Synthesis of Magnetic Nanoparticle/Polymer Matrix Nanocomposites with Induced Magnetic Performance
Abstract
1. Introduction
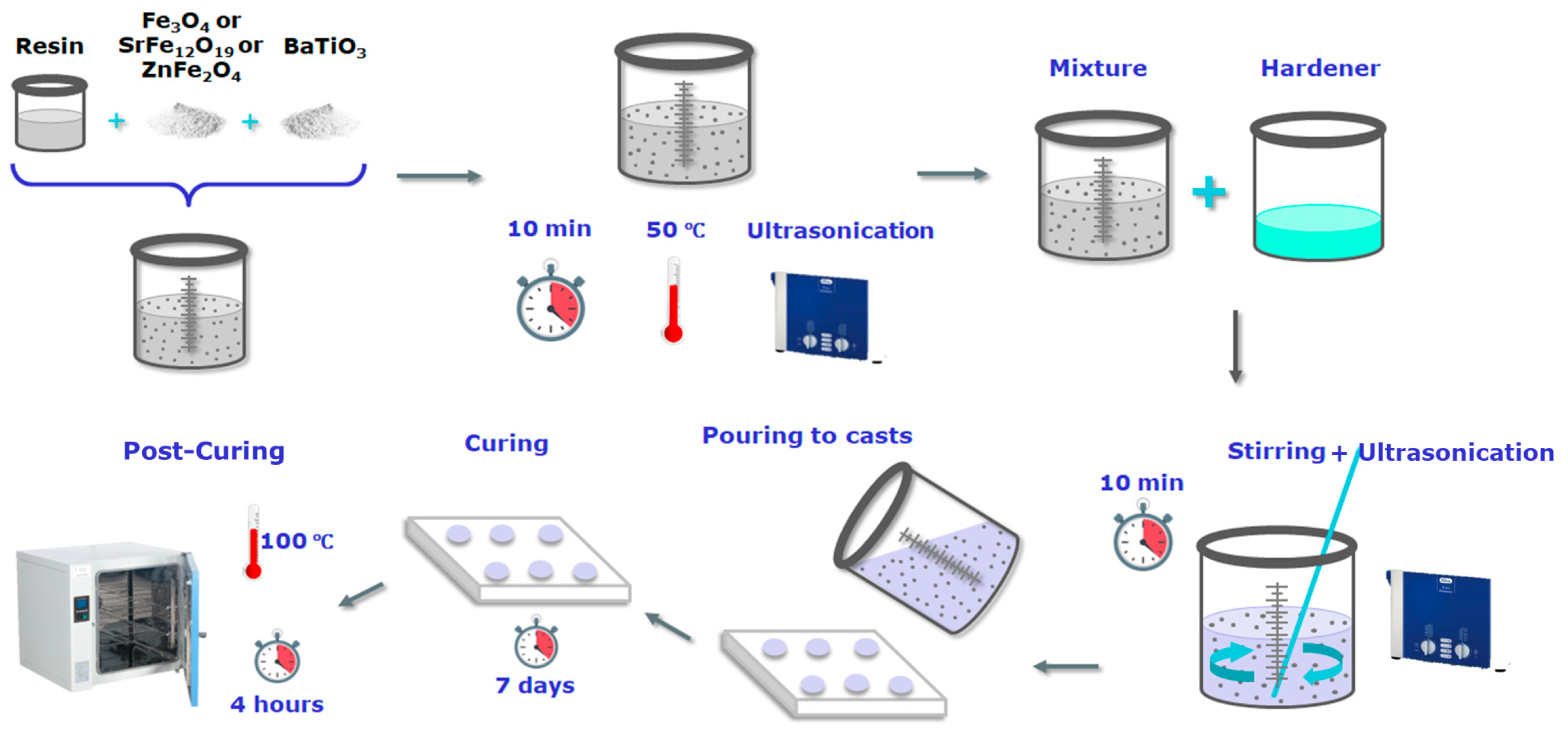
2. Materials and Methods
3. Results
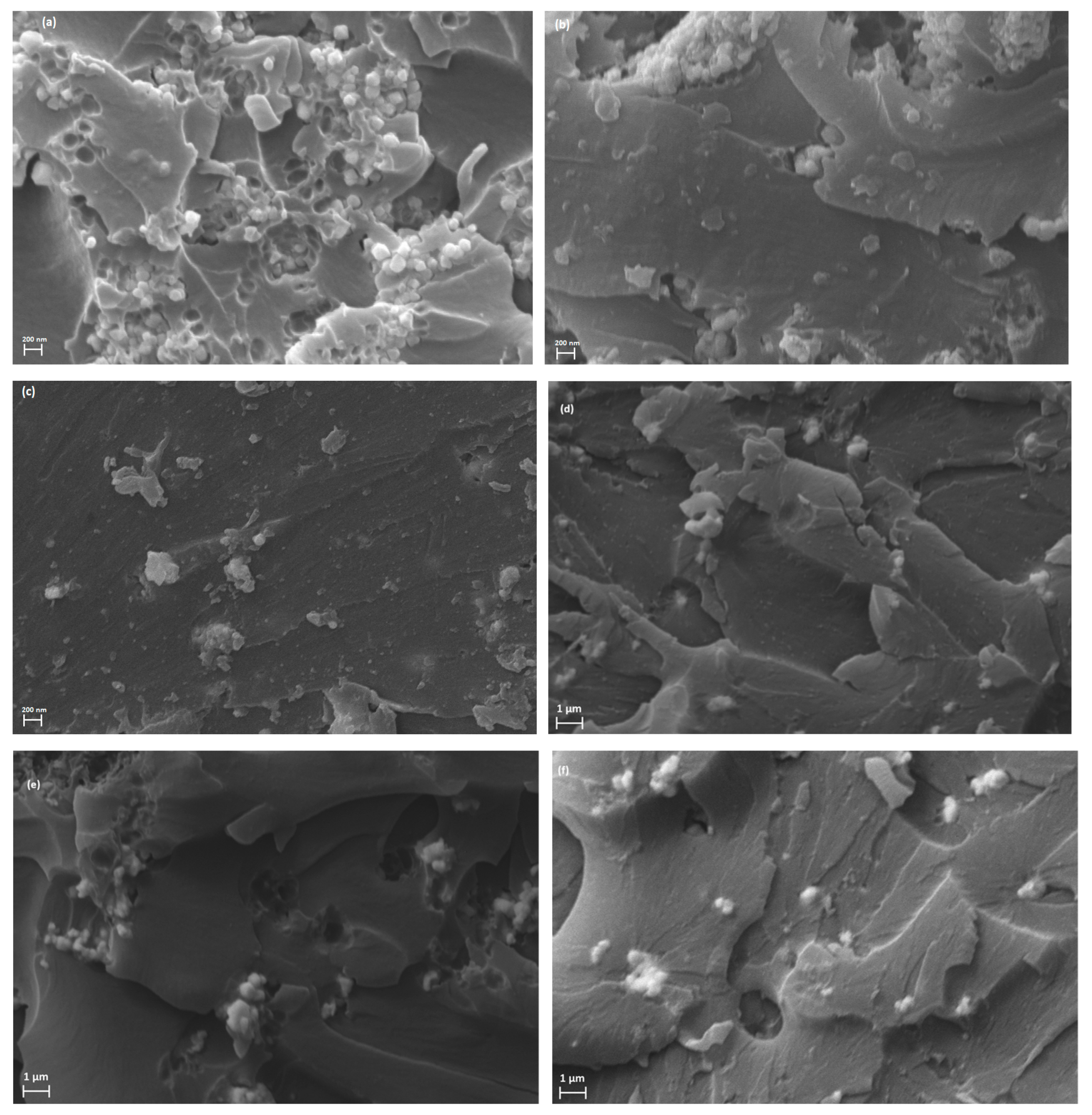
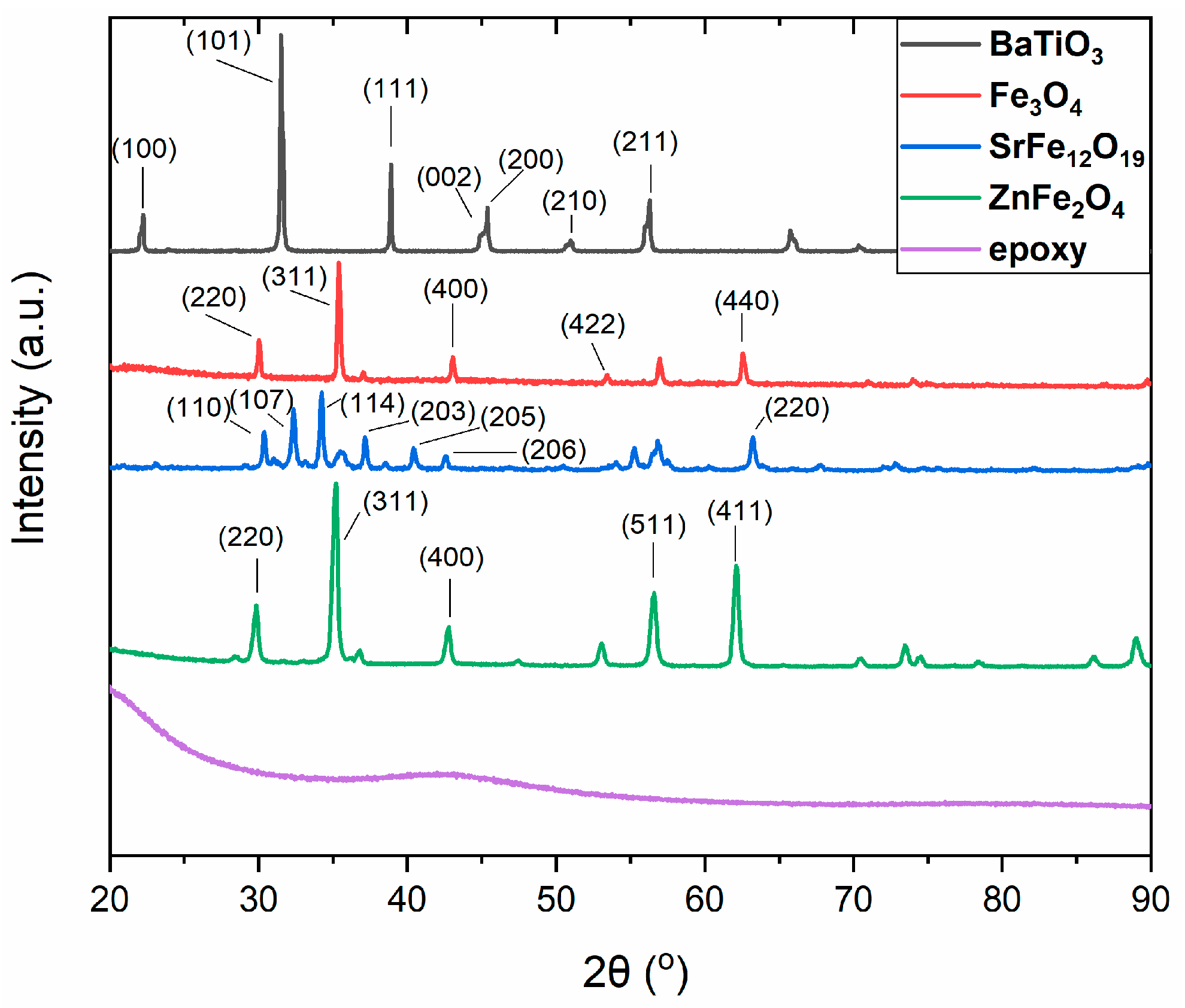
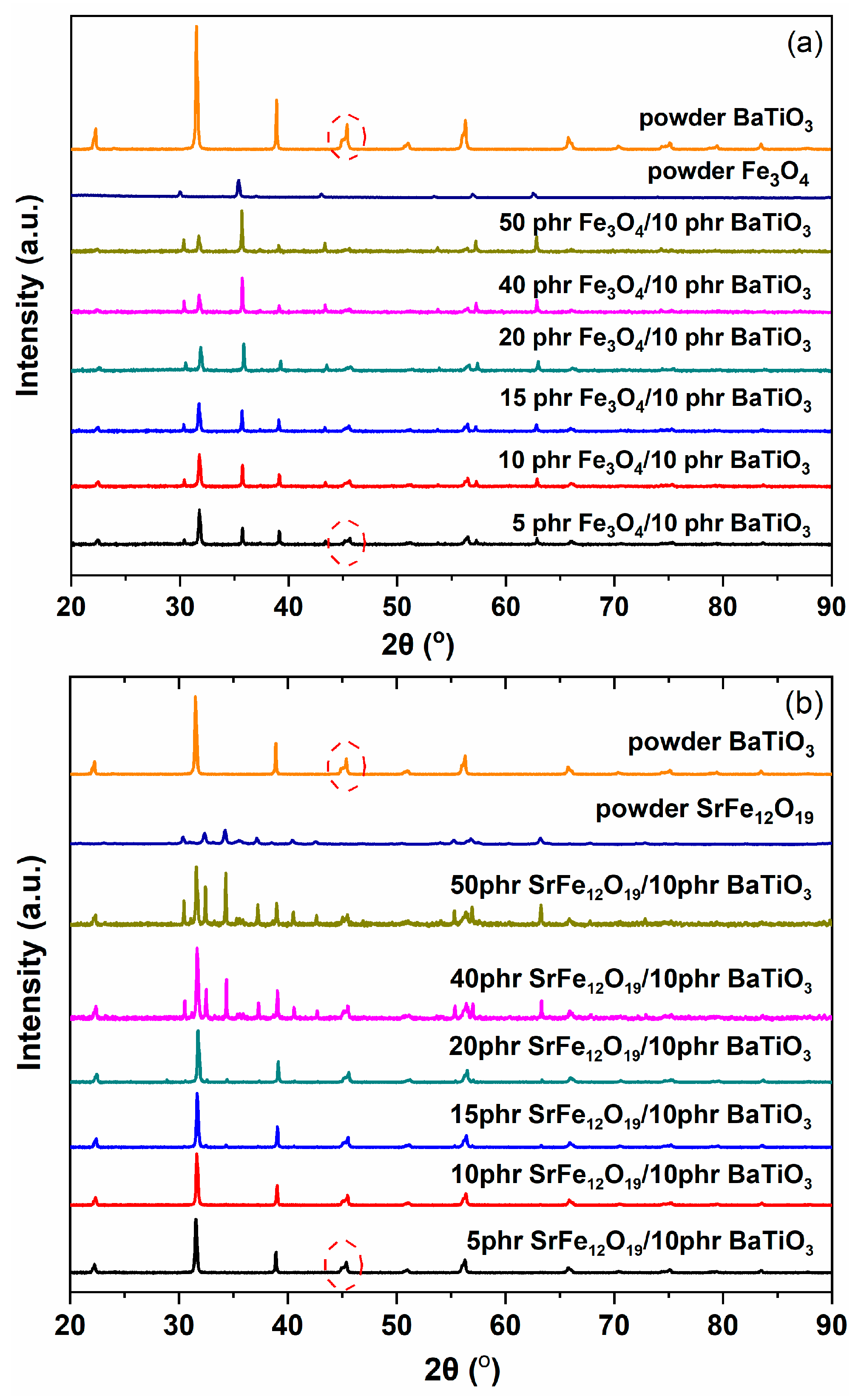
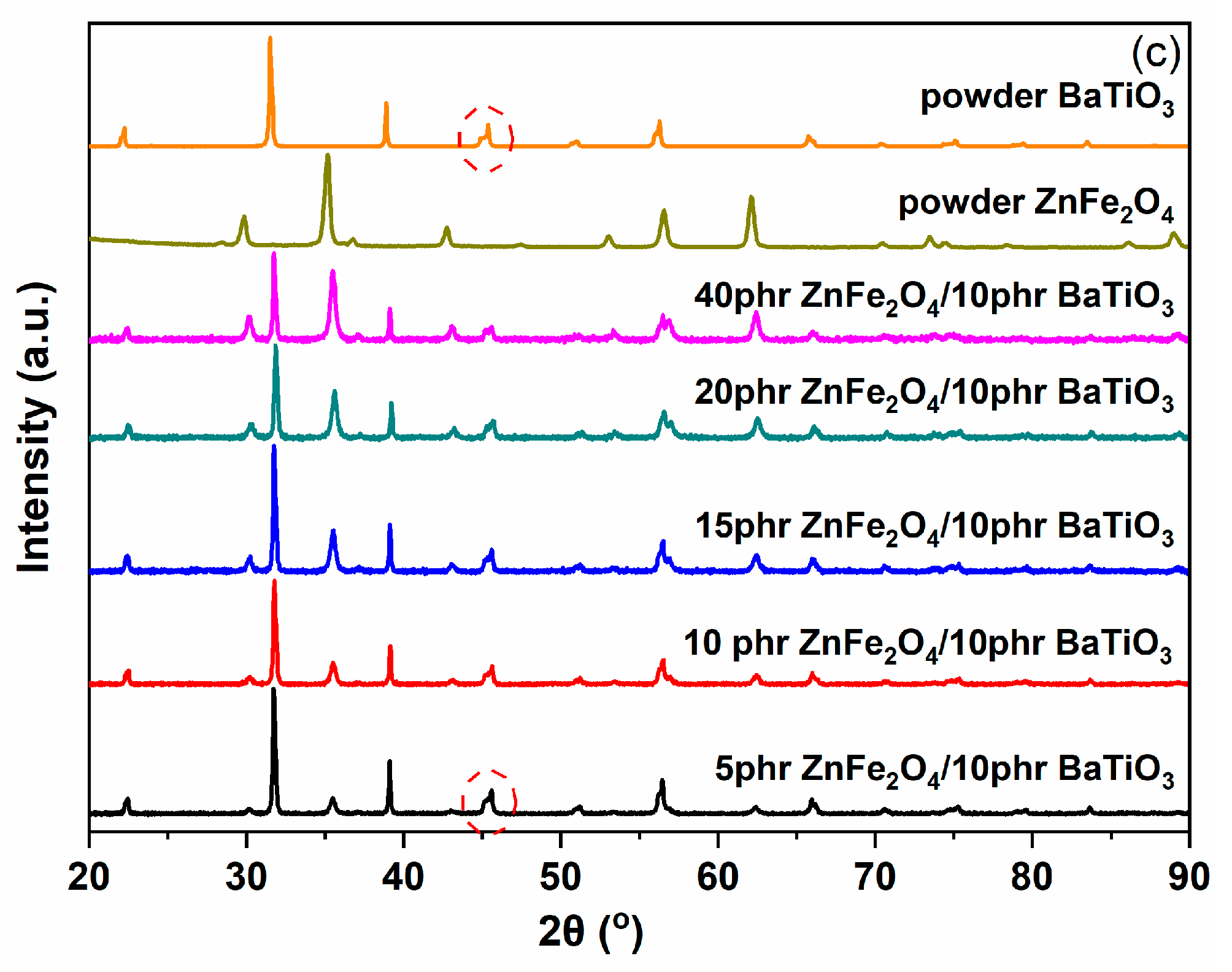
3.1. Morphological/Structural Characterization
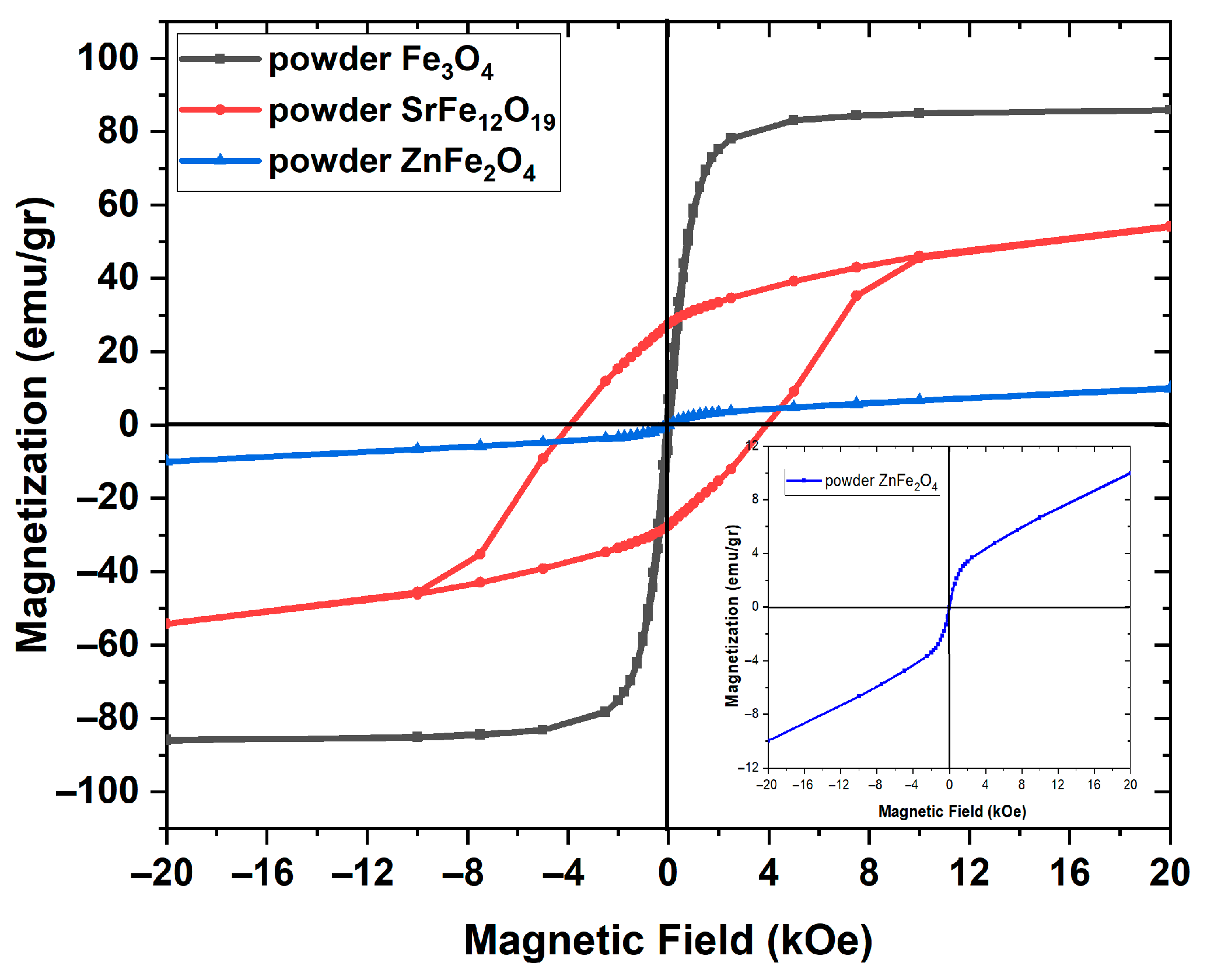
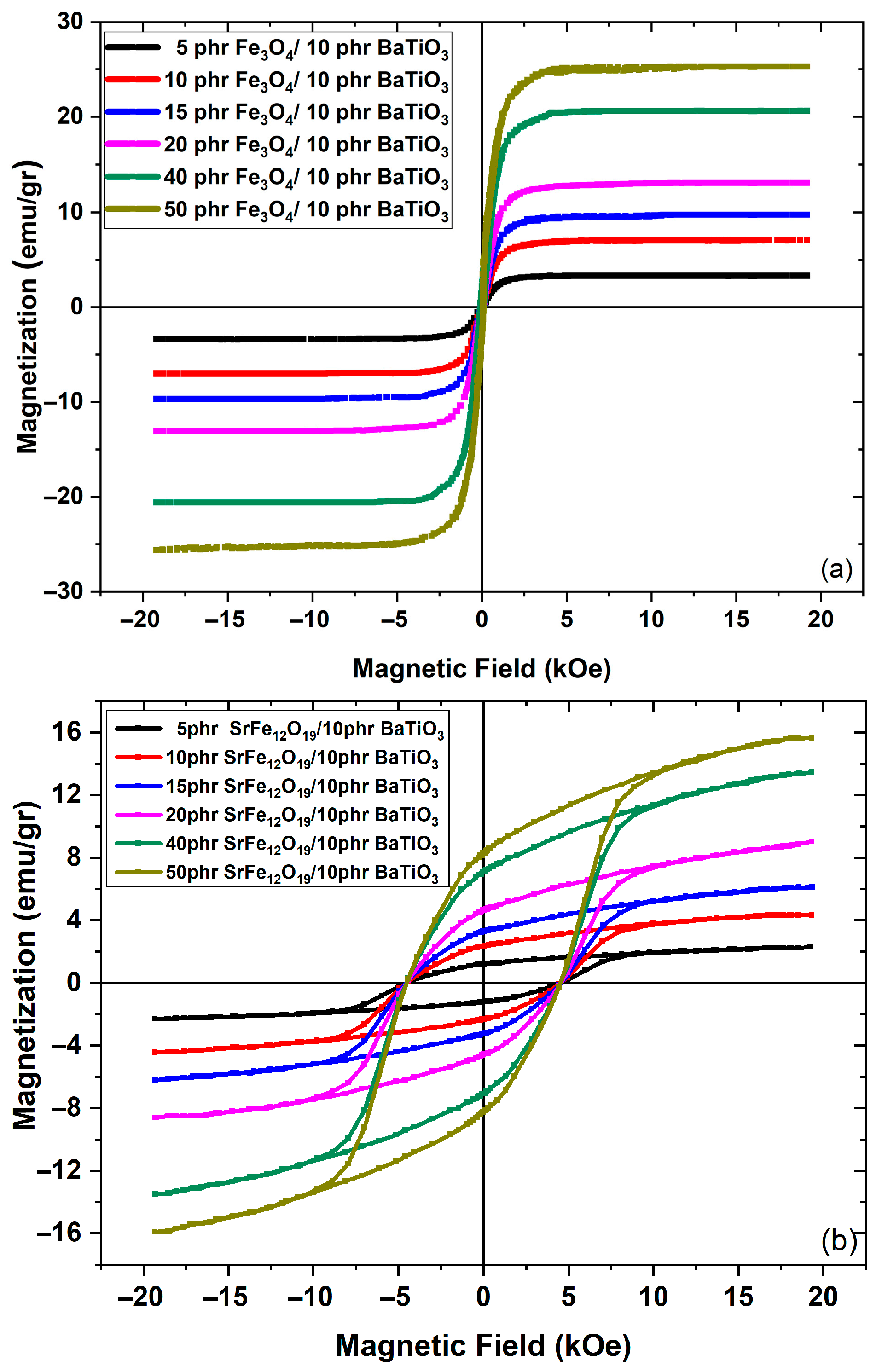
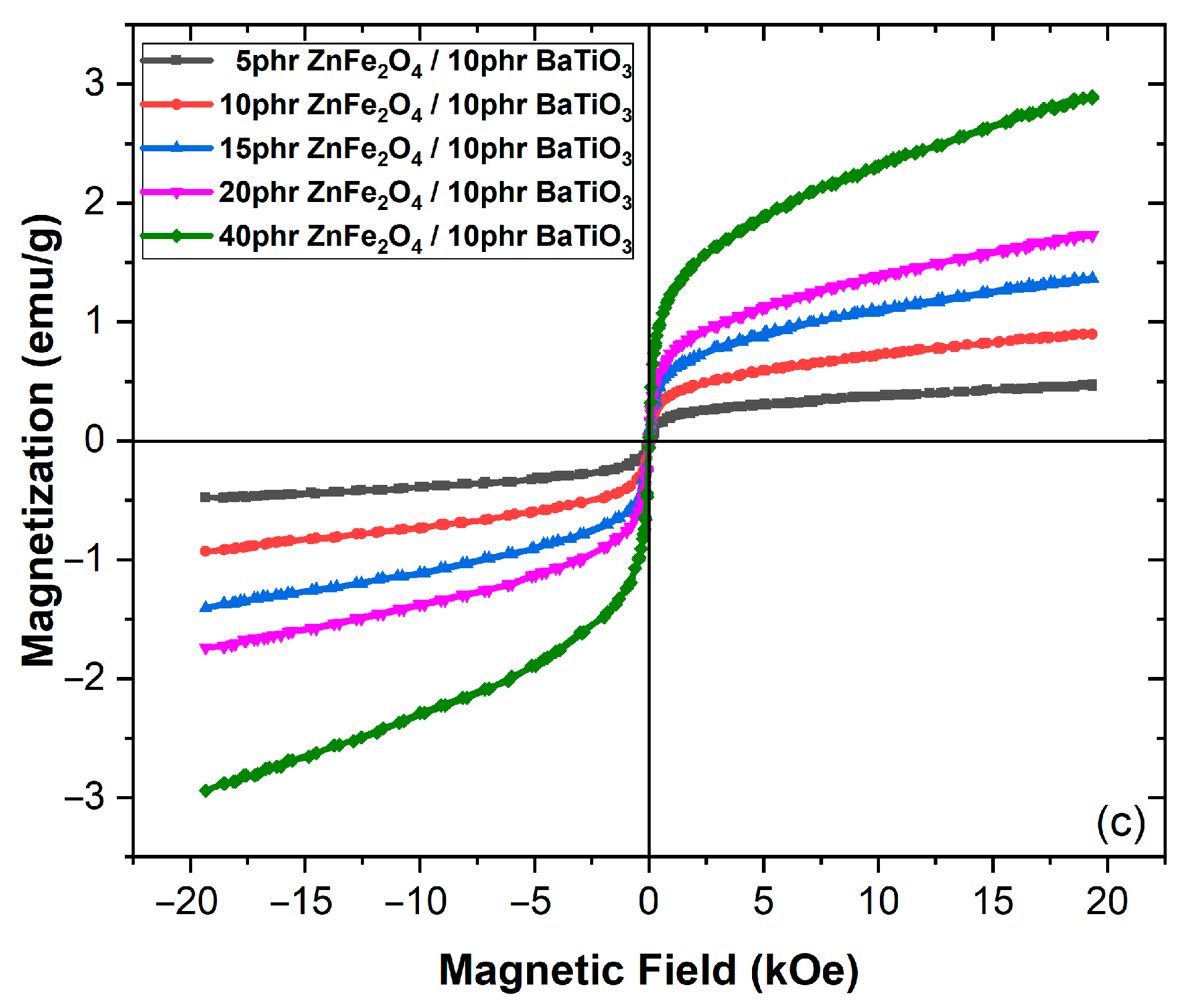
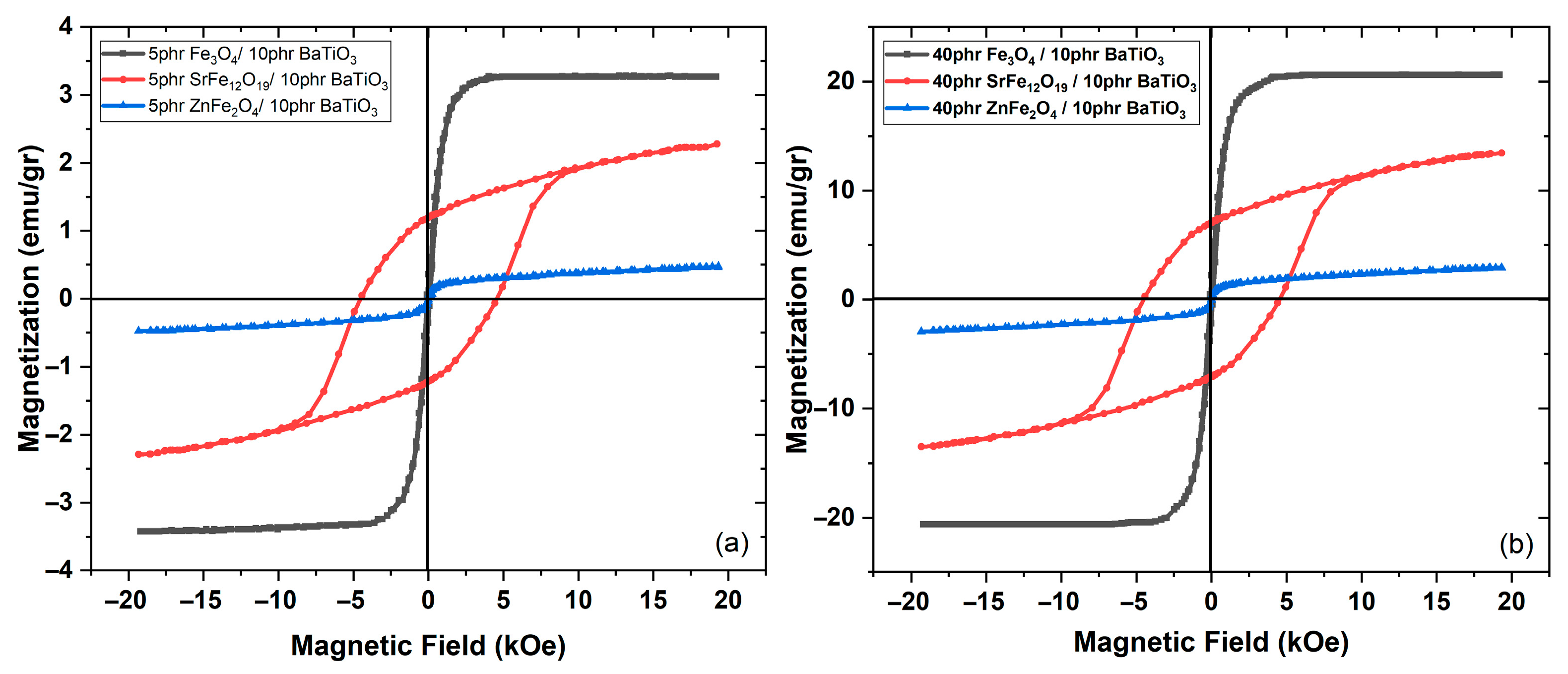
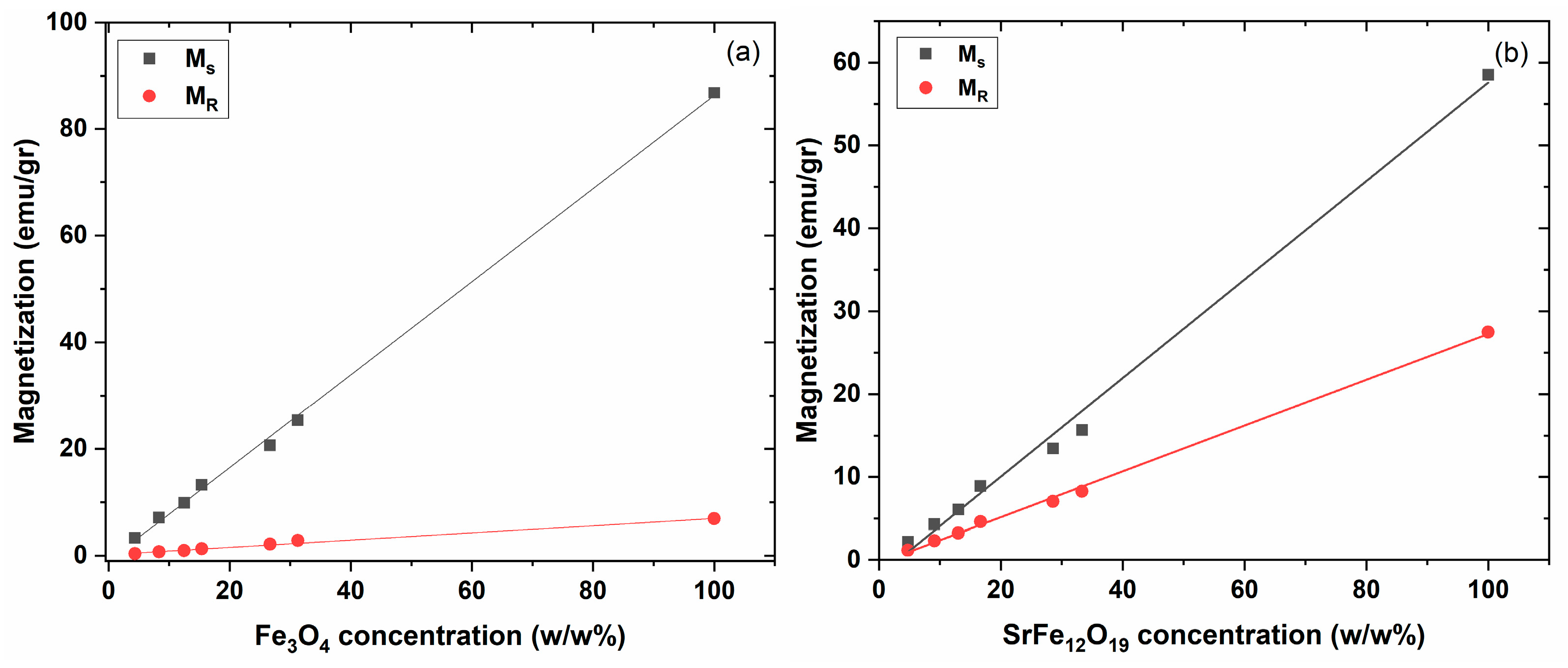
3.2. Magnetic Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, K.; Guo, J.Z.; Liu, C. Polymer-Based Multifunctional Nanocomposites and Their Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128150672. [Google Scholar]
- Friedrich, K. Routes for achieving multifunctionality in reinforced polymers and composite structures. In Multifunctionality of Polymer Composites; Friedrich, K., Breuer, U., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–41. ISBN 978-0-323-26434-1. [Google Scholar]
- Psarras, G.C. Ceramic/Polymer Nanodielectrics: Towards a Multifunctional or Smart Performance. MatSci Express 2024, 1, 162–169. [Google Scholar] [CrossRef]
- Gioti, S.; Sanida, A.; Mathioudakis, G.N.; Patsidis, A.C.; Speliotis, T.; Psarras, G.C. Multitasking Performance of Fe3O4/BaTiO3/Epoxy Resin Hybrid Nanocomposites. Materials 2022, 15, 1784. [Google Scholar] [CrossRef] [PubMed]
- Manika, G.C.; Gioti, S.; Sanida, A.; Mathioudakis, G.N.; Speliotis, T.; Patsidis, A.C.; Psarras, G.C. Multifunctional Performance of Hybrid SrFe12O19/BaTiO3/Epoxy Resin Nanocomposites. Polymers 2022, 14, 4817. [Google Scholar] [CrossRef]
- Dang, Z.-M.; Yuan, J.-K.; Yao, S.-H.; Liao, R.-J. Flexible Nanodielectric Materials with High Permittivity for Power Energy Storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef]
- Li, B.; Randall, C.A.; Manias, E. Polarization mechanism underlying strongly enhanced dielectric permittivity in polymer composites with conductive fillers. J. Phys. Chem. C 2022, 126, 7596–7604. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alhassan, S.; Iraqi, A.; Saad, S.A.; Taha, T.A.M. Dielectric relaxation investigations of polyester/CoFe2O4 composites. J. Mater. Sci. Mater. Electron. 2023, 34, 2132. [Google Scholar] [CrossRef]
- Barman, S.; Paul, S.; Ranjan, P.; Das, S.; Datta, A. Emerging ferroelectricity and piezoelectric energy harvesting properties in lead-free zinc titanate nanocrystals. J. Mat. Sci. 2023, 58, 7060–7075. [Google Scholar] [CrossRef]
- Su, X.; Wang, R.; Li, X.; Araby, S.; Kuan, H.-C.; Naeem, M.; Ma, J. A comparative study of polymer nanocomposites containing multi-walled carbon nanotubes and graphene nanoplatelets. Nano Mater. Sci. 2022, 4, 185–204. [Google Scholar] [CrossRef]
- Schenk, V.; Labastie, K.; Destarac, M.; Guerre, M. Vitrimer composites: Current status and future challenges. Mater. Adv. 2022, 3, 8012–8029. [Google Scholar] [CrossRef]
- Ezzeddine, I.; Ilsouk, M.; Arous, M.; Atlas, S.; Lahcini, M.; Raihane, M. Molecular dynamics of poly(ε-caprolactone)/beidellite organoclay bionanocomposites obtained by in-situ polymerization highlighted by dielectric relaxation spectroscopy. Polym. Eng. Sci. 2024, 64, 3765–3779. [Google Scholar] [CrossRef]
- Jamal, E.M.A.; Joy, P.A.; Kurian, P.; Anantharaman, M.R. On the magnetic and dielectric properties of nickel–neoprene nanocomposites. Mater. Chem. Phys. 2010, 121, 154–160. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.G.; Speliotis, T.; Psarras, G.C. Evaluating the multifunctional performance of polymer matrix nanodielectrics incorporating magnetic nanoparticles: A comparative study. Polymer 2021, 236, 124311. [Google Scholar] [CrossRef]
- Romero-Fierro, D.; Bustamante-Torres, M.; Bucio, E. Polymer-Magnetic Semiconductor Nanocomposites for Industrial Electronic Applications. Polymers 2022, 14, 2467. [Google Scholar] [CrossRef] [PubMed]
- Vega-Garcia, A.; Marino-Castellanos, P.; Fernandez-Santiesteban, E.; Velazquez-Infante, J.; Guerrero, F.; Pena-Garcia, R. Structural, magnetic, and electrical properties of a SrFe12O19/PLA composite. Ceram. Int. 2023, 49, 38877–38884. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.G.; Speliotis, T.; Psarras, G.C. Probing the magnetoelectric response and energy efficiency in Fe3O4/epoxy nanocomposites. Polym. Test. 2020, 88, 106560. [Google Scholar] [CrossRef]
- Zhao, Y.; Dunnill, C.W.; Zhu, Y.; Gregory, D.H.; Kockenberger, W.; Li, Y.; Hu, W.; Ahmad, I.; McCartney, D.G. Low-temperature magnetic properties of hematite nanorods. Chem. Mater. 2007, 19, 916–921. [Google Scholar] [CrossRef]
- Díaz-Guerra, C.; Pérez, L.; Piqueras, J.; Chioncel, M.F. Magnetic transitions in—Fe2O3 nanowires. J. Appl. Phys. 2009, 106, 104302. [Google Scholar] [CrossRef]
- Xu, K.; Feng, J.S.; Liu, Z.P.; Xiang, H. Origin of Ferrimagnetism and Ferroelectricity in Room-Temperature Multiferroic ε-Fe2O3. J. Phys. Rev. Appl. 2018, 9, 044011. [Google Scholar] [CrossRef]
- Buscaglia, V.; Buscaglia, M.T.; Viviani, M.; Mitoseriu, L.; Nanni, P.; Trefiletti, V.; Piaggio, P.; Gregora, I.; Ostapchuk, T.; Pokorný, J.; et al. Grain size and grain boundary-related effects on the properties of nanocrystalline barium titanate ceramics. J. Eur. Ceram. Soc. 2006, 26, 2889–2898. [Google Scholar] [CrossRef]
- Mandal, T.K. Characterization of tetragonal BaTiO3 nanopowders prepared with a new soft chemistry route. Mater. Lett. 2007, 61, 850–854. [Google Scholar] [CrossRef]
- Ram, S.; Jana, A.; Kundu, T.K. Ferroelectric BaTiO3 phase of orthorhombic crystal structure contained in nanoparticles. J. Appl. Phys. 2007, 102, 054107. [Google Scholar] [CrossRef]
- Smith, M.B.; Page, K.; Siegrist, T.; Et, A. Crystal structure and the paraelectric-to-ferroelectric phase transition of nanoscale BaTiO3. J. Am. Chem. Soc. 2008, 130, 6955–6963. [Google Scholar] [CrossRef]
- Chávez, E.; Fuentes, S.; Zarate, R.A.; Padilla-Campos, L. Structural analysis of nanocrystalline BaTiO3. J. Mol. Struct. 2010, 984, 131–136. [Google Scholar] [CrossRef]
- Patsidis, A.C.; Psarras, G.C. Structural transition, dielectric properties and functionality in epoxy resin—Barium titanate nanocomposites. Smart Mater. Struct. 2013, 22, 115006. [Google Scholar] [CrossRef]
- Manika, G.C.; Andrikopoulos, K.S.; Psarras, G.C. On the Ferroelectric to Paraelectric Structural Transition of BaTiO3 Micro-/Nanoparticles and Their Epoxy Nanocomposites. Molecules 2020, 25, 2686. [Google Scholar] [CrossRef]
- Manika, G.C.; Psarras, G.C. Barium titanate/epoxy resin composite nanodielectrics as compact capacitive energy storing systems. Express Polym. Lett. 2019, 13, 749–758. [Google Scholar] [CrossRef]
- Drakopoulos, S.X.; Yang, J.; Vryonis, O.; Williams, L.; Psarras, G.C.; Mele, E. Flexible Polymer-Based Nanodielectrics Reinforced with Electrospun Composite Nanofibers for Capacitive Energy Storage. ACS Appl. Polym. Mater. 2022, 4, 8203–8215. [Google Scholar] [CrossRef]
- Patsidis, A.C.; Koufakis, E.I.; Mathioudakis, G.N.; Vryonis, O.; Psarras, G.C. Development, Dielectric Response, and Functionality of ZnTiO3/BaTiO3/Epoxy Resin Hybrid Nanocomposites. J. Compos. Sci. 2024, 8, 225. [Google Scholar] [CrossRef]
- Patsidis, A.; Psarras, G.C. Dielectric behaviour and functionality of polymer matrix—Ceramic BaTiO3 composites. Express Polym. Lett. 2008, 2, 718–726. [Google Scholar] [CrossRef]
- Patsidis, A.C.; Kalaitzidou, K.; Psarras, G.C. Dielectric response, functionality and energy storage in epoxy nanocomposites: Barium titanate vs exfoliated graphite nanoplatelets. Mater. Chem. Phys. 2012, 135, 798–805. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; ISBN 3527302743. [Google Scholar] [CrossRef]
- Goldman, A. Modern Ferrite Technology, 2nd ed.; Springer: Pittsburgh, PA, USA, 2005; ISBN 9780387294131. [Google Scholar]
- Özgür, Ü.; Alivov, Y.; Morkoç, H. Microwave ferrites, part 1: Fundamental properties. J. Mater. Sci. Mater. Electron. 2009, 20, 789–834. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.-Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Adam, J.D.; Davis, L.E.; Dionne, G.F.; Schloemann, E.F.; Stitzer, S.N. Ferrite devices and materials. IEEE Trans. Microw. Theory Tech. 2002, 50, 721–737. [Google Scholar] [CrossRef]
- Kalia, S.; Kango, S.; Kumar, A.; Haldorai, Y.; Kumari, B.; Kumar, R. Magnetic polymer nanocomposites for environmental and biomedical applications. Colloid Polym. Sci. 2014, 292, 2025–2052. [Google Scholar] [CrossRef]
- Yao, C.; Zeng, Q.; Goya, G.F.; Torres, T.; Liu, J.; Wu, H.; Ge, M.; Zeng, Y.; Wang, Y.; Jiang, J.Z. ZnFe2O4 nanocrystals: Synthesis and magnetic properties. J. Phys. Chem. C 2007, 111, 12274–12278. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Xue, J.M.; Chan, H.S.O.; Wang, J. Transparent magnetic composites of ZnFe2O4 nanoparticles in silica. J. Appl. Phys. 2001, 90, 4169–4174. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, Y.; Hahn, E.J. Size dependence of the magnetic properties in superparamagnetic zinc-ferrite nanoparticles. J. Korean Phys. Soc. 2008, 53, 2090–2094. [Google Scholar] [CrossRef]
- Wu, K.; Li, J.; Zhang, C. Zinc ferrite based gas sensors: A review. Ceram. Int. 2019, 45, 11143–11157. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.G.; Speliotis, T.; Psarras, G.C. Investigating the Effect of Zn Ferrite Nanoparticles on the Thermomechanical, Dielectric and Magnetic Properties of Polymer Nanocomposites. Materials 2019, 12, 3015. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Gerber, R.; Atkinson, R.; Simsa, Z. Magnetism and magneto-optics of hexaferrite layers. J. Magn. Magn Mater. 1997, 175, 79–89. [Google Scholar] [CrossRef]
- Swanson, H.E.; McMurdie, H.F.; Morris, M.C.; Evans, E.H. Standard X-Ray Diffraction Powder Patterns: NBS Monograph 25—Section 5; National Bureau of Standards Reports: Gaithersburg, MD, USA; Government Printing Office: Washington, DC, USA, 1967.
- Cheng, W.; Tang, K.; Qi, Y.; Sheng, J.; Liu, Z. One-step synthesis of superparamagnetic monodisperse porous Fe3O4 hollow and core-shell spheres. J. Mater. Chem. 2010, 20, 1799–1805. [Google Scholar] [CrossRef]
- Prateek; Thakur, V.K.; Gupta, R.K. Recent Progress on Ferroelectric Polymer-Based Nanocomposites for High Energy Density Capacitors: Synthesis, Dielectric Properties, and Future Aspects. Chem. Rev. 2016, 116, 4260–4317. [Google Scholar] [CrossRef]
- Li, Q.; Song, J.; Saura-Múzquiz, M.; Besenbacher, F.; Christensen, M.; Don, M. Magnetic Properties of Strontium Hexaferrite Nanostructures Measured with Magnetic Force Microscopy. Sci. Rep. 2016, 6, 25985. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, C.N.; Narayanasamy, A.; Ponpandian, N.; Chattopadhyay, K.; Guerault, H.; Greneche, J.-M. Magnetic properties of nanostructured ferrimagnetic zinc ferrite. J. Phys. Condens. Matter. 2000, 12, 7795–7805. [Google Scholar] [CrossRef]
- Chithra, M.; Anumol, C.N.; Sahu, B.; Sahoo, S.C. Magnetic properties of Zn-ferrite nanoparticles prepared by sol-gel and coprecipitation methods. Mater. Res. Express 2019, 6, 125059. [Google Scholar] [CrossRef]
- Sahoo, P.; Choudhary, P.; Laha, S.S.; Dixit, A.; Mefford, O.T. Recent advances in zinc ferrite (ZnFe2O4) based nanostructures for magnetic hyperthermia applications. Chem. Commun. 2023, 59, 12065. [Google Scholar] [CrossRef]
- Sanida, A.; Stavropoulos, S.; Speliotis, T.; Psarras, G.C. Magneto-Dielectric Behaviour of M-Type Hexaferrite/Polymer Nanocomposites. Materials 2018, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Ramajo, L.A.; Cristóbal, A.A.; Botta, P.M.; Porto López, J.M.; Reboredo, M.M.; Castro, M.S. Dielectric and magnetic response of Fe3O4/epoxy composites. Compos. Part A 2009, 40, 388–393. [Google Scholar] [CrossRef]
- Kaadhm, E.Q.; Salman, K.D.; Reja, A.H. Magnetic and Dielectric Properties of Epoxy Composites Reinforced with Hybrid Nanoparticle iron oxide (Fe3O4) and nickel (Ni). J. Phys. Conf. Ser. 2021, 1973, 012052. [Google Scholar] [CrossRef]
- Lotgerlng, F.K. The influence of Fe3+ ions at tetrahedral sites on the magnetic properties of ZnFe2O4. J. Chem. Solids 1966, 27, 139–145. [Google Scholar] [CrossRef]
- Blum, P. Physical Properties Handbook; Texas A & M University Press: College Station, TX, USA, 1997. [Google Scholar]
- Kletetschka, G.; Wasilewski, P.J.; Taylor, P.T. Hematite vs. magnetite as the signature for planetary magnetic anomalies? Phys. Earth Planet. Inter. 2000, 119, 259–267. [Google Scholar] [CrossRef]
- Ghasemi, A.; Morisako, A.; Liu, X. Magnetic properties of hexagonal strontium ferrite thick film synthesized by sol–gel processing using SrM nanoparticles. J. Magn. Magn. Mater. 2008, 320, 2300–2304. [Google Scholar] [CrossRef]
- Psarras, G.C. Fundamentals of dielectric theories. In Dielectric Polymer Materials for High-Density Energy Storage; Dang, Z.M., Ed.; Elsevier Inc.: Cambridge, MA, USA, 2018; pp. 11–57. [Google Scholar]

| Fe3O4/BaTiO3 | SrFe12O19/BaTiO3 | ZnFe2O4/BaTiO3 | |||
|---|---|---|---|---|---|
| Content (phr) | x (m3/kg) | Content (phr) | x (m3/kg) | Content (phr) | x (m3/kg) |
| Neat epoxy | - | Neat epoxy | - | Neat epoxy | - |
| 5/10 | 2.864 × 10−5 | 5/10 | 2.126 × 10−6 | 5/10 | 2.022 × 10−6 |
| 10/10 | 6.104 × 10−5 | 10/10 | 4.082 × 10−6 | 10/10 | 6.580 × 10−6 |
| 15/10 | 9.269 × 10−5 | 15/10 | 4.283 × 10−6 | 15/10 | 6.720 × 10−6 |
| 20/10 | 1.226 × 10−4 | 20/10 | 9.809 × 10−6 | 20/10 | 7.342 × 10−6 |
| 40/10 | 1.966 × 10−4 | 40/10 | 1.557 × 10−5 | 40/10 | 1.216 × 10−5 |
| 50/10 | 1.997 × 10−4 | 50/10 | 1.696 × 10−5 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patsidis, A.C.; Sanida, A.; Manika, G.C.; Gioti, S.; Mathioudakis, G.N.; Petropoulos, N.; Kanapitsas, A.; Tsonos, C.; Speliotis, T.; Psarras, G.C. Synthesis of Magnetic Nanoparticle/Polymer Matrix Nanocomposites with Induced Magnetic Performance. Polymers 2025, 17, 1913. https://doi.org/10.3390/polym17141913
Patsidis AC, Sanida A, Manika GC, Gioti S, Mathioudakis GN, Petropoulos N, Kanapitsas A, Tsonos C, Speliotis T, Psarras GC. Synthesis of Magnetic Nanoparticle/Polymer Matrix Nanocomposites with Induced Magnetic Performance. Polymers. 2025; 17(14):1913. https://doi.org/10.3390/polym17141913
Chicago/Turabian StylePatsidis, Anastasios C., Aikaterini Sanida, Georgia C. Manika, Sevasti Gioti, Georgios N. Mathioudakis, Nicholas Petropoulos, Athanasios Kanapitsas, Christos Tsonos, Thanassis Speliotis, and Georgios C. Psarras. 2025. "Synthesis of Magnetic Nanoparticle/Polymer Matrix Nanocomposites with Induced Magnetic Performance" Polymers 17, no. 14: 1913. https://doi.org/10.3390/polym17141913
APA StylePatsidis, A. C., Sanida, A., Manika, G. C., Gioti, S., Mathioudakis, G. N., Petropoulos, N., Kanapitsas, A., Tsonos, C., Speliotis, T., & Psarras, G. C. (2025). Synthesis of Magnetic Nanoparticle/Polymer Matrix Nanocomposites with Induced Magnetic Performance. Polymers, 17(14), 1913. https://doi.org/10.3390/polym17141913










