Effect of Oxidation and Silane Modifications Applied to the Bonded Material and Fibers in Carbon-Fiber-Reinforced Composite Adhesive Joints
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
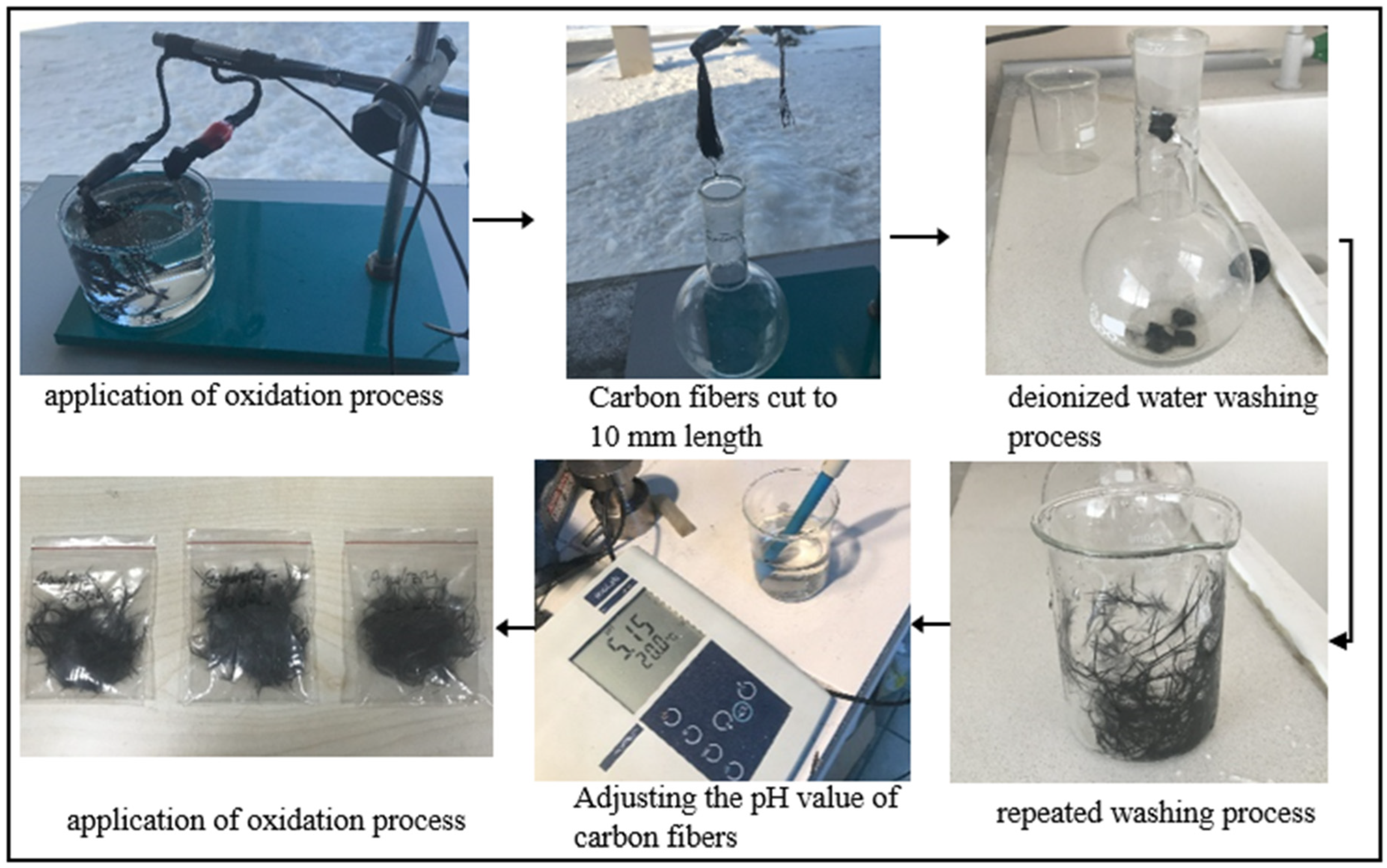
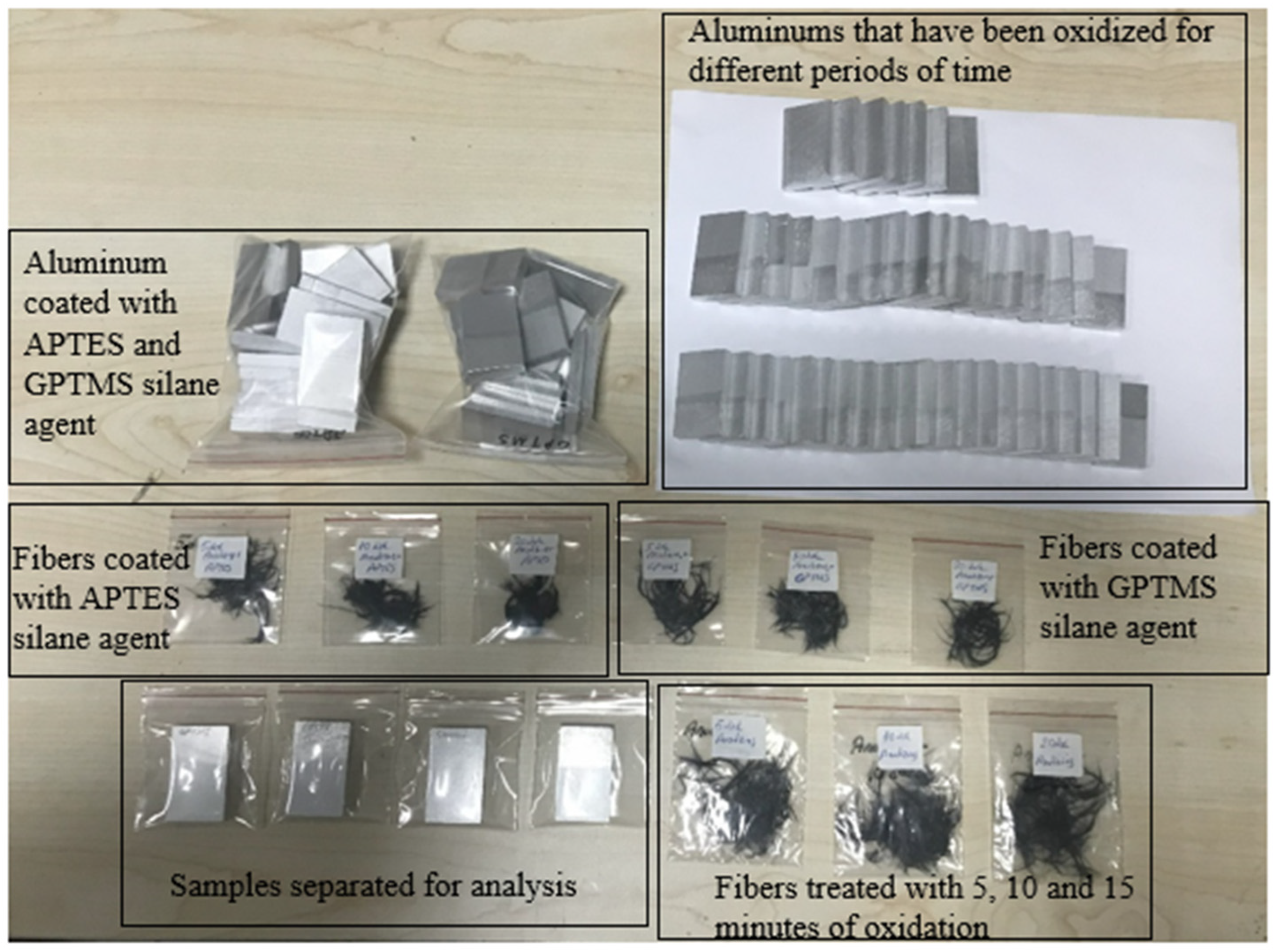
2.2. Application of Chemical Surface Treatments
2.3. Characterization of Surface-Treated Carbon Fibers and Aluminum Parts
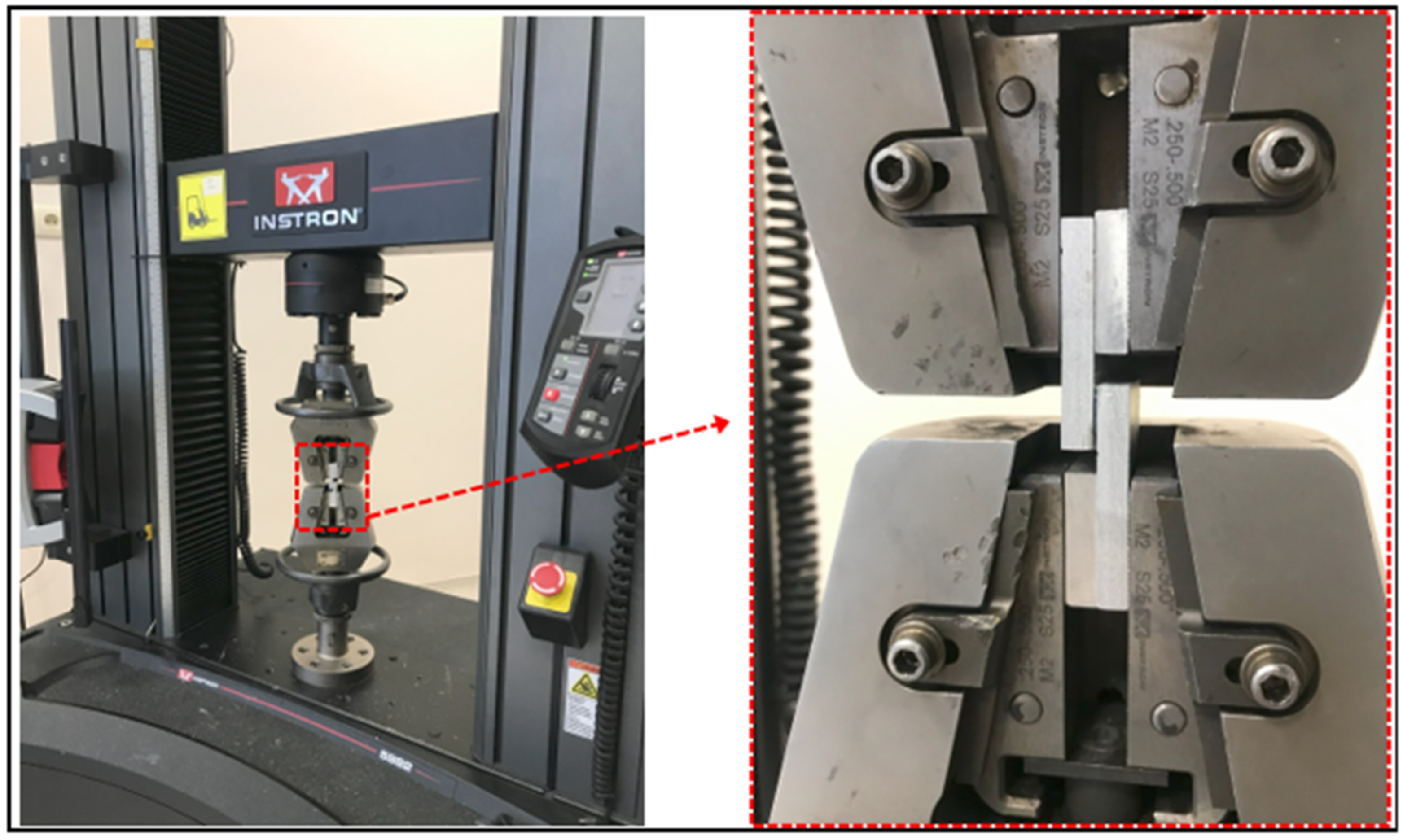
2.4. Single-Lap Joint Production
2.5. Mechanical Properties of Sing-Lap Joints
3. Results and Discussion
3.1. Surface Morphologies of Carbon Fibers
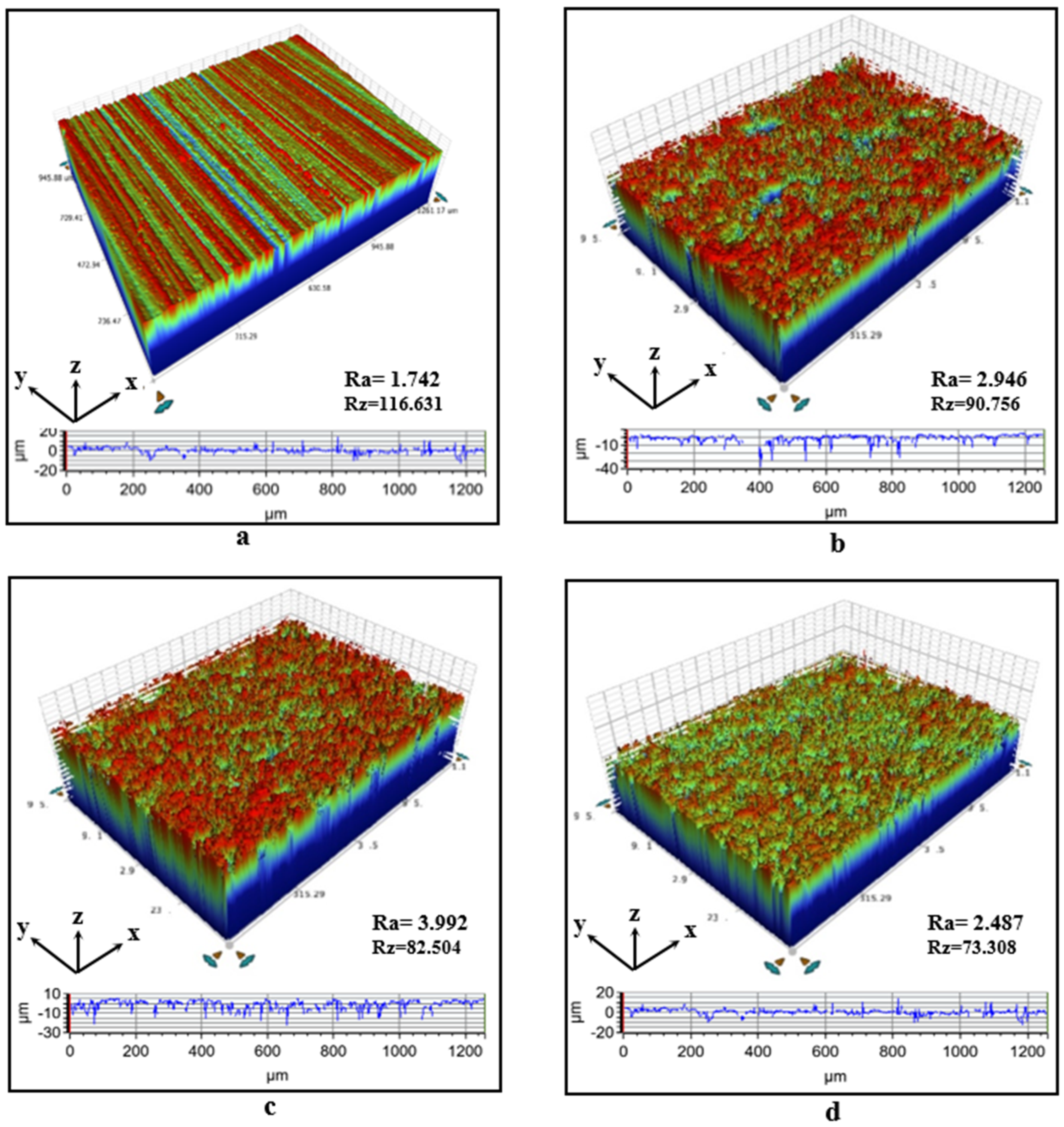
3.2. Surface Roughness
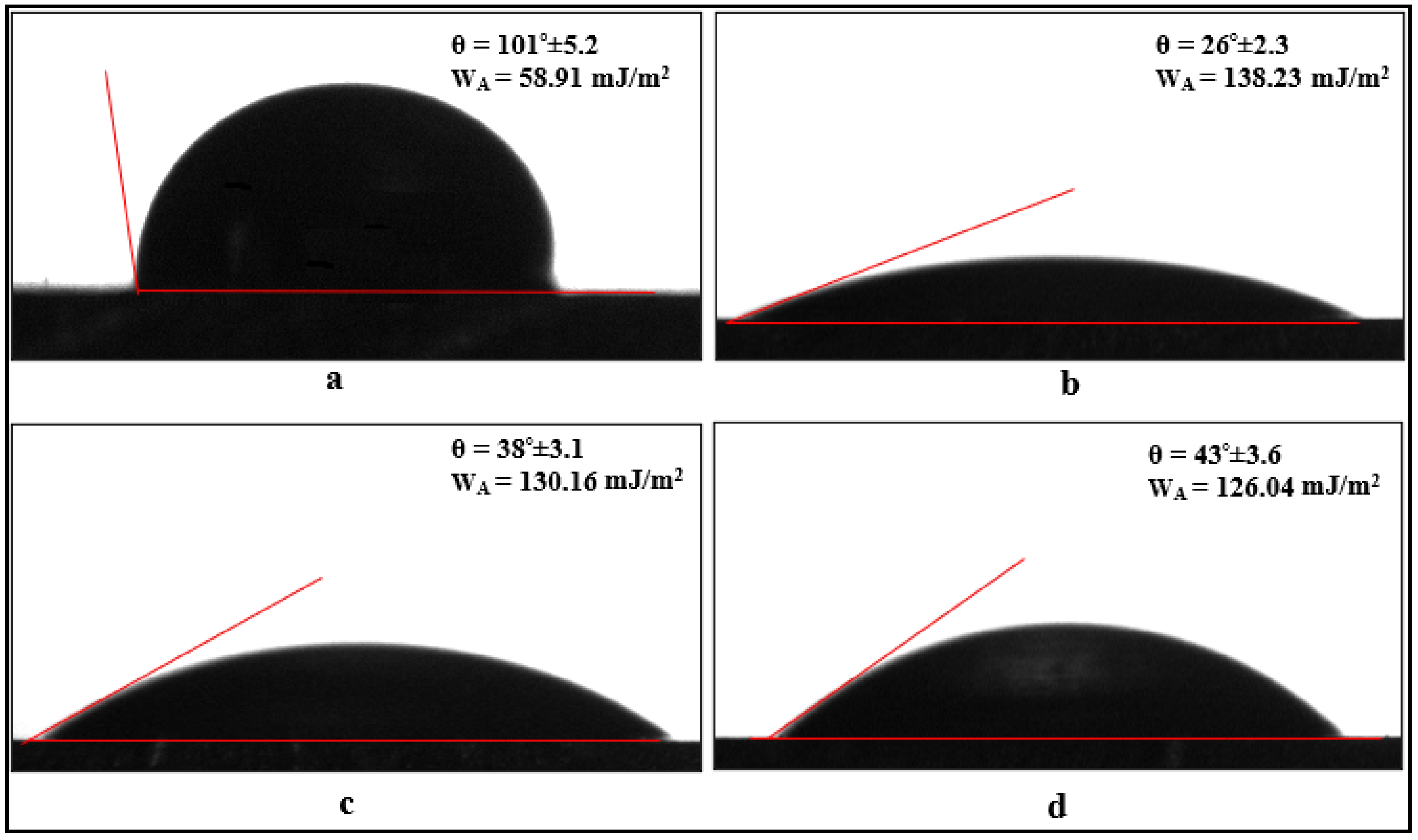
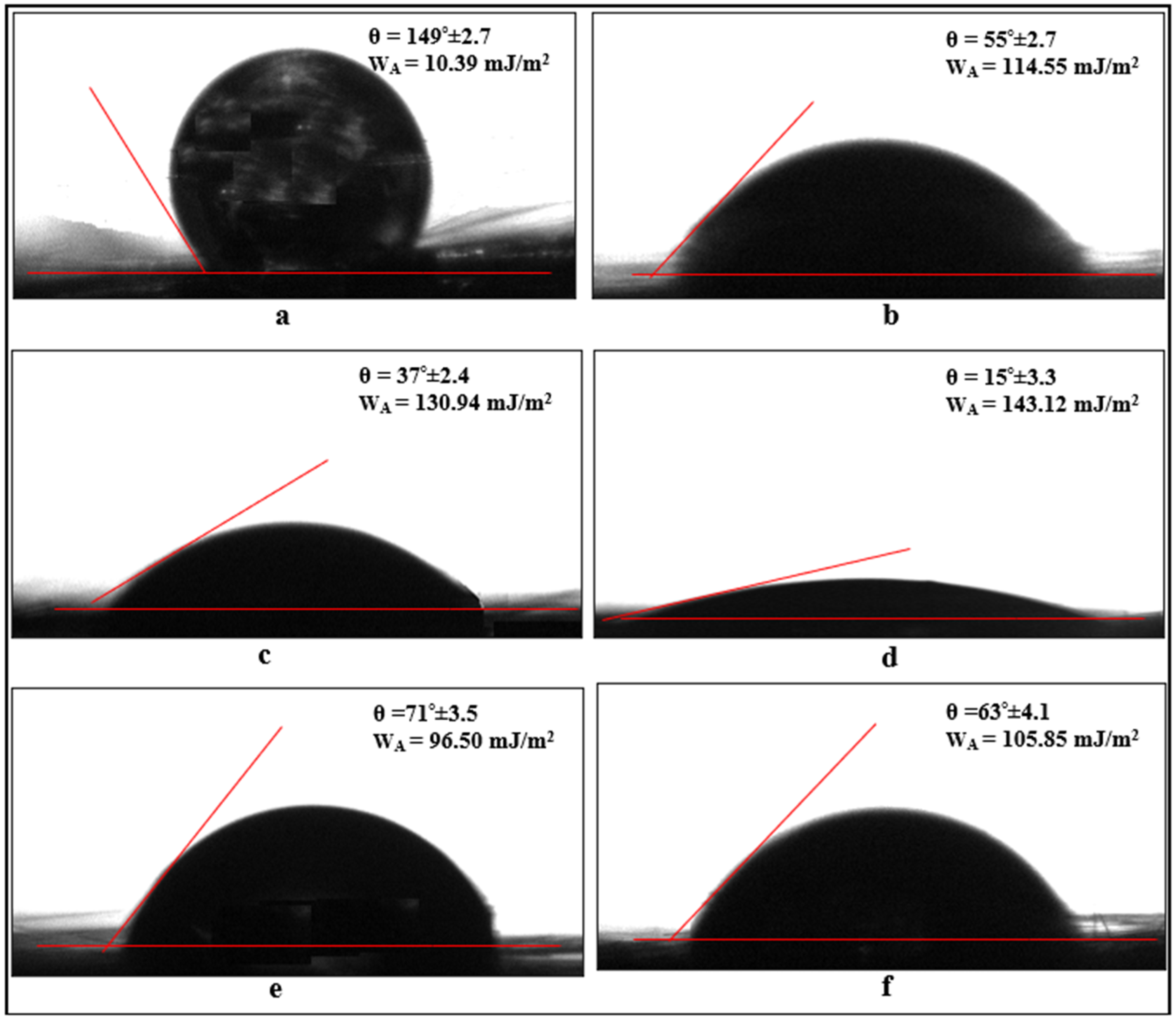
3.3. Contact Angle Measurements
3.4. Mechanical Strengths of Single-Lap Joints
4. Conclusions
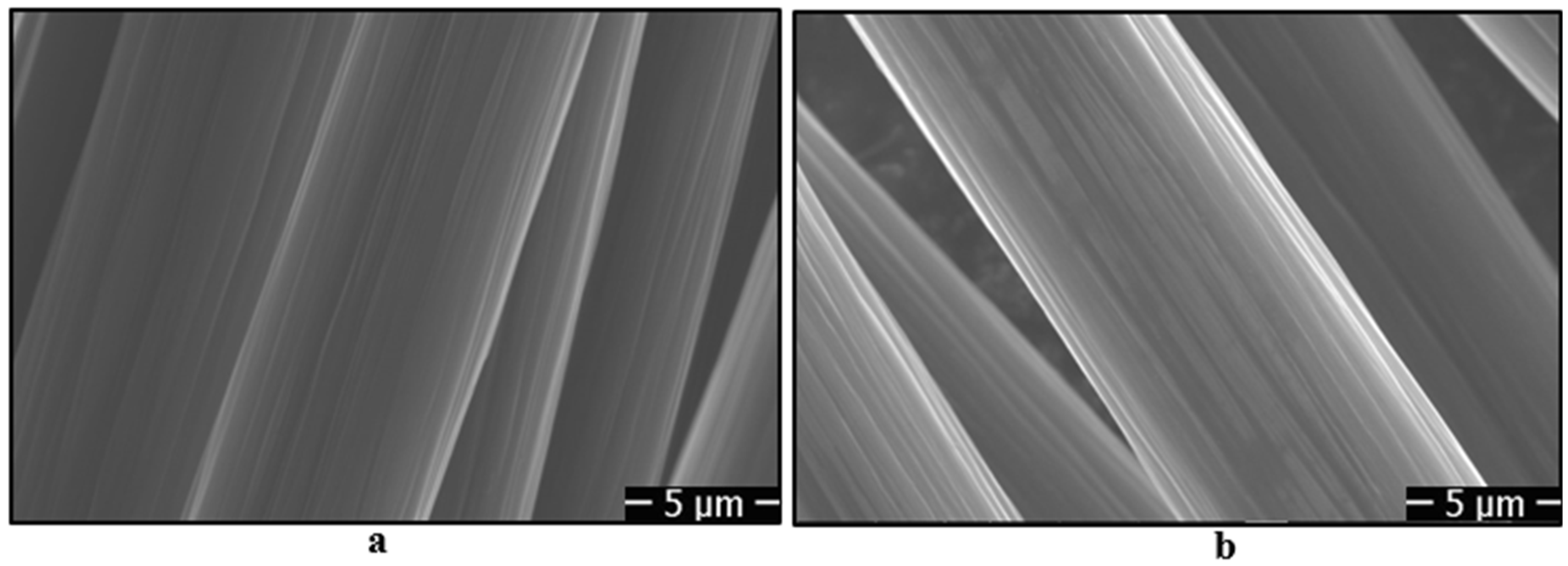
- When the carbon fibers were cleaned with acetone, longitudinal linear micro-grooves were observed on the surface of the fibers. However, when oxidation treatments were applied for different durations, the micro-grooves on the fiber surface were reduced, as observed from SEM images. Additionally, when silanization treatments were applied to the fiber surfaces, a thin film layer formed by the silane agents was observed on the surface.
- The application of oxidation and silanization treatments to the aluminum surfaces increased the surface energy of the aluminum, as revealed by contact angle tests. The adhesion work obtained using contact angle tests showed that the adhesion work of surfaces subjected to oxidation treatment increased by approximately 135%.
- The contact angle on the surface of carbon fibers without chemical surface treatment is 149°. However, when electrochemical oxidation is applied to the fibers for 5, 10, and 20 min, the hydroxyl and carboxyl (-OH and -COOH) groups formed on the fiber surface reduce the contact angle values to 55°, 37°, and 15°, respectively. This indicates that oxidation treatment increases the wettability (hydrophilicity) of the fiber surface, and the wettability changes depending on the oxidation duration. However, when the fiber surface is coated with APTES and GPTMS agents, the contact angle values increase to an average of 71° and 63°, respectively, balancing the surface wettability form with the silane agents.
- The effect of applying oxidation treatments for 5, 10, and 20 min to carbon fibers added to the adhesive results in an increase in the failure strength of the connection by 4% to 10%. However, in addition to the oxidation treatments, coating the fibers with APTES increases this failure strength by approximately 20%, while GPTMS coating increases it by 16%.
- Compared to the failure strength obtained from the basic adhesive connection type without fibers and without chemical surface treatments, the application of oxidation treatments to the aluminum and carbon fiber surfaces, in addition to coating with APTES silane agent, increases the failure strength of the connection by approximately 66.8% to 74.2%. When GPTMS is used as the silane agent; this increase ranges from 61.2% to 66.1%.
- When the aluminum parts’ surface is cleaned only with a chemical degreasing process, adhesive failure occurs at the joint, while the oxidation treatment of the aluminum parts’ surface results in cohesive failure in all joints. These damage modes demonstrate that the oxidation treatment alters the surface energy of the aluminum, improving epoxy adhesion and increasing mechanical interlocking. Additionally, the surface damage modes observed after the test are in strong agreement with the failure strengths obtained from the tensile tests of the joints.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heidary, H.; Karimi, N.Z.; Minak, G. Investigation on delamination and flexural properties in drilling of carbon nanotube/polymer composites. Compos. Struct. 2018, 201, 112–120. [Google Scholar] [CrossRef]
- Banea, M.D.; de Queiroz, H.F.M. Structural Adhesives; Wiley: Hoboken, NJ, USA, 2023; pp. 375–396. [Google Scholar]
- Akhavan-Safar, A.; Bozchaloei, G.E.; Jalali, S.; Beygi, R.; Ayatollahi, M.R.; da Silva, L.F.M. Impact Fatigue Life of Adhesively Bonded Composite-Steel Joints Enhanced with the Bi-Adhesive Technique. Materials 2023, 16, 419. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.M.; Noormohammed, S.; Sarkar, D.K.; Chen, X.-G. Polymer–Aluminum Lightweight Multi-Material Joints Bonded with Mixed Adhesive. Eng. Proc. 2023, 43, 29. [Google Scholar]
- Borghei, R.; Behjat, H.; Yazdani, B. The Impact of Graphene Nanoparticle Additives on the Strength of Simple and Hybrid Adhesively Bonded Joints. J. Compos. Mater. 2019, 53, 3335–3346. [Google Scholar] [CrossRef]
- Hadjez, F.; Necib, B. Experimental Characterization and Numerical Modelling Analyses of Nano-Adhesive-Bonded Joints. Frat. Integrita Strutt. 2018, 12, 94–105. [Google Scholar] [CrossRef]
- Akpinar, S.; Akpinar, I.A. Effect of Nanostructured Reinforcement of Adhesive on Thermal Cycling Performance of a Single-Lap Joint with Composite Adherends. Compos. Part B Eng. 2019, 175, 107106. [Google Scholar] [CrossRef]
- Razavi, S.M.J.; Ayatollahi, M.; Giv, R.A.N.; Khoramishad, H. Single Lap Joints Bonded with Structural Adhesives Reinforced with a Mixture of Silica Nanoparticles and Multi-Walled Carbon Nanotubes. Int. J. Adhes. Adhes. 2018, 80, 76–86. [Google Scholar] [CrossRef]
- Marami, G.; Nazari, S.A.; Faghidian, S.A.; Tahami, F.V. Improving the Mechanical Behavior of the Adhesively Bonded Joints Using RGO Additive. Int. J. Adhes. Adhes. 2016, 70, 277–286. [Google Scholar] [CrossRef]
- Simaafrookhteh, S.; Tsokanas, P.; Loutas, T.; Lomov, S.V.; Ivens, J. Measuring the Interlaminar Fracture Toughness of Thin Carbon Fiber/Polyamide6 Composites Using Adhesively Bonded Stiffeners. Compos. Part A Appl. Sci. Manuf. 2024, 176, 107841. [Google Scholar] [CrossRef]
- Ladani, R.B.; Wu, S.; Kinloch, A.J.; Ghorbani, K.; Mouritz, A.P.; Wang, C.H. Enhancing Fatigue Resistance and Damage Characterisation in Adhesively-Bonded Composite Joints by Carbon Nanofibres. Compos. Sci. Technol. 2017, 149, 116–126. [Google Scholar] [CrossRef]
- Mrzljak, S.; Zanghellini, B.; Gerdes, L.; Helwing, R.; Schuller, R.; Sinn, G.; Rennhofer, H. Effect of Carbon Nanofibre Orientation on Fatigue Properties of Carbon Fibre-Reinforced Polymers. J. Compos. Mater. 2023, 57, 1149–1164. [Google Scholar] [CrossRef]
- Huang, Y.; Kinloch, I.A.; Vallés, C. Static and Dynamic Mechanical Behavior of Carbon Fiber Reinforced Plastic (CFRP) Single-Lap Shear Joints Joule-Bonded with Conductive Epoxy Nanocomposites. J. Compos. Sci. 2024, 8, 112. [Google Scholar] [CrossRef]
- Andideh, M.; Esfandeh, M. Effect of Surface Modification of Electrochemically Oxidized Carbon Fibers by Grafting Hydroxyl and Amine Functionalized Hyperbranched Polyurethanes on Interlaminar Shear Strength of Epoxy Composites. Carbon 2017, 123, 233–242. [Google Scholar] [CrossRef]
- Kiss, P.; Glinz, J.; Stadlbauer, W.; Burgstaller, C.; Archodoulaki, V.M. The Effect of Thermally Desized Carbon Fibre Reinforcement on the Flexural and Impact Properties of PA6, PPS and PEEK Composite Laminates: A Comparative Study. Compos. Part B: Eng. 2021, 215, 108844. [Google Scholar] [CrossRef]
- Xiao, J.; Tian, S.Y.; Zhou, H.; Wang, Y.; Xu, L.H. Andic Oxidation of High-Modulus Carbon Fibers in Sulfuric Acid Under Different Electric Fields. Cailiao Gongcheng-J. Mater. Eng. 2024, 52, 122–131. [Google Scholar]
- Adwani, S.; Elsubeihi, E.; Zebari, A.; Aljanahi, M.; Moharamzadeh, K.; Elbishari, H. Effect of Different Silane Coupling Agents on the Bond Strength between Hydrogen Peroxide-Etched Epoxy-Based- Fiber-Reinforced Post and Composite Resin Core. Dent. J. 2023, 11, 142. [Google Scholar] [CrossRef]
- Roseno, S.; Ammarullah, M.I.; Rohman, S.; Kurniawati, F.; Wahyudi, T.; Wargadipura, A.H.S.; Juwono, A.L. The Effects of Carbon Fiber Surface Treatment by Oxidation Process for Enhanced Mechanical Properties of Carbon Fiber/Epoxy Composites for Biomedical Application. AIP Adv. 2024, 14, 015044. [Google Scholar] [CrossRef]
- Liu, J.; Tian, Y.; Chen, Y.; Liang, J. Interfacial and Mechanical Properties of Carbon Fibers Modified by Electrochemical Oxidation in (NH4HCO3)/(NH4)2C2O4·H2O Aqueous Compound Solution. Appl. Surf. Sci. 2010, 256, 6199–6204. [Google Scholar] [CrossRef]
- Du, H.; Xian, G.; Tian, J.; Ma, Z.; Li, C.; Xin, M.; Zhang, Y. Effect of fiber surface treatment with silane coupling agents and carbon nanotubes on mechanical properties of carbon fiber reinforced polyamide 6 composites. Polym. Compos. 2025, 46, 1267–1283. [Google Scholar] [CrossRef]
- Wen, Z.; Xu, C.; Qian, X.; Zhang, Y.; Wang, X.; Song, S.; Zhang, C. A Two-Step Carbon Fiber Surface Treatment and Its Effect on the Interfacial Properties of CF/EP Composites: The Electrochemical Oxidation Followed by Grafting of Silane Coupling Agent. Appl. Surf. Sci. 2019, 486, 546–554. [Google Scholar] [CrossRef]
- Wu, X.; Zhan, L.; Zhao, X.; Wang, X.; Chang, T. Effects of Surface Pre-Treatment and Adhesive Quantity on Interface Characteristics of Fiber Metal Laminates. Compos. Interfaces 2020, 27, 829–843. [Google Scholar] [CrossRef]
- Jing, C.; Kunpeng, D.; Xiumin, C.; Youbing, L.; Jin, H.; Yutao, W.; Chaolong, Y.; Xiaochao, X. Influence of Surface Microstructure on Bonding Strength of Modified Polypropylene/Aluminum Alloy Direct Adhesion. Appl. Surf. Sci. 2019, 489, 392–402. [Google Scholar]
- Akpinar, A. The Effect of Chemical Etching and Nanostructure Additive Epoxy Coating Technique on Adhesion Strength in Aluminum Joints Bonded with Nanostructure Additive Adhesive. Int. J. Adhes. Adhes. 2024, 129, 103584. [Google Scholar] [CrossRef]
- Yiwei, X.; Huaguan, L.; Yizhou, S.; Senyun, L.; Wentao, W.; Jie, T. Improvement of Adhesion Performance Between Aluminum Alloy Sheet and Epoxy Based on Anodizing Technique. Int. J. Adhes. Adhes. 2016, 70, 74–80. [Google Scholar]
- Baldan, A. Adhesion Phenomena in Bonded Joints. Int. J. Adhes. Adhes. 2012, 38, 95–116. [Google Scholar] [CrossRef]
- Gultekin, K.; Akpinar, S.; Gürses, A.; Eroglu, Z.; Cam, S.; Akbulut, H.; Keskin, Z.; Ozel, A. The effects of graphene nanostructure reinforcement on the adhesive method and the graphene reinforcement ratio on the failure load in adhesively bonded joints. Compos. Part B 2016, 67, 170–178. [Google Scholar] [CrossRef]
- Akpinar, S.; Ozel, A. Experimental and numerical determination of the thermal cycle performance of joints obtained with nanostructure-doped nanocomposite adhesives. Compos. Part B Eng. 2019, 174, 106959. [Google Scholar] [CrossRef]
- Avinc Akpinar, I.; Gultekin, K.; Akpinar, S.; Akbulut, H.; Ozel, A. Research on strength of nanocomposite adhesively bonded composite joints. Compos. Part B Eng. 2017, 126, 143–152. [Google Scholar] [CrossRef]
- Akpinar, I.A.; Gürses, A.; Akpinar, S.; Gültekin, K.; Akbulut, H.; Ozel, A. Investigation of Mechanical and Thermal Properties of Nanostructure-Doped Bulk Nanocomposite Adhesives. J. Adhes. 2018, 94, 847–866. [Google Scholar] [CrossRef]
- ISO 10365; Adhesives-Designation of Main Failure Patterns. ISO: Geneva, Switzerland, 1992.







| Carbon Fiber | AA2024-T3 | DP 460 | |
|---|---|---|---|
| σt (MPa) | 3760±380 | 458±13 | 37.2±0.9 |
| εt (%) | 1.85 | 16.2 | 4.5 |
| ν | - | 0.32 | 0.38 |
| E (MPa) | 226,000±2700 | 71,050±535 | 1963±57 |
| Element | Cu | Mg | Mn | Fe | Zn | Si | Ti | Others | Al |
|---|---|---|---|---|---|---|---|---|---|
| % by weight | 4.48 | 1.57 | 0.58 | 0.17 | 0.16 | 0.06 | 0.03 | ≤0.04 | Remainder |
| Sample Code | Aluminum Surface Treatment | Fiber Surface Treatment | Fiber Ratio (%) |
|---|---|---|---|
| Ae | chemical etching | - | - |
| Ao | oxidation | - | - |
| AoCF | oxidation | cleaned with acetone | 2 |
| AoCFo/5 | oxidation | 5 min oxidation | 2 |
| AoCFo/10 | oxidation | 10 min oxidation | 2 |
| AoCFo/20 | oxidation | 20 min oxidation | 2 |
| Ao/ACFo/5 | oxidation and APTES | 5 min oxidation | 2 |
| Ao/ACFo/10 | oxidation and APTES | 10 min oxidation | 2 |
| Ao/ACFo/20 | oxidation and APTES | 20 min oxidation | 2 |
| Ao/GCFo/5 | oxidation and GPTMS | 5 min oxidation | 2 |
| Ao/GCFo/10 | oxidation and GPTMS | 10 min oxidation | 2 |
| Ao/GCFo/20 | oxidation and GPTMS | 20 min oxidation | 2 |
| AoCFo/5A | oxidation | 5 min oxidation and APTES | 2 |
| AoCFo/10A | oxidation | 10 min oxidation and APTES | 2 |
| AoCFo/20A | oxidation | 20 min oxidation and APTES | 2 |
| AoCFo/5G | oxidation | 5 min oxidation and GPTMS | 2 |
| AoCFo/10G | oxidation | 10 min oxidation and GPTMS | 2 |
| AoCFo/20G | oxidation | 20 min oxidation and GPTMS | 2 |
| Ao/ACFo/5A | oxidation and APTES | 5 min oxidation and APTES | 2 |
| Ao/ACFo/10A | oxidation and APTES | 10 min oxidation and APTES | 2 |
| Ao/ACFo/20A | oxidation and APTES | 20 min oxidation and APTES | 2 |
| Ao/GCFo/5G | oxidation and GPTMS | 5 min oxidation and GPTMS | 2 |
| Ao/GCFo/10G | oxidation and GPTMS | 10 min oxidation and GPTMS | 2 |
| Ao/GCFo/20G | oxidation and GPTMS | 20 min oxidation and GPTMS | 2 |
| Sample Code | Average Failure Strenght (MPa) | % Increase Rate |
|---|---|---|
| Ae | 21.07 ± 0.43 | |
| Ao | 24.11 ± 0.59 | 14.4 |
| AoCF | 27.54 ± 0.87 | 30.7 |
| AoCFo/5 | 28.38 ± 0.60 | 34.7 |
| AoCFo/10 | 29.35 ± 0.46 | 38.3 |
| AoCFo/20 | 29.54 ± 0.66 | 40.2 |
| Ao/ACFo/5 | 30.17 ± 0.67 | 43.2 |
| Ao/ACFo/10 | 30.99 ± 0.61 | 47.1 |
| Ao/ACFo/20 | 31.50 ± 0.56 | 49.5 |
| Ao/GCFo/5 | 29.77 ± 0.33 | 41.3 |
| Ao/GCFo/10 | 30.45 ± 0.51 | 44.6 |
| Ao/GCFo/20 | 30.70 ± 0.71 | 45.7 |
| AoCFo/5A | 32.91 ± 0.65 | 56.2 |
| AoCFo/10A | 33.40 ± 0.63 | 58.5 |
| AoCFo/20A | 33.56 ± 0.61 | 59.3 |
| AoCFo/5G | 31.98 ± 0.53 | 51.8 |
| AoCFo/10G | 32.68 ± 0.50 | 55.1 |
| AoCFo/20G | 33.06 ± 0.67 | 56.9 |
| Ao/ACFo/5A | 35.15 ± 0.74 | 66.8 |
| Ao/ACFo/10A | 36.71 ± 0.66 | 74.2 |
| Ao/ACFo/20A | 35.92 ± 0.59 | 70.5 |
| Ao/GCFo/5G | 33.97 ± 0.55 | 61.2 |
| Ao/GCFo/10G | 34.42 ± 0.69 | 63.4 |
| Ao/GCFo/20G | 34.99 ± 0.61 | 66.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akpinar, I.A.; Koçyiğit, Ö.F.; Atasoy, S. Effect of Oxidation and Silane Modifications Applied to the Bonded Material and Fibers in Carbon-Fiber-Reinforced Composite Adhesive Joints. Polymers 2025, 17, 1893. https://doi.org/10.3390/polym17141893
Akpinar IA, Koçyiğit ÖF, Atasoy S. Effect of Oxidation and Silane Modifications Applied to the Bonded Material and Fibers in Carbon-Fiber-Reinforced Composite Adhesive Joints. Polymers. 2025; 17(14):1893. https://doi.org/10.3390/polym17141893
Chicago/Turabian StyleAkpinar, Iclal Avinc, Ömer Faruk Koçyiğit, and Selcuk Atasoy. 2025. "Effect of Oxidation and Silane Modifications Applied to the Bonded Material and Fibers in Carbon-Fiber-Reinforced Composite Adhesive Joints" Polymers 17, no. 14: 1893. https://doi.org/10.3390/polym17141893
APA StyleAkpinar, I. A., Koçyiğit, Ö. F., & Atasoy, S. (2025). Effect of Oxidation and Silane Modifications Applied to the Bonded Material and Fibers in Carbon-Fiber-Reinforced Composite Adhesive Joints. Polymers, 17(14), 1893. https://doi.org/10.3390/polym17141893







