Starch Films Reinforced with Pistachio Shell Particles: A Sustainable Biocomposite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Reinforcer
2.3. Composite Preparation
2.4. Characterization Methods
3. Results and Discussion
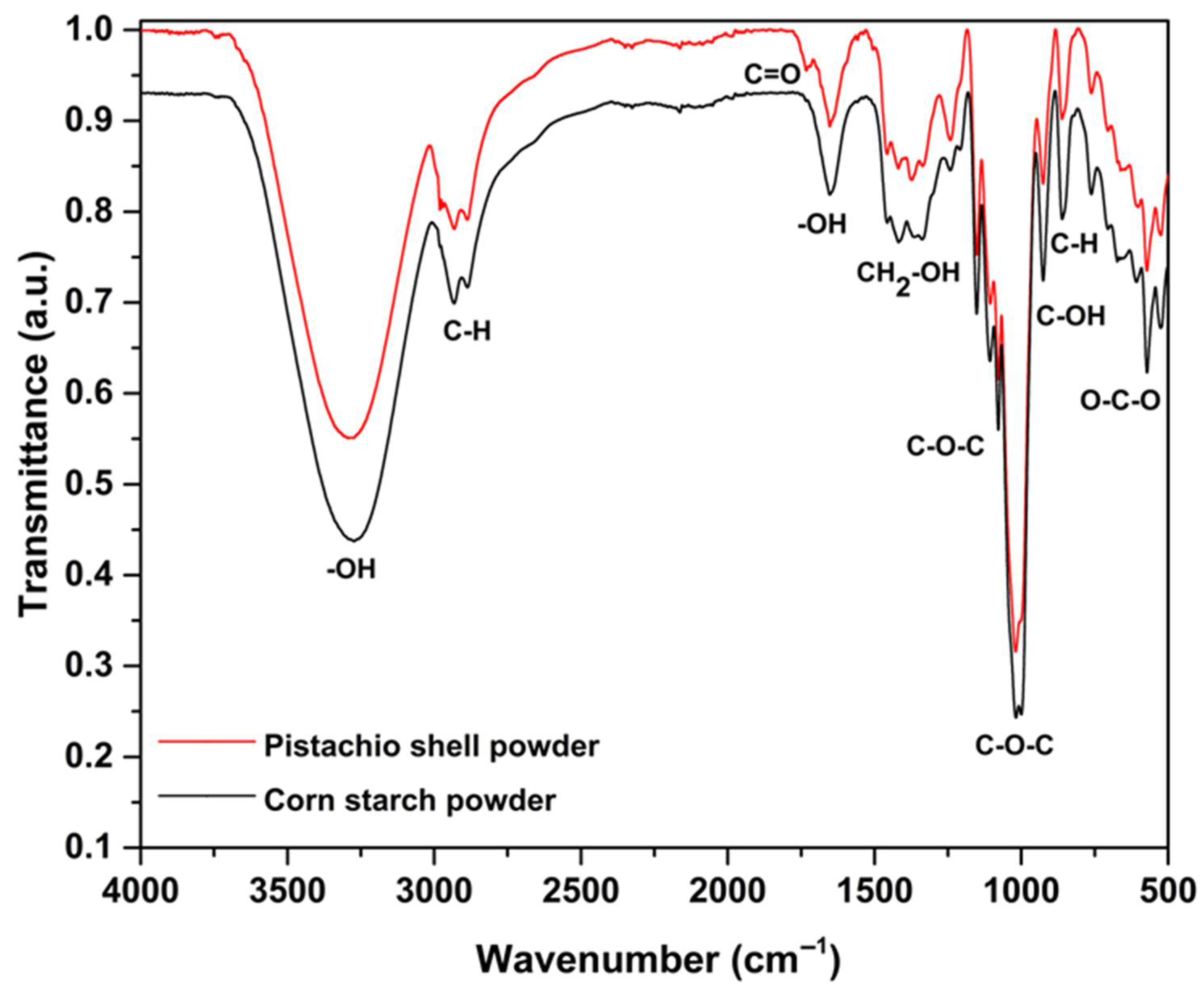
3.1. Infrared Spectroscopy (FTIR)
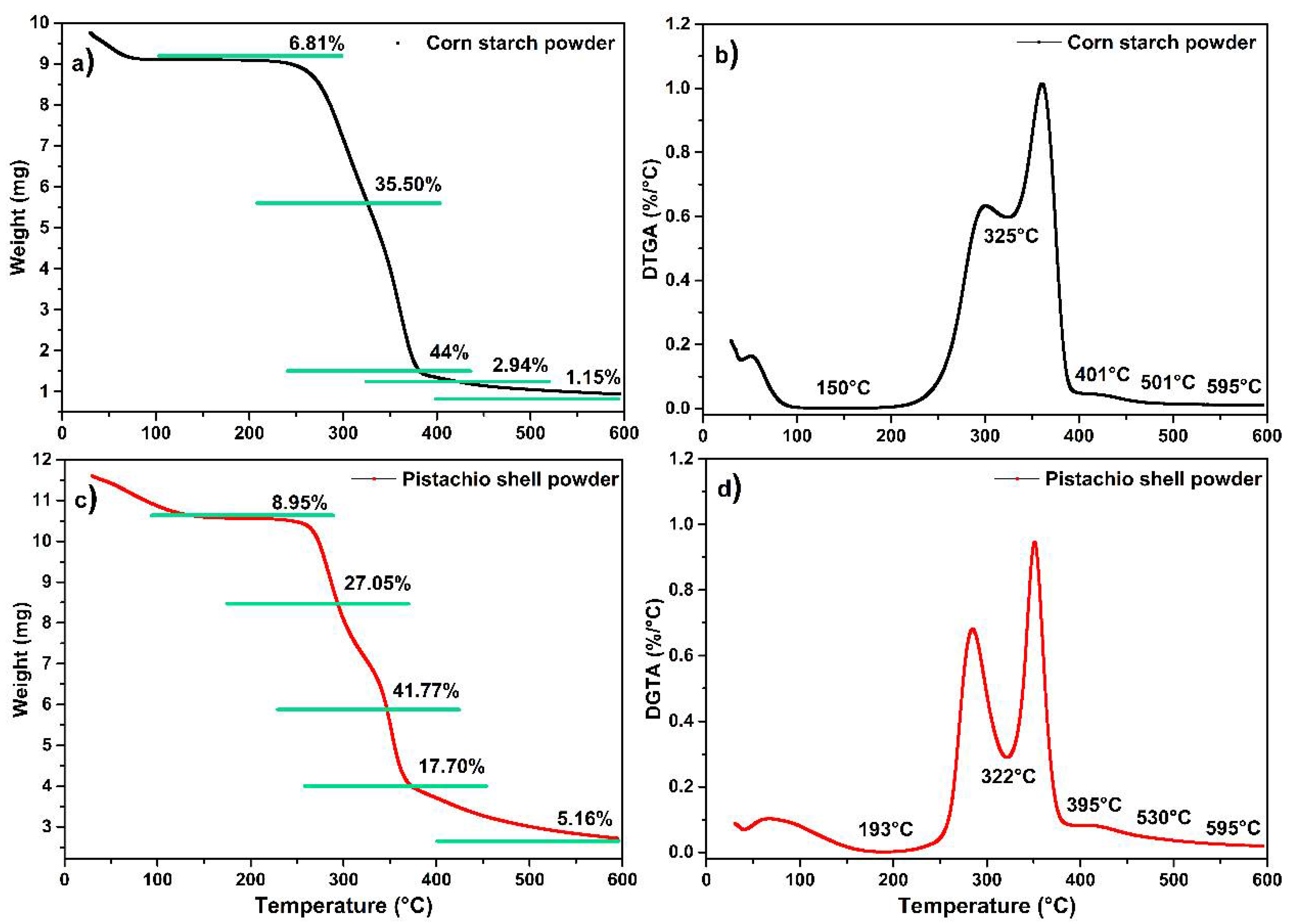
3.2. Thermogravimetric Analysis (TGA)
3.3. X-Ray Diffraction
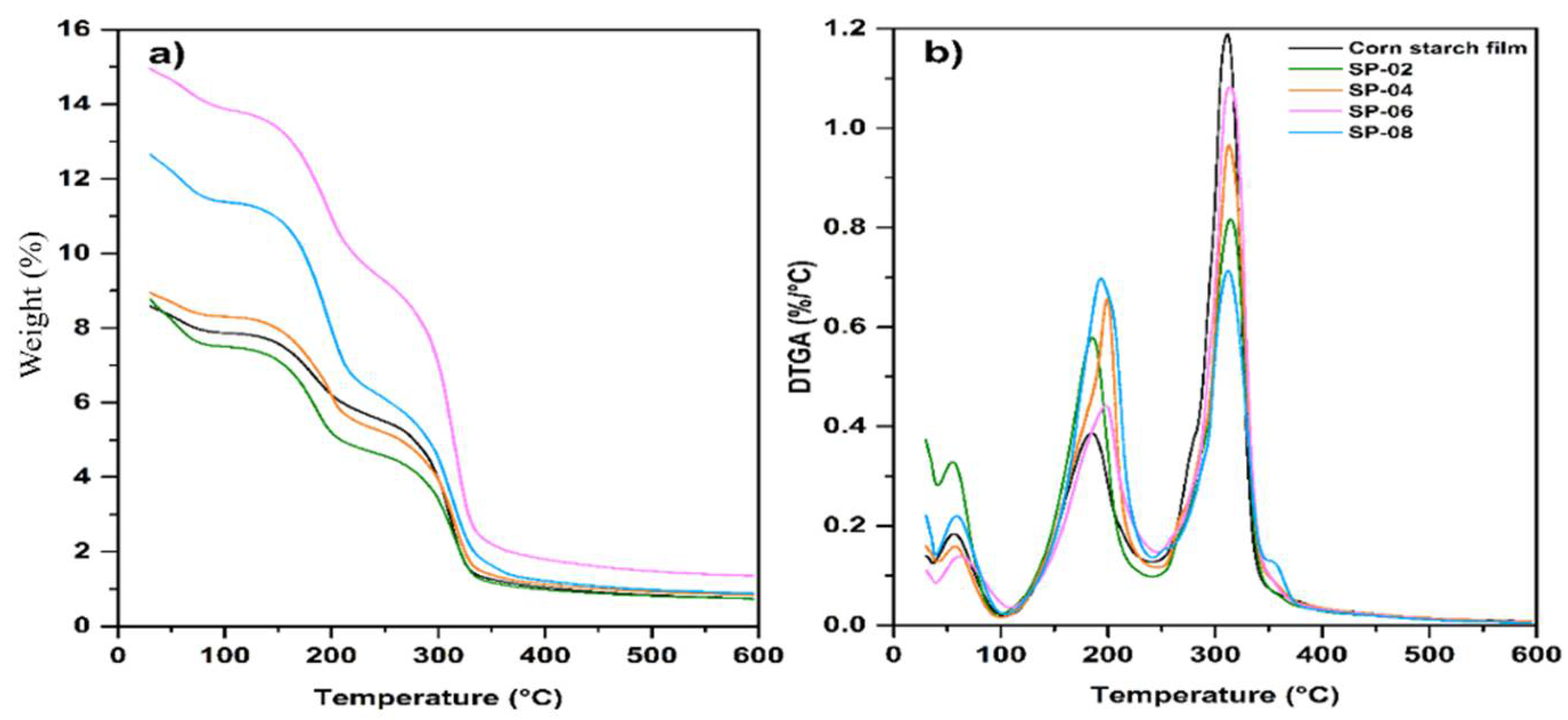
3.4. TGA of Composites
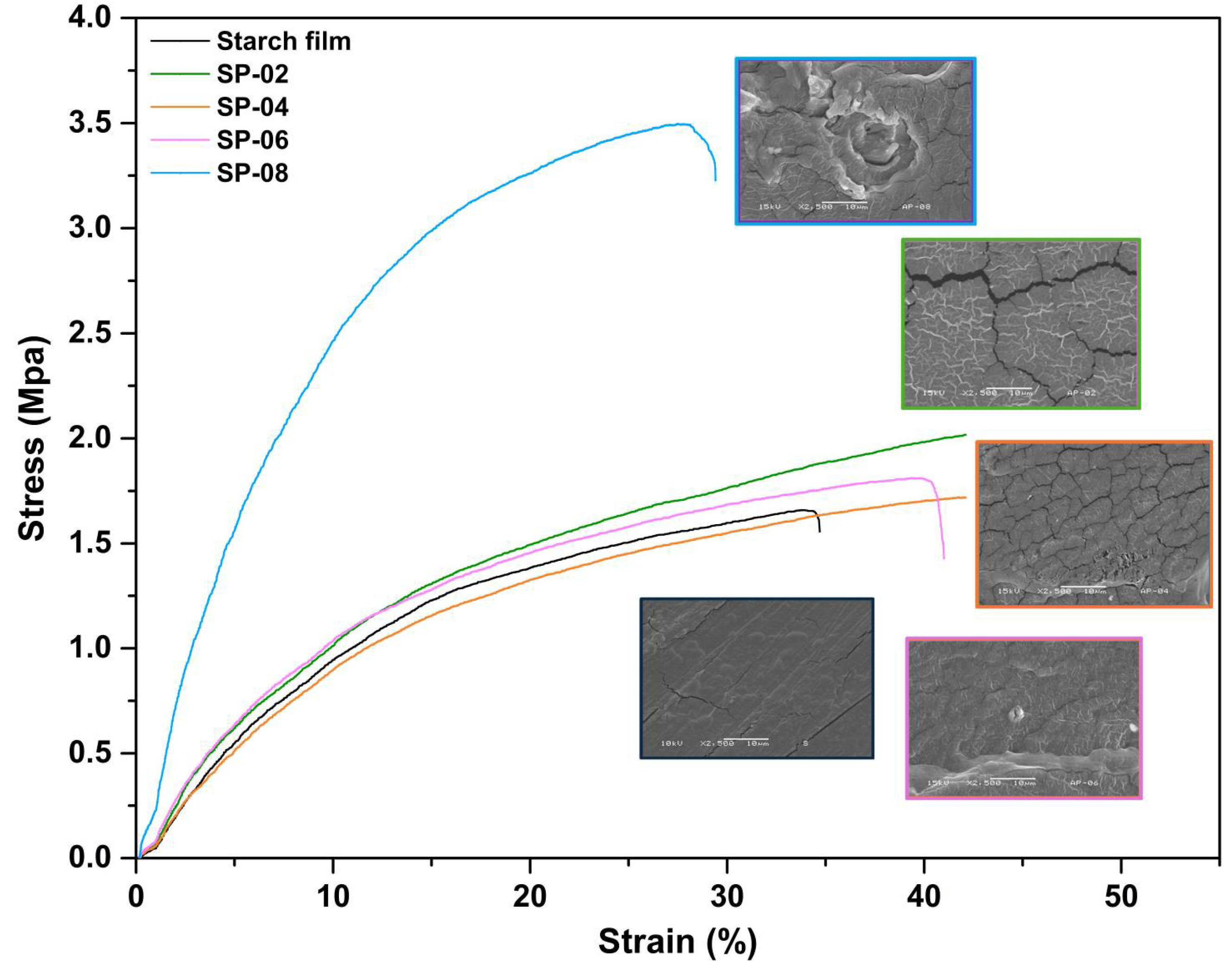
3.5. Tensile Test for Composites
3.6. Scanning Electron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortuño-López, M.B.; Salazar-Cruz, B.A.; del Real, A.; Almendarez-Camarillo, A.; Lopez-Barroso, J.; Rivera-Armenta, J.L.; Flores-Hernández, C.G. Physical Properties of Thermoplastic Cornstarch/Hibiscus sabdariffa Fiber Obtained by Evaporation Casting. Starch/Staerke 2023, 75, 2200242. [Google Scholar] [CrossRef]
- Flores-Hernández, C.G.; López-Barroso, J.; Salazar-Cruz, B.A.; Saucedo-Rivalcoba, V.; Almendarez-Camarillo, A.; Rivera-Armenta, J.L. Evaluation of Starch–Garlic Husk Polymeric Composites through Mechanical, Thermal, and Thermo-Mechanical Tests. Polymers 2024, 16, 289. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Brahmakumar, M.; Abraham, T.E. Microstructural imaging and characterization of the mechanical, chemical, thermal, and swelling properties of starch-chitosan blend films. Biopolymers 2006, 82, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Schutz, G.F.; de Ávila Gonçalves, S.; Alves, R.M.V.; Vieira, R.P. A review of starch-based biocomposites reinforced with plant fibers. Int. J. Biol. Macromol. 2024, 261, 129916. [Google Scholar] [CrossRef]
- Tacha, S.; Somord, K.; Rattanawongkun, P.; Intatha, U.; Tawichai, N.; Soykeabkaew, N. Bio-nanocomposite foams of starch reinforced with bacterial nanocellulose fibers. Mater. Today Proc. 2023, 75, 119–123. [Google Scholar] [CrossRef]
- Flores-Hernandez, C.G.; Martinez-Hernandez, A.L.; Colin-Cruz, A.; Martínez-Bustos, F.; Castaño, V.M.; Olivas-Armendariz, I.; Almendarez-Camarillo, A.; Velasco-Santos, C. Starch Modified with Chitosan and Reinforced with Feather Keratin Materials Produced by Extrusion Process: An Alternative to Starch Polymers. Starch/Staerke 2018, 70, 1700295. [Google Scholar] [CrossRef]
- Omar, F.N.; Hafid, H.S.; Zhu, J.; Bahrin, E.K.; Nadzri, F.Z.M.; Wakisaka, M. Starch-based composite film reinforcement with modified cellulose from bamboo for sustainable packaging application. Mater. Today Commun. 2022, 33, 104392. [Google Scholar] [CrossRef]
- Salazar-Cruz, B.A.; Chávez-Cinco, M.Y.; Morales-Cepeda, A.B.; Ramos-Galván, C.E.; Rivera-Armenta, J.L. Evaluation of Thermal Properties of Composites Prepared from Pistachio Shell Particles Treated Chemically and Polypropylene. Molecules 2022, 27, 426. [Google Scholar] [CrossRef]
- Babapour, H.; Jalali, H.; Nafchi, A.M.; Jokar, M. Effects of active packaging based on potato starch/nano zinc oxide/fennel (Foeniculum vulgare miller) essential oil on fresh pistachio during cold storage. J. Nuts 2022, 13, 105–123. [Google Scholar]
- Harrazi, N.; Özbek, H.N.; Yanık, D.K.; Zaghbib, I.; Göğüş, F. Development and characterization of gelatin-based biodegradable films incorporated with pistachio shell hemicellulose. J. Food Sci. Technol. 2024, 61, 1919–1929. [Google Scholar] [CrossRef]
- Sharayei, P.; Niazmand, R.; Sarabi Jamab, M.; Sabeghi, Y.; Einafshar, S.; Azarpazhooh, E.; Sharif, V.; Sharif, F. Sustainable Bioactive Films from Saffron Corm and Corn Husk: A Transformative Solution for Antioxidant and Antimicrobial Pistachio Packaging. LWT Food Sci. Technol. 2025, 228, 118097. [Google Scholar] [CrossRef]
- Raj, V.A.; Sankar, K.; Narayanasamy, P.; Moorthy, I.G.; Sivakumar, N.; Rajaram, S.K.; Karuppiah, P.; Shaik, M.R.; Alwarthan, A.; Oh, T.H.; et al. Development and characterization of Bio-based Composite films for Food Packing Applications using boiled Rice Water and Pistacia vera Shells. Polymers 2023, 15, 3456. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ceron, Z.; Flores-Silva, P.C.; Saucedo-Salazar, E.; Gallardo-Vega, C.; Hernandez-Gamez, F.; Hernandez-Hernandez, E.; Sifuentes-Nieves, I. Modified pistachio shell waste: Structure, chemical interaction, and viscoelastic performance in biopolymer composites-based starch. Polym. Compos. 2025, 46, 8025–8036. [Google Scholar] [CrossRef]
- Kepekci, R.A.; Şekeroğlu, G.; Alhveis, I. Development of bioactive and environmentally friendly chitosan-based film using waste of pistachio dehulling process as a novel promising food packaging material. Int. J. Biol. Macromol. 2024, 272, 132866. [Google Scholar] [CrossRef] [PubMed]
- Taye, A.; Kalita, U.; Dutta, P.P.; Haloi, D. From Waste to Wrap: Pistachio Shell Powder–Reinforced Chitosan Biocomposite for Eco-Friendly Packaging. Pack. Technol. Sci. 2025; early view. [Google Scholar]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films- A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Johar, N.; Ahmad, I. Starch biocomposite film reinforced by multiscale rice husk fiber. Compos. Sci. Technol. 2017, 151, 147–155. [Google Scholar] [CrossRef]
- Marett, J.; Aning, A.; Foster, E.J. The isolation of cellulose nanocrystals from pistachio shells via acid hydrolysis. Ind. Crops Prod. 2017, 109, 869–874. [Google Scholar] [CrossRef]
- Kasiri, N.; Fathi, M. Production of cellulose nanocrystals from pistachio shells and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2018, 106, 1023–1031. [Google Scholar] [CrossRef]
- Robles, E.; Izaguirre, N.; Martin, A.; Moschou, D.; Labidi, J. Assessment of Bleached and Unbleached Nanofibers from Pistachio Shells for Nanopaper Making. Molecules 2021, 26, 1371. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Vafakish, B.; Patel, R.; Falua, K.J.; Dunlop, M.J.; Acharya, B. Cellulose nanocrystals in the development of biodegradable materials: A review on CNC resources, modification, and their hybridization. Int. J. Biol. Macromol. 2024, 258, 128834. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Water barrier properties of starch films reinforced with cellulose nanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Netravali, A.N. High-performance green nanocomposites using aligned bacterial cellulose and soy protein. Compos. Sci. Technol. 2017, 146, 183–190. [Google Scholar] [CrossRef]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Noreen, A.; Zuber, M. Polysaccharide based bionanocomposites, properties and applications: A review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef]
- de Azevedo, A.R.G.; Cruz, A.S.A.; Marvila, M.T.; de Oliveria, L.B.; Monteiro, S.N.; Vieira, C.M.F.; Fediuk, R.; Timokhin, R.; Vatin, N.; Daironas, M. Natural Fibers as an Alternative to Synthetic Fibers in Reinforcement of Geopolymer Matrices: A Comparative Review. Polymers 2021, 13, 2493. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernández, C.G.; Colín-Cruz, A.; Velasco-Santos, C.; Castaño, V.M.; Rivera-Armenta, J.L.; Almendarez Camarillo, A.; García-Casillas, P.E. All green composites from fully renewable biopolymers: Chitosan-Starch reinforced with Keratin from feathers. Polymers 2014, 6, 686–705. [Google Scholar] [CrossRef]
- Rumi, S.S.; Liyanage, S.; Abidi, N. Conversion of low-quality cotton to bioplastics. Cellulose 2021, 28, 2021–2038. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, S.; Stephen, R. Nanocellulose extracted from pistachio nut shells as a template for nanosilver synthesis and its antimicrobial activity. Cellulose 2024, 31, 293–308. [Google Scholar] [CrossRef]
- Ghanbari, A.; Tabarsa, T.; Ashori, A.; Shakeri, A.; Mashkour, M. Preparation and characterization of thermoplastic starch and cellulose nanofibers as green nanocomposites: Extrusion processing. Int. J. Biol. Macromol. 2018, 112, 442–447. [Google Scholar] [CrossRef]
- Vârban, R.; Crișan, I.; Vârban, D.; Ona, A.; Olar, L.; Stoie, A.; Stefan, R. Comparative FT-IR Prospecting for Cellulose in Stems of Some Fiber Plants: Flax, Velvet Leaf, Hemp and Jute. Appl. Sci. 2021, 11, 8570. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & Sons: West Sussex, UK, 2004. [Google Scholar]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H. FTIR spectral band shifts explained by OM–cation interactions. J. Plant Nutrit. Soil Sci. 2021, 184, 388–397. [Google Scholar] [CrossRef]
- Kian, L.K.; Saba, N.; Jawaid, M.; Alothman, O.Y.; Fouad, H. Properties and characteristics of nanocrystalline cellulose isolated from olive fiber. Carbohyd. Polym. 2020, 241, 116423. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.M.; Popescu, M.C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral characterization of eucalyptus wood. Appl. Spectros. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; D’Alessio, A.; Licursi, D.; Antonetti, C.; Valentini, G.; Galia, A.; Nassi o Di Nasso, N. Midinfrared FT-IR as a Tool for Monitoring Herbaceous Biomass Composition and Its Conversion to Furfural. J. Spectros. 2015, 2015, 719042. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Barbosa, L.S.; Ramos, I.G.; Coelho, R.E.; Druzian, J.I. Characterization of amylose and amylopectin fractions separated from potato, banana, corn, and cassava starches. Int. J. Biol. Macromol. 2019, 132, 32–42. [Google Scholar] [CrossRef]
- Faleeva, Y.M.; Lavrenov, V.A.; Zaichenko, V.M. Investigation of plant biomass two-stage pyrolysis based on three major components: Cellulose, hemicellulose, and lignin. Biomass Convers. Biorefinery 2024, 14, 14519–14529. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, H.; Ban, X.; Li, C.; Gu, Z.; Li, Z. Rice noodle quality is structurally driven by the synergistic effect between amylose chain length and amylopectin unit-chain ratio. Carbohydr. Polym. 2022, 295, 119834. [Google Scholar] [CrossRef]
- Farrag, Y.; Malmir, S.; Montero, B.; Rico, M.; Rodríguez-Llamazares, S.; Barral, L.; Bouza, R. Starch edible films loaded with donut-shaped starch microparticles. LWT 2018, 98, 62–68. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibbi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Aladpoosh, R.; Montazer, M. In Situ Nanoassembly of Mg-Al Layered Double Hydroxide on Polyester Fabric Surface: Mechanism, Tunable Wettability, and Boosted Thermal Features. Ind. Eng. Chem. Res. 2019, 58, 16532–16540. [Google Scholar] [CrossRef]
- Wei, D.; Lv, S.; Liu, J.; Zuo, J.; Mu, Y.; Liu, L.; He, T.; Zeng, Q. Esterification modified corn starch composite with chitosan and PVP for enhanced mechanical properties and antimicrobial freshness preservation of starch-based films. React. Funct. Polym. 2024, 202, 105991. [Google Scholar] [CrossRef]
- Jebalia, I.; Kristiawan, M.; Charalambides, M.N.; Humphry-Baker, S.; Valle, G.D.; Guessasma, S. Microstructure and local mechanical properties of pea starch/protein composites. Compos. Part C Open Access 2022, 8, 100272. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Cai, J. Robust design of starch composite nanofibrous films for active food packaging: Towards improved mechanical, antioxidant, and antibacterial properties. Int. J. Biol. Macromol. 2024, 260, 129329. [Google Scholar] [CrossRef]



| Percentage of PSP Particles (% wt) | Composites Code |
|---|---|
| 0 | Starch |
| 2 | SP02 |
| 4 | SP04 |
| 6 | SP06 |
| 8 | SP08 |
| Starch Powder | Pistachio Shell Powder | ||
|---|---|---|---|
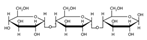 | 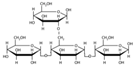 |  |  |
| Amylose | amylopectin | Cellulose | hemicellulose |
| 3262 | -OH stretching of hydroxy group [31,32] | 3280 | -OH stretching of hydroxy group a,b and stretching from intermolecular hydrogen bonds [31] |
| 2948 | C-H asymmetric stretching of CH2-OH [31,32] | 2950 | C-H asymmetric stretching of CH2-OH [31,32] |
| 2930 | C-H symmetric stretching of CH2-OH [31] | 2929 | C-H symmetric stretching of CH2-OH [31] |
| 2885 | C-H symmetric stretching [31] | 2886 | C-H symmetric stretching [31] |
| 1733 | C=O stretching carboxylic acid [31] | ||
| 1647 | -OH angular bending | 1652 | -OH bending and carboxylate anions [33] |
| 1434 | -CH2 deformation of CH2-OH [31] | 1456 | CH2 deformation of CH2-OH [31] |
| 1415 | C-H symmetric stretching of CH2-OH [31] | 1419 | C-H symmetric stretching of CH2-OH [31] |
| 1368 | β-glycosidic linkage and asymmetric C-H deformation [34] | ||
| 1337 | C-OH in-plane bending of α-anomer [32] | 1334 | C-OH in-plane bending of β-anomer [31] |
| 1221 | C-O bending of α-anomer [32] | 1241 | CH2 twisting of CH2-OH [31] |
| 1149 | C-O-C stretching in α-1,4 glycosidic linkage [32] | 1152 | C-O-C stretching in β-1,4 glycosidic linkage |
| 1087 | C-O deformation in secondary alcohols and aliphatic ethers [35] | ||
| 1052 | C-O-C in α 1,6 glycosidic linkage | 1058 | C-O-C β-1,3 glycosidic linkage [36] |
| 1019 | C-O and C-C stretching bonds in the C–6 position [36] | ||
| 1004 | C-O-C in-plane bending of α 1,4 glycosidic linkage [32] | ||
| 996 | C-O stretching in COC α 1,4 glycosidic linkage [32] | ||
| 927 | C-OH in plane bending [31] | 926 | C-OH in plane bending [31] |
| 860 | C-H deformation pyranose ring [31] | 862 | C-H deformation in pyranose ring [31] |
| 762 | Symmetric breathing of the pyranose ring [31,32] | 760 | Symmetric breathing of the pyranose ring [31,32] |
| 707 | -OH out-of-plane bending [32] | 684 | -OH out-of-plane bending [32] |
| 586 | OCO in-plane bending [32] | 584 | OCO in plane bending [32] |
| 524 | COC in-plane bending in glycosidic linkage and skeletal modes [32] | 523 | COC in-plane bending in glycosidic linkage, skeletal modes [32] |
| Glycerol 200 °C | Hemicellulose 268 °C | Amylose 306 °C | Amylopectin 364 °C | Cellulose 353 °C | |
|---|---|---|---|---|---|
| Starch film | 200 °C | 282 °C | 318 °C | ||
| SP02 | 189 °C | 264 °C | 286 °C | 317 °C | |
| SP04 | 201 °C | 267 °C | 288 °C | 315 °C | |
| SP06 | 201 °C | 260 °C | 279 °C | 316 °C | |
| SP08 | 195 °C | 253 °C | 279 °C | 314 °C | 350 °C |
| Material Code | Elong (%) | Standard Deviation | Elastic Modulus [MPa] | Standard Deviation | Tension (MPa) | Standard Deviation |
|---|---|---|---|---|---|---|
| Starch | 33.61 | 0.54 | 20.73 | 7.23 | 1.66 | 0.84 |
| SP02 | 53.22 | 6.55 | 34.38 | 7.63 | 2.42 | 0.22 |
| SP04 | 40.2 | 4.90 | 20.3 | 3.67 | 1.5 | 0.28 |
| SP06 | 41.19 | 3.57 | 22.13 | 2.16 | 1.64 | 0.06 |
| SP08 | 27.04 | 2.43 | 87.5 | 2.78 | 3.39 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Hernandez, C.G.; Del Real, A.; Cornejo-Villegas, M.d.l.Á.; Millán-Malo, B.; Fonseca-Hernández, G.A.; Rivera-Armenta, J.L. Starch Films Reinforced with Pistachio Shell Particles: A Sustainable Biocomposite. Polymers 2025, 17, 1907. https://doi.org/10.3390/polym17141907
Flores-Hernandez CG, Del Real A, Cornejo-Villegas MdlÁ, Millán-Malo B, Fonseca-Hernández GA, Rivera-Armenta JL. Starch Films Reinforced with Pistachio Shell Particles: A Sustainable Biocomposite. Polymers. 2025; 17(14):1907. https://doi.org/10.3390/polym17141907
Chicago/Turabian StyleFlores-Hernandez, Cynthia G., Alicia Del Real, María de los Ángeles Cornejo-Villegas, Beatriz Millán-Malo, Gerardo A. Fonseca-Hernández, and José Luis Rivera-Armenta. 2025. "Starch Films Reinforced with Pistachio Shell Particles: A Sustainable Biocomposite" Polymers 17, no. 14: 1907. https://doi.org/10.3390/polym17141907
APA StyleFlores-Hernandez, C. G., Del Real, A., Cornejo-Villegas, M. d. l. Á., Millán-Malo, B., Fonseca-Hernández, G. A., & Rivera-Armenta, J. L. (2025). Starch Films Reinforced with Pistachio Shell Particles: A Sustainable Biocomposite. Polymers, 17(14), 1907. https://doi.org/10.3390/polym17141907











