Sulfonated Biopolymer Derived from Wheat Straw for the Recovery of Au(III)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experiment Procedure
2.3. Characterization of the Adsorbent
3. Results and Discussion
3.1. Effect of Acid Concentration
3.2. Effect of Contact Time
3.3. Kinetic Study of the Adsorption
3.4. Instrumental Analysis
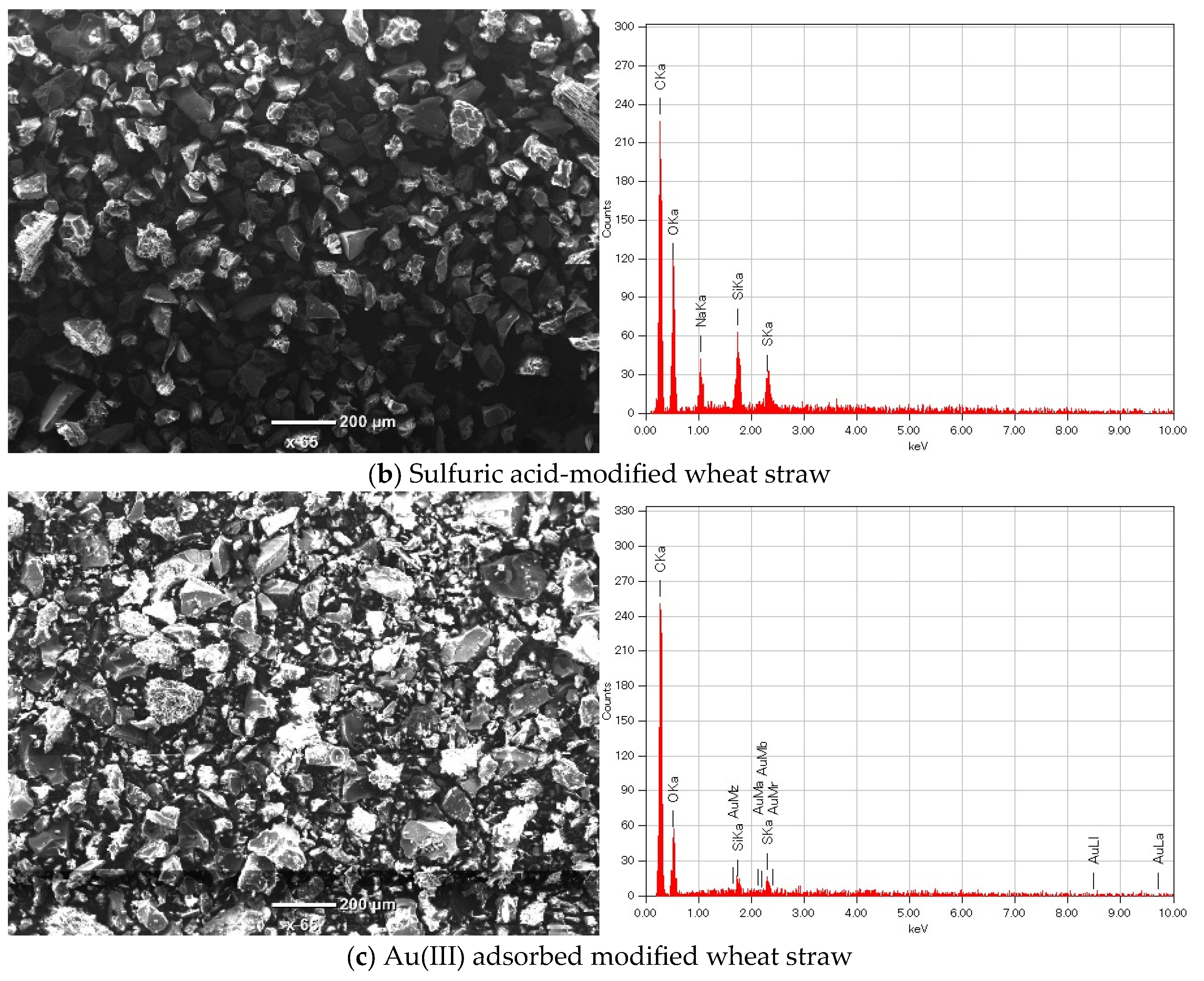
3.4.1. SEM/EDS Analysis
3.4.2. XRD Analysis
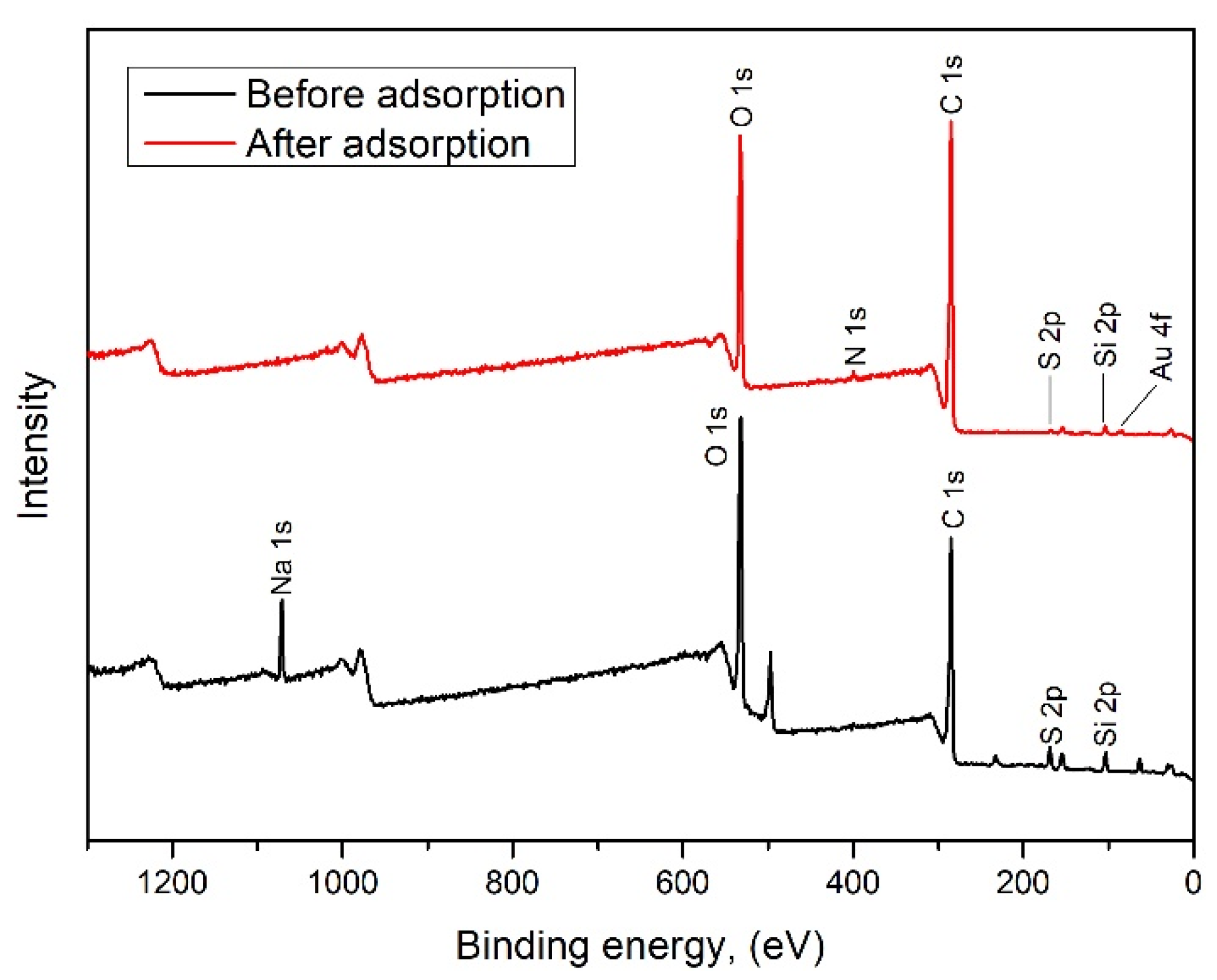
3.4.3. XPS Analysis
- The significant decrease in carbonyl (C = O) groups suggests their active role in the reduction and coordination of Au3+.
- The increase in carboxylic/ester (O–C = O) groups may result from oxidation reactions occurring during the reduction of gold.
- An increase in ether (C–O–C) groups suggests the exposure or rearrangement of these groups during treatment.
- The decrease in sulfonyl (S = O) and sulfonic (S–O–S) groups indicates their participation in gold coordination, likely through direct bonding or redox activity.
- The sharp increase in new S–O environments is attributed to gold interactions with sulfur species.
- Moreover, the appearance of Au 4f peaks after adsorption confirms the deposition of gold, either in its elemental (Au0) or partially reduced form, indicating a redox reaction (Au3+ → Au0).
- Electrostatic Attraction: In an acidic medium, sulfonated lignin possesses protonated –SO3H groups that electrostatically attract negatively charged AuCl4− ions.
- Complexation and Chelation: Au3+ ions coordinate with electron-donating functional groups such as carbonyl (C = O), carboxyl (O–C = O), sulfonate (S–O–C), and sulfate (S = O) groups on the modified wheat straw.
- Redox Reaction: Phenolic and carboxylic groups facilitate the reduction of Au3+ to metallic Au0. Sulfuric acid treatment enhances this effect by increasing the availability of reactive sites.
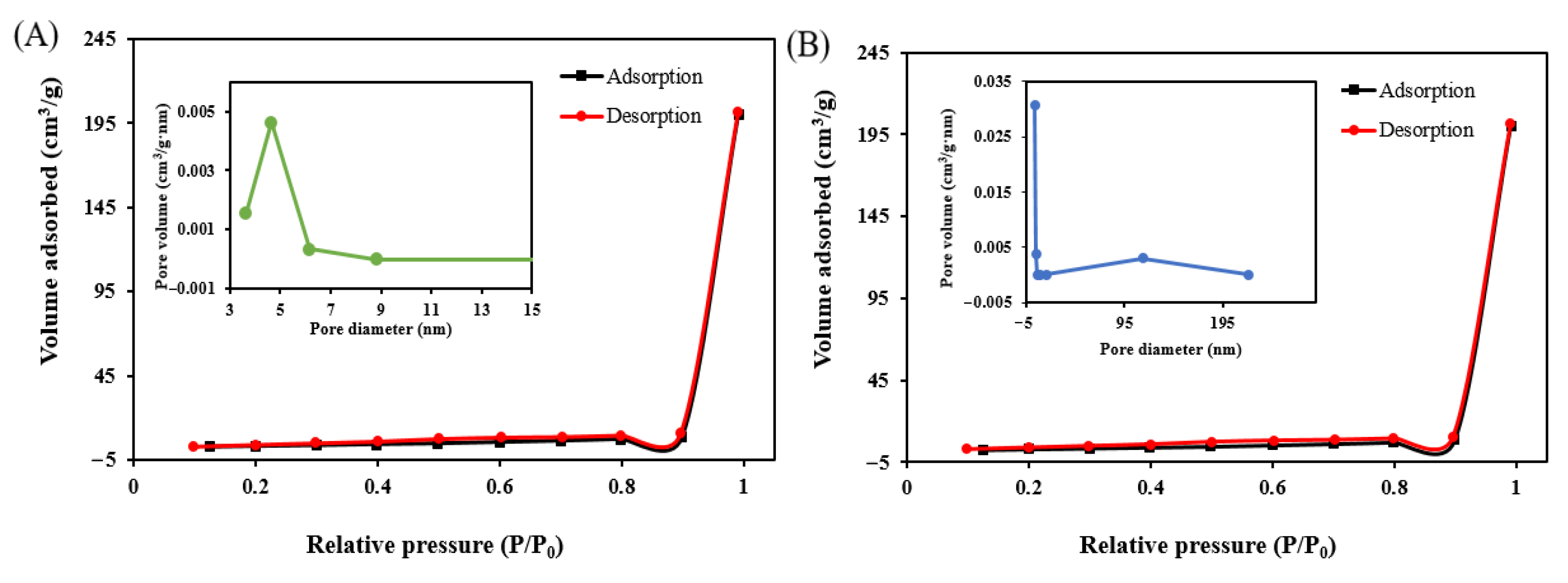
3.4.4. BET Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manigandan, S.; Rajmohan, K.S.; Varjani, S. Current trends in gold recovery from electronic wastes. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 307–325. [Google Scholar]
- Syed, S. Recovery of gold from secondary sources—A review. Hydrometallurgy 2012, 115–116, 30–51. [Google Scholar] [CrossRef]
- Nhan Hau Nguyen, T.; Seung Lee, M. Recovery of Au and Pd from the etching solution of printed circuit boards by cementation, solvent extraction, reduction, and precipitation. J. Ind. Eng. Chem. 2023, 126, 214–223. [Google Scholar] [CrossRef]
- Rocky, M.M.H.; Rahman, I.M.M.; Endo, M.; Hasegawa, H. Comprehensive insights into aqua regia-based hybrid methods for efficient recovery of precious metals from secondary raw materials. Chem. Eng. J. 2024, 495, 153537. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Chen, S.; Zi, F.; Hu, X. Highly Efficient Recovery of Au(I) from Gold Leaching Solution Using Sodium Dimethyldithiocarbamate. ACS Omega 2024, 9, 20547–20556. [Google Scholar] [CrossRef]
- Zhang, H.; Ritchie, I.M.; La Brooy, S.R. The adsorption of gold thiourea complex onto activated carbon. Hydrometallurgy 2004, 72, 291–301. [Google Scholar] [CrossRef]
- Hayat, M.; Waseem, M.; Arif, S.; Ali, J.; Sattar, A.; Dilpazir, S.; Hussain, K.; Tabassam, L. Turning trash into Treasure: Extracting precious metals from e-waste with Electrochemically Exfoliated graphene derivatives. Chem. Eng. J. 2024, 500, 156957. [Google Scholar] [CrossRef]
- Torrinha, M.B.Q.L.F.; Bacelo, H.A.M.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Uptake and Recovery of Gold from Simulated Hydrometallurgical Liquors by Adsorption on Pine Bark Tannin Resin. Water 2020, 12, 3456. [Google Scholar] [CrossRef]
- Grad, O.; Ciopec, M.; Negrea, A.; Duțeanu, N.; Vlase, G.; Negrea, P.; Dumitrescu, C.; Vlase, T.; Vodă, R. Precious metals recovery from aqueous solutions using a new adsorbent material. Sci. Rep. 2021, 11, 214072. [Google Scholar] [CrossRef]
- Hilal, R.H. Removal of Precious Metals from Electronic-Waste by Using Composite Material. IOP Conf. Ser. Mater. Sci. Eng. 2020, 881, 012089. [Google Scholar] [CrossRef]
- Peydayesh, M.; Boschi, E.; Donat, F.; Mezzenga, R. Gold Recovery from E-Waste by Food-Waste Amyloid Aerogels. Adv. Mater. 2024, 36, e2310642. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.; Yavuz, C.T.; Nam, Y.S. Sunlight-boosted recovery of precious metal ions from E-waste using tannin-grafted mesoporous silica. Chem. Eng. J. 2024, 487, 150529. [Google Scholar] [CrossRef]
- Son, H.Y.; Kim, I.; Nam, Y.S. On-surface synthesis of metal nanostructures on solid and hydrated polymer nanofibers coated with polydopamine. J. Ind. Eng. Chem. 2015, 30, 220–224. [Google Scholar] [CrossRef]
- Ganchimeg, Y.; Burmaa, G.; Naoki, K.; Hee Joon, K. Recovery of Gold from Aqueous Solution Containing Au(III) by Silicon Organic Polymer. J. Chem. Chem. Eng. 2017, 11, 15–21. [Google Scholar] [CrossRef][Green Version]
- Amuda, O.S.; Giwa, A.A.; Bello, I.A. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Matouq, M.; Jildeh, N.; Qtaishat, M.; Hindiyeh, M.; Al Syouf, M.Q. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J. Environ. Chem. Eng. 2015, 3, 775–784. [Google Scholar] [CrossRef]
- Pan, H.-W.; Iizuka, A.; Shibata, E. Gold recovery from dilute aqueous solution by a biosorbent derived from woody biomass. Chem. Eng. Commun. 2020, 208, 1711–1724. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, M.; Tay, C.C.; Liu, Z.; Wang, W.; Hu, B. Efficient adsorption and reduction of Au(III) to gold particles using cost-effective chitosan functionalized cellulose nanofiber. Carbon Res. 2025, 4, 12. [Google Scholar] [CrossRef]
- Liu, F.; Peng, G.; Li, T.; Yu, G.; Deng, S. Au(III) adsorption and reduction to gold particles on cost-effective tannin acid immobilized dialdehyde corn starch. Chem. Eng. J. 2019, 370, 228–236. [Google Scholar] [CrossRef]
- Pangeni, B.; Paudyal, H.; Inoue, K.; Kawakita, H.; Ohto, K.; Alam, S. Selective recovery of gold(III) using cotton cellulose treated with concentrated sulfuric acid. Cellulose 2011, 19, 381–391. [Google Scholar] [CrossRef]
- Tasdelen, C.; Aktas, S.; Acma, E.; Guvenilir, Y. Gold recovery from dilute gold solutions using DEAE-cellulose. Hydrometallurgy 2009, 96, 253–257. [Google Scholar] [CrossRef]
- Cecchi, T.; Gao, Z.; Clement, C.; Camus, A.; Karim, A.; Girard, O.; Santato, C. Recovery of gold from e-waste via food waste byproducts. Nanotechnology 2022, 34, 065302. [Google Scholar] [CrossRef] [PubMed]
- Golnaraghi Ghomi, A.; Asasian-Kolur, N.; Sharifian, S.; Golnaraghi, A. Biosorpion for sustainable recovery of precious metals from wastewater. J. Environ. Chem. Eng. 2020, 8, 103996. [Google Scholar] [CrossRef]
- Rubcumintara, T. Adsorptive Recovery of Au(III) from Aqueous Solution Using Modified Bagasse Biosorbent. Int. J. Chem. Eng. Appl. 2015, 6, 95–100. [Google Scholar] [CrossRef]
- Xiong, Y.; Adhikari, C.R.; Kawakita, H.; Ohto, K.; Inoue, K.; Harada, H. Selective recovery of precious metals by persimmon waste chemically modified with dimethylamine. Bioresour. Technol. 2009, 100, 4083–4089. [Google Scholar] [CrossRef]
- Losev, V.N.; Buyko, O.V.; Borodina, E.V.; Samoilo, A.S.; Zhyzhaev, A.M.; Velichko, B.A. Biosorbents based on pine sawdust and malt sprouts for preconcentration and ICP-OES determination of nonferrous, heavy, and precious metals in the environmental samples. Sep. Sci. Technol. 2018, 53, 1654–1665. [Google Scholar] [CrossRef]
- Saman, N.; Tan, J.-W.; Mohtar, S.S.; Kong, H.; Lye, J.W.P.; Johari, K.; Hassan, H.; Mat, H. Selective biosorption of aurum(III) from aqueous solution using oil palm trunk (OPT) biosorbents: Equilibrium, kinetic and mechanism analyses. Biochem. Eng. J. 2018, 136, 78–87. [Google Scholar] [CrossRef]
- Deng, K.; Yin, P.; Liu, X.; Tang, Q.; Qu, R. Modeling, analysis and optimization of adsorption parameters of Au(III) using low-cost agricultural residuals buckwheat hulls. J. Ind. Eng. Chem. 2014, 20, 2428–2438. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vazvani, M.G.; Hassanisaadi, M.; Thakur, V.K. Agricultural wastes: A practical and potential source for the isolation and preparation of cellulose and application in agriculture and different industries. Ind. Crops Prod. 2024, 208, 117904. [Google Scholar] [CrossRef]
- Deng, A.; Lin, Q.; Yan, Y.; Li, H.; Ren, J.; Liu, C.; Sun, R. A feasible process for furfural production from the pre-hydrolysis liquor of corncob via biochar catalysts in a new biphasic system. Bioresour. Technol. 2016, 216, 754–760. [Google Scholar] [CrossRef]
- Huang, L.-Z.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. Recent Developments and Applications of Hemicellulose From Wheat Straw: A Review. Front. Bioeng. Biotechnol. 2021, 9, 690773. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Liang, M.; Xu, R.; Yu, Z.; Duan, B.; Lu, L.; Si, C. Conversion of waste lignocellulose to furfural using sulfonated carbon microspheres as catalyst. Waste Manag. 2020, 108, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Azadeh, E.; Seyed, F.; Ardovan, Y. Surfactant-Modified Wheat Straw: Preparation, Characterization and its Application for Methylene Blue Adsorption from Aqueous Solution. J. Chem. Eng. Process Technol. 2015, 6, 231. [Google Scholar] [CrossRef]
- Bui, T.H.; Lee, W.; Jeon, S.-B.; Kim, K.-W.; Lee, Y. Enhanced Gold(III) adsorption using glutaraldehyde-crosslinked chitosan beads: Effect of crosslinking degree on adsorption selectivity, capacity, and mechanism. Sep. Purif. Technol. 2020, 248, 116989. [Google Scholar] [CrossRef]
- Hurskainen, A.; Bediako, J.K.; Ouardi, Y.E.; Lamsayah, M.; Frimodig, J.; Repo, E. Understanding the mechanisms of gold(III) adsorption onto additively manufactured polyamide adsorbent, AM-N12. Chem. Eng. Sci. 2025, 305, 121130. [Google Scholar] [CrossRef]
- Zhou, S.; Mo, X.; Zhu, W.; Xu, W.; Tang, K.; Lei, Y. Selective adsorption of Au(III) with ultra-fast kinetics by a new metal-organic polymer. J. Mol. Liq. 2020, 319, 114125. [Google Scholar] [CrossRef]
- An, F.-Q.; Li, M.; Guo, X.-D.; Wang, H.-Y.; Wu, R.-Y.; Hu, T.-P.; Gao, J.-F.; Jiao, W.-Z. Selective adsorption of AuCl4—On chemically modified D301 resin with containing N/S functional polymer. J. Environ. Chem. Eng. 2017, 5, 10–15. [Google Scholar] [CrossRef]
- Yang, L.; Jia, F.; Song, S. Recovery of [Au(CN)2]—From gold cyanidation with graphene oxide as adsorbent. Sep. Purif. Technol. 2017, 186, 63–69. [Google Scholar] [CrossRef]
- Hepperle, P.; Herman, A.; Khanbabaee, B.; Baek, W.Y.; Nettelbeck, H.; Rabus, H. XPS Examination of the Chemical Composition of PEGMUA-Coated Gold Nanoparticles. Part. Part. Syst. Charact. 2022, 39, 2200070. [Google Scholar] [CrossRef]
- Yu, H.; Zi, F.; Hu, X.; Nie, Y.; Chen, Y.; Cheng, H. Adsorption of gold from thiosulfate solutions with chemically modified activated carbon. Adsorpt. Sci. Technol. 2017, 36, 408–428. [Google Scholar] [CrossRef]
- Mendsaikhan, E.; Bat-Amgalan, M.; Yunden, G.; Miyamoto, N.; Kano, N.; Kim, H.J. Modified Urtica dioica Leaves as a Low-Cost and Effective Adsorbent for the Simultaneous Removal of Pb(II), Cu(II), Cd(II), and Zn(II) from Aqueous Solution. Int. J. Mol. Sci. 2025, 26, 2639. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Lin, J.; Lu, L. Hydrothermal synthesis of MnO2/CNT nanocomposite with a CNT core/porous MnO2 sheath hierarchy architecture for supercapacitors. Nanoscale Res. Lett. 2011, 6, 1–10. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Dong, Y.; Zhao, J.; Sun, G.; Hu, J.; Xu, Q.; Zhang, X.; Li, L.; Toyao, T.; et al. Photothermal CO2 hydrogenation to CO on CeO2 catalyst via redox mechanism. Chem. Eng. J. 2025, 510, 161609. [Google Scholar] [CrossRef]




mg/l | qe (exp) | Pseudo-First Order Model | Pseudo-Second Order Model | Weber–Morris Model | |||||
|---|---|---|---|---|---|---|---|---|---|
| K1 × 10−2 (min−1) |
(cal) mg/g | R2 | K2 × 10−4 mg/g·min | (cal) mg/g | R2 | Kid (mg/g·min 0.5) | R2 | ||
| 60.36 | 29.79 | 3.9 | 5.86 | 0.939 | 13.6 | 31.56 | 0.996 | 0.5 | 0.916 |
| 90 | 59.97 | 5.4 | 9.87 | 0.868 | 15.2 | 60.98 | 0.995 | 0.6 | 0.815 |
| 150 | 135.70 | 2.1 | 3.33 | 0.817 | 28.1 | 136.99 | 0.999 | 0.2 | 0.720 |
| Biosorbent | Modification Agent | Adsoption Capacity, mmol/g (mg/g) | Reference |
| Pine (Pinus sylvestris) sawdust-based biosorbent | Chemical grafted thiourea groups | 0.4 (78.79) | [23,26] |
| Sugarcane bagasse | Concentrated sulfuric acid | 7.6 (1497.50) | [24] |
| Persimmon waste | Dimethylamine | 5.63 (1108.92) | [25] |
| Malt sprout | Ortho-phosphoric acid + carbamide | 0.065 (12.80) | [26,27] |
| Wheat straw | Concentrated sulfuric acid | 0.69 (135.70) | In this study |
| Element | Atomic, % | Sub-Peak | Surface Area, % | ||
|---|---|---|---|---|---|
| Before Adsorption | After Adsorption | Before Adsorption | After Adsorption | ||
| C1s | 57.07 | 72.94 | C-C | 64.04 | 65.84 |
| C-O | 21.74 | 20.79 | |||
| C = O | 8.53 | 1.92 | |||
| O-C = O | 5.69 | 11.45 | |||
| O1s | 30.85 | 22.44 | C-O-C | 48.36 | 54.40 |
| C = O | 44.42 | 40.30 | |||
| O-C = O | 7.22 | 5.30 | |||
| S2p | 2.98 | 0.4 | S = O | 64.89 | 57.89 |
| S-O-C | 27.89 | 13.98 | |||
| S-O | 7.23 | 28.13 | |||
| Au4f | - | 0.04 | 4f 7/2 | - | 55.69 |
| 4f 5/2 | - | 44.31 | |||
| Sample | Surface Area (m2·g−1) | Pore Volume (cc·g−1) | Pore Size (Å) |
|---|---|---|---|
| Before adsorption | 10.420 | 0.310 | 641.771 |
| After adsorption | 14.847 | 0.313 | 18.179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lkhamtogmid, N.; Gunchin, B.; Dashdendev, B.; Punsantsogvoo, M.; Bat-Amgalan, M.; Yunden, G. Sulfonated Biopolymer Derived from Wheat Straw for the Recovery of Au(III). Polymers 2025, 17, 1914. https://doi.org/10.3390/polym17141914
Lkhamtogmid N, Gunchin B, Dashdendev B, Punsantsogvoo M, Bat-Amgalan M, Yunden G. Sulfonated Biopolymer Derived from Wheat Straw for the Recovery of Au(III). Polymers. 2025; 17(14):1914. https://doi.org/10.3390/polym17141914
Chicago/Turabian StyleLkhamtogmid, Nyamjargal, Burmaa Gunchin, Burmaa Dashdendev, Munkhbaatar Punsantsogvoo, Munkhpurev Bat-Amgalan, and Ganchimeg Yunden. 2025. "Sulfonated Biopolymer Derived from Wheat Straw for the Recovery of Au(III)" Polymers 17, no. 14: 1914. https://doi.org/10.3390/polym17141914
APA StyleLkhamtogmid, N., Gunchin, B., Dashdendev, B., Punsantsogvoo, M., Bat-Amgalan, M., & Yunden, G. (2025). Sulfonated Biopolymer Derived from Wheat Straw for the Recovery of Au(III). Polymers, 17(14), 1914. https://doi.org/10.3390/polym17141914







