Strong Amphoteric Adsorption of Reactive Red-141 onto Modified Orange Peel Derivatives: Optimization, Characterization, and Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Usage in the Adsorption Process
2.2. Preparation of ROP, NOOP, and CE
2.3. Characterization of ROP, NOOP, and CE
2.4. Adsorption and Desorption Experiments
3. Results
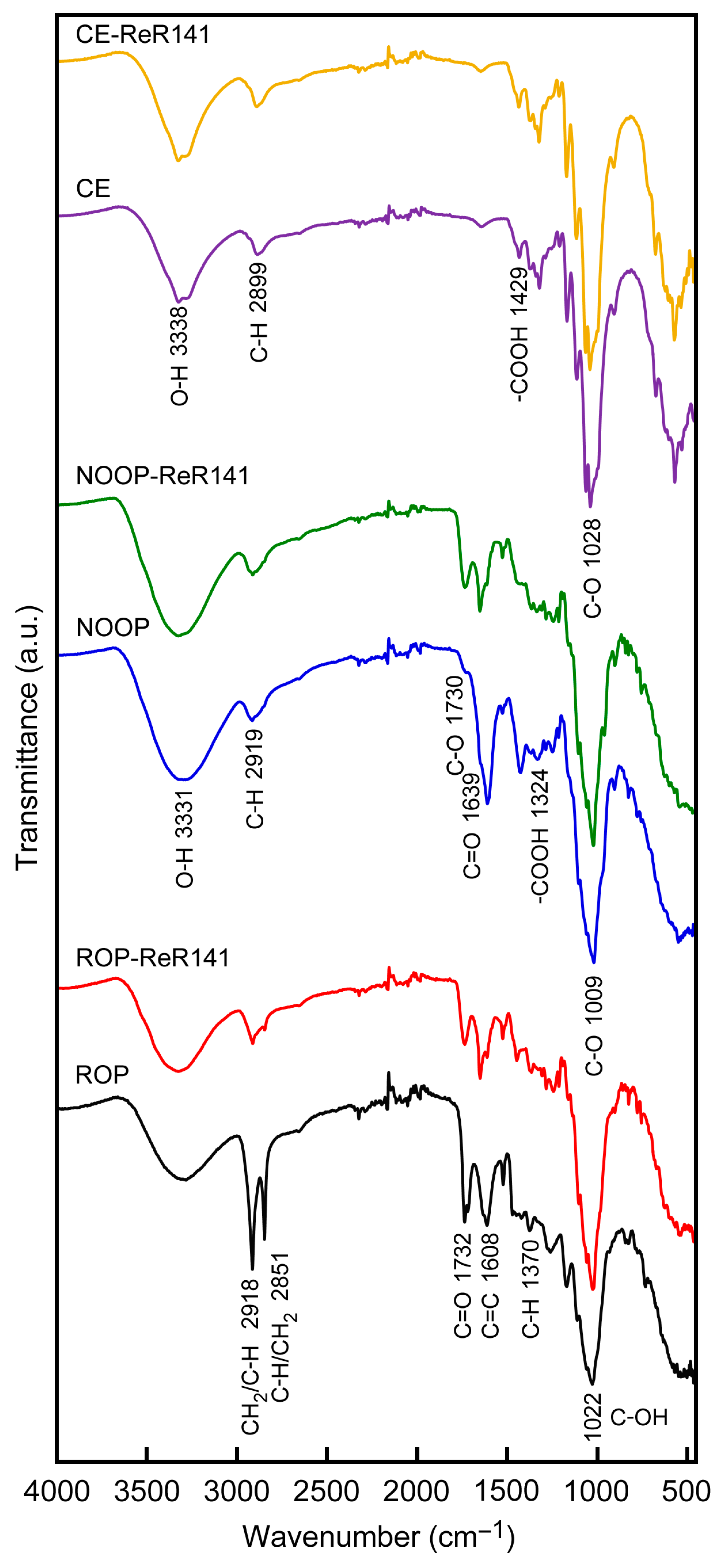
3.1. Functional Group on the Surface of ROP, NOOP, and CE
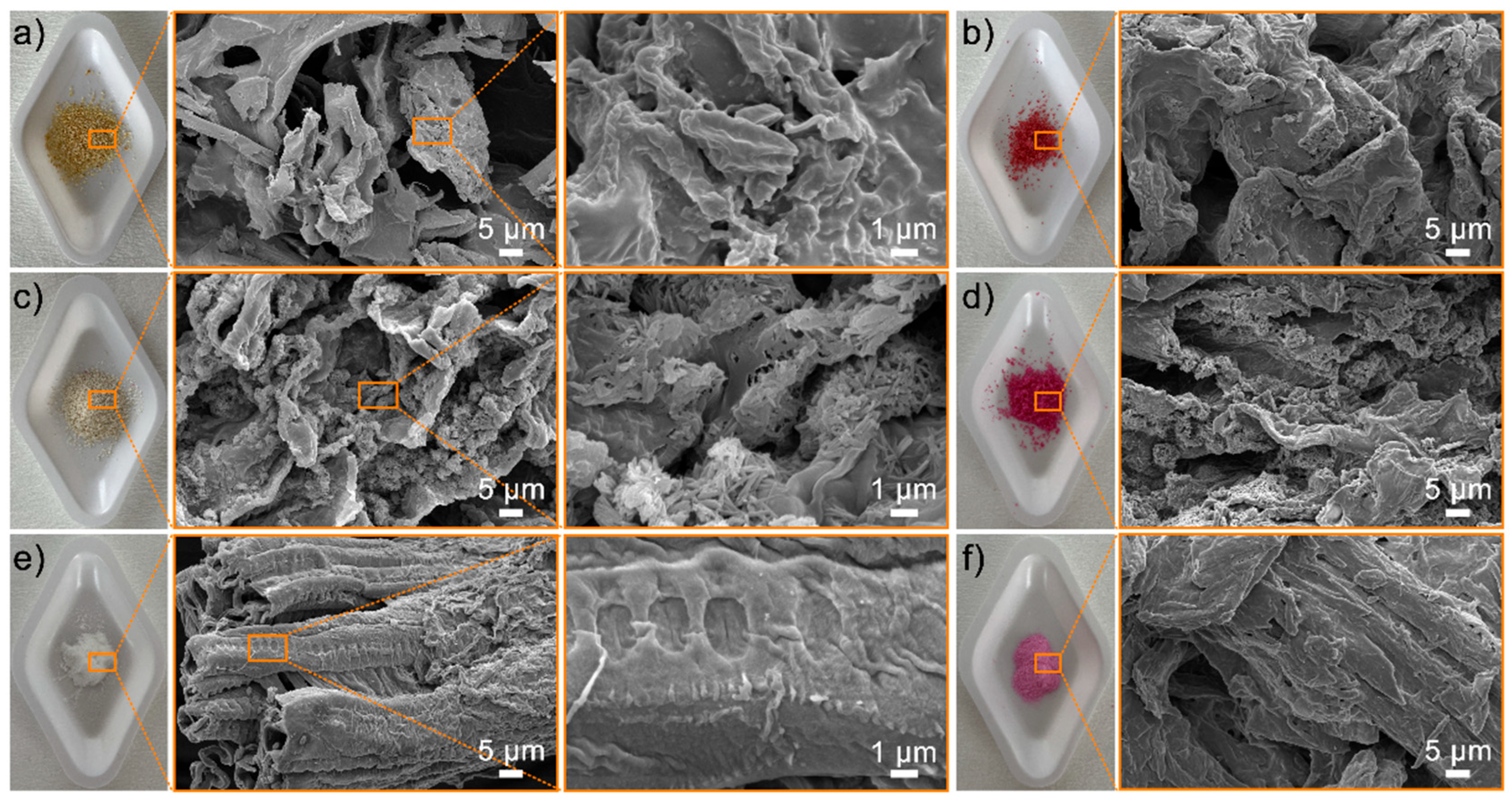
3.2. Surface Morphology and Element Component of the ROOP, NOOP, and CE
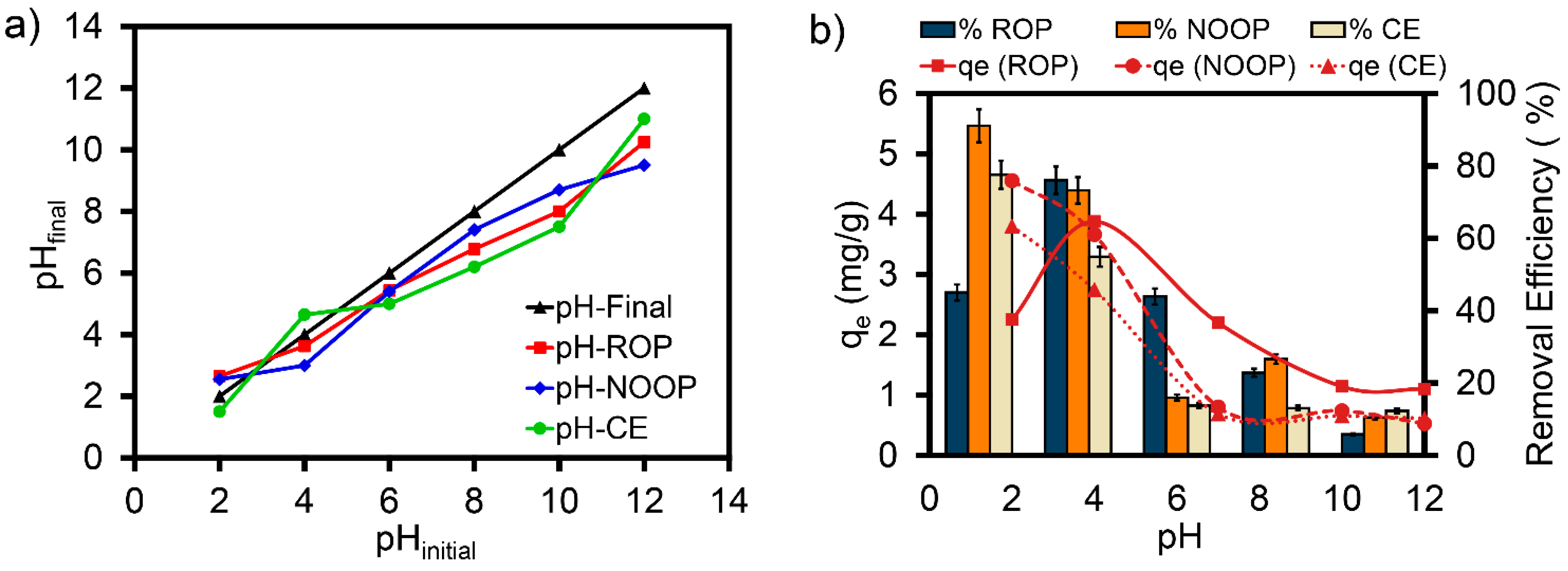
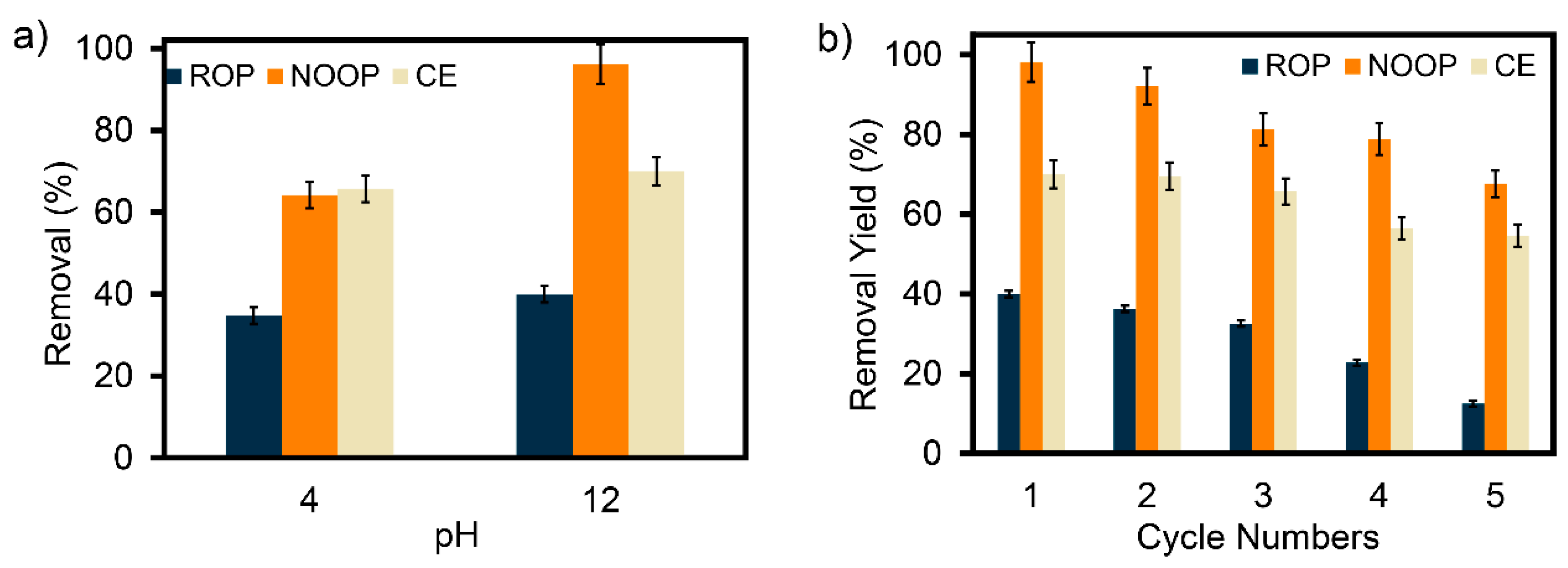
3.3. Effect of pH on Adsorption of ReR-141 Dyestuff
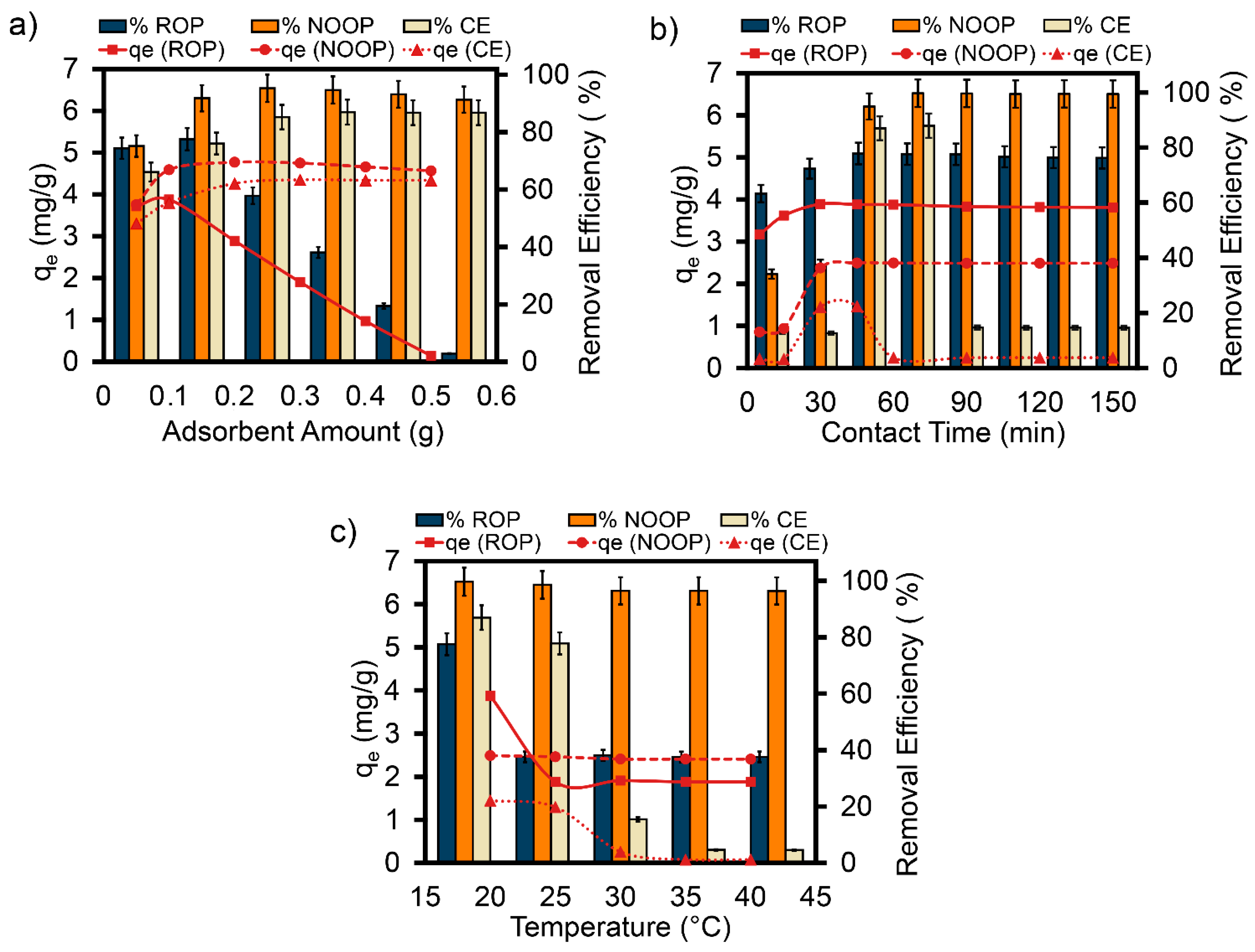
3.4. Impact of Adsorbent Dosage
3.5. Impact of Contact Time
3.6. Thermodynamic Theory Dependent on Temperature
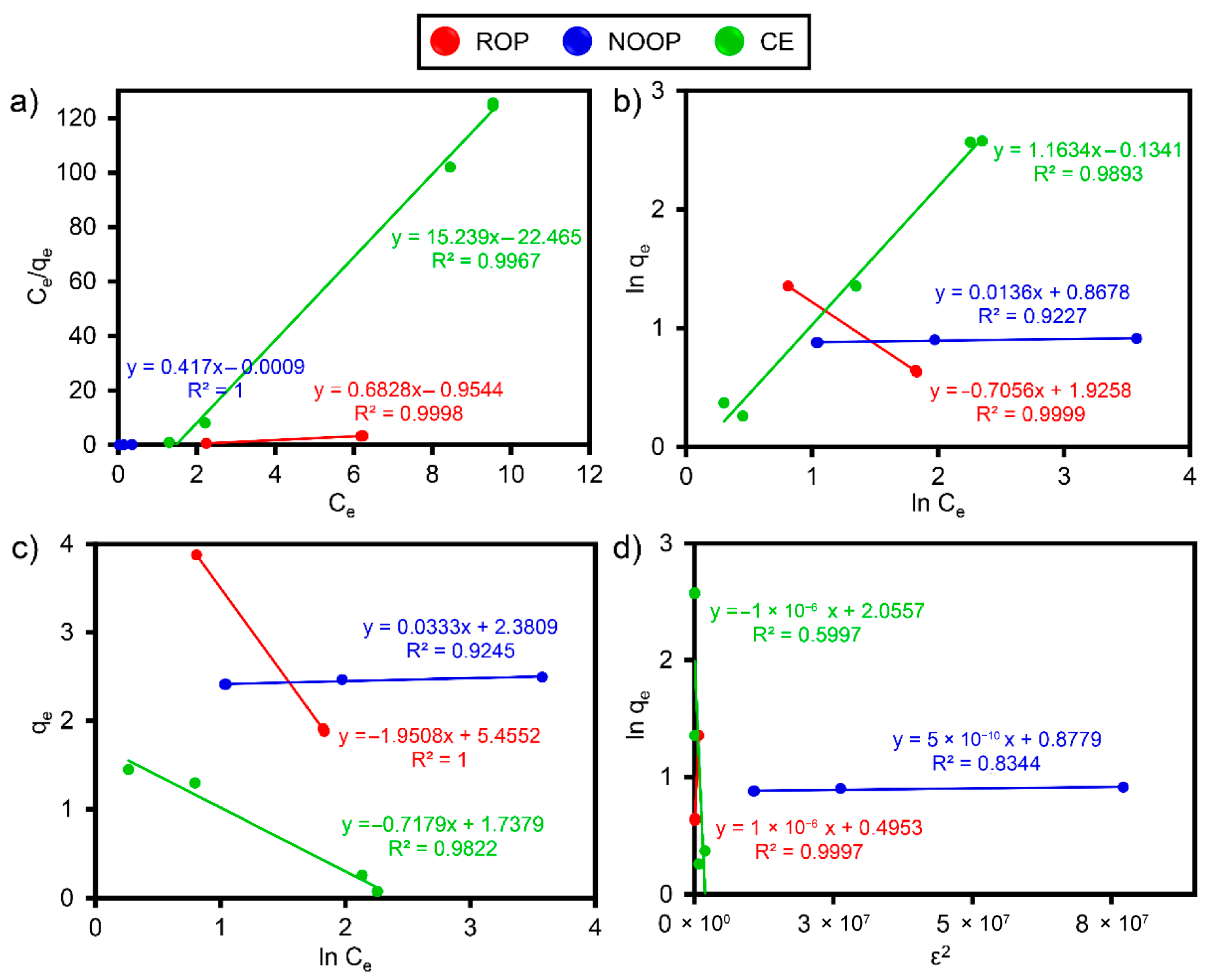
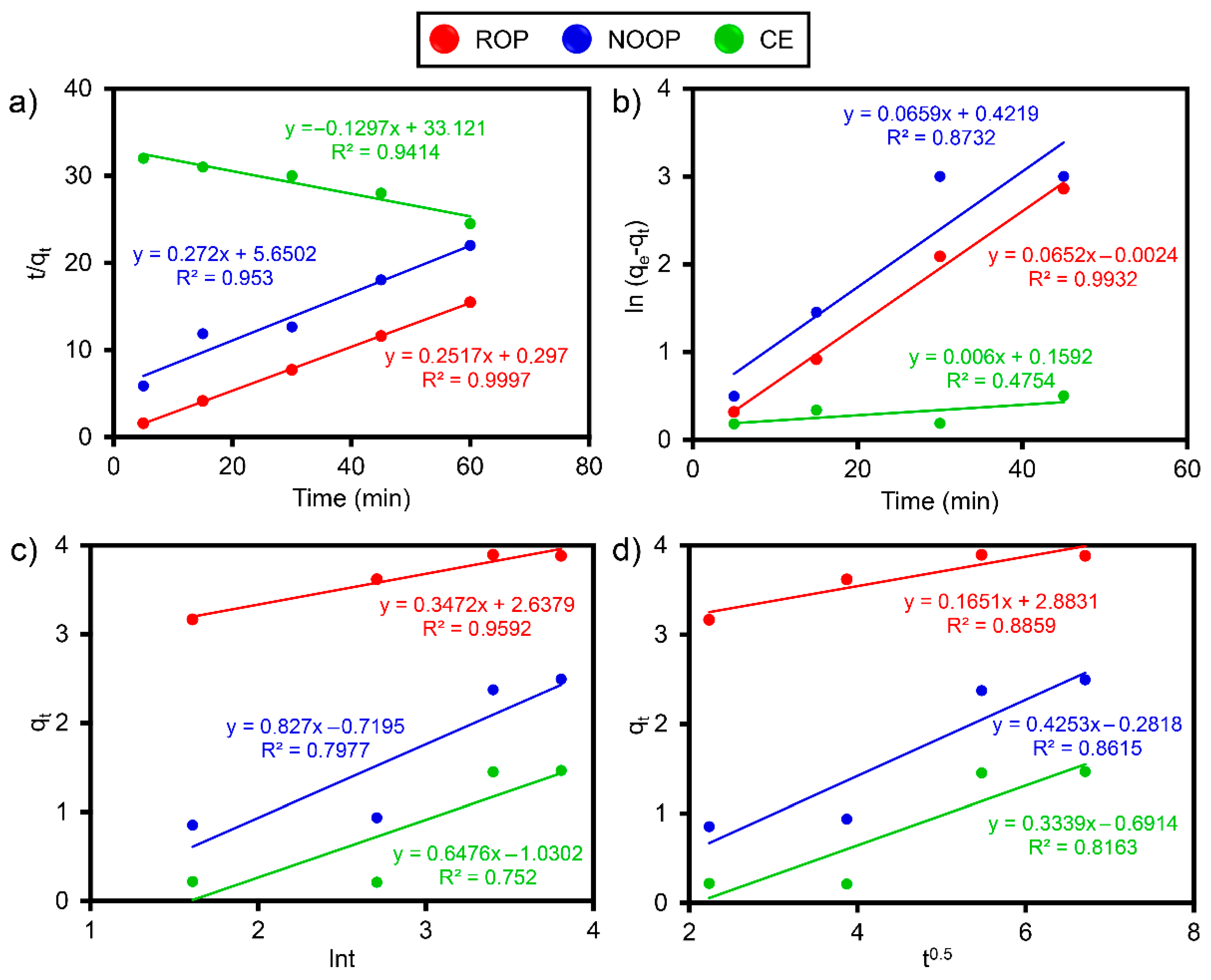
3.7. Adsorption Isotherms and Kinetics
3.8. Evaluation in Relation to Other Adsorbents
3.9. Potential Mechanism of Adsorption
3.10. Desorption Capacity of ROP, NOOP, and CE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Abdelrazek, E.J.; Gahlan, A.A.; Gouda, G.A.; Ahmed, A.S. Cost-effective adsorption of cationic dyes using ZnO nanorods supported by orange peel-derived carbon. Sci. Rep. 2025, 15, 4123. [Google Scholar] [CrossRef]
- Yang, L.; Bao, L.; Zhong, Y.; Hao, C.; Chen, J.; Wu, J.; Wang, X. Fabrication of in situ metal-organic framework grown on sodium lignosulphonate hydrogel for removal of Pb2+, methylene blue and crystal violet from aqueous solution. J. Clean. Prod. 2024, 434, 139831. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater treatment and reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Şimşek, İ.; Bahadir, T.; Çelebi, H.; Tulun, Ş. Selective adsorption of single and binary dyestuffs by citrus peel: Characterization, and adsorption performance. Chemosphere 2024, 352, 141475. [Google Scholar] [CrossRef]
- Ahmadian, M.; Derakhshankhah, H.; Jaymand, M. Biosorptive removal of organic dyes using natural gums-based materials: A comprehensive review. J. Ind. Eng. Chem. 2023, 124, 102–131. [Google Scholar] [CrossRef]
- Aouaini, F.; Dhaouadi, F.; Georgin, J.; Franco, D.S.P.; Alyousef, H.; Bonilla-Petriciolet, A.; Lamine, A.B. Statistical physics analysis of the adsorption of reactive red 141 dye on residual avocado peel (Persea americana) chemically modified with H2SO4 and HNO3. Inorg. Chem. Commun. 2024, 165, 112547. [Google Scholar] [CrossRef]
- Iqbal, M.; Hanif, M.A.; Rashid, U.; Jilani, M.I.; Alharthi, F.A.; Kazerooni, E.A. Optimization and kinetic study of treating dye-contaminated wastewater using bio-composite synthesized from natural waste. Separations 2023, 10, 386. [Google Scholar] [CrossRef]
- Oueslati, K.; Naifar, A.; Al-mugren, K.S.; Aouaini, F.; Lamine, A.B. Theoretical assessment of the adsorption mechanism of Reactive Red 141 on metal hydroxide: Water remediation via statistical physics modelling. Surf. Interfaces 2024, 51, 104631. [Google Scholar] [CrossRef]
- Chen, X.; Memon, H.A.; Wang, Y.; Marriam, I.; Tebyetekerwa, M. Circular economy and sustainability of the clothing and textile industry. Mater. Circ. Econ. 2021, 3, 12. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent advances in the remediation of textile-dye-containing wastewater: Prioritizing human health and sustainable wastewater treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Behloul, H.; Ferkous, H.; Bougdah, N.; Djellali, S.; Alam, M.; Djilani, C.; Sedik, A.; Lerari, D.; Jeon, B.-H.; Benguerba, Y. New insights on the adsorption of CI-Reactive Red 141 dye using activated carbon prepared from the ZnCl2-treated waste cotton fibers: Statistical physics, DFT, COSMO-RS, and AIM studies. J. Mol. Liq. 2022, 364, 119956. [Google Scholar] [CrossRef]
- Hafdi, H.; Joudi, M.; Mouldar, J.; Hatimi, B.; Nasrellah, H.; El Mhammedi, M.; Bakasse, M. Design of a new low cost natural phosphate doped by nickel oxide nanoparticles for capacitive adsorption of reactive red 141 azo dye. Environ. Res. 2020, 184, 109322. [Google Scholar] [CrossRef]
- Fatima, S.K.; Ceesay, A.S.; Khan, M.S.; Sarwar, R.; Bilal, M.; Uddin, J.; Ul-Hamid, A.; Khan, A.; Riaz, N.; Al-Harrasi, A. Visible light-induced reactive yellow 145 discoloration: Structural and photocatalytic studies of graphene quantum dot-incorporated TiO2. ACS Omega 2023, 8, 3007–3016. [Google Scholar] [CrossRef]
- Recio-Colmenares, C.L.; Flores-Gómez, J.; Morales Rivera, J.P.; Palacios Hinestroza, H.; Sulbarán-Rangel, B. Green Materials for Water and Wastewater Treatment: Mechanisms and Artificial Intelligence. Processes 2025, 13, 566. [Google Scholar] [CrossRef]
- Valle, C.; Voss, M.; Calcio Gaudino, E.; Forte, C.; Cravotto, G.; Tabasso, S. Harnessing agri-food waste as a source of biopolymers for agriculture. Appl. Sci. 2024, 14, 4089. [Google Scholar] [CrossRef]
- Manakas, P.; Balafoutis, A.T.; Kottaridi, C.; Vlysidis, A. Sustainability assessment of orange peel waste valorization pathways from juice industries. Biomass Conv. Bioref. 2024, 15, 6525–6544. [Google Scholar] [CrossRef]
- de Castro, S.C.; Stanisic, D.; Tasic, L. Sequential extraction of hesperidin, pectin, lignin, and cellulose from orange peels: Towards valorization of agro-Waste. Biofuels Bioprod. Bior. 2024, 18, 804–817. [Google Scholar] [CrossRef]
- Michael-Igolima, U.; Abbey, S.J.; Ifelebuegu, A.O.; Eyo, E.U. Modified orange peel waste as a sustainable material for adsorption of contaminants. Materials 2023, 16, 1092. [Google Scholar] [CrossRef]
- Matsedisho, B.; Otieno, B.; Kabuba, J.; Leswifi, T.; Ochieng, A. Removal of Ni (II) from aqueous solution using chemically modified cellulose nanofibers derived from orange peels. Int. J. Environ. Sci. Technol. 2024, 22, 2905–2916. [Google Scholar] [CrossRef]
- Akinhanmi, T.F.; Ofudje, E.A.; Adeogun, A.I.; Aina, P.; Joseph, I.M. Orange peel as low-cost adsorbent in the elimination of Cd (II) ion: Kinetics, isotherm, thermodynamic and optimization evaluations. Bioresour. Bioprocess. 2020, 7, 34. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Bitar-Castro, N.; Villabona-Ortíz, Á.; Tejada-Tovar, C.; González-Delgado, Á.D. Nickel adsorption from aqueous solution using lemon peel biomass chemically modified with TiO2 nanoparticles. Sustain. Chem. Pharm. 2020, 17, 100299. [Google Scholar] [CrossRef]
- El Gheriany, I.A.; El Saqa, F.A.; Amer, A.A.E.R.; Hussein, M. Oil spill sorption capacity of raw and thermally modified orange peel waste. Alex. Eng. J. 2020, 59, 925–932. [Google Scholar] [CrossRef]
- Praipipat, P.; Ngamsurach, P.; Rattanavaru, M.; Choothai, N.; Phongsa, A.; Saengwijit, P. Synthesis and characterization of metal oxide dopped beaded sugarcane bagasse fly ash for direct red 28 dye removal. J. Ind. Eng. Chem. 2023, 128, 495–514. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Razeghi, J.; Ramavandi, B. Performance of algal activated carbon/Fe3O4 magnetic composite for cationic dyes removal from aqueous solutions. Algal. Res. 2019, 40, 101509. [Google Scholar] [CrossRef]
- Bushra, R.; Mohamad, S.; Alias, Y.; Jin, Y.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review. Micropor. Mesopor. Mat. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Guediri, A.; Bouguettoucha, A.; Tahraoui, H.; Chebli, D.; Amrane, A.; Zhang, J. Thermodynamic study and the development of a support vector machine model for predicting adsorption behavior of orange peel-derived beads in wastewater treatment. J. Mol. Liq. 2024, 403, 124860. [Google Scholar] [CrossRef]
- Hamad, N.; Galhoum, A.A.; Saad, A.; Wageh, S. Efficient adsorption of cationic and anionic dyes using hydrochar nanoparticles prepared from orange peel. J. Mol. Liq. 2024, 409, 125349. [Google Scholar] [CrossRef]
- Sabry, N.W.; Naeem, I.; Hassanien, S.A.; Abuzalat, O.; Baraka, A. Alkali-treatment of Lupine peels for valorizing adsorption of organic cationic pollutants from wastewater: Kinetics, isotherm, thermodynamic, regeneration, and mechanism. Next Mat. 2025, 8, 100568. [Google Scholar] [CrossRef]
- Ediati, R.; Hidayat, A.R.P.; Syukrie, T.D.; Zulfa, L.L.; Jannah, M.; Harmami, H.; Fansuri, H.; Ali, B.T.I. Investigation the adsorption kinetic and isotherm studies of Remazol Red 5B dye on benzoic acid modified Al2O3/UiO-66 composite. Arab. J. Chem. 2024, 18, 106078. [Google Scholar] [CrossRef]
- Simion, A.-I.; Grigoraș, C.-G.; Favier, L. Batch Adsorption of Orange II Dye on a New Green Hydrogel—Study on Working Parameters and Process Enhancement. Gels 2025, 11, 79. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Benalia, A.; Selama, Z.; Hammoud, A.; Derbal, K.; Panico, A.; Pizzi, A. Removal of chromium (VI) from water using orange peel as the biosorbent: Experimental, modeling, and kinetic studies on adsorption isotherms and chemical structure. Water 2024, 16, 742. [Google Scholar] [CrossRef]
- Bingöl, M.S. Efficient removal of congo red and cytotoxicity evaluation of biosorbents prepared from chitosan-added watermelon pulp. Sci. Rep. 2024, 14, 23170. [Google Scholar] [CrossRef]
- Wang, H.; Luo, W.; Guo, R.; Li, D.; Xue, B. Effective adsorption of Congo red dye by magnetic chitosan prepared by solvent-free ball milling. Mat. Chem. Phys. 2022, 292, 126857. [Google Scholar] [CrossRef]
- Maanane, F.; Saufi, H.; El Alouani, M.; Tabyaoui, M.; Bellaouchou, A. Super-efficient removal of yellow 161 anionic dye from water by activated carbon based on coffee grounds. Desalin Water Treat. 2024, 320, 100713. [Google Scholar] [CrossRef]
- Bonyadi, Z.; Fouladi, Z.; Robatjazi, A.; Zahmatkesh Anbarani, M. Reactive red-141 removal from synthetic solutions by γ-Al2O3 nanoparticles: Process modeling, kinetic, and isotherm studies. Appl. Water Sci. 2023, 13, 52. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Cr2O3/Al2O3 as adsorbent: Physicochemical properties and adsorption behaviors towards removal of Congo red dye from water. J. Environ. Chem. Eng. 2019, 7, 102848. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Anjum, M.N.; Ali, A.; Ijaz, R.; Iqbal, S.; Al-Salahi, R.; Abuelizz, H.A.; Delattre, C. Water pollution remediation: Synthesis, characterization and adsorption studies of poly (o-anisidine)/MWCNTs nanocomposite for the elimination of direct yellow 26 dye. Kuwait J. Sci. 2025, 52, 100368. [Google Scholar] [CrossRef]
- Ahmad, M.N.; Hussain, A.; Anjum, M.N.; Hussain, T.; Mujahid, A.; Hammad Khan, M.; Ahmed, T. Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water. Open Chem. 2020, 18, 843–849. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Cheikh, S.; Hadadi, A.; Hamri, N.; Bollinger, J.-C.; Amrane, A.; Tahraoui, H.; Manseri, A.; Mouni, L. Adsorption performance of zeolite for the removal of Congo red dye: Factorial design experiments, kinetic, and equilibrium studies. Separations 2023, 10, 57. [Google Scholar] [CrossRef]
- Ishak, Z.; Kumar, D. Adsorption of Methylene blue and reactive black 5 by activated carbon derived from tamarind seeds. Trop. Aqua. Soil Pollut. 2022, 2, 1–12. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Sillanpää, M. Removal of reactive black 5 dye by banana peel biochar and evaluation of its phytotoxicity on tomato. Sustainability 2022, 14, 4176. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Boffito, D.C.; Ramavandi, B. Sono-photocatalytic activity of cloisite 30B/ZnO/Ag2O nanocomposite for the simultaneous degradation of crystal violet and methylene blue dyes in aqueous media. Nanomaterials 2022, 12, 3103. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Esvandi, Z.; Khatooni, H.; Ramavandi, B. Evaluation of two cationic dyes removal from aqueous environments using CNT/MgO/CuFe2O4 magnetic composite powder: A comparative study. J. Environ. Chem. Eng. 2021, 9, 104752. [Google Scholar] [CrossRef]
- Nasoudari, E.; Ameri, M.; Shams, M.; Ghavami, V.; Bonyadi, Z. The biosorption of Alizarin Red S by Spirulina platensis; process modelling, optimisation, kinetic and isotherm studies. Int. J. Environ. Anal. Chem. 2023, 103, 633–647. [Google Scholar] [CrossRef]
- Tsoutsa, E.K.; Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. An update on agricultural wastes used as natural adsorbents or coagulants in single or combined systems for the removal of dyes from wastewater. Water Air Soil Pollut. 2024, 235, 178. [Google Scholar] [CrossRef]
- Babakir, B.A.; Abd Ali, L.I.; Ismail, H.K. Rapid removal of anionic organic dye from contaminated water using a poly (3-aminobenzoic acid/graphene oxide/cobalt ferrite) nanocomposite low-cost adsorbent via adsorption techniques. Arab. J. Chem. 2022, 15, 104318. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M. A review of avocado waste-derived adsorbents: Characterizations, adsorption characteristics, and surface mechanism. Chemosphere 2022, 296, 134036. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; da Gama, B.M.V.; da Silva Gonçalves, A.H.; Medeiros, J.A.; de Souza Abud, A.K. Basic-dye adsorption in albedo residue: Effect of pH, contact time, temperature, dye concentration, biomass dosage, rotation and ionic strength. J. King Saud Univ. Eng. Sci. 2020, 32, 351–359. [Google Scholar] [CrossRef]
- Fraga, T.J.M.; de Lima, L.E.M.; de Souza, Z.S.B.; Carvalho, M.N.; Freire, E.M.P.d.L.; Ghislandi, M.G.; da Motta, M.A. Amino-Fe 3 O 4-functionalized graphene oxide as a novel adsorbent of Methylene Blue: Kinetics, equilibrium, and recyclability aspects. Environ. Sci. Pollut. Res. 2019, 26, 28593–28602. [Google Scholar] [CrossRef]
- Ebelegi, A.N.; Ayawei, N.; Wankasi, D. Interpretation of adsorption thermodynamics and kinetics. Open J. Phys. Chem. 2020, 10, 166–182. [Google Scholar] [CrossRef]
- Aichour, A.; Zaghouane-Boudiaf, H. Single and competitive adsorption studies of two cationic dyes from aqueous mediums onto cellulose-based modified citrus peels/calcium alginate composite. Int. J. Biol. Macromol. 2020, 154, 1227–1236. [Google Scholar] [CrossRef]
- Beyan, S.M.; Prabhu, S.V.; Ambio, T.A.; Gomadurai, C. A statistical modeling and optimization for Cr (VI) adsorption from aqueous media via teff straw-based activated carbon: Isotherm, kinetics, and thermodynamic studies. Adsorp. Sci. Technol. 2022, 2022, 7998069. [Google Scholar] [CrossRef]
- Baştürk, E.; Işık, M.; Karataş, M. Usage of titanium nanomaterial for the decolorization of Methylene blue and Reactive Red 198 dyes by sonocatalysis. Desalin. Water Treat. 2021, 224, 389–394. [Google Scholar] [CrossRef]
- Bessaha, F.; Bessaha, G.; Benhouria, A.; Benalioua, B.; Bendahma, F.; Boucif, F.; Mahrez, N.; Ziane, S.; Çoruh, A.; Khelifa, A. Highly efficient batch adsorption of anionic dye in wastewater using nanocomposite: Experimental and theoretical studies. Desalin. Water Treat. 2024, 317, 100292. [Google Scholar] [CrossRef]
- Hambisa, A.A.; Regasa, M.B.; Ejigu, H.G.; Senbeto, C.B. Adsorption studies of methyl orange dye removal from aqueous solution using Anchote peel-based agricultural waste adsorbent. Appl. Water Sci. 2023, 13, 24. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Albahly, E.F.; Mohamed, N.A. Kinetics, isotherm and thermodynamic studies for efficient adsorption of Congo Red dye from aqueous solution onto novel cyanoguanidine-modified chitosan adsorbent. Polymers 2021, 13, 4446. [Google Scholar] [CrossRef]
- Abolghasemi, S.; Nasiri, A.; Hashemi, M.; Rajabi, S.; Rahimi, F. Magnetic nanocomposites: Innovative adsorbents for antibiotics removal from aqueous environments–a narrative review. Appl. Water. Sci. 2025, 15, 30. [Google Scholar] [CrossRef]
- Mahajan, T.; Paikaray, S.; Mahajan, P. Applicability of the Equilibrium Adsorption Isotherms and the Statistical Tools on to Them: A Case Study for the Adsorption of Fluoride Onto Mg-Fe-CO3 LDH. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2023. [Google Scholar]
- Aguilar, M.I.; Lloréns, M.; Ortuño, J.F.; Meseguer, V.F.; Pérez-Marín, A.B.; Cases, A. Use of the Box–Behnken experimental design for the optimization of Orange II (Acid Orange 7) adsorption on Aloe vera. Sustainability 2023, 15, 15727. [Google Scholar] [CrossRef]
- Jedynak, K.; Repelewicz, M.; Kurdziel, K.; Wideł, D. Removal of orange II from aqueous solutions using micro-mesoporous carbon materials: Kinetic and equilibrium studies. Desalin. Water Treat. 2020, 190, 294–311. [Google Scholar] [CrossRef]
- Gunay Gurer, A.; Aktas, K.; Ozkaleli Akcetin, M.; Erdem Unsar, A.; Asilturk, M. Adsorption isotherms, thermodynamics, and kinetic modeling of methylene blue onto novel carbonaceous adsorbent derived from bitter orange peels. Water Air Soil Pollut. 2021, 232, 138. [Google Scholar] [CrossRef]
- Grigoraș, C.-G.; Simion, A.-I.; Favier, L. Exploration of Reactive Black 5 Dye Desorption from Composite Hydrogel Beads—Adsorbent Reusability, Kinetic and Equilibrium Isotherms. Gels 2023, 9, 299. [Google Scholar] [CrossRef]
- Kifetew, M.; Alemayehu, E.; Fito, J.; Worku, Z.; Prabhu, S.V.; Lennartz, B. Adsorptive removal of reactive yellow 145 dye from textile industry effluent using teff straw activated carbon: Optimization using central composite design. Water 2023, 15, 1281. [Google Scholar] [CrossRef]
- Alver, A.; Baştürk, E.; Altaş, L.; Işık, M. A solution of taste and odor problem with activated carbon adsorption in drinking water: Detailed kinetics and isotherms. Desalin. Water Treat. 2022, 252, 300–318. [Google Scholar] [CrossRef]
- Zazycki, M.A.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions. J. Clean. Prod. 2018, 171, 57–65. [Google Scholar] [CrossRef]
- Mouldar, J.; Hatimi, B.; Hafdi, H.; Joudi, M.; Belghiti, M.E.A.; Nasrellah, H.; El Mhammedi, M.A.; El Gaini, L.; Bakasse, M. Pyrrhotite ash waste for capacitive adsorption and fixed-bed column studies: Application for reactive red 141 dye. Water Air Soil Pollut. 2020, 231, 205. [Google Scholar] [CrossRef]
- Bakshi, A. Adsorption of reactive dyes from aqueous solution using activated carbon prepared from plantain leaf sheath waste. Chem. Biochem. Eng. Q. 2020, 34, 169–180. [Google Scholar]
- Aarfane, A.; Bensemlali, M.; Byadi, S.; Hatimi, B.; Baraket, A.; El Alami El Hassani, N.; Bakasse, M.; Nasrellah, H. Adsorption of Red 141 and methylene blue by cuttlebone: Experimental and molecular dynamics study. J. Umm. Al-Qura Univ. Appll. Sci. 2024, 1–11. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Tsoutsa, E.K.; Katsoyiannis, I.A.; Kyzas, G.Z. Simultaneous removal of anionic and cationic dyes on quaternary mixtures by adsorption onto banana, orange and pomegranate peels. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133176. [Google Scholar] [CrossRef]
- Kouzoutzoglou-Efremidou, A.A.; Tolkou, A.K.; Maroulas, K.N.; Kosheleva, R.I.; Katsoyiannis, I.A.; Kyzas, G.Z. Interfacial Adsorption Interactions of Dyes and Chitosan/Activated Carbon@ Curcumin Derivatives in Single-Component and Binary Solutions. Langmuir 2025, 41, 3603–3622. [Google Scholar] [CrossRef]
- Dubinin, M. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR. 1947, 327–329. [Google Scholar]
- Elovich, S.Y.; Larinov, O. Theory of adsorption from solutions of non electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form,(II) verification of the equation of adsorption isotherm from solutions. Izv. Akad. Nauk. SSSR Otd. Khim. Nauk 1962, 2, 209–216. [Google Scholar]
- Freundlich, H. Über die adsorption in lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Hall, K.R.; Eagleton, L.C.; Acrivos, A.; Vermeulen, T. Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam. 1966, 5, 212–223. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe, Kungliga svenska vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Temkin, M.J.; Pyzhev, V. Recent modifications to Langmuir isotherms. Acta Physiochim. URSS 1940, 12, 217–225. [Google Scholar]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
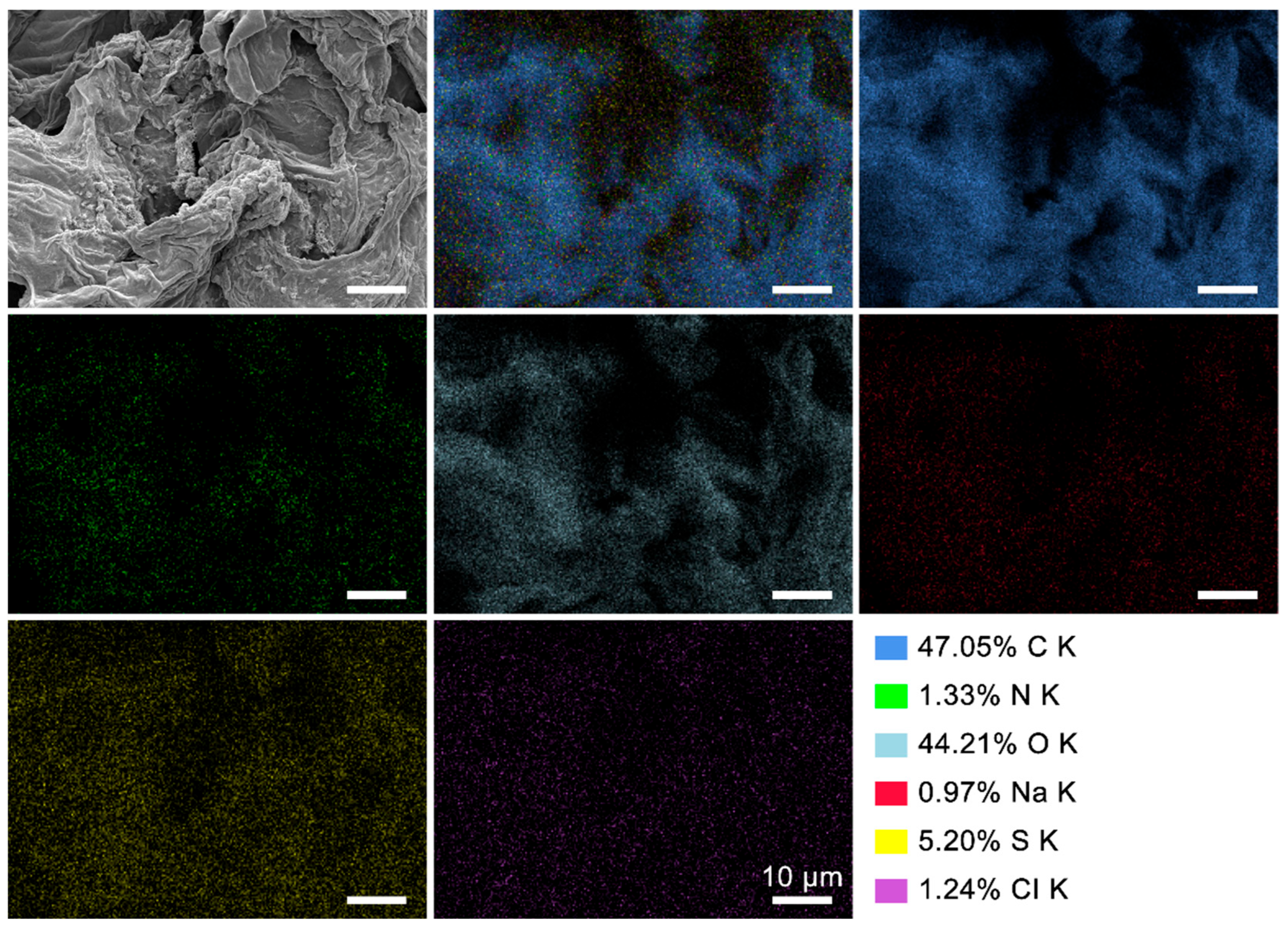

| Samples | C K (%) | N K (%) | O K (%) | Na K (%) | S K (%) | CI K (%) |
|---|---|---|---|---|---|---|
| ROP | 52.64 | 4.91 | 42.45 | - | - | - |
| ROP-R141 | 50.22 | 2.57 | 43.12 | 0.31 | 2.94 | 0.84 |
| NOOP | 53.53 | 3.23 | 43.24 | - | - | - |
| NOOP-R141 | 47.05 | 1.33 | 44.21 | 0.97 | 5.20 | 1.24 |
| CE | 50.70 | 3.99 | 45.31 | - | - | - |
| CE-R141 | 47.54 | 2.05 | 46.06 | 0.40 | 3.14 | 0.81 |
| Parameters | Unit | ROP | NOOP | CE |
|---|---|---|---|---|
| ΔH° | (kJ/mol) | −24.28 | −30.15 | −25.29 |
| ΔS° | (J/mol/K) | 9.08 | 7.91 | 8.63 |
| ΔG°293.15 | (kJ/mol) | −5.95 | −14.07 | −18.11 |
| ΔG°298.15 | (kJ/mol) | −5.84 | −10.97 | −14.97 |
| ΔG°303.15 | (kJ/mol) | −5.72 | −9.40 | −13.93 |
| ΔG°308.15 | (kJ/mol) | −5.64 | −9.35 | −13.30 |
| ΔG°318.15 | (kJ/mol) | −4.87 | −9.32 | −12.26 |
| Ea | (kJ/mol) | 15.97 | 14.67 | 24.43 |
| Optimum Kinetics Results for ReR-141 | ||||
| Parameter | ROP | NOOP | CE | |
| PSO | k2 (g/mg/min) | 2.13 × 10−1 ± 0.044 | 1.31 × 10−1 ± 0.072 | 5.08 × 10−5 ± 0.147 |
| qe (mg/g) | 3.973 ± 0.043 | 3.976 ± 0.051 | 3.312 ± 0.0360 | |
| R2 | 0.999 | 0.953 | 0.941 | |
| PFO | k1 (1/min) | 0.065 ± 0.013 | 0.066 ± 0.016 | 0.006 ± 0.001 |
| R2 | 0.993 | 0.873 | 0.475 | |
| Elovich | α | 1.99 × 103 ± 1.0045 × 104 | 2.39 × 100± 2.15 × 106 | 4.91 × 100 ± 2.32 × 1010 |
| β | 0.3472 ± 0.175 | 0.8270 ± 0.416 | 0.6476 ± 0.341 | |
| R2 | 0.752 | 0.797 | 0.959 | |
| IPD | kipd | 0.1651 ± 0.075 | 0.4253 ± 0.192 | 0.3339 ± 0.153 |
| R2 | 0.816 | 0.861 | 0.886 | |
| Optimum Isotherm Results for ReR-141 | ||||
| Parameter | ROP | NOOP | CE | |
| Langmuir | qm, mg/g | 3.863 ± 1.032 | 4.796 ± 1.304 | 4.657 ± 1.266 |
| KL, 1/mg | 0.715 ± 0.399 | 4.633 ± 2.586 | 0.678 ± 0.378 | |
| RL | 0.123 ± 0.033 | 0.021 ± 0.005 | 0.128 ± 0.047 | |
| R2 | 0.999 | 1.000 | 0.997 | |
| Freundlich | KF, ((mg/g)(L/mg)1/n) | 6.861 ± 3.816 | 2.382 ± 1.614 | 1.144 ± 0.775 |
| n | 1.417 ± 0.129 | 3.646 ± 0.210 | 1.020 ± 0.093 | |
| 1/n | 0.706 ± 0.064 | 0.274 ± 0.016 | 0.980 ± 0.089 | |
| R2 | 0.999 | 0.923 | 0.989 | |
| D-R | KDR, (mol k)−1)2 | 1.00 × 10−4 ± 0.022 | 5.00 × 10−4 ± 0.058 | 1.00 × 10−3 ± 0.026 |
| R2 | 0.997 | 0.834 | 0.599 | |
| Temkin | KT, L/mg | 16.39 ± 4.097 | 13.91 ± 3.477 | 1.27 ± 0.711 |
| B (kcal/mol) | 12.486 ± 2.687 | 7.316 ± 1.574 | 3.393 ± 0.736 | |
| R2 | 1.000 | 0.925 | 0.982 | |
| Adsorbent | pH | Adsorbent (g) | Time (min) | Temperature (°C) | % Removal | References |
|---|---|---|---|---|---|---|
| Cotton fibers | 3.00 | 0.25 | 70 | 20 | 96.87 | [11] |
| Pecan nutshell | 3.00 | 0.05 | - | 25 | 85.00 | [65] |
| Ash Waste | 6.00 | 0.50 | 60 | 25 | 97.00 | [66] |
| Leaf sheath waste | 2.00 | 1.00 | 180 | - | 72.70 | [67] |
| γ-Al2O3 nanopart. | 4.81 | 0.38 | 51.61 | - | 97.74 | [35] |
| Cuttlebone | 6.50 | 1.20 | 120 | 24 | 90.00 | [68] |
| ROP | 4.00 | 0.10 | 30 | 20 | 77.85 | This study |
| NOOP | 2.00 | 0.20 | 45 | 20 | 99.72 | |
| CE | 2.00 | 0.30 | 45 | 20 | 87.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koc-Bilican, B.; Bilican, I.; Çelebi, H. Strong Amphoteric Adsorption of Reactive Red-141 onto Modified Orange Peel Derivatives: Optimization, Characterization, and Mechanism. Polymers 2025, 17, 1875. https://doi.org/10.3390/polym17131875
Koc-Bilican B, Bilican I, Çelebi H. Strong Amphoteric Adsorption of Reactive Red-141 onto Modified Orange Peel Derivatives: Optimization, Characterization, and Mechanism. Polymers. 2025; 17(13):1875. https://doi.org/10.3390/polym17131875
Chicago/Turabian StyleKoc-Bilican, Behlul, Ismail Bilican, and Hakan Çelebi. 2025. "Strong Amphoteric Adsorption of Reactive Red-141 onto Modified Orange Peel Derivatives: Optimization, Characterization, and Mechanism" Polymers 17, no. 13: 1875. https://doi.org/10.3390/polym17131875
APA StyleKoc-Bilican, B., Bilican, I., & Çelebi, H. (2025). Strong Amphoteric Adsorption of Reactive Red-141 onto Modified Orange Peel Derivatives: Optimization, Characterization, and Mechanism. Polymers, 17(13), 1875. https://doi.org/10.3390/polym17131875






