Recent Advances in Liquid Crystal Polymer-Based Circularly Polarized Luminescent Materials: A Review
Abstract
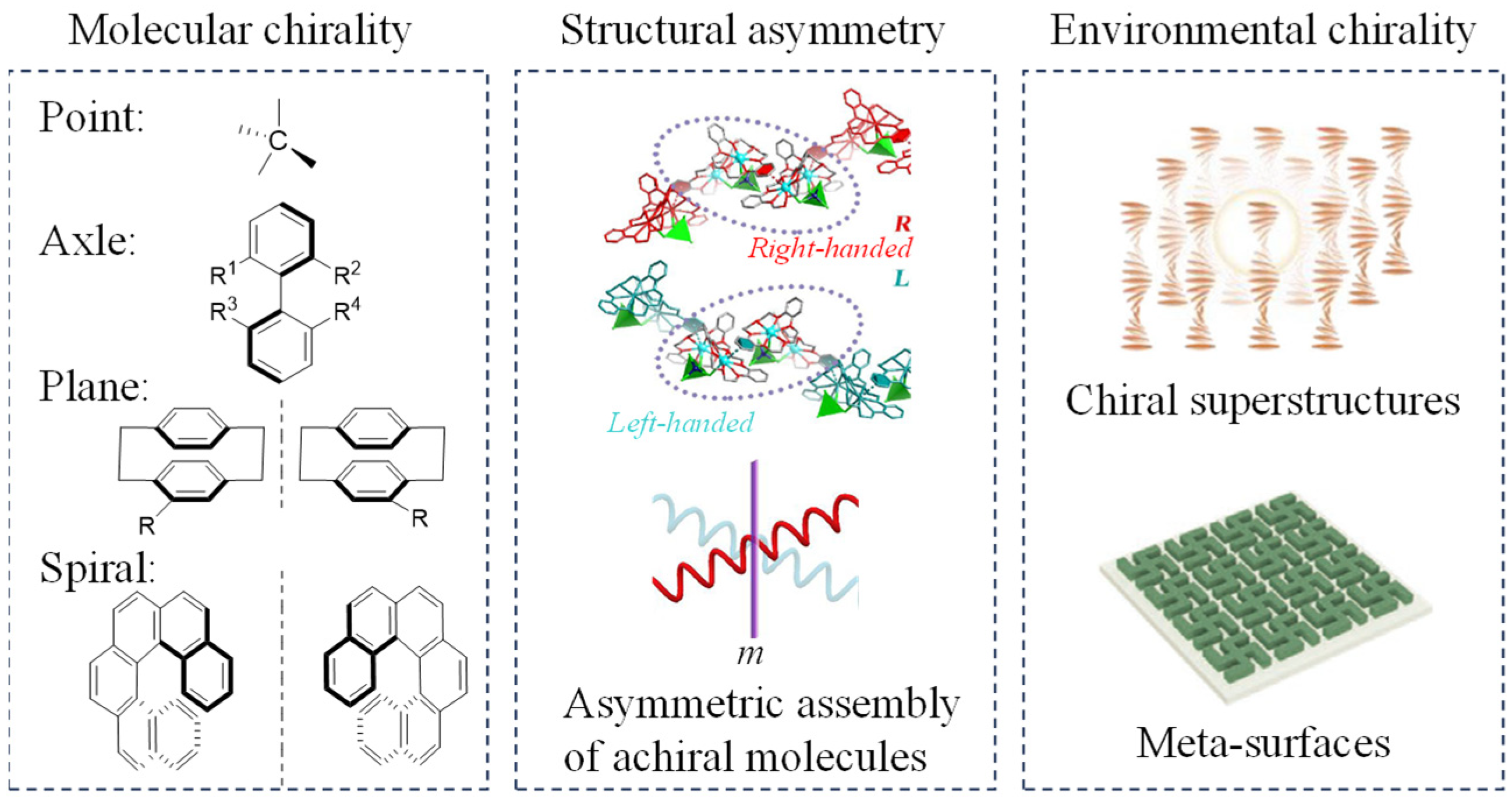
1. Introduction
2. CPL Materials Based on Liquid Crystal Polymers
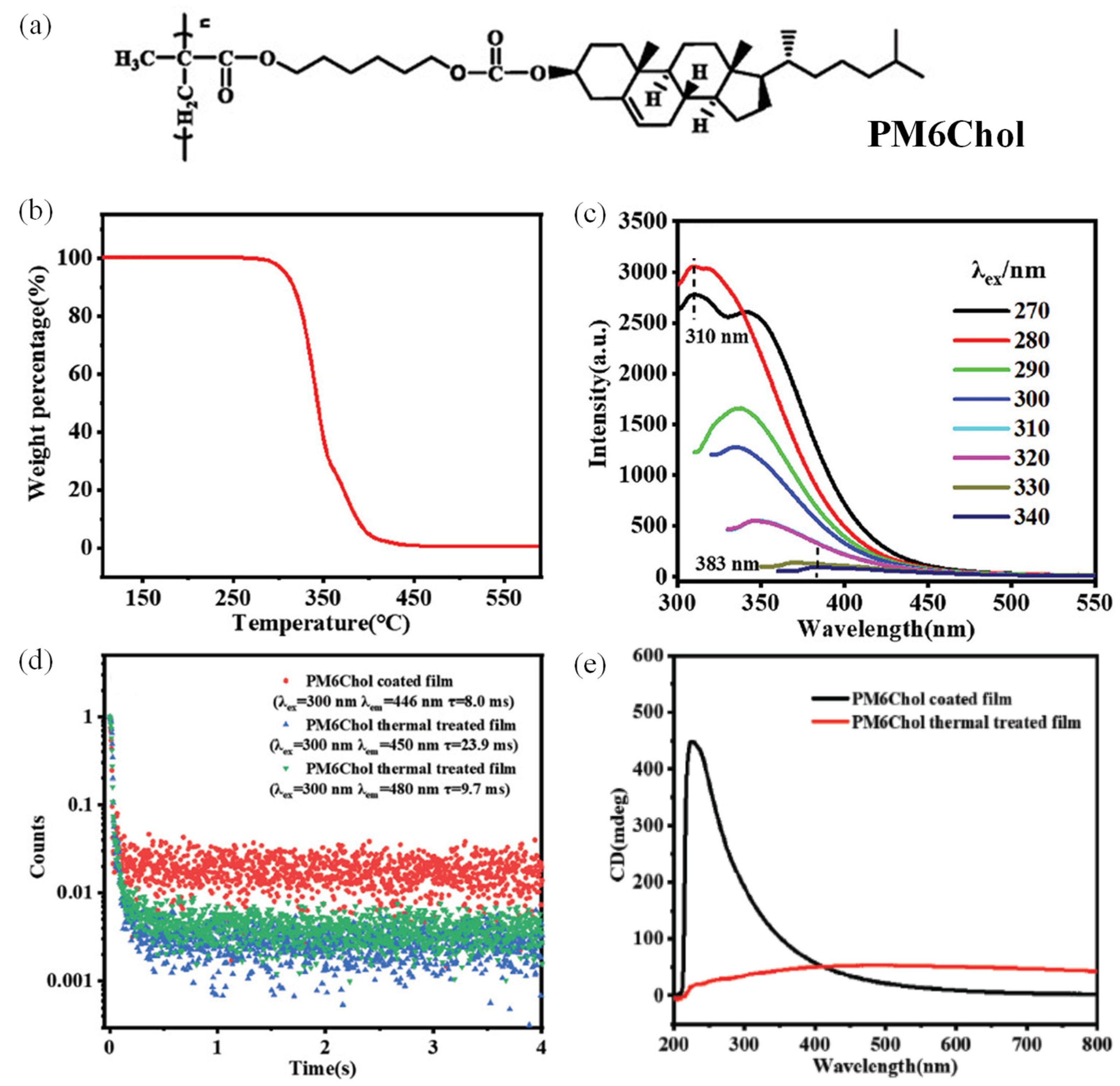
2.1. LCP-Based CPL Materials Constructed by Covalently Grafting of Cholesterol Clusteroluminogens with Methacrylic Acids and Radical Polymerization
2.2. CPL Materials Constructed by Noncovalent Interactions Between LCPs and Luminescent Sources
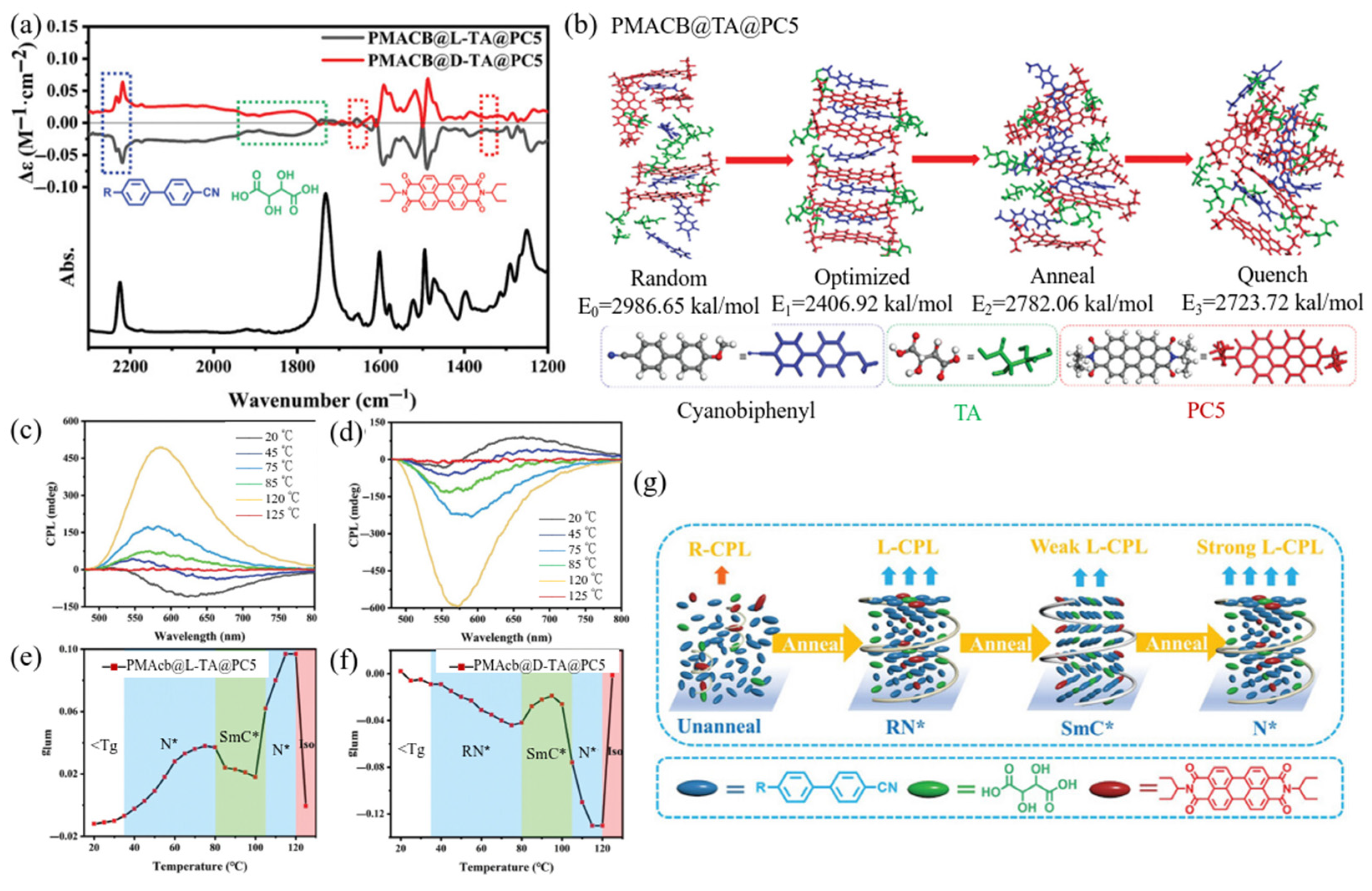
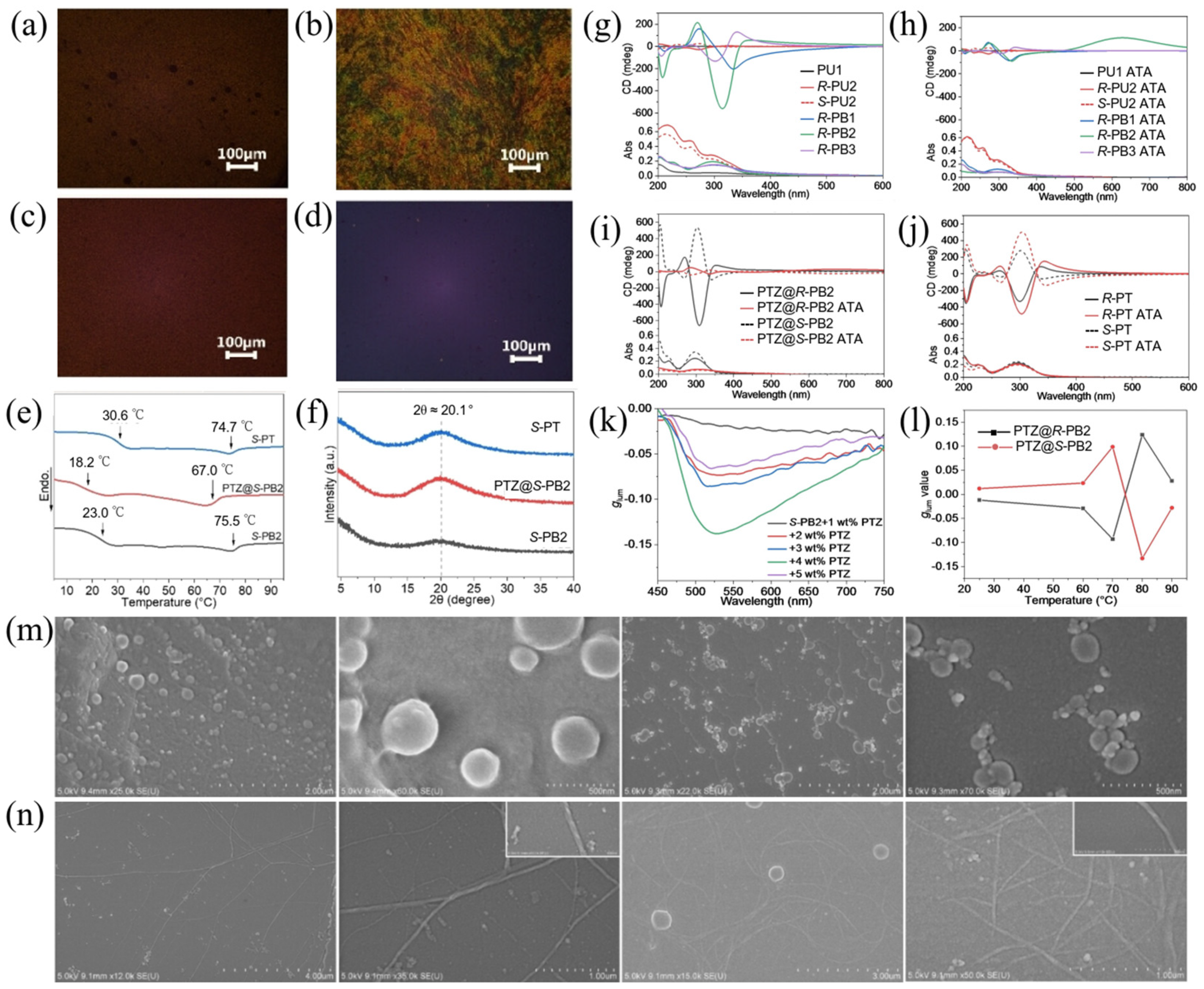
2.2.1. LCP-Based Hybrid Films Prepared by Host–Guest Co-Assembly of Achiral Dyes with LCPs for Controllable CPL
2.2.2. High-Performance CPL Materials Prepared by Chirality–Induction Co-Assembly of Aggregate-Induced-Emission (AIE) Molecules with LCPs
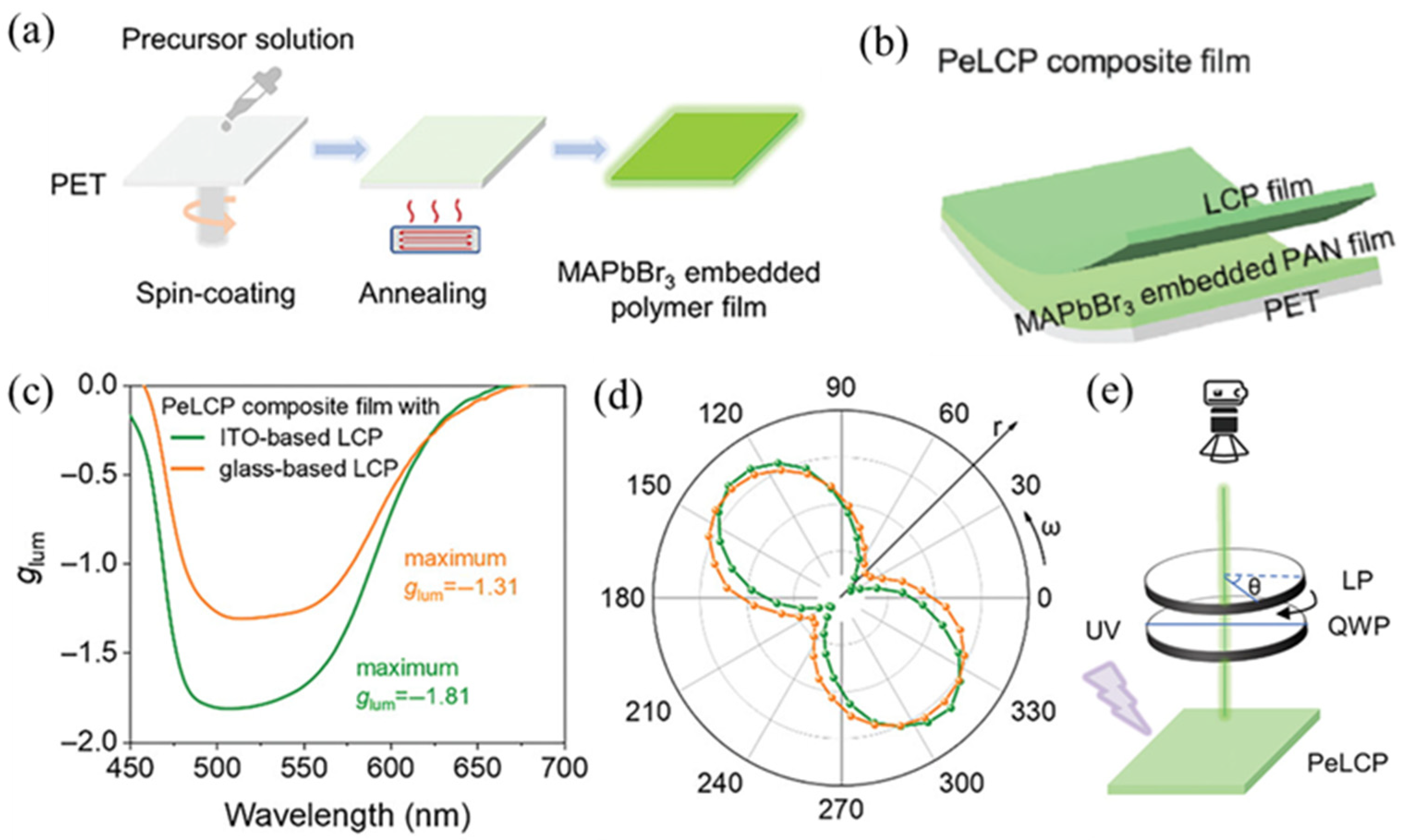
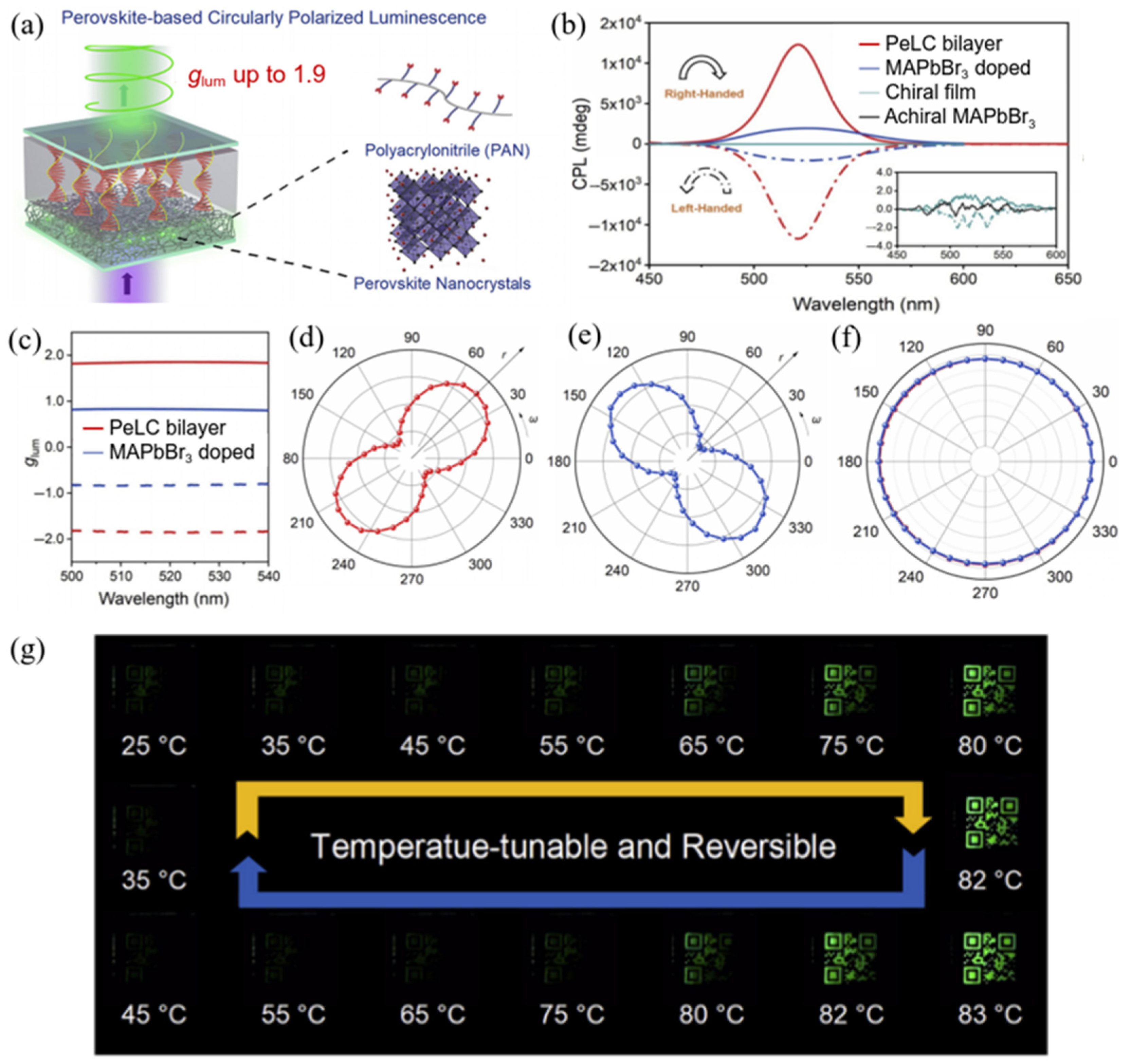
2.2.3. Perovskite Quantum Dots–LCPs Composites as Flexible and Stable CPL Materials Prepared by a Double-Layer Construction Strategy
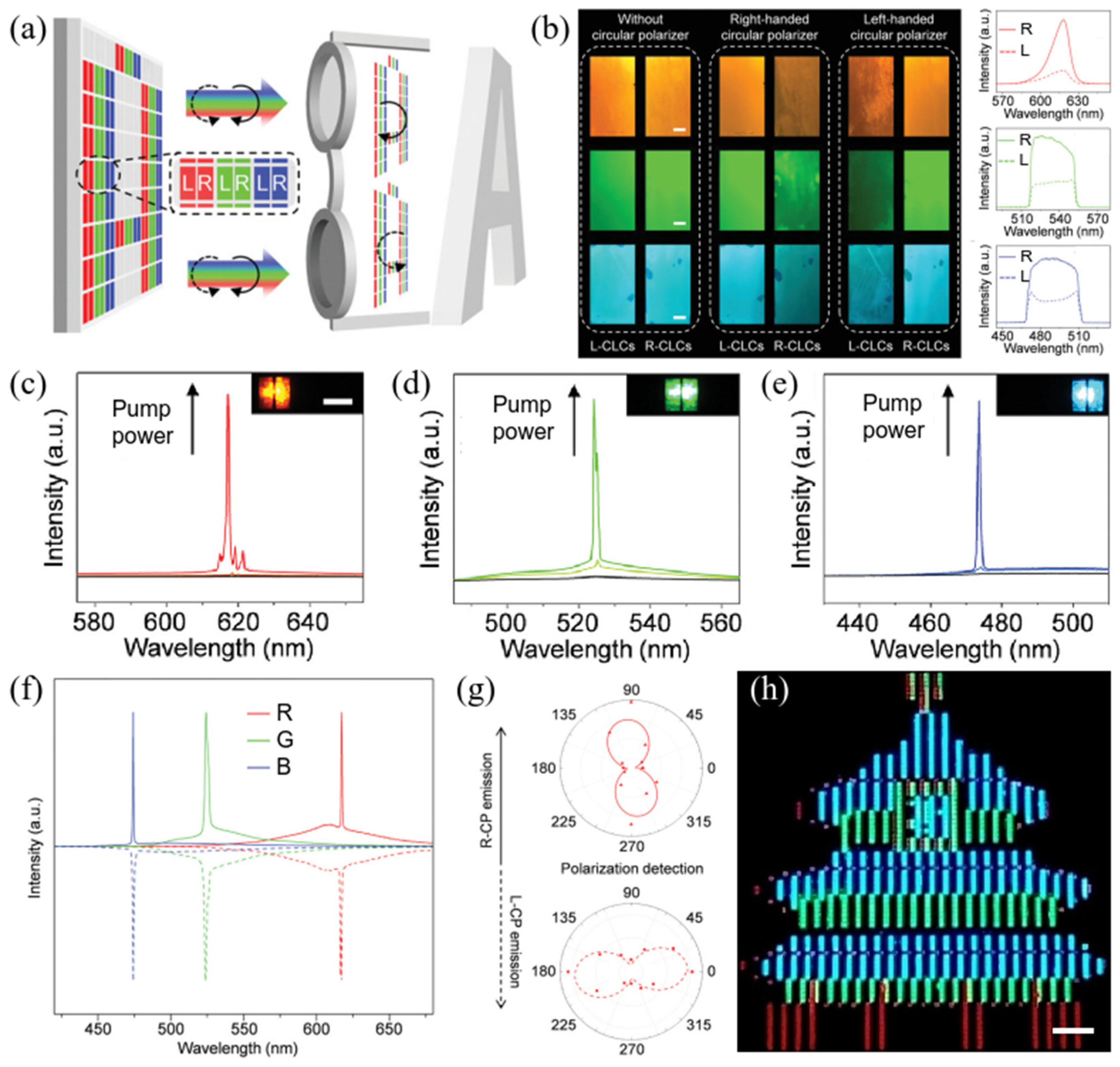
2.2.4. Full-Color CPL Materials Constructed by Combining Chiral Helical Polymer and Liquid Crystal Monomers via Photopolymerization
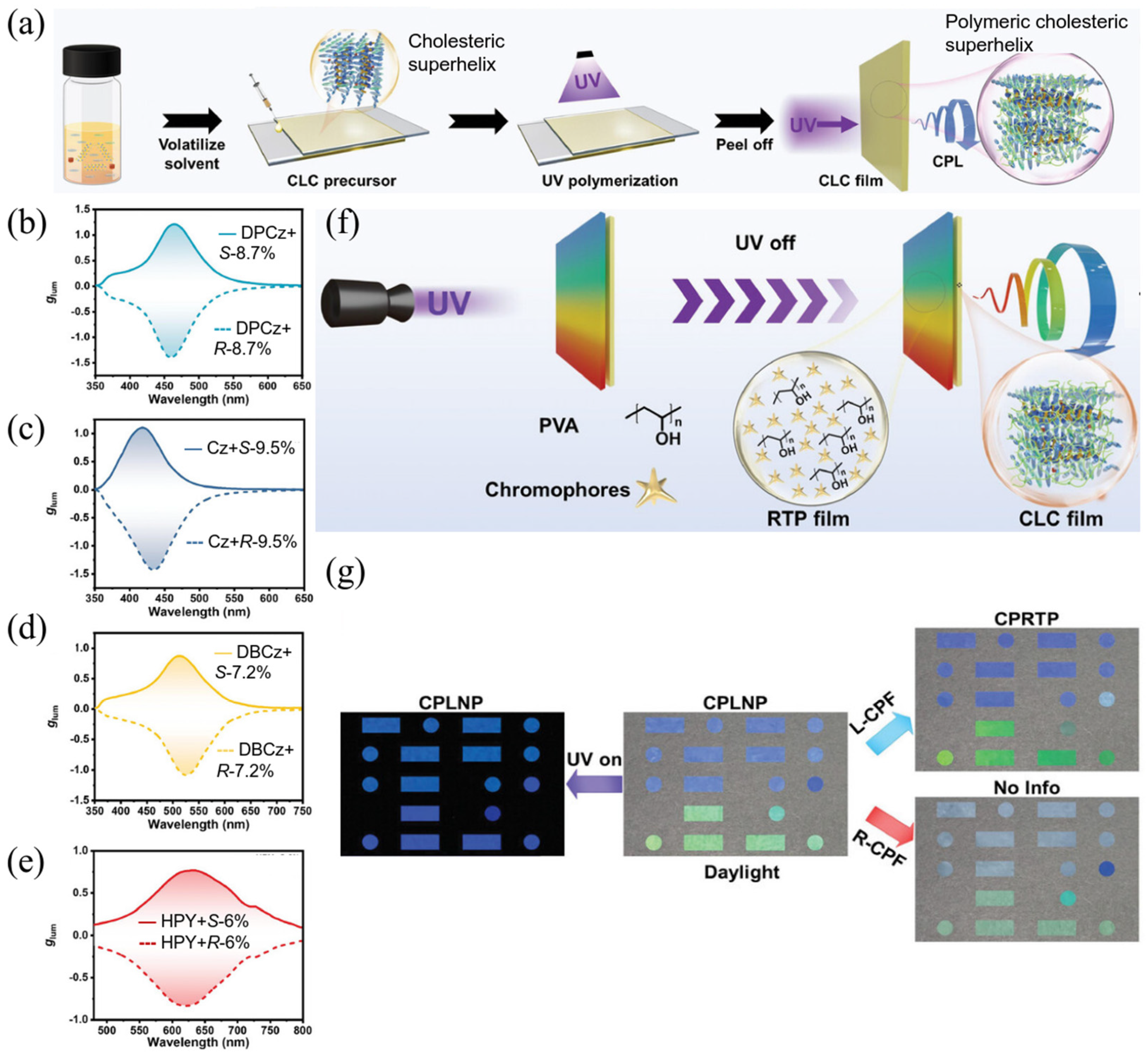
2.3. Other CPL Hybrid Systems Containing LCs or/and LCPs for Future Possible CPL-Based Applications
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Wang, L.; Urbas, A.M.; Li, Q. Nature-inspired emerging chiral liquid crystal nanostructures: From molecular self-assembly to DNA mesophase and nanocolloids. Adv. Mater. 2020, 32, 1801335. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.H.; Zhang, L.; Wang, T.Y. Supramolecular chirality in self-assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Han, J.; Zhao, T.; Duan, P.; Liu, M.H. Circularly polarized luminescence in nanoassemblies: Generation, amplication, and application. Adv. Mater. 2020, 32, 1900110. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; Mccall, A.; Clark, S.; Ménard, F.; Tamura, M. Circular polarization in star-formation regions: Implications for biomolecular homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.H.; Guida, W.C.; Daniel, K.G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 2011, 11, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.T.; Pau, S. Circularly and elliptically polarized light under water and the Umov effect. Light Sci. Appl. 2019, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Lin, H.Y.; Tsai, C.H. Designs of broadband and wide-view patterned polarizers for stereoscopic 3D displays. Opt. Express 2010, 18, 27079–27094. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Xu, L.; Sun, M.; Zhang, H.; Kuang, H.; Xu, C. Circularly polarized light triggers biosensing based on chiral assemblies. Chemistry 2019, 25, 12235–12240. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Costa, R.C.; Fuchter, M.J.; Campbell, A.J. Circularly polarized light detection by a chiral organic semiconductor transistor. Nat. Photonics 2013, 7, 634–638. [Google Scholar] [CrossRef]
- Xu, L.R.; Feng, Y.; Yu, D.; Zheng, Z.; Chen, X.; Hong, W. Recoverable photolithographic patterning for polarized display and encryption. Adv. Mater. Technol. 2020, 5, 2000373. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, M.; Zhuang, Y.; Liu, S.; Huang, W.; Zhao, Q. Circularly polarized luminescence from organic micro-/nano-structures. Light Sci. Appl. 2021, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Li, Z.; Zhu, X.; Ouyang, G.; Liu, M. Hierarchically self-assembled homochiral helical microtoroids. Nat. Nanotechnol. 2022, 17, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Nakashima, T.; Tsumatori, H.; Mori, M.; Naito, M.; Kawai, T. Circularly polarized luminescence in supramolecular assemblies of chiral bichromophoric perylene bisimides. Chemistry 2013, 19, 14090–14097. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Ding, Z.; Shen, Y.; Yang, B.; Liu, M.; Jiang, S. Multi-color tunable circularly polarized luminescence in one single AIE system. Chem. Sci. 2020, 11, 2169–2174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, J.; Wang, Y.; Li, D.; Jia, X.; Yuan, Y.; Cheng, Y. Tunable aggregation-induced circularly polarized luminescence of chiral AIEgens via the regulation of mono-/di-substituents of molecules or nanostructures of self-assemblies. Mater. Chem. Front. 2019, 3, 2066–2071. [Google Scholar] [CrossRef]
- Furlan, F.; Moreno-Naranjo, J.M.; Gasparini, N.; Feldmann, S.; Wade, J.; Fuchter, M.J. Chiral materials and mechanisms for circularly polarized light-emitting diodes. Nat. Photonics 2024, 18, 658–668. [Google Scholar] [CrossRef]
- Wan, S.-P.; Lu, H.-Y.; Li, M.; Chen, C.-F. Advances in circularly polarized luminescent materials based on axially chiral compounds. J. Photochem. Photobiol. C Photochem. Rev. 2022, 50, 100500. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Gong, Z.-L.; Liang, T.; Bernhard, S.; Zhong, Y.-W.; Yao, J. Circularly polarized phosphorescence and photon transport of micro/nanocrystals of ruthenium and iridium complexes with chiral anions. Sci. China Chem. 2023, 66, 2892–2902. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Song, Y.; Zhao, S.; Zhang, M.; Li, G.; Guo, Q.; Tong, Z.; Li, Z.; Jin, S.; et al. Helical-caging enables single-emitted large asymmetric full-color circularly polarized luminescence. Nat. Comm. 2024, 15, 251. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-T.; Chen, K.; Xu, P.; Yeung, F.; Kwok, H.-S.; Li, G. Fully chiral light emission from CsPbX3 perovskite nanocrystals enabled by cholesteric superstructure stacks. Adv. Funct. Mater. 2019, 29, 1903155. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Luo, J.; Quan, Y.; Cheng, Y. Light-Driven sign inversion of circularly polarized luminescence enabled by dichroism modulation in cholesteric liquid crystals. Adv. Mater. 2024, 36, 2312331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Chang, W.; Liu, L.; Li, J. Recent progress in circularly polarized luminescent materials based on cyclodextrins. Polymers 2024, 16, 2140. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Kotov, N.A. Circular polarized light emission in chiral inorganic nanomaterials. Adv. Mater. 2023, 35, e2108431. [Google Scholar] [CrossRef] [PubMed]
- Han, D.X.; Li, C.; Jiang, C.; Jin, X.; Wang, X.; Chen, R.; Cheng, J.; Duan, P. Endowing inorganic nanomaterials with circularly polarized luminescence. Aggregate 2022, 3, e148. [Google Scholar] [CrossRef]
- Mendoza-Carreño, J.; Molet, P.; Otero-Martínez, C.; Alonso, M.I.; Polavarapu, L.; Mihi, A. Nanoimprinted 2D-chiral perovskite nanocrystal metasurfaces for circularly polarized photoluminescence. Adv. Mater. 2023, 35, 2210477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, T.; Dong, X.-Y.; Sun, M.-E.; Zhang, C.; Li, X.; Zhao, Y.S.; Zang, S. Circularly polarized luminescence from achiral single crystals of hybrid manganese halides. J. Am. Chem. Soc. 2019, 141, 15755–15760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, Y.; Valenzuela, C.; Zhang, X.; Wang, L.; Feng, W.; Li, Q. Liquid crystal-templated chiral nanomaterials: From chiral plasmonics to circularly polarized luminescence. Light Sci. Appl. 2022, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jin, X.; Zhao, T.; Duan, P. Circularly polarized luminescence in chiral nematic liquid crystals: Generation and amplification. Mater. Chem. Front. 2021, 5, 4821–4832. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Chen, Y.; Valenzuela, C.; Liu, Y.; Yang, Y.; Feng, Y.; Wang, L.; Feng, W. Mechanically tunable circularly polarized luminescence of liquid crystal-templated chiral perovskite quantum dots. Angew. Chem. Int. Ed. 2024, 63, e202404202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, C.Y. Liquid Crystal Polymers; Spinger: Cham, Switzerland, 2020; pp. 1–619. [Google Scholar]
- Lyu, X.; Xiao, A.; Shi, D.; Li, Y.; Shen, Z.; Chen, E.-Q.; Zheng, S.; Fan, X.-H.; Zhou, Q.-F. Liquid crystalline polymers: Discovery, development, and the future. Polymer 2020, 202, 122740. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, J.; Ichikawa, T.; Soberats, B. Functional liquid-crystalline polymers and supramolecular liquid crystals. Polymer J. 2018, 50, 149–166. [Google Scholar] [CrossRef]
- Akram, A.; Shahzady, T.G.; Hussain, S.; Saad, N.A.; Islam, M.T.; Ikram, M. Liquid crystal polymers: Overview of characteristics and applications in communication and biomedical technologies. Russ. J. Appl. Chem. 2021, 94, 1585–1593. [Google Scholar] [CrossRef]
- Han, A.; Guo, S.; Lu, H.; Liu, S.; Zhao, Q.; Huang, W. Recent progress on circularly polarized luminescent materials for organic optoelectronic devices. Adv. Opt. Mater. 2018, 6, 1800538. [Google Scholar] [CrossRef]
- Gong, Z.-L.; Zhu, X.; Zhou, Z.; Zhang, S.-W.; Yang, D.; Zhao, B.; Zhang, Y.-P.; Deng, J.; Cheng, Y.; Zheng, Y.-X.; et al. Frontiers in circularly polarized luminescence: Molecular design, self-assembly, nanomaterials, and applications. Sci. China Chem. 2021, 64, 2060–2104. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Han, B.; Zhou, Y.; Zhang, X.; Gao, X.; Tang, Z. Circularly polarized luminescence in chiral materials. Matter 2022, 5, 837–875. [Google Scholar] [CrossRef]
- Zhong, H.; Zhao, B.; Deng, J. Polymer-based circularly polarized luminescent materials. Adv. Opt. Mater. 2023, 11, 2202787. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, S.; Hu, F.; Han, J.; Li, F. Circularly polarized luminescence polymers: From design to applications. Coord. Chem. Rev. 2023, 485, 215116. [Google Scholar] [CrossRef]
- Zhao, T.; Han, J.; Duan, P.; Liu, M. New perspectives to trigger and modulate circularly polarized luminescence of complex and aggregated systems: Energy transfer, photon upconversion, charge transfer, and organic radical. Acc. Chem. Res. 2020, 53, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Gao, X.; Zhao, B.; Deng, J. “Matching rule“ for generation, modulation and amplification of circularly polarized luminescence. Acc. Chem. Res. 2024, 57, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, H.; Wei, J.; Zhang, D.; Liu, F.; Pan, G.; Zhao, D.; He, W.; Deng, J. Polymer stabilized liquid crystal films reflecting both right- and left-circularly polarized light. Appl. Phys. Lett. 2008, 93, 201901. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Chen, Y.; Chen, Z.; Yuan, Y.; Gong, Y.; Zhang, H. Clustering-triggered emission liquid crystalline polymer bearing cholesterol: Tunable circularly polarized luminescence and room-temperature phosphorescence. Macromol. Rapid Commun. 2023, 44, 2300449. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Huang, J.; Yan, Y.; Tang, B.Z. Clusterization-triggered emission (CTE): One for all, all for one. Mater. Chem. Front. 2021, 5, 6693–6717. [Google Scholar] [CrossRef]
- Zhou, M.; Lu, P.; Xu, Z.; Wang, Y.; Yuan, Y.; Gong, Y.; Zhang, H. Circularly polarized luminescent liquid crystal copolymers constructed by triplet-singlet Förster-resonance energy transfer: Achieving large glum values, long lifetime, and high photoluminescence quantum yield. Adv. Opt. Mater. 2024, 12, 2401208. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Geng, Z.; Zheng, W.-H.; Quan, Y.; Cheng, Y. Inverted circularly polarized luminescence behavior by helical nanofibers through chiral co-assembly from achiral liquid crystal polymers and chiral inducers. ACS Nano 2022, 16, 3173–3181. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-W.; Tao, L.; Zhong, C.-L.; Li, Z.-X.; Lan, K.; Feng, Y.; Wang, P.; Xie, H.-L. High-Efficiency circularly polarized luminescence from chiral luminescent liquid crystalline polymers with aggregation-induced emission properties. Macromolecules 2020, 53, 9758–9768. [Google Scholar] [CrossRef]
- Luo, Z.-W.; Huang, A.; Luo, H.-Y.; Chen, J.-K.; Huang, J.; Xie, H.-L. Regulating circularly polarized luminescent behavior of luminescent liquid crystalline polymers through resonance energy transfer. Macromolecules 2023, 56, 2700–2708. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, B.; Deng, J. Liquid crystals doped with chiral fluorescent polymers: Multi-color circularly polarized fluorescence and room-temperature phosphorescence with high dissymmetry factor and anti-counterfeiting application. Adv. Mater. 2023, 35, 2304405. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yuan, J.; He, X.; Zheng, F.; Lu, X.; Xiang, S.; Lu, Q. Controllable circularly polarized luminescence with high dissymmetry factor via co-assembly of achiral dyes in liquid crystal polymer films. Small Methods 2024, 8, 2301517. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, J.; Liu, C.; Yu, W.; Cheng, Y. Strong circularly polarized luminescence promoted by AIE-active chiral co-assemblies in liquid crystal polymer films. Chem. Eur. J. 2024, 30, e202303852. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Q.; Wang, Y.; Quan, Y.; Chen, D.; Cheng, Y. Strong aggregation-induced CPL response promoted by chiral emissive nematic liquid crystals (N*-LCs). Chem. Eur. J. 2018, 24, 12607–12612. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Li, H.; Liu, C.; Li, L.; Quan, Y.; Cheng, Y. Achiral dichroic dyes-mediated circularly polarized emission regulated by orientation order parameter through cholesteric liquid crystals. Angew. Chem. Int. Ed. 2023, 62, e202312159. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhou, Z.; Ni, B.; Li, H.; Li, Y.; Li, B.; Liu, W.; Wang, Y.; Yang, Y. Tuning the circularly polarized luminescence of polymer-stabilized cholesteric liquid crystal films using chiral dopants. J. Mater. Chrm. C 2022, 10, 8246–8253. [Google Scholar] [CrossRef]
- Shi, F.; Han, F.; Zhang, W.; Yang, Y.; Li, H. Color-tunable circularly polarized luminescence from liquid crystalline polymer networks. Dyes Pigments 2024, 222, 111910. [Google Scholar] [CrossRef]
- Yu, F.; Liu, S.; Liu, X.; Zhang, H.; Zheng, Z.; Zhu, W.-H.; Wu, Y. Circularly polarized perovskite luminescence in composite films with high flexibility and stability. Adv. Opt. Mater. 2024, 12, 2400411. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Zhao, B.; Deng, J. Polymeric cholesteric superhelix induced by chiral helical polymer for achieving full-color circularly polarized room-temperature phosphorescence with ultra-high dissymmetry factor. Small 2024, 20, 2404576. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.; Wu, Y.; Zhang, D.; Wu, Y.; Tian, H.; Zheng, Z.; Zhu, W.-H. Circularly polarized perovskite luminescence with dissymmetry factor up to 1.9 by soft helix bilayer device. Matter 2022, 5, 2319–2333. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Zheng, Z.-G.; Yu, Z.-Q.; Zhu, W.-H. Liquid crystal assembly for ultra-dissymmetric circularly polarized luminescence and beyond. J. Am. Chem. Soc. 2023, 145, 12951–12966. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ji, L.; Jiang, C.; Duan, P.; Ouyang, G.; Liu, M. A cooperative noncovalent-covalent strategy for amplified circularly polarized luminescence and multiple information encryption within chiral liquid crystals. CCS Chem. 2025, 1–12. [Google Scholar] [CrossRef]
- Zhan, X.; Xu, F.-F.; Zhou, Z.; Yan, Y.; Yao, J.; Zhao, Y.S. 3D laser displays based on circularly polarized lasing from cholesteric liquid crystal arrays. Adv. Mater. 2021, 33, 2104418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.-F.; Qin, J.; Zhong, Y.-W.; Gao, D.; Dong, Y.; Feng, H. Recent Advances in Liquid Crystal Polymer-Based Circularly Polarized Luminescent Materials: A Review. Polymers 2025, 17, 1961. https://doi.org/10.3390/polym17141961
Xu F-F, Qin J, Zhong Y-W, Gao D, Dong Y, Feng H. Recent Advances in Liquid Crystal Polymer-Based Circularly Polarized Luminescent Materials: A Review. Polymers. 2025; 17(14):1961. https://doi.org/10.3390/polym17141961
Chicago/Turabian StyleXu, Fa-Feng, Jingzhou Qin, Yu-Wu Zhong, Dandan Gao, Yaping Dong, and Haitao Feng. 2025. "Recent Advances in Liquid Crystal Polymer-Based Circularly Polarized Luminescent Materials: A Review" Polymers 17, no. 14: 1961. https://doi.org/10.3390/polym17141961
APA StyleXu, F.-F., Qin, J., Zhong, Y.-W., Gao, D., Dong, Y., & Feng, H. (2025). Recent Advances in Liquid Crystal Polymer-Based Circularly Polarized Luminescent Materials: A Review. Polymers, 17(14), 1961. https://doi.org/10.3390/polym17141961







