Temperature-Responsive and Self-Healing Hydrogel: A Novel Approach to Combat Postoperative Adhesions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 4-(2-(Acryloyloxy)ethoxy)-4-oxobutanoic Acid (AEOA)
2.3. Synthesis of Acryloyloxyethyl Pentafluorobenzoate (AOEPFB)
2.4. Synthesis of Poly {(N-Isopropyl acrylamide)-co-[4-(2-(acryloyloxy)ethoxy)-4-oxobutanoic acid]-co-(acryloyloxyethyl pentafluorobenzoate)}-b-PEO-b-Poly {(N-isopropyl acrylamide)-co-[4-(2-(acryloyloxy)ethoxy)-4-oxobutanoic acid]-co-(acryloyloxyethyl pentafluorobenzoate)} (APOAP)
2.5. In Vitro Hemolysis Ratios of the APOAP Hydrogels
2.6. Blood Clotting Index of the APOAP Hydrogels
2.7. In Vitro Cytocompatibility
2.8. In Vivo Hemostasis Experiment in Rats
2.9. In Vivo Anti-Adhesion Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Hydrogel
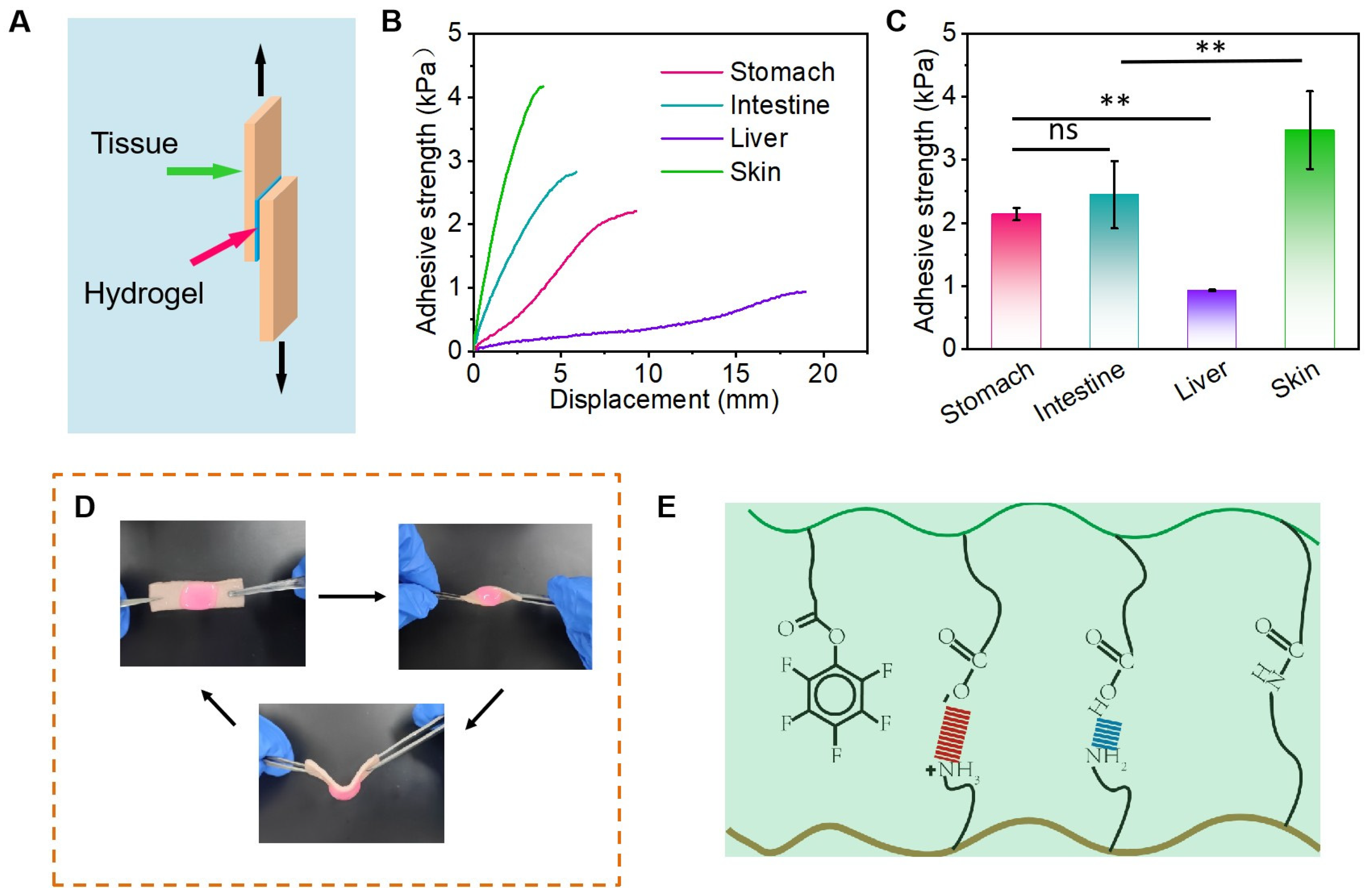
3.2. Adhesion Properties of Hydrogels
3.3. Hemocompatibility, Cytocompatibility, and Hemostatic Properties of Hydrogels
3.4. In Vivo Anti-Adhesive Efficacy of APOAP Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PNIPAM | Poly (N-isopropyl acrylamide) |
| PEG | Polyethylene glycol |
| RAFT | Reversible addition–fragmentation chain transfer |
| PA | Postoperative adhesion |
| APOAP | Poly {(N-isopropyl acrylamide)-co-[4-(2-(acryloyloxy) ethoxy)-4-oxobutanoic acid]-co-(acryloyloxyethyl pentafluorobenzoate)}-b-PEO-b-Poly {(N-isopropyl acrylamide)-co-[4-(2-(acryloyloxy)ethoxy)-4-oxobutanoic acid]-co-(acryloyloxyethyl pentafluorobenzoate)} |
| DMAP | 4-Dimethylaminopyridine |
| EDC·HCl | 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride |
| THF | Tetrahydrofuran |
| DCM | Dichloromethane |
| AIBN | 2,2′-Azobisisobutyronitrile |
| NIPAM | N-isopropylacrylamide |
| CTA | Chain transfer agent |
| ATCC | American type culture collection |
| SPF | Specific-pathogen-free |
| SD | Standard deviation |
| GPC | Gel permeation chromatography |
| H&E | Hematoxylin and eosin |
References
- Wang, A.Y.; Lam, C.W.; Wang, M.; Woo, J.; Chan, I.H.; Lui, S.F.; Sanderson, J.E.; Li, P.K. Circulating soluble vascular cell adhesion molecule 1: Relationships with residual renal function, cardiac hypertrophy, and outcome of peritoneal dialysis patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2005, 45, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Chakedis, J.; Rahnemai-Azar, A.A.; Wilson, A.; Hennessy, M.M.; Athanasiou, A.; Beal, E.W.; Argyrou, C.; Felekouras, E.; Pawlik, T.M. Postoperative Abdominal Adhesions: Clinical Significance and Advances in Prevention and Management. J. Gastrointest. Surg. 2017, 21, 1713–1722. [Google Scholar] [CrossRef]
- Soltany, S. Postoperative peritoneal adhesion: An update on physiopathology and novel traditional herbal and modern medical therapeutics. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 317–336. [Google Scholar] [CrossRef]
- Duron, J.J. Postoperative intraperitoneal adhesion pathophysiology. Color. Dis. Off. J. Assoc. Coloproctology Great Br. Irel. 2007, 9 (Suppl. S2), 14–24. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Kearns, S.; Kelly, J.; Zeugolis, D.I. Battling adhesions: From understanding to prevention. BMC Biomed. Eng. 2019, 1, 5. [Google Scholar] [CrossRef]
- Ensan, B.; Bathaei, P.; Nassiri, M.; Khazaei, M.; Hassanian, S.M.; Abdollahi, A.; Ghorbani, H.R.; Aliakbarian, M.; Ferns, G.A.; Avan, A. The Therapeutic Potential of Targeting Key Signaling Pathways as a Novel Approach to Ameliorating Post-Surgical Adhesions. Curr. Pharm. Des. 2022, 28, 3592–3617. [Google Scholar] [PubMed]
- Chen, J.; Tang, X.; Wang, Z.; Perez, A.; Yao, B.; Huang, K.; Zhang, Y.; King, M.W. Techniques for navigating postsurgical adhesions: Insights into mechanisms and future directions. Bioeng. Transl. Med. 2023, 8, e10565. [Google Scholar] [CrossRef]
- Hellebrekers, B.W.; Kooistra, T. Pathogenesis of postoperative adhesion formation. Br. J. Surg. 2011, 98, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- ten Broek, R.P.; Issa, Y.; van Santbrink, E.J.; Bouvy, N.D.; Kruitwagen, R.F.; Jeekel, J.; Bakkum, E.A.; Rovers, M.M.; van Goor, H. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met-analysis. BMJ (Clin. Res. Ed.) 2013, 347, f5588. [Google Scholar] [CrossRef]
- Kucukozkan, T.; Ersoy, B.; Uygur, D.; Gundogdu, C. Prevention of adhesions by sodium chromoglycate, dexamethasone, saline and aprotinin after pelvic surgery. ANZ J. Surg. 2004, 74, 1111–1115. [Google Scholar] [CrossRef]
- Yang, L.; Li, Z.; Chen, Y.; Chen, F.; Sun, H.; Zhao, M.; Chen, Y.; Wang, Y.; Li, W.; Zeng, L.; et al. Elucidating the Novel Mechanism of Ligustrazine in Preventing Postoperative Peritoneal Adhesion Formation. Oxidative Med. Cell. Longev. 2022, 2022, 9226022. [Google Scholar] [CrossRef]
- Fang, Y.; Huang, S.; Gong, X.; King, J.A.; Wang, Y.; Zhang, J.; Yang, X.; Wang, Q.; Zhang, Y.; Zhai, G.; et al. Salt sensitive purely zwitterionic physical hydrogel for prevention of postoperative tissue adhesion. Acta Biomater. 2023, 158, 239–251. [Google Scholar] [CrossRef]
- Li, J.; Yu, H.; Kang, Y.; Niu, K.; Wang, M.; Jiang, Y.; Jiang, N.; Ding, Z.; Gan, Z.; Yu, Q. STING Membrane Prevents Post-Surgery Tissue Adhesion and Tumor Recurrence of Colorectal Cancer. Adv. Mater. 2024, 36, e2309655. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Wu, Z.; Jiao, Z.X.; Wang, Y.; Wang, Z.L.; Guo, M.; Li, G.; Wang, L.Q.; Zhang, P.B. A rapid crosslinking injectable polygalacturonic acid barrier modified with zwitterion bottlebrush for preventing postoperative adhesion. Chem. Eng. J. 2024, 482, 148932. [Google Scholar] [CrossRef]
- Brochhausen, C.; Schmitt, V.H.; Planck, C.N.; Rajab, T.K.; Hollemann, D.; Tapprich, C.; Krämer, B.; Wallwiener, C.; Hierlemann, H.; Zehbe, R.; et al. Current strategies and future perspectives for intraperitoneal adhesion prevention. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract. 2012, 16, 1256–1274. [Google Scholar] [CrossRef] [PubMed]
- Ouaïssi, M.; Gaujoux, S.; Veyrie, N.; Denève, E.; Brigand, C.; Castel, B.; Duron, J.J.; Rault, A.; Slim, K.; Nocca, D. Post-operative adhesions after digestive surgery: Their incidence and prevention: Review of the literature. J. Visc. Surg. 2012, 149, e104–e114. [Google Scholar] [CrossRef]
- Liu, B.; Kong, Y.; Alimi, O.A.; Kuss, M.A.; Tu, H.; Hu, W.; Rafay, A.; Vikas, K.; Shi, W.; Lerner, M.; et al. Multifunctional Microgel-Based Cream Hydrogels for Postoperative Abdominal Adhesion Prevention. ACS Nano 2023, 17, 3847–3864. [Google Scholar] [CrossRef]
- Ma, P.; Liang, W.; Huang, R.; Zheng, B.; Feng, K.; He, W.; Huang, Z.; Shen, H.; Wang, H.; Wu, D. Super-Structured Wet-Adhesive Hydrogel with Ultralow Swelling, Ultrahigh Burst Pressure Tolerance, and Anti-Postoperative Adhesion Properties for Tissue Adhesion. Adv. Mater. 2024, 36, e2305400. [Google Scholar] [CrossRef]
- Peng, W.; Lai, Y.; Jiang, Y.; Zhang, Y.; Kan, Z.; Dai, C.; Shen, J.; Liu, P. Charge balance transition enabled Janus hydrogel for robust wet-tissue adhesion and anti-postoperative adhesion. Bioact. Mater. 2025, 52, 123–138. [Google Scholar] [CrossRef]
- Gao, J.; Wen, J.; Hu, D.; Liu, K.; Zhang, Y.; Zhao, X.; Wang, K. Bottlebrush inspired injectable hydrogel for rapid prevention of postoperative and recurrent adhesion. Bioact. Mater. 2022, 16, 27–46. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, H.; Li, Z.; Zhangji, A.; Guo, B. Bioinspired Injectable Self-Healing Hydrogel Sealant with Fault-Tolerant and Repeated Thermo-Responsive Adhesion for Sutureless Post-Wound-Closure and Wound Healing. Nanomicro. Lett. 2022, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, G.; Pan, Z.; Zhang, K.; Xian, Y.; Zhu, Z.; Hong, Y.; Zhang, C.; Wu, D. An Injectable Hydrogel with Ultrahigh Burst Pressure and Innate Antibacterial Activity for Emergency Hemostasis and Wound Repair. Adv. Mater. 2024, 36, e2404811. [Google Scholar] [CrossRef]
- Hemati, H.; Haghiralsadat, F.; Hemati, M.; Sargazi, G.; Razi, N. Design and Evaluation of Liposomal Sulforaphane-Loaded Polyvinyl Alcohol/Polyethylene Glycol (PVA/PEG) Hydrogels as a Novel Drug Delivery System for Wound Healing. Gels 2023, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xu, G.; Huang, H.; Wang, K.; Wang, H.; Lang, M.; Gao, H.; Zhao, S. Sequential Release of Small Extracellular Vesicles from Bilayered Thiolated Alginate/Polyethylene Glycol Diacrylate Hydrogels for Scarless Wound Healing. ACS Nano 2021, 15, 6352–6368. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, X.; Liu, B.; Yu, Y.; Sun, L.; Liu, T.; Wang, Y.; Ding, J.; Chen, X. Polymer materials for prevention of postoperative adhesion. Acta Biomater. 2017, 61, 21–40. [Google Scholar] [CrossRef]
- Olivier, S.; Derue, L.; Geffroy, B.; Maindron, T.; Ishow, E. Small molecule-based photocrosslinkable fluorescent materials toward multilayered and high-resolution emissive patterning. J. Mater. Chem. C 2015, 3, 8403–8412. [Google Scholar] [CrossRef]
- Morgese, G.; Siegmann, K.; Winkler, M. Specific, nondestructive, and durable adhesion primer for polyolefins. J. Coat. Technol. Res. 2024, 21, 1921–1930. [Google Scholar] [CrossRef]
- Nair, S.K.; Bhat, I.K.; Aurora, A.L. Role of proteolytic enzyme in the prevention of postoperative intraperitoneal adhesions. Arch. Surg. 1974, 108, 849–853. [Google Scholar] [CrossRef]
- Keddie, D.J. A guide to the synthesis of block copolymers using reversible-addition fragmentation chain transfer (RAFT) polymerization. Chem. Soc. Rev. 2014, 43, 496–505. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, Y.; Huang, S.; Guo, B. Chitosan-based self-healing hydrogel dressing for wound healing. Adv. Colloid Interface Sci. 2024, 332, 103267. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Ji, T.; Xue, Y.; Zhao, L.; Zhao, K.; Jia, B.; Wang, B.; Wang, J.; Zhang, S.; et al. Self-Healing Hydrogel Bioelectronics. Adv. Mater. 2024, 36, e2306350. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, H.; Davoodi, E.; Baidya, A.; Baghdasarian, S.; Sarikhani, E.; Meyer, C.E.; Haghniaz, R.; Badv, M.; Annabi, N.; Khademhosseini, A.; et al. Engineered Hemostatic Biomaterials for Sealing Wounds. Chem. Rev. 2022, 122, 12864–12903. [Google Scholar] [CrossRef]
- Ouyang, C.; Yu, H.; Wang, L.; Ni, Z.; Liu, X.; Shen, D.; Yang, J.; Shi, K.; Wang, H. Tough adhesion enhancing strategies for injectable hydrogel adhesives in biomedical applications. Adv. Colloid Interface Sci. 2023, 319, 102982. [Google Scholar] [CrossRef]
- Poehnert, D.; Abbas, M.; Kreipe, H.H.; Klempnauer, J.; Winny, M. High reproducibility of adhesion formation in rat with meso-stitch approximation of injured cecum and abdominal wall. Int. J. Med. Sci. 2015, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sultana, T.; Van Hai, H.; Park, M.; Lee, S.Y.; Lee, B.T. Controlled release of Mitomycin C from modified cellulose based thermo-gel prevents post-operative de novo peritoneal adhesion. Carbohydr. Polym. 2020, 229, 115552. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.Y.; Park, S.H.; Kim, M.J.; Song, B.R.; Yun, H.W.; Kang, T.W.; Choi, H.S.; Kim, Y.J.; Min, B.H.; et al. Cross-linked electrospun cartilage acellular matrix/poly(caprolactone-co-lactide-co-glycolide) nanofiber as an antiadhesive barrier. Acta Biomater. 2018, 74, 192–206. [Google Scholar] [CrossRef]
- Hindocha, A.; Beere, L.; Dias, S.; Watson, A.; Ahmad, G. Adhesion prevention agents for gynaecological surgery: An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2015, 1, Cd011254. [Google Scholar] [CrossRef]
- Hu, Q.; Xia, X.; Kang, X.; Song, P.; Liu, Z.; Wang, M.; Lu, X.; Guan, W.; Liu, S. A review of physiological and cellular mechanisms underlying fibrotic postoperative adhesion. Int. J. Biol. Sci. 2021, 17, 298–306. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Y.; Zhao, X.; He, C.; Bi, S.; Liu, R.; Gu, J.; Yan, B. Temperature-Responsive and Self-Healing Hydrogel: A Novel Approach to Combat Postoperative Adhesions. Polymers 2025, 17, 1925. https://doi.org/10.3390/polym17141925
Zhan Y, Zhao X, He C, Bi S, Liu R, Gu J, Yan B. Temperature-Responsive and Self-Healing Hydrogel: A Novel Approach to Combat Postoperative Adhesions. Polymers. 2025; 17(14):1925. https://doi.org/10.3390/polym17141925
Chicago/Turabian StyleZhan, Yujia, Xueshan Zhao, Changyuan He, Siwei Bi, Ruiqi Liu, Jun Gu, and Bin Yan. 2025. "Temperature-Responsive and Self-Healing Hydrogel: A Novel Approach to Combat Postoperative Adhesions" Polymers 17, no. 14: 1925. https://doi.org/10.3390/polym17141925
APA StyleZhan, Y., Zhao, X., He, C., Bi, S., Liu, R., Gu, J., & Yan, B. (2025). Temperature-Responsive and Self-Healing Hydrogel: A Novel Approach to Combat Postoperative Adhesions. Polymers, 17(14), 1925. https://doi.org/10.3390/polym17141925






