysis
3. Results and Discussion
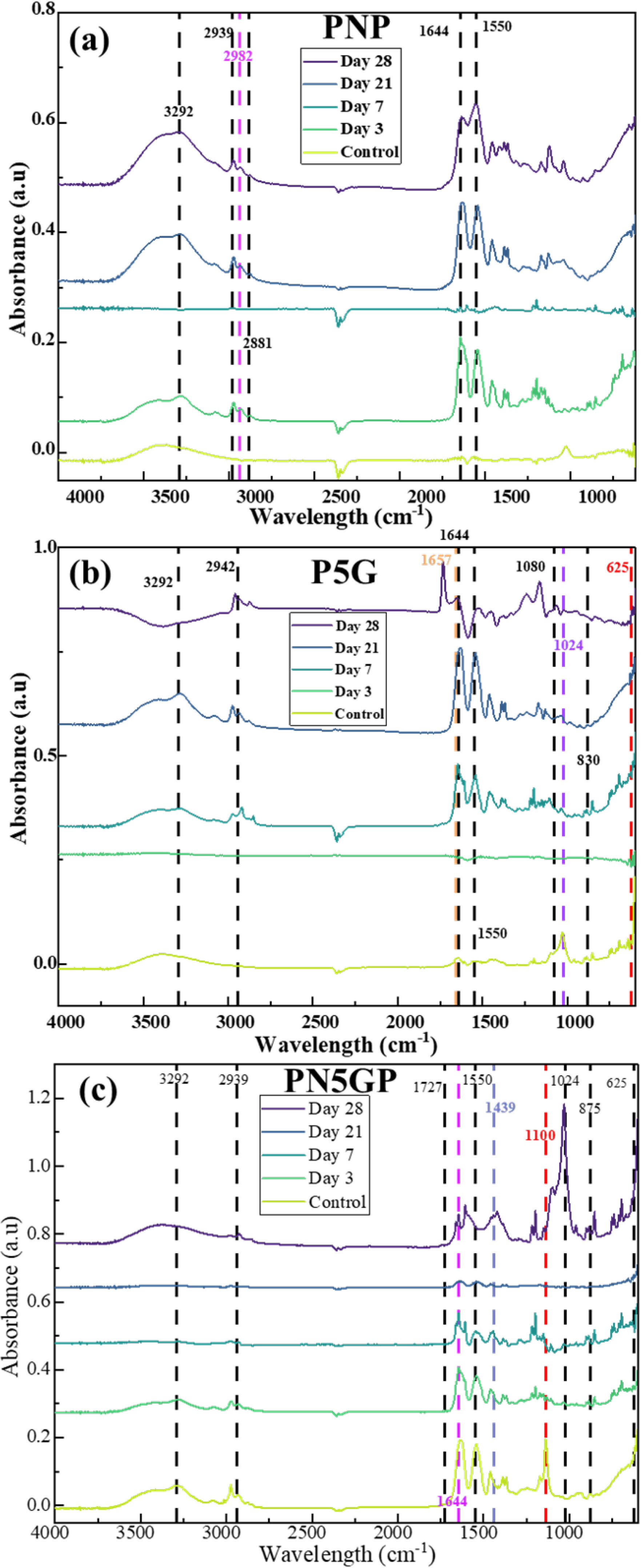
3.1. Chemical Analysis
3.2. Thermoresponsiveness and LCST
3.3. Mass Loss
3.4. Biomineralization and Surface Morphology
3.5. Antioxidant Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALP | Alkaline Phosphatase |
| ATR | Attenuated Total Reflectance |
| Ca | Calcium |
| Cu | Copper |
| CuSO4 | Copper (II) Sulfate |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DSC | Differential Scanning Calorimetry |
| EDS | Energy-Dispersive Spectrometry |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| GO | Graphene Oxide |
| GONS | Graphene Oxide Nanoscrolls |
| H2O2 | Hydrogen Peroxide |
| KCl | Potassium Chloride |
| LAC-SBF | Lactate-Modified Simulated Body Fluid |
| LCST | Lower Critical Solution Temperature |
| nHA | Nanohydroxyapatite |
| NIPAAm | N-isopropylacrylamide |
| P5G | PNIPAAm + 5 wt.% nHA-GONS |
| PCL | Polycaprolactone |
| PEG | Polyethylene Glycol |
| PN5GP | PNIPAAm + 5 wt.% nHA-GONS + PCL Microspheres |
| PNP | Pure PNIPAAm |
| PNIPAAm | Poly(N-isopropylacrylamide) |
| PBS | Phosphate-Buffered Saline |
| Q | Swelling Ratio |
| ROS | Reactive Oxygen Species |
| RSA | Radical Scavenging Activity |
| SBF | Simulated Body Fluid |
| SEM | Scanning Electron Microscopy |
| SLA | Stereolithography |
| sp2 | sp2-Hybridized Carbon (conjugated carbon domain of graphene) |
| UV | Ultraviolet (used implicitly in photo-initiation context) |
| wt.% | Weight Percent |
References
- Liang, X.; Yang, X.; Liu, J.; Tu, L.; Wei, W.; Wang, H.; Wu, M.; Cai, L.; Zheng, Y.; Chen, Y. ROS-scavenging bioactive scaffold orchestrates bone regeneration for osteoporotic bone defect repair. Compos. Part B Eng. 2024, 281. [Google Scholar] [CrossRef]
- Mambiri, L.T.; Depan, D. Degradation Kinetics, Mechanisms, and Antioxidant Activity of PCL-Based Scaffolds with In Situ Grown Nanohydroxyapatite on Graphene Oxide Nanoscrolls. C 2025, 11, 5. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold degradation in bone tissue engineering: An overview. Int. Biodeterior. Biodegradation 2023, 180, 105599. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, M.; Fu, Y.; Castro, N.J.; Fu, S.W.; Zhang, L.G. Engineering a biomimetic three-dimensional nanostructured bone model for breast cancer bone metastasis study. Acta Biomater. 2015, 14, 164–174. [Google Scholar] [CrossRef]
- Cerqueni, G.; Scalzone, A.; Licini, C.; Gentile, P.; Mattioli-Belmonte, M. Insights into oxidative stress in bone tissue and novel challenges for biomaterials. Mater. Sci. Eng. C 2021, 130, 112433. [Google Scholar] [CrossRef]
- Vakil, A.U.; Petryk, N.M.; Du, C.; Howes, B.; Stinfort, D.; Serinelli, S.; Gitto, L.; Ramezani, M.; Beaman, H.T.; Monroe, M.B.B. In vitro and in vivo degradation correlations for polyurethane foams with tunable degradation rates. J. Biomed. Mater. Res. A 2023, 111, 580–595. [Google Scholar] [CrossRef]
- De Oliveira-Marques, V.; Cyrne, L.; Marinho, H.S.; Antunes, F. A Quantitative Study of NF-κB Activation by H2O2: Relevance in Inflammation and Synergy with TNF-α. J. Immunol. 2007, 178, 3893–3902. [Google Scholar] [CrossRef]
- Feng, P.; He, J.; Peng, S.; Gao, C.; Zhao, Z.; Xiong, S.; Shuai, C. Characterizations and interfacial reinforcement mechanisms of multicomponent biopolymer based scaffold. Mater. Sci. Eng. C 2019, 100, 809–825. [Google Scholar] [CrossRef]
- Hu, H.; Liu, X.; Chen, J.; Cui, S.; Yi, H.; Wang, G.; Wang, R.; Zheng, T.; Wan, B.; Zhou, Z.; et al. Regulation of hypoxic stress and oxidative stress in bone grafting: Current trends and future perspectives. J. Mater. Sci. Technol. 2023, 157, 144–153. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, X.; Yang, C.; Su, P.; Yin, C.; Qian, A.-R. The Impact of Oxidative Stress on the Bone System in Response to the Space Special Environment. Int. J. Mol. Sci. 2017, 18, 2132. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Q.; Ma, X.; Zhong, Y.; Tang, H.; Mai, S. The mechanism of biomineralization: Progress in mineralization from intracellular generation to extracellular deposition. Jpn. Dent. Sci. Rev. 2023, 59, 181–190. [Google Scholar] [CrossRef]
- Mambiri, L.T.; Broussard, G.; Smith, J.; Depan, D. In-Situ Grown Nanohydroxyapatite on Graphene Oxide Nanoscrolls for Modulated Physicochemical Properties of Poly (Caprolactone) Composites. Macromol 2024, 4, 285–303. [Google Scholar] [CrossRef]
- Ashraf, S.; Park, H.-K.; Park, H.; Lee, S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: Role in drug delivery and tissue engineering. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Wu, K.; Hu, Y.; Feng, H. Investigation of 3D-printed PNIPAM-based constructs for tissue engineering applications: A review. J. Mater. Sci. 2023, 58, 17727–17750. [Google Scholar] [CrossRef]
- Yang, G.; Mahadik, B.; Choi, J.Y.; Fisher, J.P. Vascularization in tissue engineering: Fundamentals and state-of-art. Progress. Biomed. Eng. 2020, 2, 012002. [Google Scholar] [CrossRef]
- Abdelhalim, A.O.; Meshcheriakov, A.A.; Maistrenko, D.N.; Molchanov, O.E.; Ageev, S.V.; Ivanova, D.A.; Iamalova, N.R.; Luttsev, M.D.; Vasina, L.V.; Sharoyko, V.V.; et al. Graphene oxide enriched with oxygen-containing groups: On the way to an increase of antioxidant activity and biocompatibility. Colloids Surf. B Biointerfaces 2022, 210, 112232. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Serna, J.A.; Debastiani, R.; Gomez, J.E.U.; Lu, L.; Yang, W.; Dong, Z.; Levkin, P.A. Enhancing Temperature Responsiveness of PNIPAM Through 3D-Printed Hierarchical Porosity. Adv. Funct. Mater. 2024, 34, 2403794. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Han, D.; Lu, Z.; Chester, S.A.; Lee, H. Micro 3D Printing of a Temperature-Responsive Hydrogel Using Projection Micro-Stereolithography. Sci. Rep. 2018, 8, 1963. [Google Scholar] [CrossRef]
- Sun, X.; Tyagi, P.; Agate, S.; McCord, M.G.; Lucia, L.A.; Pal, L. Highly tunable bioadhesion and optics of 3D printable PNIPAm/cellulose nanofibrils hydrogels. Carbohydr. Polym. 2020, 234, 115898. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Yilmaz, B.; Pazarceviren, A.E.; Tezcaner, A.; Evis, Z. Historical development of simulated body fluids used in biomedical applications: A review. Microchem. J. 2020, 155, 104713. [Google Scholar] [CrossRef]
- George, K.; Depan, D.; Khattab, A.; Chirdon, W.M. Accelerated weathering of EPON-IPD thermosets reinforced with carbon black. Polym. Degrad. Stab. 2025, 232, 111143. [Google Scholar] [CrossRef]
- García, A.V.; Serrano, N.J.; Sanahuja, A.B.; Garrigós, M.C. Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds. Antioxidants 2020, 9, 629. [Google Scholar] [CrossRef]
- Dueramae, I.; Tanaka, F.; Shinyashiki, N.; Yagihara, S.; Kita, R. UV-Crosslinked Poly(N-isopropylacrylamide) Interpenetrated into Chitosan Structure with Enhancement of Mechanical Properties Implemented as Anti-Fouling Materials. Gels 2023, 10, 20. [Google Scholar] [CrossRef]
- Liu, M.; Bian, F.; Sheng, F. FTIR study on molecular structure of poly(N-isopropylacrylamide) in mixed solvent of methanol and water. Eur. Polym. J. 2005, 41, 283–291. [Google Scholar] [CrossRef]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012. [Google Scholar] [CrossRef]
- Nahi, O.; Kulak, A.N.; Zhang, S.; He, X.; Aslam, Z.; Ilett, M.A.; Ford, I.J.; Darkins, R.; Meldrum, F.C. Polyamines Promote Aragonite Nucleation and Generate Biomimetic Structures. Adv. Sci. 2023, 10, e2203759. [Google Scholar] [CrossRef]
- Honold, T.; Skrybeck, D.; Wagner, K.G.; Karg, M. Fully Reversible Quantitative Phase Transfer of Gold Nanoparticles Using Bifunctional PNIPAM Ligands. Langmuir 2017, 33, 253–261. [Google Scholar] [CrossRef]
- Wong, S.; Eaton, A.; Krywka, C.; Nair, A.; Deymier, A. The location of cationic substitutions in carbonated biomimetic apatites significantly affects crystal nanomechanics. Sci. Rep. 2024, 14, 22625. [Google Scholar] [CrossRef]
- Tan, J.; Yang, Q.; Hu, G.; Zhang, H.; Pei, L.; Wang, J. Experimental study on the temperature-sensitive behavior of poly-n-isopropylacrylamide/graphene oxide composites and the flexible conductive cotton fabrics. Polym. Test. 2022, 110, 107563. [Google Scholar] [CrossRef]
- Hruschka, V.; Saeed, A.; Slezak, P.; Al Ghanami, R.C.; Feichtinger, G.A.; Alexander, C.; Redl, H.; Shakesheff, K.; Wolbank, S. Evaluation of a Thermoresponsive Polycaprolactone Scaffold for In Vitro Three-Dimensional Stem Cell Differentiation. Tissue Eng. Part A 2015, 21, 310–319. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, H.; Du, T.; Yang, D. The impact of copper on bone metabolism. J. Orthop. Transl. 2024, 47, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Lee, B.H.; Pauken, C.; Vernon, B.L. Degradation, cytotoxicity, and biocompatibility of NIPAAm-based thermosensitive, injectable, and bioresorbable polymer hydrogels. J. Biomed. Mater. Res. A 2011, 98A, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bingham, N.M.; Nisa, Q.U.; Chua, S.H.L.; Fontugne, L.; Spick, M.P.; Roth, P.J. Thioester-Functional Polyacrylamides: Rapid Selective Backbone Degradation Triggers Solubility Switch Based on Aqueous Lower Critical Solution Temperature/Upper Critical Solution Temperature. ACS Appl. Polym. Mater. 2020, 2, 3440–3449. [Google Scholar] [CrossRef]
- Babu, A.; Maji, S.; Sivakumar, G.; Hoogenboom, R. Design of poly(N-isopropylacrylamide) coated MnO 2 nanoparticles for thermally regulated catalytic decomposition of H2O2. Polym. Chem. 2024, 15, 2763–2772. [Google Scholar] [CrossRef]
- Valencia, L.; Enríquez, F.J.; Valencia, M.; Díaz, R. Tuning the LCST of PNIPAM via Random Oxidation-Sensitive Thioether Functionalities. Macromol. Chem. Phys. 2017, 218, 1600556. [Google Scholar] [CrossRef]
- Apsite, I.; Stoychev, G.; Zhang, W.; Jehnichen, D.; Xie, J.; Ionov, L. Porous Stimuli-Responsive Self-Folding Electrospun Mats for 4D Biofabrication. Biomacromolecules 2017, 18, 3178–3184. [Google Scholar] [CrossRef]
- Damonte, G.; Cozzani, M.; Di Lisa, D.; Pastorino, L.; Mariani, A.; Monticelli, O. Mechanically-reinforced biocompatible hydrogels based on poly(N-isopropylacrylamide) and star-shaped polycaprolactones. Eur. Polym. J. 2023, 195, 112239. [Google Scholar] [CrossRef]
- Rakshit, A.; Khatua, K.; Shanbhag, V.; Comba, P.; Datta, A. Cu 2+ selective chelators relieve copper-induced oxidative stress in vivo. Chem. Sci. 2018, 9, 7916–7930. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, Z.; Wang, P.; Chen, F.; Luo, X. CuS-PNIPAm nanoparticles with the ability to initiatively capture bacteria for photothermal treatment of infected skin. Regen. Biomater. 2022, 9, rbac026. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Liu, S.-T.; Lin, W.-R.; Lin, C.-K.; Huang, S.-M. The Mechanisms Underlying the Cytotoxic Effects of Copper Via Differentiated Embryonic Chondrocyte Gene 1. Int. J. Mol. Sci. 2019, 20, 5225. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Sun, J.; Yang, P.; Li, S.; Liu, Z.; Liu, C.; Shen, C. Efficient adsorption of uranyl from seawater by Nitrogen-Oxygen synergistic bidentate Coordination: Deciphering the mechanism from experimental and Real-Space functional aspects. Chem. Eng. J. 2024, 497, 154480. [Google Scholar] [CrossRef]
- Lu, M.; Liu, P.; Wang, F.; Ding, Y.; Zhang, S.; Yang, M. Synthesis of nanoparticle-immobilized antioxidants and their antioxidative performances in polymer matrices: A review. Polym. Int. 2018, 67, 356–373. [Google Scholar] [CrossRef]
- Sánchez-Abella, L.; Ruiz, V.; Vicente, A.P.-S.; Grande, H.-J.; Loinaz, I.; Dupin, D. Reactive oxygen species (ROS)-responsive biocompatible polyethylene glycol nanocomposite hydrogels with different graphene derivatives. J. Mater. Sci. 2021, 56, 10041–10052. [Google Scholar] [CrossRef]








| Sample | PNP | P5G | PN5GP | |||
|---|---|---|---|---|---|---|
| Ca (wt.%) | P (wt.%) | Ca (wt.%) | P (wt.%) | Ca (wt.%) | P (wt.%) | |
| Day | ||||||
| 0 | 0 | 0 | 0.1 | 0 | 0.1 | 0 |
| 1 | 0 | 0 | 2.7 | 0.2 | 1.6 | 3.6 |
| 3 | 1.3 | 0 | 3.6 | 8 | 1.2 | 4.2 |
| 7 | 4.2 | 0 | 9 | 1.8 | 5 | 0.1 |
| 14 | 4.3 | 0 | 9.7 | 4.9 | 10 | 3 |
| 21 | 3.8 | 0 | 1.8 | 0 | 4.3 | 2 |
| 28 | 3.8 | 0 | 1.8 | 0 | 4.3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mambiri, L.T.; Guillory, R.; Depan, D. Thermoresponsive Behavior, Degradation, and Bioactivity of Nanohydroxyapatite on Graphene Oxide Nanoscroll-Enhanced Poly(N-isopropylacrylamide)-Based Scaffolds. Polymers 2025, 17, 2014. https://doi.org/10.3390/polym17152014
Mambiri LT, Guillory R, Depan D. Thermoresponsive Behavior, Degradation, and Bioactivity of Nanohydroxyapatite on Graphene Oxide Nanoscroll-Enhanced Poly(N-isopropylacrylamide)-Based Scaffolds. Polymers. 2025; 17(15):2014. https://doi.org/10.3390/polym17152014
Chicago/Turabian StyleMambiri, Lillian Tsitsi, Riley Guillory, and Dilip Depan. 2025. "Thermoresponsive Behavior, Degradation, and Bioactivity of Nanohydroxyapatite on Graphene Oxide Nanoscroll-Enhanced Poly(N-isopropylacrylamide)-Based Scaffolds" Polymers 17, no. 15: 2014. https://doi.org/10.3390/polym17152014
APA StyleMambiri, L. T., Guillory, R., & Depan, D. (2025). Thermoresponsive Behavior, Degradation, and Bioactivity of Nanohydroxyapatite on Graphene Oxide Nanoscroll-Enhanced Poly(N-isopropylacrylamide)-Based Scaffolds. Polymers, 17(15), 2014. https://doi.org/10.3390/polym17152014








