Machine Learning in Predicting and Optimizing Polymer Printability for 3D Bioprinting
Abstract
1. Introduction
2. Printability
2.1. Evaluation of Printability
2.2. Factors Affecting Printability
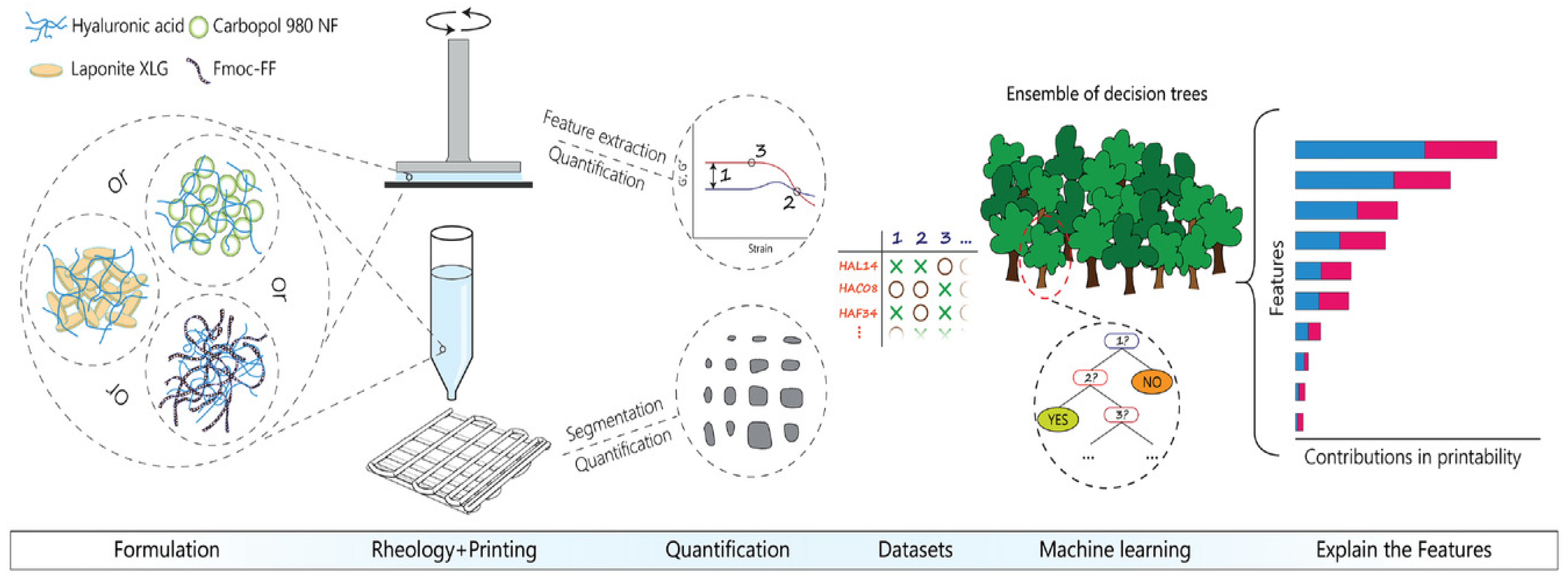
3. Machine Learning
3.1. Introduction to Common Algorithms
3.1.1. Bayesian Optimization
3.1.2. Neural Network
3.1.3. Random Forest
3.1.4. Hierarchical Machine Learning
3.1.5. Linear Regression
3.1.6. XGBoost
4. The Application of Machine Learning in the Evaluation of Printability
4.1. Optimization of Ink Material Performance
4.2. Optimization of Printing Parameters
4.3. Applications in Predicting Cell Viability
5. Challenges and Opportunities
- a.
- At the data level
- b.
- At the model level
- c.
- At the application level
6. Future Outlook
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.; Ng, D.W.H.; Park, H.S.; McAlpine, M.C. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater. 2020, 6, 27–47. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, X.; Fang, Y.; Xiong, Z.; Zhang, T. AI-driven 3D bioprinting for regenerative medicine: From bench to bedside. Bioact. Mater. 2025, 45, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yao, K.; An, J.; Jing, L.; Huang, K.; Huang, D. Machine learning and 3D bioprinting. Int. J. Bioprint. 2024, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Guvendiren, M. Complex 3D bioprinting methods. APL Bioeng. 2021, 5, 011508. [Google Scholar] [CrossRef]
- Ramesh, S.; Deep, A.; Tamayol, A.; Kamaraj, A.; Mahajan, C.; Madihally, S. Advancing 3D bioprinting through machine learning and artificial intelligence. Bioprinting 2024, 38, e00331. [Google Scholar] [CrossRef]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef]
- Abu Owida, H. Developments and Clinical Applications of Biomimetic Tissue Regeneration using 3D Bioprinting Technique. Appl. Bion. Biomech. 2022, 2022, 2260216. [Google Scholar] [CrossRef]
- Wang, H.; Bi, S.; Shi, B.; Ma, J.; Lv, X.; Qiu, J.; Wei, Y. Recent Advances in Engineering Bioinks for 3D Bioprinting. Adv. Eng. Mater. 2023, 25, 2300648. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef]
- Li, H.; Yin, Y.; Xiang, Y.; Liu, H.; Guo, R. A novel 3D printing PCL/GelMA scaffold containing USPIO for MRI-guided bile duct repair. Biomed. Mater. 2020, 15, 045004. [Google Scholar] [CrossRef]
- Mendoza-Cerezo, L.; Rodríguez-Rego, J.M.; Macías-García, A.; Callejas-Marín, A.; Sánchez-Guardado, L.; Marcos-Romero, A.C. Three-Dimensional Bioprinting of GelMA Hydrogels with Culture Medium: Balancing Printability, Rheology and Cell Viability for Tissue Regeneration. Polymers 2024, 16, 1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, Y.; Liu, Y.; Chen, B.; Li, M.; Yuan, C.; Wang, P. 3D bioprinting of DPSCs with GelMA hydrogel of various concentrations for bone regeneration. Tissue Cell 2024, 88, 102418. [Google Scholar] [CrossRef]
- Moon, S.H.; Choi, H.N.; Yang, Y.J. Natural/Synthetic Polymer Materials for Bioink Development. Biotechnol. Bioprocess Eng. 2022, 27, 482–493. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Goodman, S.B.; Kumar, M.; Bose, S. Improving biocompatibility for next generation of metallic implants. Prog. Mater. Sci. 2023, 133, 101053. [Google Scholar] [CrossRef]
- Rotella, G.; Morano, C.; Saffioti, M.R.; Umbrello, D. Surface functionalization of titanium screws for orthopaedic implant applications. CIRP Ann. 2024, 73, 453–456. [Google Scholar] [CrossRef]
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.-W.; Mohd Ariffin, M.K.A.; Seng Kim, C.L.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef]
- Kim, S.H.; Ki, M.-R.; Han, Y.; Pack, S.P. Biomineral-Based Composite Materials in Regenerative Medicine. Int. J. Mol. Sci. 2024, 25, 6147. [Google Scholar] [CrossRef]
- Ferrández-Montero, A.; Ortega-Columbrans, P.; Eguiluz, A.; Sanchez-Herencia, A.J.; Detsch, R.; Boccaccini, A.R.; Ferrari, B. Biocompatible colloidal feedstock for material extrusion processing of bioceramic-based scaffolds. Polym. Compos. 2024, 45, 7237–7255. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Rezvaninejad, R.; Rezvaninejad, R.; França, R. Biomaterials in bone and mineralized tissue engineering using 3D printing and bioprinting technologies. Biomed. Phys. Eng. Express 2021, 7, 062001. [Google Scholar] [CrossRef] [PubMed]
- Ana, I.D.; Satria, G.A.P.; Dewi, A.H.; Ardhani, R. Bioceramics for Clinical Application in Regenerative Dentistry. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1077, pp. 309–316. ISBN 978-981-13-0946-5. [Google Scholar]
- Joddar, B.; Ito, Y. Biological modifications of materials surfaces with proteins for regenerative medicine. J. Mater. Chem. 2011, 21, 13737. [Google Scholar] [CrossRef]
- Kuperkar, K.; Atanase, L.; Bahadur, A.; Crivei, I.; Bahadur, P. Degradable Polymeric Bio(nano)materials and Their Biomedical Applications: A Comprehensive Overview and Recent Updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, J.; Hartanto, Y.; Durham, M.; Tang, J.; Zhang, H.; Hooper, G.; Lim, K.; Woodfield, T. Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks. Adv. Healthc. Mater. 2020, 9, 1901648. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Shan, M.; Hao, Z.; Zhang, X.; Meng, L.; Zhai, Z.; Zhang, L.; Liu, X.; Wang, X. Convergence of 3D Bioprinting and Nanotechnology in Tissue Engineering Scaffolds. Biomimetics 2023, 8, 94. [Google Scholar] [CrossRef]
- Loukelis, K.; Helal, Z.A.; Mikos, A.G.; Chatzinikolaidou, M. Nanocomposite Bioprinting for Tissue Engineering Applications. Gels 2023, 9, 103. [Google Scholar] [CrossRef]
- Gómez-Blanco, J.C.; Pagador, J.B.; Galván-Chacón, V.P.; Sánchez-Peralta, L.F.; Matamoros, M.; Marcos, A.; Sánchez-Margallo, F.M. Computational simulation-based comparative analysis of standard 3D printing and conical nozzles for pneumatic and piston-driven bioprinting. Int. J. Bioprint. 2024, 9, 730. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Lin, X.; Xie, L.; Ivone, R.; Shen, J.; Yang, G. A tunable extruded 3D printing platform using thermo-sensitive pastes. Int. J. Pharm. 2020, 583, 119360. [Google Scholar] [CrossRef]
- Koch, F.; Thaden, O.; Conrad, S.; Tröndle, K.; Finkenzeller, G.; Zengerle, R.; Kartmann, S.; Zimmermann, S.; Koltay, P. Mechanical properties of polycaprolactone (PCL) scaffolds for hybrid 3D-bioprinting with alginate-gelatin hydrogel. J. Mech. Behav. Biomed. Mater. 2022, 130, 105219. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhang, X.; Huang, X.; Zheng, L.; Ye, X.; Li, Y. Effect of extrusion on viscoelastic slurry 3D print quality: Numerical analysis and experiment validation. SN Appl. Sci. 2019, 1, 1036. [Google Scholar] [CrossRef]
- Yogeshwaran, S.; Goodarzi Hosseinabadi, H.; Gendy, D.E.; Miri, A.K. Design considerations and biomaterials selection in embedded extrusion 3D bioprinting. Biomater. Sci. 2024, 12, 4506–4518. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef]
- Curti, F.; Drăgușin, D.-M.; Serafim, A.; Iovu, H.; Stancu, I.-C. Development of thick paste-like inks based on superconcentrated gelatin/alginate for 3D printing of scaffolds with shape fidelity and stability. Mater. Sci. Eng. C 2021, 122, 111866. [Google Scholar] [CrossRef]
- Khattati, M.; Abarghooei, E.; Hemasian Etefagh, A.; Khajehzadeh, M.; Razfar, M.R. Experimental investigation of pre-crosslinking methods and employing nano-hydroxyapatite powder on alginate/carboxymethyl cellulose hydrogel printability via 3D bioprinting. Rapid Prototyp. J. 2025, 31, 1249–1263. [Google Scholar] [CrossRef]
- Freeman, S.; Calabro, S.; Williams, R.; Jin, S.; Ye, K. Bioink Formulation and Machine Learning-Empowered Bioprinting Optimization. Front. Bioeng. Biotechnol. 2022, 10, 913579. [Google Scholar] [CrossRef]
- Yu, C.; Jiang, J. A Perspective on Using Machine Learning in 3D Bioprinting. Int. J. Bioprinting 2020, 6, 253. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Y.; Terpenny, J. Surface Roughness Prediction in Additive Manufacturing Using Machine Learning. In Volume 3: Manufacturing Equipment and Systems, Proceedings of the ASME 2018 13th International Manufacturing Science and Engineering Conference, College Station, TX, USA, 18–22 June 2018; American Society of Mechanical Engineers: New York, NY, USA, 2018; p. V003T02A018. [Google Scholar]
- Ege, D.; Boccaccini, A.R. Investigating the Effect of Processing and Material Parameters of Alginate Dialdehyde-Gelatin (ADA-GEL)-Based Hydrogels on Stiffness by XGB Machine Learning Model. Bioengineering 2024, 11, 415. [Google Scholar] [CrossRef]
- Shi, J.; Song, J.; Song, B.; Lu, W.F. Multi-Objective Optimization Design through Machine Learning for Drop-on-Demand Bioprinting. Engineering 2019, 5, 586–593. [Google Scholar] [CrossRef]
- Entekhabi, E.; Haghbin Nazarpak, M.; Sedighi, M.; Kazemzadeh, A. Predicting degradation rate of genipin cross-linked gelatin scaffolds with machine learning. Mater. Sci. Eng. 2020, 107, 110362. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadi, M.; Goushchi, S.J.; Keshtiban, P.M. Optimization of 3D printing process parameters to minimize surface roughness with hybrid artificial neural network model and particle swarm algorithm. Prog. Addit. Manuf. 2021, 6, 199–215. [Google Scholar] [CrossRef]
- Saad, M.S.; Nor, A.M.; Baharudin, M.E.; Zakaria, M.Z.; Aiman, A. Optimization of surface roughness in FDM 3D printer using response surface methodology, particle swarm optimization, and symbiotic organism search algorithms. Int. J. Adv. Manuf. Technol. 2019, 105, 5121–5137. [Google Scholar] [CrossRef]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and Chemical Factors Influencing the Printability of Hydrogel-based Extrusion Bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef]
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in extrusion bioprinting. Biofabrication 2021, 13, 033001. [Google Scholar] [CrossRef]
- Wilson, S.A.; Cross, L.M.; Peak, C.W.; Gaharwar, A.K. Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2017, 9, 43449–43458. [Google Scholar] [CrossRef]
- Roehm, K.D.; Madihally, S.V. Bioprinted chitosan-gelatin thermosensitive hydrogels using an inexpensive 3D printer. Biofabrication 2017, 10, 015002. [Google Scholar] [CrossRef]
- Perin, F.; Spessot, E.; Famà, A.; Bucciarelli, A.; Callone, E.; Mota, C.; Motta, A.; Maniglio, D. Modeling a Dynamic Printability Window on Polysaccharide Blend Inks for Extrusion Bioprinting. ACS Biomater. Sci. Eng. 2023, 9, 1320–1331. [Google Scholar] [CrossRef]
- Kang, K.H.; Hockaday, L.A.; Butcher, J.T. Quantitative optimization of solid freeform deposition of aqueous hydrogels. Biofabrication 2013, 5, 035001. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, B.; Xue, Q.; Zhao, C.; Luo, Y.; Zhou, H.; Ma, L.; Yang, H.; Bai, D. A Systematic Thermal Analysis for Accurately Predicting the Extrusion Printability of Alginate–Gelatin-Based Hydrogel Bioinks. Int. J. Bioprint. 2021, 7, 394. [Google Scholar] [CrossRef]
- Coşkun, S.; Akbulut, S.O.; Sarıkaya, B.; Çakmak, S.; Gümüşderelioğlu, M. Formulation of chitosan and chitosan-nanoHAp bioinks and investigation of printability with optimized bioprinting parameters. Int. J. Biol. Macromol. 2022, 222, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.; Izadifar, M.; Schreyer, D.; Chen, X. Influence of ionic crosslinkers (Ca2+/Ba2+/Zn2+) on the mechanical and biological properties of 3D Bioplotted Hydrogel Scaffolds. J. Biomater. Sci. Polym. Ed. 2018, 29, 1126–1154. [Google Scholar] [CrossRef] [PubMed]
- Alarçin, E.; Tutar, R.; Titi, K.; Kocaaga, B.; Guner, F.S.; Bal-Öztürk, A. Optimization of methacrylated gelatin/layered double hydroxides nanocomposite cell-laden hydrogel bioinks with high printability for 3D extrusion bioprinting. J. Biomed. Mater. Res. 2023, 111, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, A.; Chen, X. Printability and Cell Viability in Extrusion-Based Bioprinting from Experimental, Computational, and Machine Learning Views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X. Printability and Cell Viability in Bioprinting Alginate Dialdehyde-Gelatin Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 2976–2987. [Google Scholar] [CrossRef]
- Naghieh, S.; Chen, X. Printability–A key issue in extrusion-based bioprinting. J. Pharm. Anal. 2021, 11, 564–579. [Google Scholar] [CrossRef]
- Naghieh, S.; Sarker, M.; Sharma, N.K.; Barhoumi, Z.; Chen, X. Printability of 3D Printed Hydrogel Scaffolds: Influence of Hydrogel Composition and Printing Parameters. Appl. Sci. 2019, 10, 292. [Google Scholar] [CrossRef]
- Limon, S.; Sarah, R.; Habib, M.A. Viscosity Inference of Hybrid Bioink Using Decision Tree-Based Machine Learning Method. In Volume 1: Additive Manufacturing; Advanced Materials Manufacturing; Biomanufacturing; Life Cycle Engineering, Proceedings of the ASME 2024 19th International Manufacturing Science and Engineering Conference, Knoxville, TN, USA, 17–21 January 2024; American Society of Mechanical Engineers: New York, NY, USA, 2024; p. V001T03A012. [Google Scholar]
- Zhu, F.; Cheng, L.; Yin, J.; Wu, Z.L.; Qian, J.; Fu, J.; Zheng, Q. 3D Printing of Ultratough Polyion Complex Hydrogels. ACS Appl. Mater. Interfaces 2016, 8, 31304–31310. [Google Scholar] [CrossRef]
- Kiyotake, E.A.; Douglas, A.W.; Thomas, E.E.; Nimmo, S.L.; Detamore, M.S. Development and quantitative characterization of the precursor rheology of hyaluronic acid hydrogels for bioprinting. Acta Biomater. 2019, 95, 176–187. [Google Scholar] [CrossRef]
- Mouser, V.H.M.; Melchels, F.P.W.; Visser, J.; Dhert, W.J.A.; Gawlitta, D.; Malda, J. Yield stress determines bioprintability of hydrogels based on gelatin-methacryloyl and gellan gum for cartilage bioprinting. Biofabrication 2016, 8, 035003. [Google Scholar] [CrossRef]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-Based Hydrogels as Suitable Bioprinting Materials for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.-J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin–alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef]
- Hu, C.; Hahn, L.; Yang, M.; Altmann, A.; Stahlhut, P.; Groll, J.; Luxenhofer, R. Improving printability of a thermoresponsive hydrogel biomaterial ink by nanoclay addition. J. Mater. Sci. 2021, 56, 691–705. [Google Scholar] [CrossRef]
- Moon, S.H.; Park, T.Y.; Cha, H.J.; Yang, Y.J. Photo-/thermo-responsive bioink for improved printability in extrusion-based bioprinting. Mater. Today Bio 2024, 25, 100973. [Google Scholar] [CrossRef] [PubMed]
- Chirianni, F.; Vairo, G.; Marino, M. Development of process design tools for extrusion-based bioprinting: From numerical simulations to nomograms through reduced-order modeling. Comput. Methods Appl. Mech. Eng. 2024, 419, 116685. [Google Scholar] [CrossRef]
- Munoz-Perez, E.; Perez-Valle, A.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. High resolution and fidelity 3D printing of Laponite and alginate ink hydrogels for tunable biomedical applications. Biomater. Adv. 2023, 149, 213414. [Google Scholar] [CrossRef]
- Ouyang, L. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Doyle, B.J. Parameter optimization for 3D bioprinting of hydrogels. Bioprinting 2017, 8, 8–12. [Google Scholar] [CrossRef]
- Li, Y.; Ma, J.; Wang, J.; Kong, Y.; Wang, F.; Zhang, P.; Fan, Y. Optimal parameter setting and evaluation for ultraviolet-assisted direct ink writing bioprinting of nHA/PEGDA scaffold. Biomed. Mater. 2025, 20, 015032. [Google Scholar] [CrossRef]
- Bin Rashid, A.; Uddin, A.S.M.N.; Azrin, F.A.; Saad, K.S.K.; Hoque, M.E. 3D bioprinting in the era of 4th industrial revolution–insights, advanced applications, and future prospects. Rapid Prototyp. J. 2023, 29, 1620–1639. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Ozcan, O.; Tasoglu, S. Machine learning-enabled optimization of extrusion-based 3D printing. Methods 2022, 206, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Gillispie, G.J. The correlation between rheological properties and extrusion-based printability in bioink artifact quantification. Mater. Des. 2023, 233, 112237. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, P.; Singh, R.; Mahto, S.K. FRESH-based 3D bioprinting of complex biological geometries using chitosan bioink. Biofabrication 2024, 16, 045007. [Google Scholar] [CrossRef] [PubMed]
- Brunel, L.G.; Hull, S.M.; Heilshorn, S.C. Engineered assistive materials for 3D bioprinting: Support baths and sacrificial inks. Biofabrication 2022, 14, 032001. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Truby, R.L.; Kim, H.; Perazzo, A.; Lewis, J.A.; Stone, H.A. Viscoplastic Matrix Materials for Embedded 3D Printing. ACS Appl. Mater. Interfaces 2018, 10, 23353–23361. [Google Scholar] [CrossRef]
- Dorati, R.; Chiesa, E.; Riva, F.; Modena, T.; Marconi, S.; Auricchio, F.; Genta, I.; Conti, B. Design and optimization of 3D-bioprinted scaffold framework based on a new natural polymeric bioink. J. Pharm. Pharmacol. 2022, 74, 57–66. [Google Scholar] [CrossRef]
- Giuseppe, M.D.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef]
- Krishna, D.V.; Sankar, M.R. Machine learning-assisted extrusion-based 3D bioprinting for tissue regeneration applications. Ann. 3D Print. Med. 2023, 12, 100132. [Google Scholar] [CrossRef]
- Qiao, Q. The use of machine learning to predict the effects of cryoprotective agents on the GelMA-based bioinks used in extrusion cryobioprinting. Bio-Des. Manuf. 2023, 6, 464–477. [Google Scholar] [CrossRef]
- Limon, S.M.; Quigley, C.; Sarah, R.; Habib, A. Advancing scaffold porosity through a machine learning framework in extrusion based 3D bioprinting. Front. Mater. 2024, 10, 1337485. [Google Scholar] [CrossRef]
- Huang, X.; Ng, W.L.; Yeong, W.Y. Predicting the number of printed cells during inkjet-based bioprinting process based on droplet velocity profile using machine learning approaches. J. Intell. Manuf. 2024, 35, 2349–2364. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Balabani, S.; Hirayama, R.; Huang, J. Machine Learning in Predicting Printable Biomaterial Formulations for Direct Ink Writing. Research 2023, 6, 0197. [Google Scholar] [CrossRef] [PubMed]
- Ege, D.; Sertturk, S.; Acarkan, B.; Ademoglu, A. Machine learning models to predict the relationship between printing parameters and tensile strength of 3D Poly (lactic acid) scaffolds for tissue engineering applications. Biomed. Phys. Eng. Express 2023, 9, 065014. [Google Scholar] [CrossRef] [PubMed]
- Etefagh, A.H.; Razfar, M.R. Bayesian optimization of 3D bioprinted polycaprolactone/magnesium oxide nanocomposite scaffold using a machine learning technique. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2023, 238, 1448–1462. [Google Scholar] [CrossRef]
- Chen, S.-L.; Senadeera, M.; Ruberu, K.; Chung, J.; Rana, S.; Venkatesh, S.; Chen, C.-Y.; Chen, G.-Y.; Wallace, G. Machine learning-generated compression modulus database for 3D printing of gelatin methacryloyl. Int. J. Bioprint. 2024, 10, 3814. [Google Scholar] [CrossRef]
- Xu, Y.; Sarah, R.; Habib, A.; Liu, Y.; Khoda, B. Constraint based Bayesian optimization of bioink precursor: A machine learning framework. Biofabrication 2024, 28, 16. [Google Scholar] [CrossRef]
- Li, Z. Predicting bone regeneration from machine learning. Nat. Comput. Sci. 2021, 1, 509–510. [Google Scholar] [CrossRef]
- Khalvandi, A.; Saber-Samandari, S.; Aghdam, M.M. Application of artificial neural networks to predict Young’s moduli of cartilage scaffolds: An in-vitro and micromechanical study. Biomater. Adv. 2022, 136, 212768. [Google Scholar] [CrossRef]
- Jin, Z.; Zhang, Z.; Shao, X.; Gu, G.X. Monitoring Anomalies in 3D Bioprinting with Deep Neural Networks. ACS Biomater. Sci. Eng. 2023, 9, 3945–3952. [Google Scholar] [CrossRef]
- Menon, A.; Póczos, B.; Feinberg, A.W.; Washburn, N.R. Optimization of Silicone 3D Printing with Hierarchical Machine Learning. 3D Print. Addit. Manuf. 2019, 6, 181–189. [Google Scholar] [CrossRef]
- Sedigh, A.; Ghelich, P.; Quint, J.; Mollocana Lara, E.C.; Samandari, M.; Tamayol, A.; Tomlinson, R.E. Approximating scaffold printability utilizing computational methods. Biofabrication 2023, 15, 025014. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, S.J.; An, S.H.; Kim, W.-D.; Kim, S.-H. Machine learning-based design strategy for 3D printable bioink: Elastic modulus and yield stress determine printability. Biofabrication 2020, 12, 035018. [Google Scholar] [CrossRef]
- Decante, G.; Costa, J.B.; Silva-Correia, J.; Collins, M.N.; Reis, R.L.; Oliveira, J.M. Engineering bioinks for 3D bioprinting. Biofabrication 2021, 13, 032001. [Google Scholar] [CrossRef]
- Nadernezhad, A.; Groll, J. Machine Learning Reveals a General Understanding of Printability in Formulations Based on Rheology Additives. Adv. Sci. 2022, 9, 2202638. [Google Scholar] [CrossRef]
- Oh, D.; Shirzad, M.; Chang Kim, M.; Chung, E.-J.; Nam, S.Y. Rheology-informed hierarchical machine learning model for the prediction of printing resolution in extrusion-based bioprinting. Int. J. Bioprint. 2023, 9, 1280. [Google Scholar] [CrossRef]
- Bone, J.M.; Childs, C.M.; Menon, A.; Póczos, B.; Feinberg, A.W.; LeDuc, P.R.; Washburn, N.R. Hierarchical Machine Learning for High-Fidelity 3D Printed Biopolymers. ACS Biomater. Sci. Eng. 2020, 6, 7021–7031. [Google Scholar] [CrossRef]
- Fu, Z.; Angeline, V.; Sun, W. Evaluation of Printing Parameters on 3D Extrusion Printing of Pluronic Hydrogels and Machine Learning Guided Parameter Recommendation. Int. J. Bioprint. 2021, 7, 434. [Google Scholar] [CrossRef]
- Ruberu, K.; Senadeera, M.; Rana, S.; Gupta, S.; Chung, J.; Yue, Z.; Venkatesh, S.; Wallace, G. Coupling machine learning with 3D bioprinting to fast track optimisation of extrusion printing. Appl. Mater. Today 2021, 22, 100914. [Google Scholar] [CrossRef]
- Chen, B.; Dong, J.; Ruelas, M.; Ye, X.; He, J.; Yao, R.; Fu, Y.; Liu, Y.; Hu, J.; Wu, T.; et al. Artificial Intelligence-Assisted High-Throughput Screening of Printing Conditions of Hydrogel Architectures for Accelerated Diabetic Wound Healing. Adv. Funct. Mater. 2022, 32, 2201843. [Google Scholar] [CrossRef]
- Habib, A.; Sarah, R.; Tuladhar, S.; Khoda, B.; Limon, S.M. Modulating rheological characteristics of bio-ink with component weight and shear rate for enhanced bioprinted scaffold fidelity. Bioprinting 2024, 38, e00332. [Google Scholar] [CrossRef]
- Hashemi, A.; Ezati, M.; Zumberg, I.; Vicar, T.; Chmelikova, L.; Cmiel, V.; Provaznik, V. Characterization and optimization of a biomaterial ink aided by machine learning-assisted parameter suggestion. Mater. Today Commun. 2024, 40, 109777. [Google Scholar] [CrossRef]
- Dai, C.; Sun, Y.; Zhang, H.; Yuan, Z.; Zhang, B.; Xie, Z.; Li, P.; Liu, H. New Strategies for High Efficiency and Precision Bioprinting by DOE Technology and Machine Learning. Adv. Mater. Technol. 2025, 10, 2401138. [Google Scholar] [CrossRef]
- Allencherry, J.; Pradeep, N.; Shrivastava, R.; Joy, L.; Imbriacco, F.; Özel, T. Investigation of Hydrogel and Gelatin Bath Formulations for Extrusion-Based 3D Bioprinting using Deep Learning. Procedia CIRP 2022, 110, 360–365. [Google Scholar] [CrossRef]
- Mohammadrezaei, D.; Podina, L.; Silva, J.D.; Kohandel, M. Cell viability prediction and optimization in extrusion-based bioprinting via neural network-based Bayesian optimization models. Biofabrication 2024, 16, 025016. [Google Scholar] [CrossRef]
- Zhang, C.; Elvitigala, K.C.M.L.; Mubarok, W.; Okano, Y.; Sakai, S. Machine learning-based prediction and optimisation framework for as-extruded cell viability in extrusion-based 3D bioprinting. Virtual Phys. Prototyp. 2024, 19, e2400330. [Google Scholar] [CrossRef]


| Biomaterials | Representative Materials | Advantages/Disadvantages | Application | Ref. |
|---|---|---|---|---|
| Natural Polymers | Collagen, Chitosan, Alginate, Silk fibroin, Gelatin, Hyaluronic Acid (HA), GelHA, Agarose | Advantages:
|
| [10,11,12,13] |
| Synthetic Polymers | Polyethylene Glycol (PEG), Polycaprolactone (PCL), Polylactic Acid (PLA), Polyvinyl Alcohol (PVA), Polyacrylamide (PAM), Polydopamine (PDA) | Advantages:
|
| [13,14,15] |
| Metallic Materials | Stainless Steel, Titanium and Titanium Alloys, Magnesium Alloys, Cobalt-Chromium Alloys, Tantalum, Nickel Titanium Alloys | Advantages:
|
| [16,17,18,19] |
| Ceramic Materials | Alumina, Bioactive Glass, β-Tricalcium Phosphate, Hydroxyapatite (HA), Calcium Silicate | Advantages:
|
| [20,21,22,23] |
| Composite Materials | PLA/HA, Gelatin/HA, Collagen/PEG, Chitosan/Hydroxyapatite Gelatin/PCL, PCL/Bioactive Glass | Advantages:
|
| [24,25,26,27] |
| Evaluation Method | Evaluation Content | Definition | Ref. |
|---|---|---|---|
| Extrudability | Forming continuous filaments | Line continuity during extrusion | [47,48,49] |
| Angular fidelity factor | AF = | Assessing complex angle variations | [50] |
| Width index | WI = | Filament change relative to nozzle diameter | [51,52] |
| Printability index | Pr = | Square pore matching in scaffold design | [53,54,55] |
| Integrity factor | I = | Thickness comparison | [56,57] |
| Irregularity | Irregularity = | 3D structural integrity index | [58,59] |
| Algorithm | Type | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Linear Regression | Supervised Learning |
|
| [83] |
| Random Forest | Supervised Learning |
|
| [84,85] |
| XGBoost | Supervised Learning |
|
| [86] |
| Bayesian Algorithm | Supervised Learning |
|
| [87,88,89] |
| Neural Network | Deep Learning |
|
| [90,91,92] |
| Hierarchical Machine Learning | Supervised Learning |
|
| [93] |
| Main Materials | Parameters | Algorithm Model | Print Assessment | Ref. |
|---|---|---|---|---|
| Alginate and CMC |
| Regression model | Filament width and porosity | [83] |
| Silicone elastomers |
| Hierarchical machine learning | Printing score based on layer fusion, stringing and filling volume components | [93] |
| Gelatin |
| Convolutional Neural Network (CNN) | Line width, droplet line | [102] |
| Alginate, CMC and TO-NFC |
| Multiple linear regression | Filament width and cell viability | [103] |
| Chitosan, agarose and gelatin |
| Bayesian optimization | Subjective evaluation of printed layer and pore structure | [104] |
| Gelatin and sodium alginate |
| Multilayer perceptron (MLP) | Evaluation of pore connectivity and shape fidelity using diffusion ratio | [105] |
| Alginate |
| Convolutional Neural Network (CNN) | Judgement based on filament thickness uniformity and hydrogel distribution uniformity | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Yao, D.; Wang, L.; Xu, M. Machine Learning in Predicting and Optimizing Polymer Printability for 3D Bioprinting. Polymers 2025, 17, 1873. https://doi.org/10.3390/polym17131873
Yu J, Yao D, Wang L, Xu M. Machine Learning in Predicting and Optimizing Polymer Printability for 3D Bioprinting. Polymers. 2025; 17(13):1873. https://doi.org/10.3390/polym17131873
Chicago/Turabian StyleYu, Junjie, Danyu Yao, Ling Wang, and Mingen Xu. 2025. "Machine Learning in Predicting and Optimizing Polymer Printability for 3D Bioprinting" Polymers 17, no. 13: 1873. https://doi.org/10.3390/polym17131873
APA StyleYu, J., Yao, D., Wang, L., & Xu, M. (2025). Machine Learning in Predicting and Optimizing Polymer Printability for 3D Bioprinting. Polymers, 17(13), 1873. https://doi.org/10.3390/polym17131873






