Regulation of MXene Membranes with β-Lactoglobulin Nanofiber-Templated CuS Nanoparticles for Photothermal Antibacterial Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amyloid β-LGNFs and β-LGNF-CuS Nanohybrids
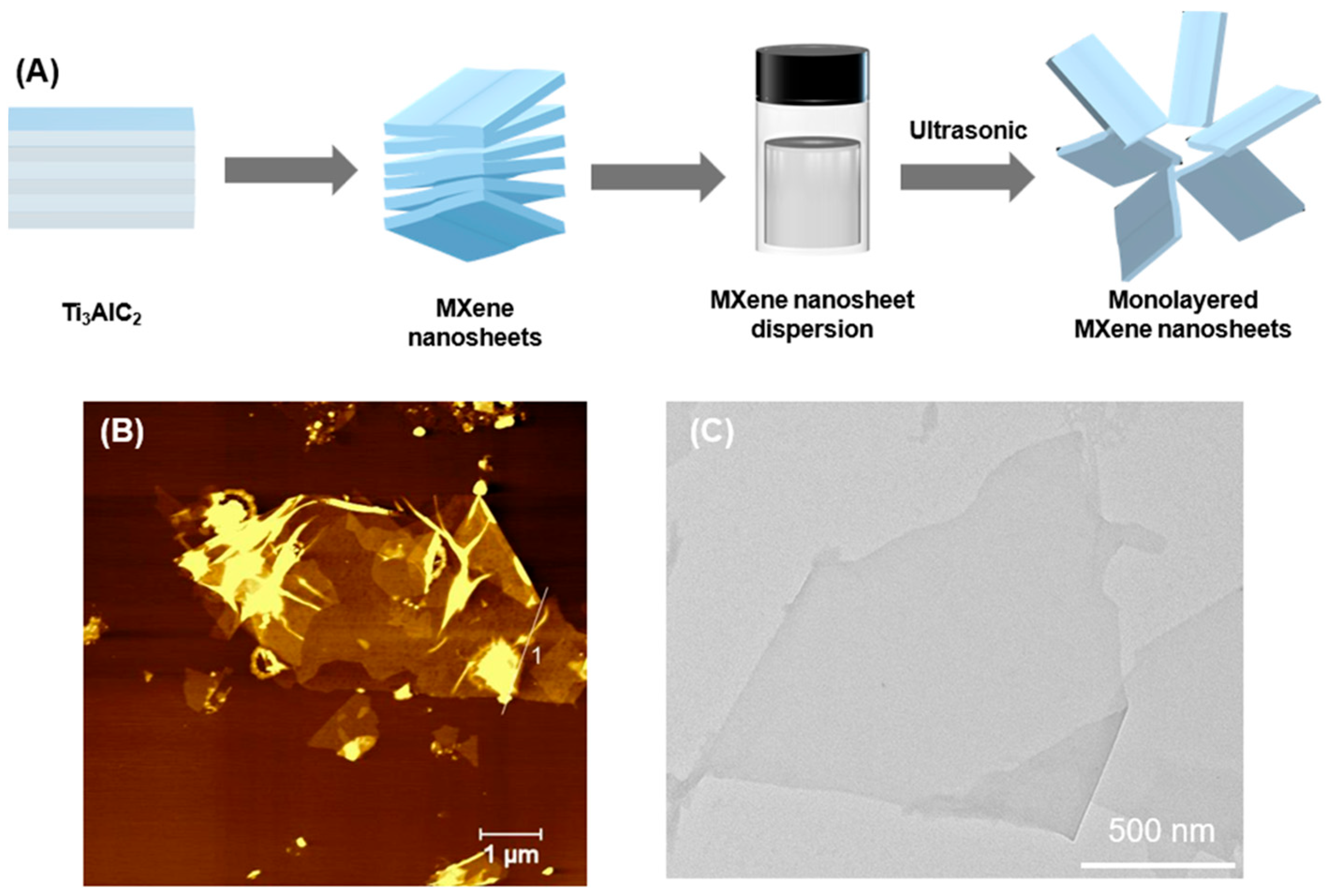
2.3. Synthesis of MXene Nanosheets
2.4. Synthesis of β-LGNF-CuS/MXene Nanohybrids and Hybrid Membranes
2.5. Characterization Techniques
2.6. Controlled Release of Cu2+
2.7. In Vitro Biocompatibility of β-LGNF-CuS/MXene Nanohybrids
2.8. In Vitro Antibacterial Assay
3. Results and Discussions
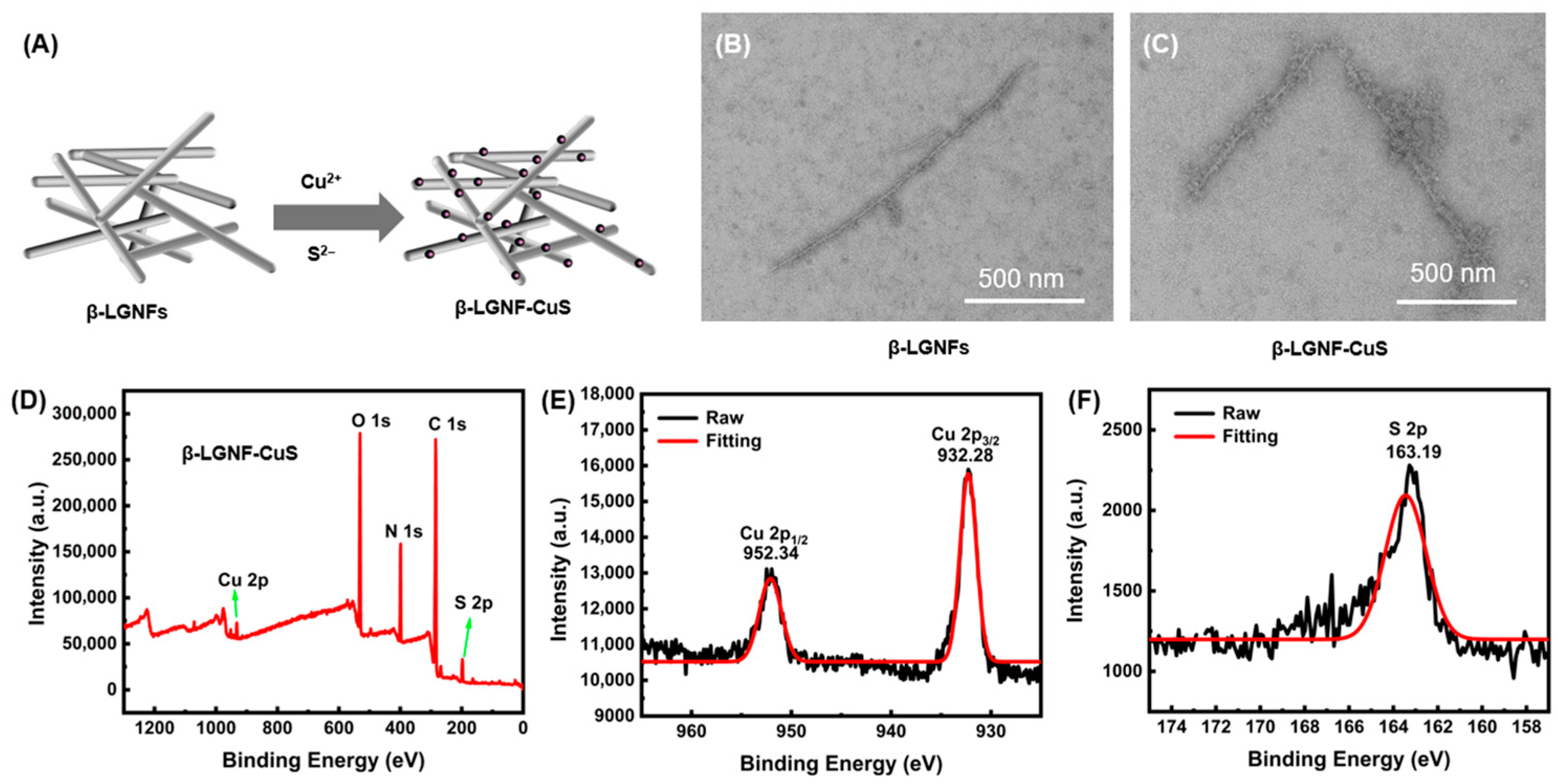
3.1. Synthesis and Characterizations of β-LGNFs
3.2. Synthesis and Characterizations of β-LGNF-CuS Nanohybrids
3.3. Synthesis and Characterization of MXene Membranes
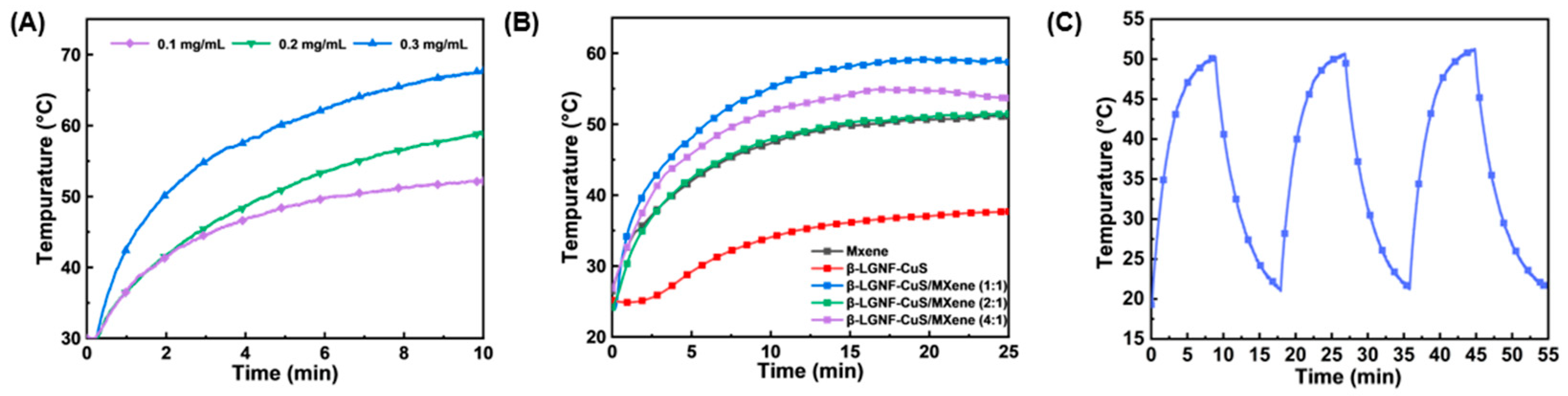
3.4. Photothermal Properties and Cu2+ Release Profile of β-LGNF-CuS/MXene Nanohybrids
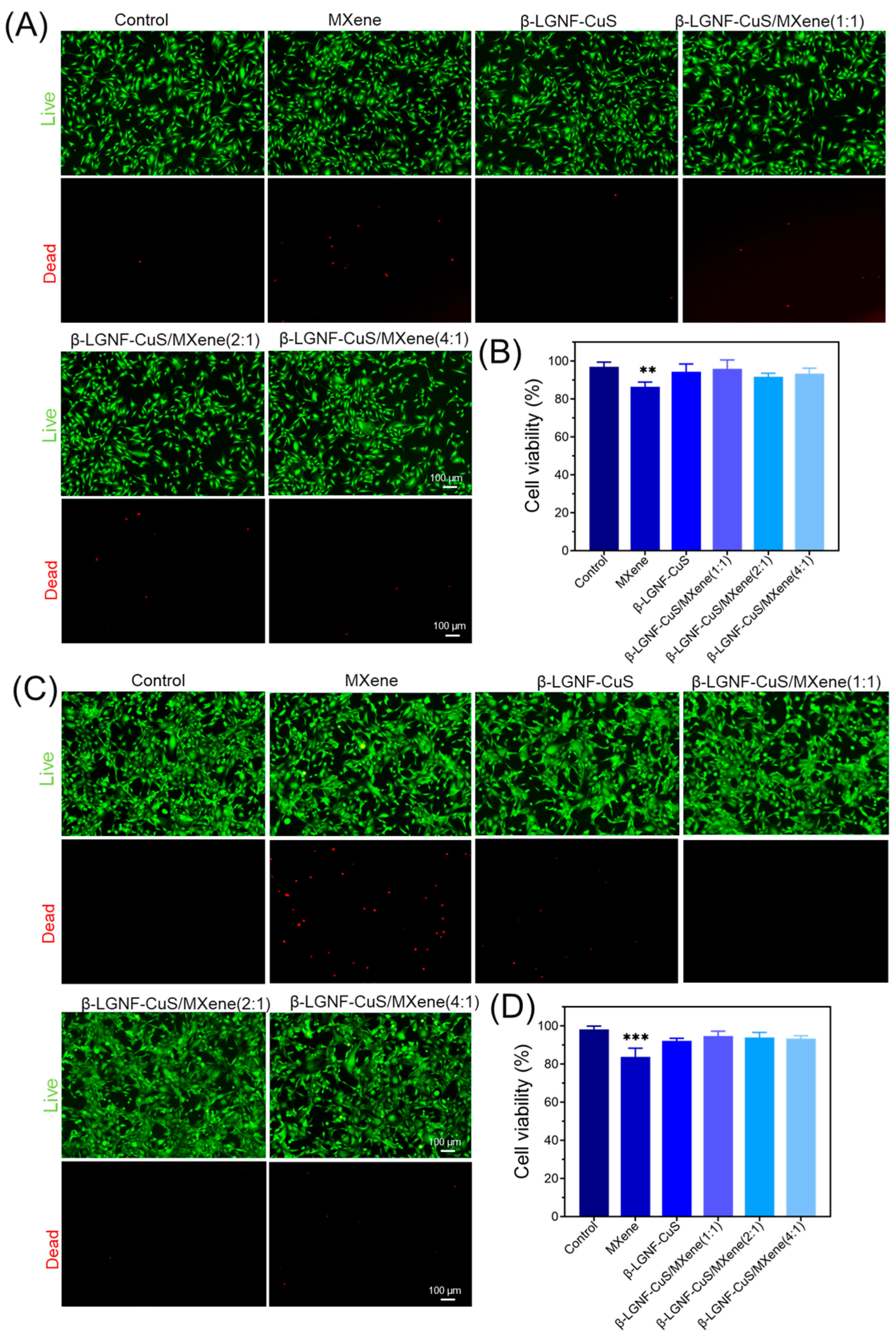
3.5. Biocompatibility of β-LGNF-CuS/MXene Nanohybrids
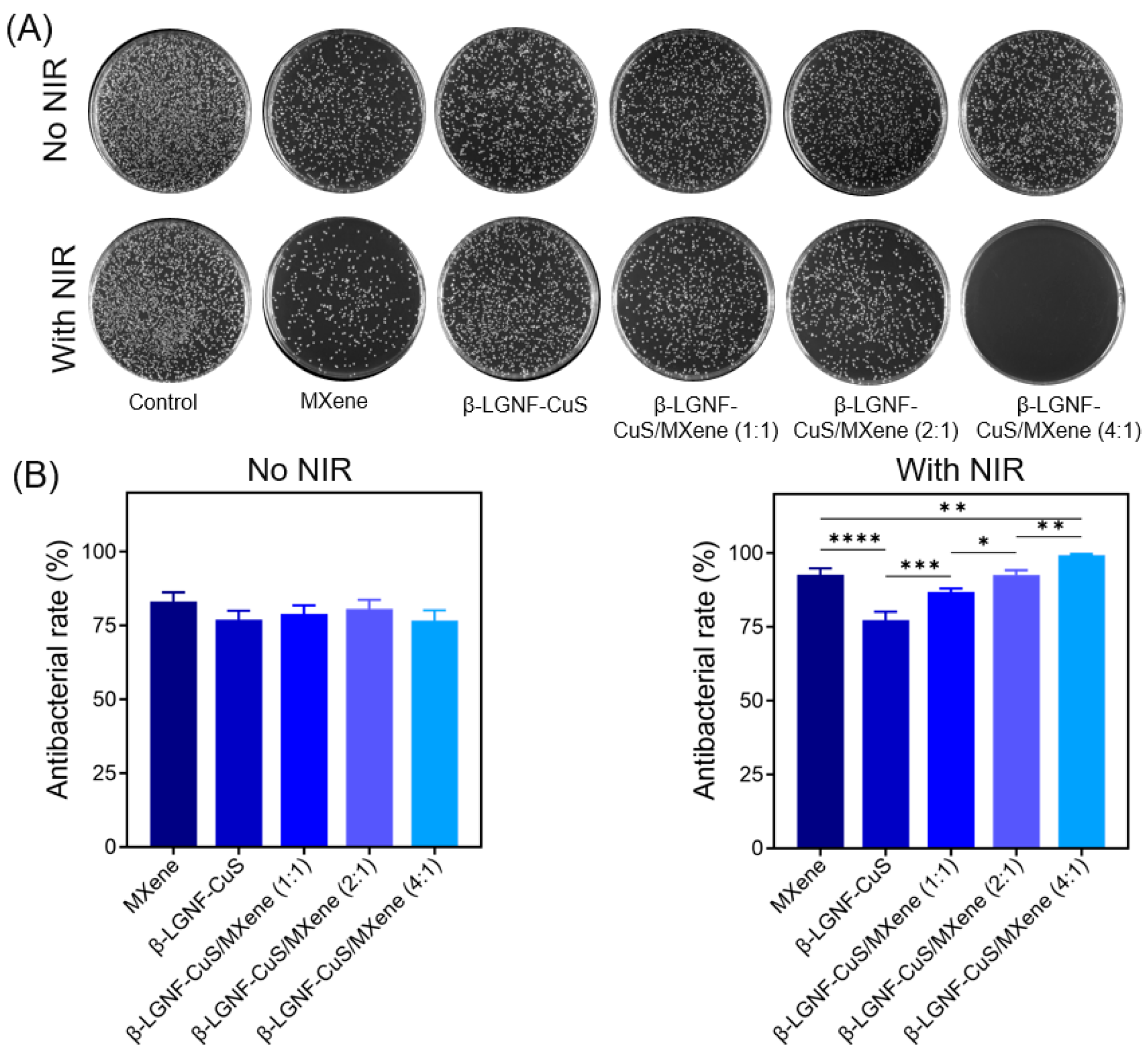
3.6. Antibacterial Properties of β-LGNF-CuS/MXene Hybrid Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Daruka, L.; Czikkely, M.S.; Szili, P.; Farkas, Z.; Balogh, D.; Grézal, G.; Maharramov, E.; Vu, T.-H.; Sipos, L.; Juhász, S.; et al. ESKAPE pathogens rapidly develop resistance against antibiotics in development in vitro. Nat. Microbiol. 2025, 10, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.-J.; Moriarty, T.F.; Morgenstern, M.; Marais, L.; Onsea, J.; O’Toole, R.V.; Depypere, M.; Obremskey, W.T.; Verhofstad, M.H.J.; McNally, M.; et al. The global burden of fracture-related infection: Can we do better? Lancet Infect. Dis. 2024, 24, e386–e393. [Google Scholar] [CrossRef] [PubMed]
- Piskovsky, V.; Oliveira, N.M. Bacterial motility can govern the dynamics of antibiotic resistance evolution. Nat. Commun. 2023, 14, 5584. [Google Scholar] [CrossRef] [PubMed]
- Panáček, D.; Belza, J.; Hochvaldová, L.; Baďura, Z.; Zoppellaro, G.; Šrejber, M.; Malina, T.; Šedajová, V.; Paloncýová, M.; Langer, R.; et al. Single Atom Engineered Antibiotics Overcome Bacterial Resistance. Adv. Mater. 2024, 36, 2410652. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Duan, Y.; Huang, Y. Infection microenvironment-related antibacterial nanotherapeutic strategies. Biomaterials 2022, 280, 121249. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Sun, H.; Lu, Q.; Sun, J.; Zhang, P.; Zeng, L.; Vasilev, K.; Zhao, Y.; Chen, Y.; Liu, P. Spontaneous formation of MXene-oxidized sono/chemo-dynamic sonosensitizer/nanocatalyst for antibacteria and bone-tissue regeneration. J. Nanobiotechnol. 2023, 21, 193. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Jiang, X.; Liu, C.; Song, S.; Zhang, J.; Shen, J. FeS@LAB-35@Ti3C2 as a high-efficiency nanozyme for near infrared light induced photothermal enhanced chemodynamic antibacterial activity and wound healing. Nano Res. 2023, 16, 2840–2850. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, Q.; Hu, Y.; He, J.; Wang, H.; Deng, C.; Li, D. MXene/zinc ion embedded agar/sodium alginate hydrogel for rapid and efficient sterilization with photothermal and chemical synergetic therapy. Talanta 2024, 266, 125101. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Feng, N.; Zhao, S.; Shi, J.; Yang, G.; Guo, W.; Liu, Y.; Fan, K. Microenvironment-Responsive Injectable Thermosensitive Hydrogel Incorporating Nanozymes for Synergistic Breast Cancer Therapy and Postsurgical Adjuvant Treatment. Adv. Funct. Mater. 2025, 2421176. [Google Scholar] [CrossRef]
- Huang, H.; Dong, C.; Feng, W.; Wang, Y.; Huang, B.; Chen, Y. Biomedical engineering of two-dimensional MXenes. Adv. Drug Delivery Rev. 2022, 184, 114178. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Qian, Y.; Wu, C.; Feng, J.; Sun, X.; Zheng, Q.; Li, X.; Shen, J. Entropy-Mediated High-Entropy MXenes Nanotherapeutics: NIR-II-Enhanced Intrinsic Oxidase Mimic Activity to Combat Methicillin-Resistant Staphylococcus Aureus Infection. Adv. Mater. 2023, 35, 2211432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yan, Y.; Lin, L.; He, Q.; Hu, H.; Luo, R.; Xian, D.; Wu, J.; Shi, Y.; Zeng, F.; et al. Titanium carbide MXene-based hybrid hydrogel for chemo-photothermal combinational treatment of localized bacterial infection. Acta Biomater. 2022, 142, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.J.; Nain, A.; Lin, Y.F.; Tseng, Y.T.; Li, Y.J.; Sangili, A.; Srivastava, P.; Yu, H.L.; Huang, Y.F.; Huang, C.C.; et al. Self-redox reaction driven in situ formation of Cu2O/Ti3C2Tx nanosheets boosts the photocatalytic eradication of multi-drug resistant bacteria from infected wound. J. Nanobiotechnol. 2022, 20, 235. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zheng, H.; Wang, W.; Wu, Q.; Zhang, Q.; Guo, J.; Pu, B.; Shi, X.; Li, J.; Chen, X.; et al. Rapid eradication of antibiotic-resistant bacteria and biofilms by MXene and near-infrared light through photothermal ablation. Sci. China Mater. 2021, 64, 748–758. [Google Scholar] [CrossRef]
- Luan, X.; Hu, H.; Sun, Z.; He, P.; Zhu, D.; Xu, Y.; Liu, B.; Wei, G. Assembling Ag2S quantum dots onto peptide nanosheet as a biomimetic two-dimensional nanoplatform for synergistic near infrared-II fluorescent imaging and photothermal therapy of tumor. J. Colloid Interface Sci. 2024, 663, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Suo, Y.; Liao, X.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Soon, C.F.; Ma, N.L.; Morsin, M.; Nayan, N.; Ahmad, M.K.; Tee, K.S. Cytotoxicity of MXene-based nanomaterials for biomedical applications: A mini review. Environ. Res. 2021, 201, 111592. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Li, S.; Jing, L.; Chen, P.-Y.; Xie, J. Synergistic Antimicrobial Titanium Carbide (MXene) Conjugated with Gold Nanoclusters. Adv. Healthcare Mater. 2020, 9, 2001007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, J.; Liao, T.; Wu, Q.; Guo, N.; Xie, G.; Lin, H.; Li, C.; Liu, Y. Ag Nanoparticles on MXene Nanosheets for Combined Ionic and Photothermal Therapy of Bacterial Infections. ACS Appl. Nano Mater. 2024, 7, 21261–21274. [Google Scholar] [CrossRef]
- Yu, C.; Sui, S.; Yu, X.; Huang, W.; Wu, Y.; Zeng, X.; Chen, Q.; Wang, J.; Peng, Q. Ti3C2Tx MXene loaded with indocyanine green for synergistic photothermal and photodynamic therapy for drug-resistant bacterium. Colloids Surf. B 2022, 217, 112663. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ma, M.; Ali, A.; Liu, Q.; Bai, R.; Zhang, K.; Guan, Y.; Wang, Y.; Liu, J.; Zhou, H. Self-assembled copper tannic acid nanoparticles: A powerful nano-bactericide by valence shift of copper. Nano Today 2024, 54, 102071. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, S.; Yang, L.; Qin, G.; Zhang, E. A nano-structured TiO2/CuO/Cu2O coating on Ti-Cu alloy with dual function of antibacterial ability and osteogenic activity. J. Mater. Sci. Technol. 2022, 97, 201–212. [Google Scholar] [CrossRef]
- Chen, G.; Yang, L.; Ma, N.; Yu, S.; Zhou, J.; Cao, R.; Zhang, Q.; Yu, H.; Zhai, M.; Wang, R.; et al. Selection and design principle of efficient antiviral nano-hybrid fiber materials for fighting pandemic viruses: A review. Nano Today 2023, 53, 102001. [Google Scholar] [CrossRef]
- Smith, J.L.; Tran, N.; Song, T.; Liang, D.; Qian, M. Robust bulk micro-nano hierarchical copper structures possessing exceptional bactericidal efficacy. Biomaterials 2022, 280, 121271. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Babu, R.P.; Zhao, W.; Johnson, C.M.; Hedström, P.; Odnevall, I.; Leygraf, C. High-Resolution Microscopical Studies of Contact Killing Mechanisms on Copper-Based Surfaces. ACS Appl. Mater. Interfaces 2021, 13, 49402–49413. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.-s. Photo-Stimuli-Responsive CuS Nanomaterials as Cutting-Edge Platform Materials for Antibacterial Applications. Pharmaceutics 2022, 14, 2343. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, X.; Qiu, L.; Zhou, X.; Hui, Z.; Ni, X.; Xuan, Y.; Lei, X.; Wang, J. Gelatinase Responsive Nanogel for Antibacterial Phototherapy and Wound Healing. Gels 2022, 8, 397. [Google Scholar] [CrossRef] [PubMed]
- Dash, K.K.; Deka, P.; Bangar, S.P.; Chaudhary, V.; Trif, M.; Rusu, A. Applications of Inorganic Nanoparticles in Food Packaging: A Comprehensive Review. Polymers 2022, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lai, W.; Liu, X.; Yang, H.; Fang, Y.; Tian, L.; Li, K.; Nie, H.; Zhang, W.; Shi, Y.; et al. Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J. Hazard. Mater. 2021, 401, 123349. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, C.; Hou, J.; Wang, P.; Ao, Y.; Li, Y.; Lv, B.; Yang, Y.; You, G.; Xu, Y. Enhanced stability and dissolution of CuO nanoparticles by extracellular polymeric substances in aqueous environment. J. Nanopart. Res. 2015, 17, 404. [Google Scholar] [CrossRef]
- Fernando, L.; Chen, W.T.; Lai, C.W.; Ye, F.Y.; Lai, P.S.; Lin, J.J.; Yasuda, K.; Song, T.T.; Song, J.M. Biocompatibility and antimicrobial activity of copper(II) oxide hybridized with nano silicate platelets. Surf. Coat. Technol. 2022, 435, 128253. [Google Scholar] [CrossRef]
- Thawari, A.G.; Rao, C.P. Peroxidase-like Catalytic Activity of Copper-Mediated Protein–Inorganic Hybrid Nanoflowers and Nanofibers of β-Lactoglobulin and α-Lactalbumin: Synthesis, Spectral Characterization, Microscopic Features, and Catalytic Activity. ACS Appl. Mater. Interfaces 2016, 8, 10392–10402. [Google Scholar] [CrossRef] [PubMed]
- Rodzik, A.; Pomastowski, P.; Buszewska-Forajta, M.; Railean, V.; Gołębiowski, A.; Buszewski, B.; Niedojadło, K.; Fijałkowski, P.; Robotnik, K.; Rafińska, K. Enhancing wound healing with zinc and silver nanocomposites synthesized with β-lactoglobulin: Antimicrobial properties, collagen deposition, and systemic effects in a C57BL/6J mouse model. Discover Nano 2024, 19, 150. [Google Scholar] [CrossRef] [PubMed]
- Navarra, G.; Tinti, A.; Di Foggia, M.; Leone, M.; Militello, V.; Torreggiani, A. Metal ions modulate thermal aggregation of beta-lactoglobulin: A joint chemical and physical characterization. J. Inorg. Biochem. 2014, 137, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Rodzik, A.; Railean-Plugaru, V.; Sprynskyy, M.; Pomastowski, P. A study of zinc ions immobilization by β-lactoglobulin. Colloids Surf. A 2020, 591, 124443. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, T.; Ng, S.W.L.; Lu, W.; Tian, G.; Ong, W.L.; Kozlov, S.M.; Ho, G.W. Polymeric Layered Films for TiO2-Au/CuS Tandem Photothermal Catalytic H2 Production in Harsh Seawater and Waste Plastic Media. Adv. Energy Mater. 2025, 15, 2404198. [Google Scholar] [CrossRef]
- Mu, R.; Zhu, D.; Abdulmalik, S.; Wijekoon, S.; Wei, G.; Kumbar, S.G. Stimuli-responsive peptide assemblies: Design, self-assembly, modulation, and biomedical applications. Bioact. Mater. 2024, 35, 181–207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kong, H.; Yang, G.; He, P.; Luan, X.; Guo, L.; Wei, G. Peptide Nanosheet-Inspired Biomimetic Synthesis of CuS Nanoparticles on Ti3C2 Nanosheets for Electrochemical Biosensing of Hydrogen Peroxide. Biosensors 2023, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, Y.; Liu, Y.; Jia, Q.; Zhao, C.; Yang, J.; Ren, W.; Liu, Q. Enhanced water purification through the double regulation of GO/MXene membranes with sodium alginate and KOH. Sep. Purif. Technol. 2025, 363, 132287. [Google Scholar] [CrossRef]
- Bolder, S.G.; Vasbinder, A.J.; Sagis, L.M.C.; van der Linden, E. Heat-induced whey protein isolate fibrils: Conversion, hydrolysis, and disulphide bond formation. Int. Dairy J. 2007, 17, 846–853. [Google Scholar] [CrossRef]
- Lara, C.; Gourdin-Bertin, S.; Adamcik, J.; Bolisetty, S.; Mezzenga, R. Self-assembly of ovalbumin into amyloid and non-amyloid fibrils. Biomacromolecules 2012, 13, 4213–4221. [Google Scholar] [CrossRef] [PubMed]
- Peydayesh, M.; Mezzenga, R. Protein nanofibrils for next generation sustainable water purification. Nat. Commun. 2021, 12, 3248. [Google Scholar] [CrossRef] [PubMed]
- Lendel, C.; Solin, N. Protein nanofibrils and their use as building blocks of sustainable materials. RSC Adv. 2021, 11, 39188–39215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Qiao, T.; Ya Hu, X. A simple hydrothermal route to nanocrystalline CuS. J. Cryst. Growth 2004, 268, 64–70. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Sarycheva, A.; Polemi, A.; Liu, Y.; Dandekar, K.; Anasori, B.; Gogotsi, Y. 2D titanium carbide (MXene) for wireless communication. Sci. Adv. 2018, 4, eaau0920. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, B.; Yang, C.; Lv, Z.; Wei, Y.; Pan, T.; Xuan, F.; Zhou, X.; Chen, H.; Shen, H.; et al. 3D Printed Pedicle Screws with Microarc Oxidation Ceramic Interfaces Enhance Osteointegration and Orthopedic Fixation Feasibility. ACS Appl. Mater. Interfaces 2024, 16, 31983–31996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Meng, L.; Hu, Y.; Yuan, Z.; Li, J.; Liu, H. Green Synthesis and Biosafety Assessment of MXene. Small 2024, 20, 2308600. [Google Scholar] [CrossRef] [PubMed]
- Rasool, K.; Helal, M.; Ali, A.; Ren, C.E.; Gogotsi, Y.; Mahmoud, K.A. Antibacterial Activity of Ti3C2Tx MXene. ACS Nano 2016, 10, 3674–3684. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Du, C.; Zhou, X.; Wei, G. Regulation of MXene Membranes with β-Lactoglobulin Nanofiber-Templated CuS Nanoparticles for Photothermal Antibacterial Effect. Polymers 2025, 17, 1960. https://doi.org/10.3390/polym17141960
Liu Z, Du C, Zhou X, Wei G. Regulation of MXene Membranes with β-Lactoglobulin Nanofiber-Templated CuS Nanoparticles for Photothermal Antibacterial Effect. Polymers. 2025; 17(14):1960. https://doi.org/10.3390/polym17141960
Chicago/Turabian StyleLiu, Zhuang, Chenxi Du, Xin Zhou, and Gang Wei. 2025. "Regulation of MXene Membranes with β-Lactoglobulin Nanofiber-Templated CuS Nanoparticles for Photothermal Antibacterial Effect" Polymers 17, no. 14: 1960. https://doi.org/10.3390/polym17141960
APA StyleLiu, Z., Du, C., Zhou, X., & Wei, G. (2025). Regulation of MXene Membranes with β-Lactoglobulin Nanofiber-Templated CuS Nanoparticles for Photothermal Antibacterial Effect. Polymers, 17(14), 1960. https://doi.org/10.3390/polym17141960






