Molecular Engineering of Carboxylated Polysulfone Membranes for Enhancing Salt Rejection
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Membrane Preparation
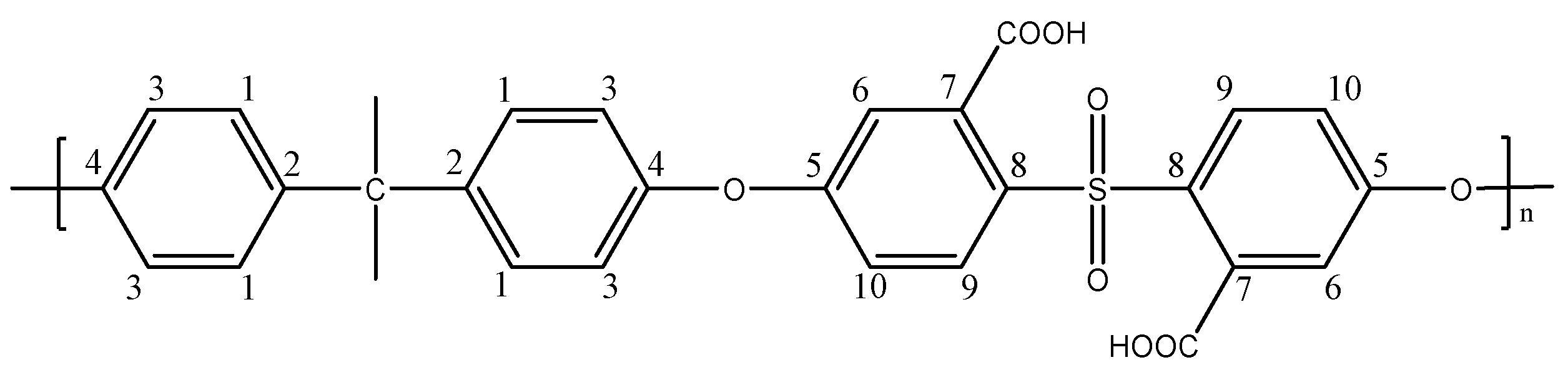
2.2.1. Preparation of a Dicarboxylated Polysulfone (PSF-COOH)
2.2.2. Method A: Dicarboxylated Membranes Crosslinked with Polyethyleneimine or Ethylenediamine
Polyethylenimine (PEI) Crosslinked Dicarboxylated Polysulfones
Ethylenediamine (EDA) Crosslinked Dicarboxylated Polysulfone
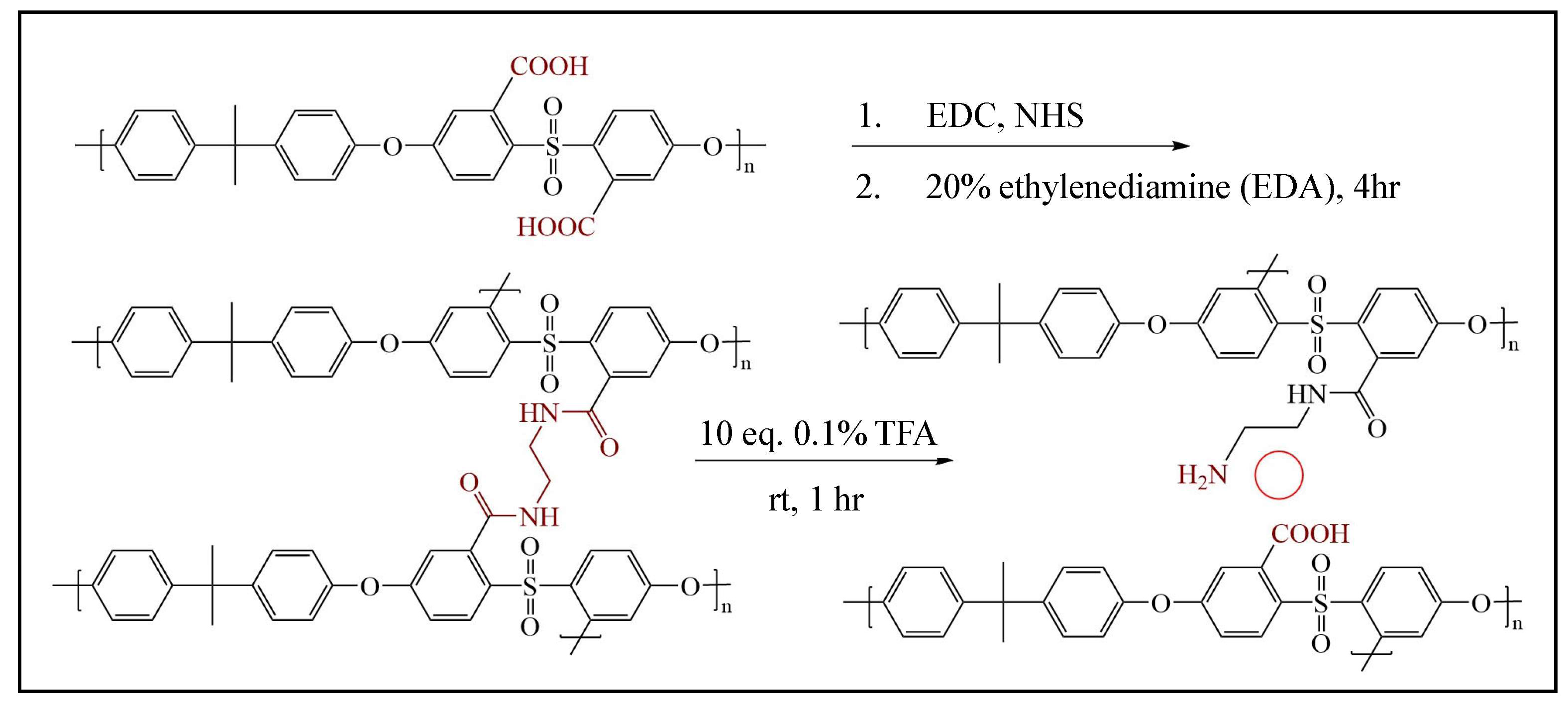
2.2.3. Method B: Partial Hydrolysis of Crosslinking Units in PSF-EDA Membranes, Forming Designed Pores and Brushes with Terminal Amine Groups
2.2.4. Method C: Synthesis of Dicarboxylated Polysulfone Membranes Containing Various Aliphatic Brushes
Preparation of Dicarboxylated Polysulfone Membranes Containing Ethyl or Hexyl Brushes (PSF-C2/PSF-C6)

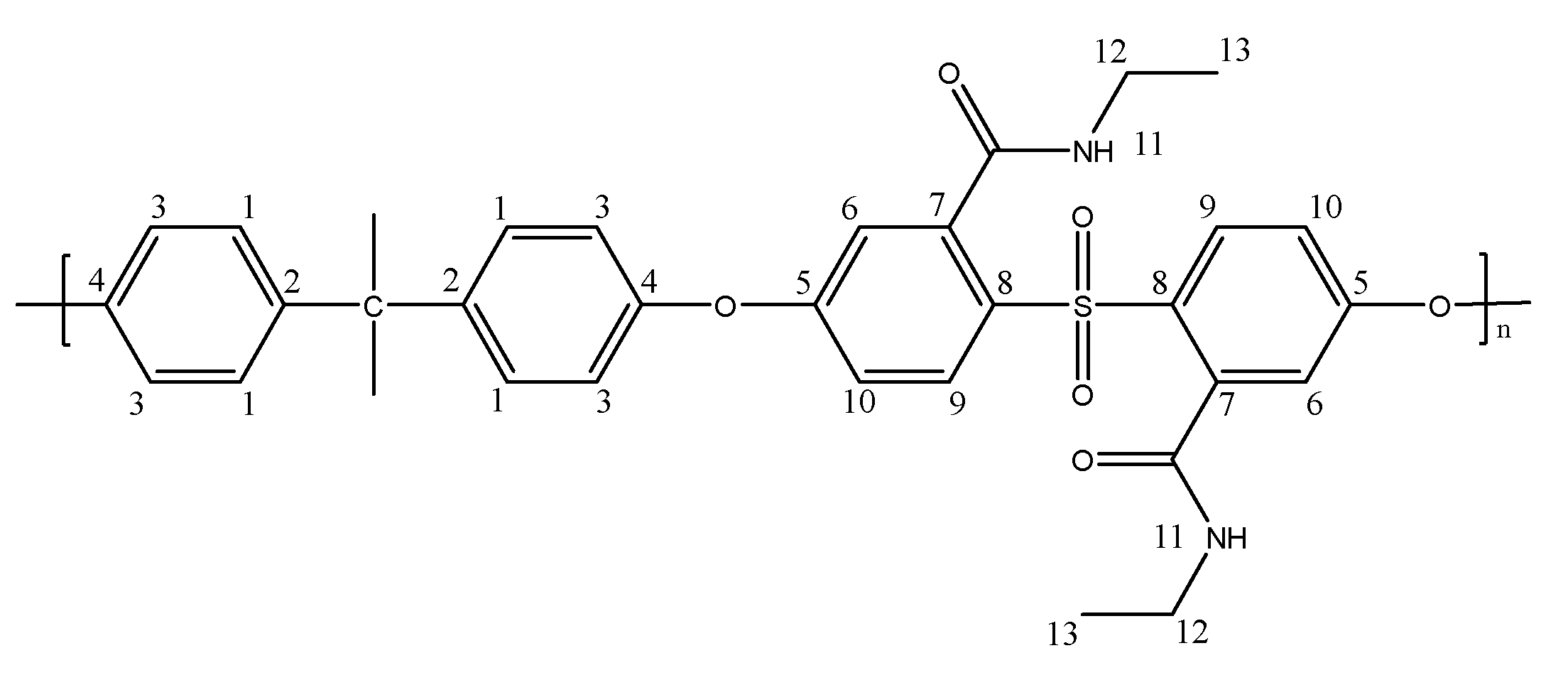
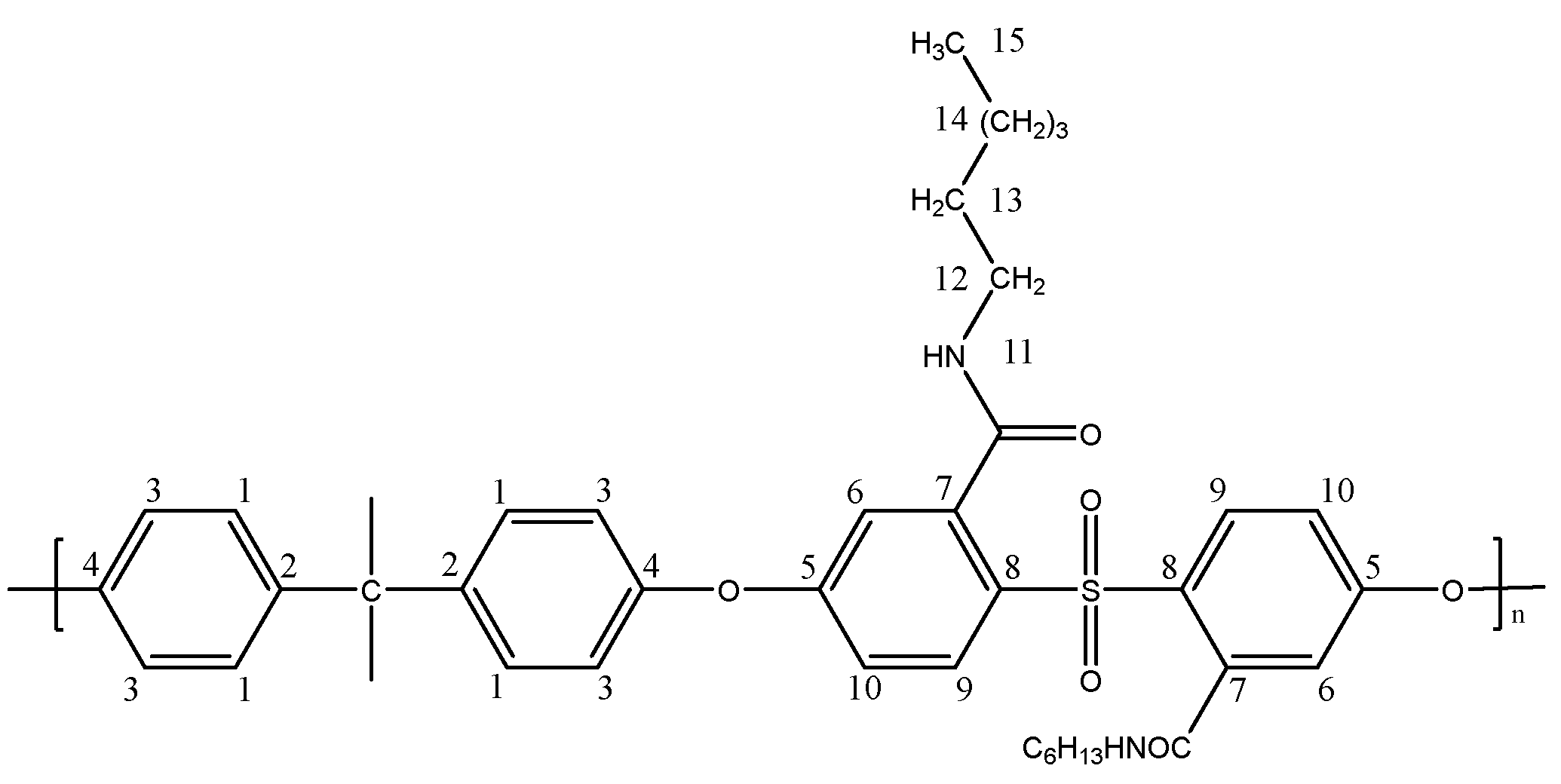
Preparation of a Dicarboxylated Polysulfone Membrane Containing Dodecylamine Brushes (PSF-C12)
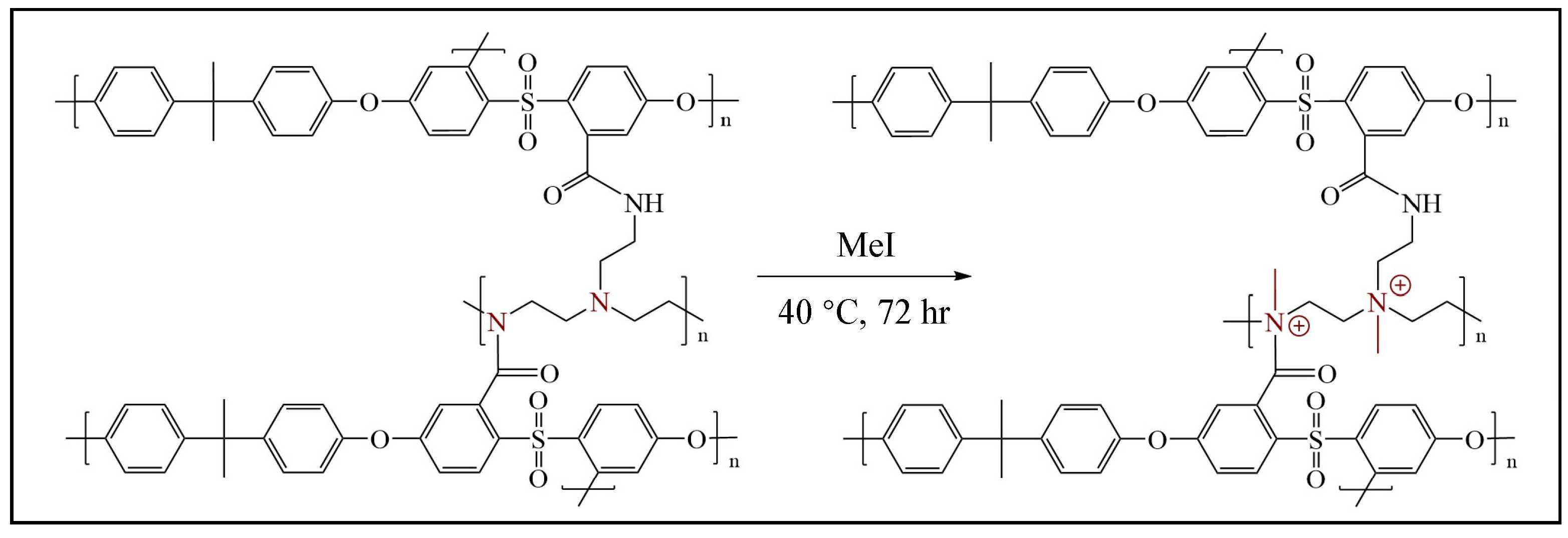
2.2.5. Method D: Formation of Quaternary Ammonium Motifs at the Surface of the Crosslinked PSF-PEI or PSF-NH2 Membranes
2.3. Membrane Characterizations
2.4. Filtration Experiments
2.5. Bacterial Attachment and Biofilm Formation
3. Results and Discussion
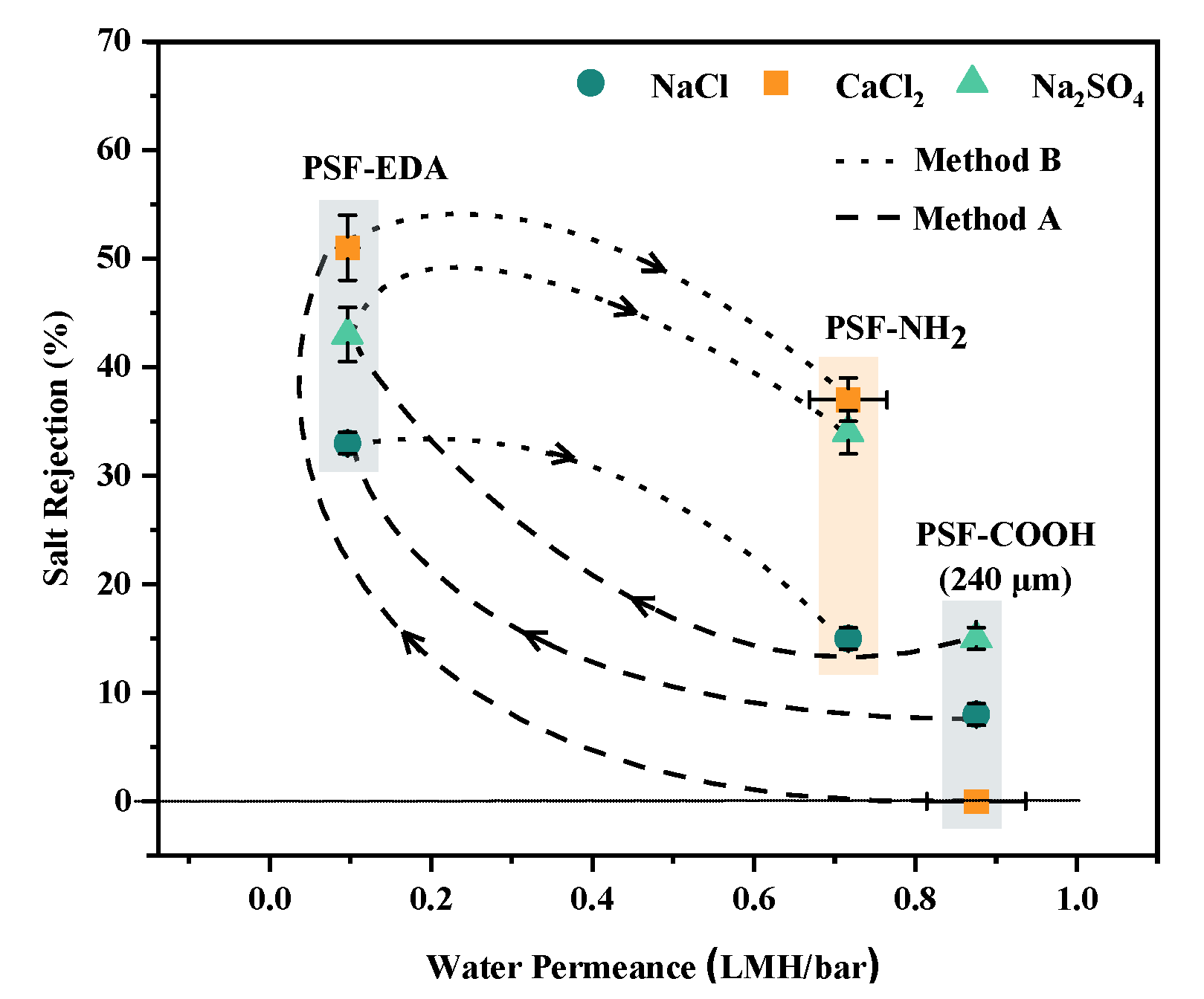
3.1. Method A: Dicarboxylated Membranes Crosslinked with Polyethyleneimine or Ethylenediamine
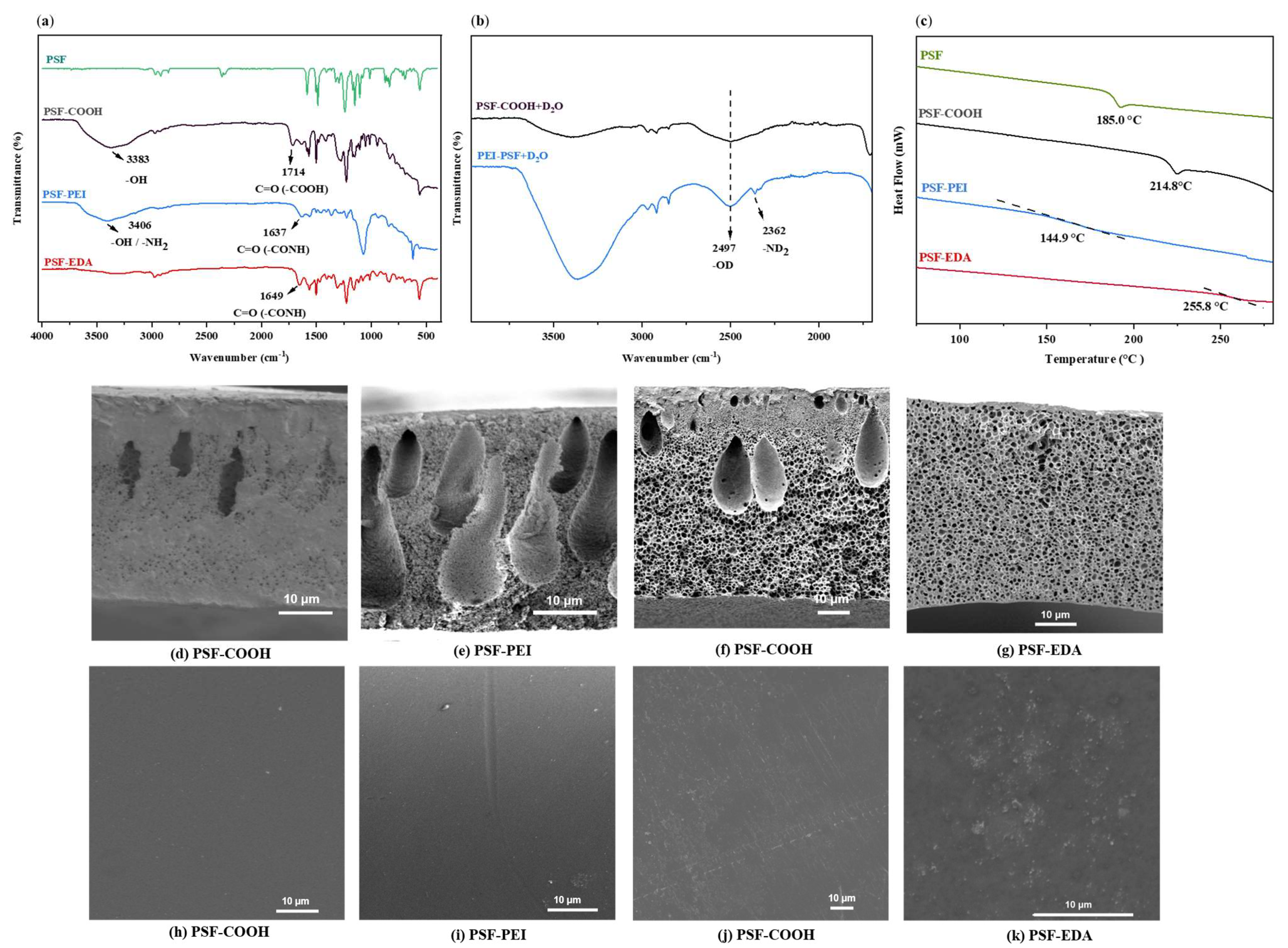
3.2. Method B: Hydrolysis of Crosslinking Units in PSF-EDA Membranes, Forming Designed Pores and Brushes with Terminal Amine Groups
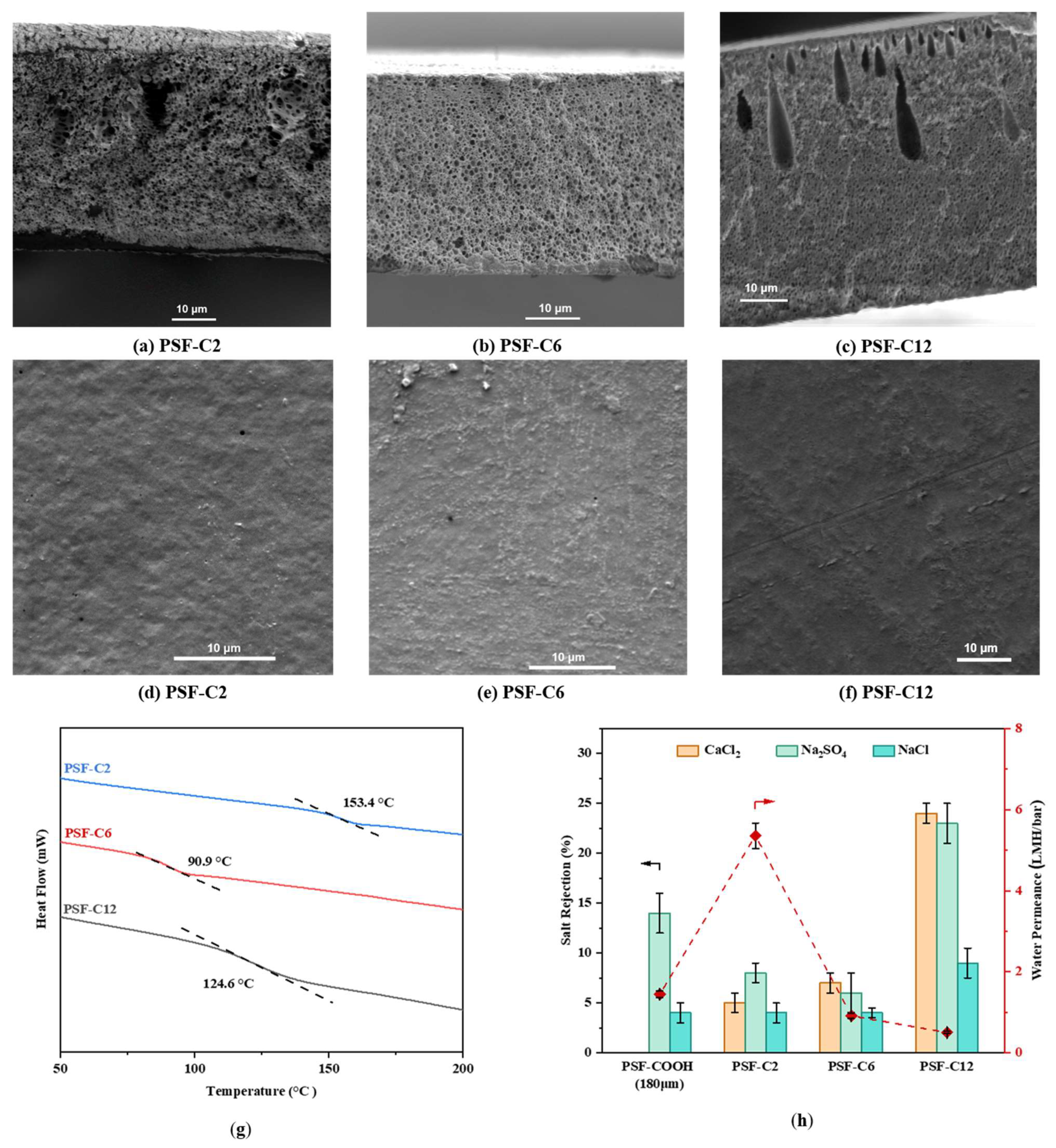
3.3. Method C: Synthesis of Dicarboxylated Polysulfone Membranes Containing Various Aliphatic Brushes
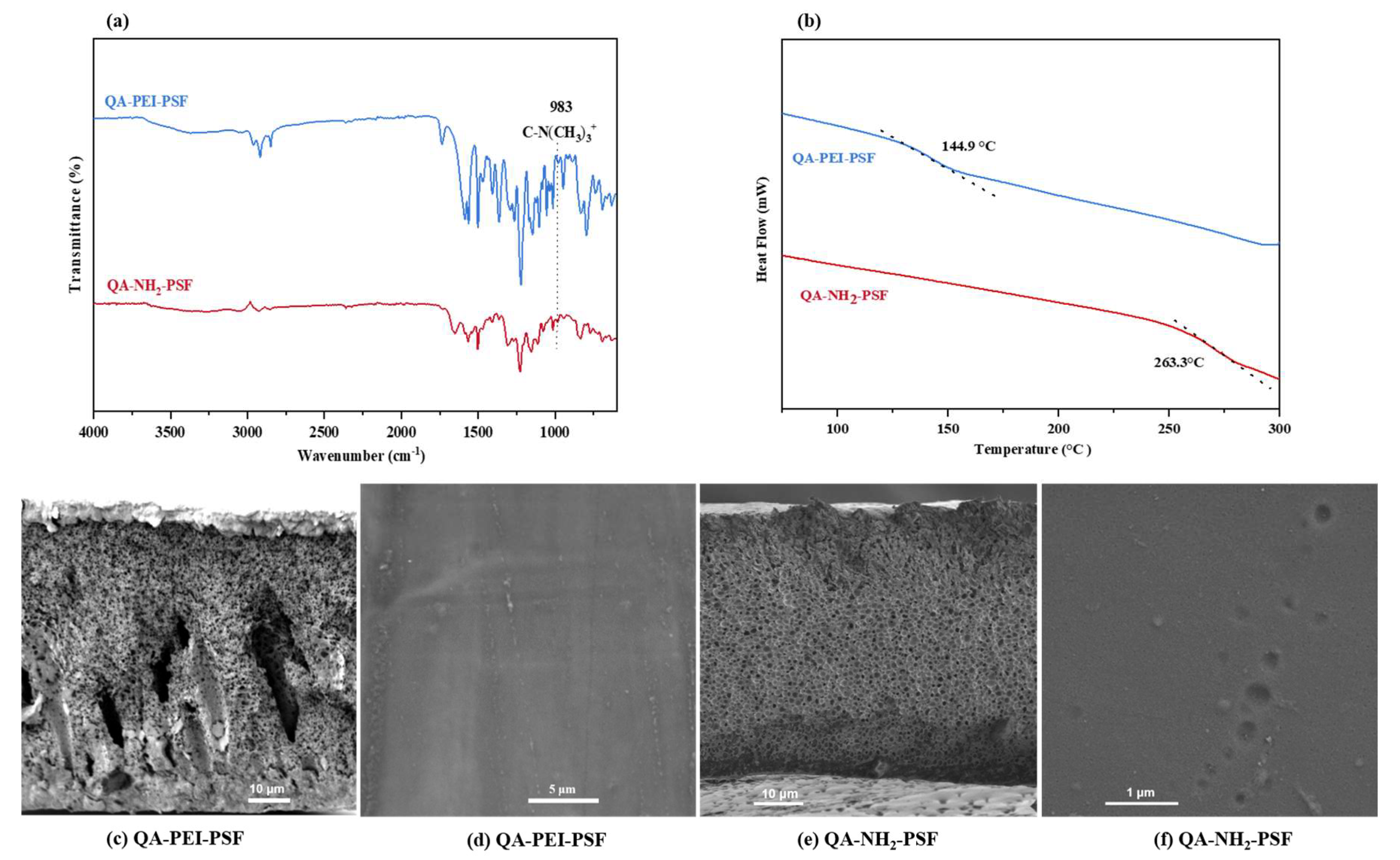
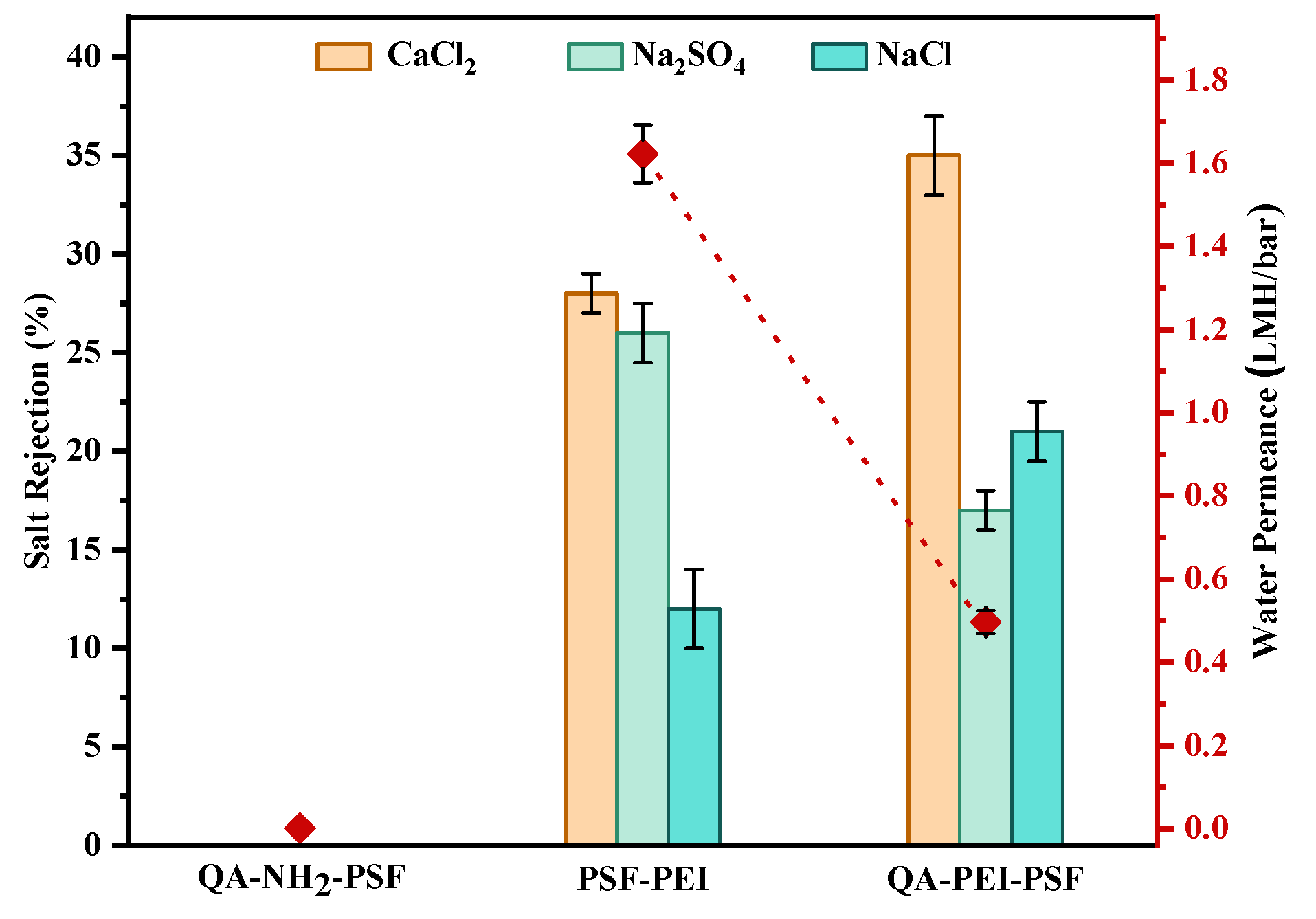
3.4. Method D: Formation of Quaternary Ammonium Motifs at the Surface of the Crosslinked PSF-PEI or PSF-NH2 Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousefi, A.; Moradi, K.; Karami, P.; Firouzjaei, M.D.; Elliott, M.; Rahimpour, A.; Sadrzadeh, M. Evaluating the Efficiency of Modified Hydrophobic PVDF Membrane for the Removal of PFOA Substances from Water by Direct Contact Membrane Distillation. Desalination 2024, 579, 117509. [Google Scholar] [CrossRef]
- Li, W.; Tian, X.; Li, X.; Liu, J.; Li, C.; Feng, X.; Shu, C.; Yu, Z.-Z. An Environmental Energy-Enhanced Solar Steam Evaporator Derived from MXene-Decorated Cellulose Acetate Cigarette Filter with Ultrahigh Solar Steam Generation Efficiency. J. Colloid. Interface. Sci. 2022, 606, 748–757. [Google Scholar] [CrossRef]
- Lu, H.; Liu, L.; Zhang, J.; Xu, J. Highly Durable Aqueous Zn Ion Batteries Based on a Zn Anode Coated by Three-Dimensional Cross-Linked and Branch-Liked Bismuth-PVDF Layer. J. Colloid. Interface. Sci. 2022, 572, 422–429. [Google Scholar] [CrossRef]
- Namdari, M.; Zokaee Ashtiani, F.; Bonyadi, E. Development of a High Flux Janus PVDF Membrane for Oily Saline Water Desalination by Membrane Distillation via PDA-TEOS-APTES Surface Modification. Desalination 2024, 572, 117139. [Google Scholar] [CrossRef]
- Pandey, R.P.; Rasheed, P.A.; Gomez, T.; Azam, R.S.; Mahmoud, K.A. A Fouling-Resistant Mixed-Matrix Nanofiltration Membrane Based on Covalently Cross-Linked Ti3C2TX (MXene)/Cellulose Acetate. J. Membr. Sci. 2020, 607, 118139. [Google Scholar] [CrossRef]
- Chang, K.; Luo, H.; Bannon, S.M.; Lin, S.Y.; Agata, W.-A.S.; Geise, G.M. Methoxy Groups Increase Water and Decrease Salt Permeability Properties of Sulfonated Polysulfone Desalination Membranes. J. Membr. Sci. 2021, 630, 119298. [Google Scholar] [CrossRef]
- Kim, K.; Jung, B.-K.; Ko, T.; Kim, T.-H.; Lee, J.-C. Comb-Shaped Polysulfones Containing Sulfonated Polytriazole Side Chains for Proton Exchange Membranes. J. Membr. Sci. 2018, 554, 232–243. [Google Scholar] [CrossRef]
- Dashtbozorg, A.; Saljoughi, E.; Mousavi, S.M.; Kiani, S. High-Performance and Robust Polysulfone Nanocomposite Membrane Containing 2D Functionalized MXene Nanosheets for the Nanofiltration of Salt and Dye Solutions. Desalination 2022, 527, 115600. [Google Scholar] [CrossRef]
- Zambare, R.S.; Dhopte, K.B.; Patwardhan, A.V.; Nemade, P.R. Polyamine Functionalized Graphene Oxide Polysulfone Mixed Matrix Membranes with Improved Hydrophilicity and Anti-Fouling Properties. Desalination 2017, 403, 24–35. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar]
- Mu, Y.; Feng, H.; Zhang, S.; Zhang, C.; Lu, N.; Luan, J.; Wang, G. Development of Highly Permeable and Antifouling Ultrafiltration Membranes Based on the Synergistic Effect of Carboxylated Polysulfone and Bio-Inspired Co-Deposition Modified Hydroxyapatite Nanotubes. J. Colloid Interface Sci. 2020, 572, 48–61. [Google Scholar] [CrossRef]
- Fahmey, M.S.; El-Aassar, A.-H.M.; Abo-Elfadel, M.M.; Orabi, A.S.; Das, R. Comparative Performance Evaluations of Nanomaterials Mixed Polysulfone: A Scale-up Approach through Vacuum Enhanced Direct Contact Membrane Distillation for Water Desalination. Desalination 2019, 451, 111–116. [Google Scholar] [CrossRef]
- Kavitha, J.; Naik, N.S.; Phani, A.R.; Arthanareeswaran, G.; Rajalakshmi, M.; Padaki, M. Renewed Physiognomies of Titanium Nanotubes for Implementation in the Polysulfone Membrane Matrix for Desalination. Desalination 2023, 552, 116444. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Altaee, A.; Déon, S.; Zhou, J. Preparation of Novel High Permeability and Antifouling Polysulfone-Vanillin Membrane. Desalination 2020, 496, 114759. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Kumoro, A.C.; Aryanti, N.; Utomo, D.P.; Hasbullah, H.; Lingga, F.F.; Widiastuti, A.; Yulfarida, M.; Dalanta, F.; Kurniawan, T.A. Photocatalytic Antifouling Nanohybrid Polysulfone Membrane Using the Synergetic Effect of Graphene Oxide and SiO2 for Effective Treatment of Natural Rubber-Laden Wastewater. J. Membr. Sci. 2022, 657, 120663. [Google Scholar] [CrossRef]
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified Polyether-Sulfone Membrane: A Mini Review. Des. Monomers. Polym. 2017, 20, 532–546. [Google Scholar] [CrossRef]
- Dumbrava, O.; Filimon, A.; Marin, L. Tailoring Properties and Applications of Polysulfone Membranes by Chemical Modification: Structure-Properties-Applications Relationship. Eur. Polym. J. 2023, 196, 112316. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone Functionalized Membranes: Properties and Challenges. Mater. Today. Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Dizman, C.; Tasdelen, M.A.; Yagci, Y. Recent Advances in the Preparation of Functionalized Polysulfones. Polym. Int. 2013, 62, 991–1007. [Google Scholar] [CrossRef]
- Guiver, M.D.; Croteau, S.; Hazlett, J.D.; Kutowy, O. Synthesis and Characterization of Carboxylated Polysulfones. Brit. Poly. J. 1990, 23, 29–39. [Google Scholar] [CrossRef]
- Vainrot, N.; Li, M.; Isloor, A.M.; Eisen, M.S. New Preparation Methods for Pore Formation on Polysulfone Membranes. Membranes 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Möckel, D.; Staude, E.; Dal-Cin, M.; Darcovich, K.; Guiver, M. Tangential Flow Streaming Potential Measurements: Hydrodynamic Cell Characterization and Zeta Potentials of Carboxylated Polysulfone Membranes. J. Membr. Sci. 1998, 145, 211–222. [Google Scholar] [CrossRef]
- Möckel, D.; Staude, E.; Guiver, M.D. Static Protein Adsorption, Ultrafiltration Behavior and Cleanability of Hydrophilized Polysulfone Membranes. J. Membr. Sci. 1999, 158, 63–75. [Google Scholar] [CrossRef]
- Wang, R.; Lin, S. Pore Model for Nanofiltration: History, Theoretical Framework, Key Predictions, Limitations, and Prospects. J. Membr. Sci. 2021, 620, 118809. [Google Scholar] [CrossRef]
- Yaroshchuk, A.E. Dielectric Exclusion of Ions from Membranes. Adv. Colloid Interface Sci. 2000, 85, 193–230. [Google Scholar] [CrossRef]
- Huang, S.; Du, P.; Min, C.; Liao, Y.; Sun, H.; Jiang, Y. Poly(1-Amino-5-Chloroanthraquinone): Highly Selective and Ultrasensitive Fluorescent Chemosensor for Ferric Ion. J. Fluoresc. 2013, 23, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Tian, M.; Zhang, Y.; Zhang, H.; Liu, J. Fabrication of a Novel “Loose” Nanofiltration Membrane by Facile Blending with Chitosan–Montmorillonite Nanosheets for Dyes Purification. Chem. Eng. J. 2015, 265, 184–193. [Google Scholar] [CrossRef]
- Bernstein, R.; Freger, V.; Lee, J.-H.; Kim, Y.-G.; Lee, J.; Herzberg, M. ‘Should I Stay or Should I Go?’ Bacterial Attachment vs Biofilm Formation on Surface-Modified Membranes. Biofouling 2014, 30, 367–376. [Google Scholar] [CrossRef]
- Perez-Gavilan, A.; De Castro, J.V.; Arana, A.; Merino, S.; Retolaza, A.; Alves, S.A.; Francone, A.; Kehagias, N.; Sotomayor-Torres, C.M.; Cocina, D.; et al. Antibacterial Activity Testing Methods for Hydrophobic Patterned Surfaces. Sci. Rep. 2021, 11, 6675. [Google Scholar] [CrossRef]
- Luo, Z.; Yang, Z.; Fei, Z.; Li, K. Effect of Crosslinking Rate on the Glass Transition Temperature of Polyimide Cross-Linked Silica Aerogels. J. Polym. Res. 2020, 27, 255. [Google Scholar] [CrossRef]
- Petersen, H.; Fechner, P.M.; Fischer, D.; Kissel, T. Synthesis, Characterization, and Biocompatibility of Polyethylenimine-graft-Poly(Ethylene Glycol) Block Copolymers. Macromolecules 2002, 35, 6867–6874. [Google Scholar] [CrossRef]
- Miao, A.; Wei, M.; Xu, F.; Wang, Y. Influence of Membrane Hydrophilicity on Water Permeability: An Experimental Study Bridging Simulations. J. Membr. Sci. 2020, 604, 118087. [Google Scholar] [CrossRef]
- Suhalim, N.S.; Kasim, N.; Mahmoudi, E.; Shamsudin, I.J.; Mohammad, A.W.; Mohamed Zuki, F.; Jamari, N.L.-A. Rejection Mechanism of Ionic Solute Removal by Nanofiltration Membranes: An Overview. Nanomaterials 2022, 12, 437. [Google Scholar] [CrossRef] [PubMed]
- Landry, K.A.; Boyer, T.H. Diclofenac Removal in Urine Using Strong-Base Anion Exchange Polymer Resins. Water. Res. 2013, 47, 6432–6444. [Google Scholar] [CrossRef] [PubMed]
- White, R.P.; Lipson, J.E.G. Polymer Free Volume and Its Connection to the Glass Transition. Macromolecules 2016, 49, 3987–4007. [Google Scholar] [CrossRef]
- Salerno, M.B.; Logan, B.E.; Velegol, D. Importance of Molecular Details in Predicting Bacterial Adhesion to Hydrophobic Surfaces. Langmuir 2004, 20, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Terada, A.; Yuasa, A.; Kushimoto, T.; Tsuneda, S.; Katakai, A.; Tamada, M. Bacterial Adhesion to and Viability on Positively Charged Polymer Surfaces. Microbiology 2006, 152, 3575–3583. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Golenser, J.; Beyth, N.; Weiss, E.I.; Domb, A.J. Quaternary Ammonium Polyethyleneimine: Antibacterial Activity. J. Nanomater. 2010, 2010, 826343. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary Ammonium Biocides: Efficacy in Application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- Mikláš, R.; Miklášová, N.; Bukovský, M.; Horváth, B.; Kubincová, J.; Devínsky, F. Synthesis, Surface and Antimicrobial Properties of Some Quaternary Ammonium Homochiral Camphor Sulfonamides. Eur. J. Pharm. Sci. 2014, 65, 29–37. [Google Scholar] [CrossRef]





| Membrane Type | Bacterial Species | CFUs (0 min) | CFUs (90 min) | Relative Change (%) |
|---|---|---|---|---|
| Parafilm | E. coli | 3 | 150 | 4900% |
| PSF | E. coli | 20 | 259 | 1195% |
| PSF | Bacillus | 19 | 88 | 363% |
| QA-PEI-PSF | E. coli | 257 | 170 | −34% |
| QA-PEI-PSF | Bacillus. | 8 | 0 | −100% |
| QA-NH2-PSF | E. coli | 190 | 91 | −52% |
| QA-NH2-PSF | Bacillus | 4 | 2 | −50% |
| Method | Functionalization | Key Effect on Membrane |
|---|---|---|
| Method A | PEI or EDA crosslinking | Improved salt rejection (CaCl2); moderate permeances |
| Method B | Partial hydrolysis of PSF-EDA and formed designed voids and brushed | Improved permeance and maintained salt rejection compared with PSF-EDA |
| Method C | Alkyl brushes attaching | Lower permeance and higher rejection with longer brushes |
| Method D | Quaternary ammonium | Biofilm prevention effect and improved CaCl2 rejection; low permeance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Eisen, M.S. Molecular Engineering of Carboxylated Polysulfone Membranes for Enhancing Salt Rejection. Polymers 2025, 17, 1840. https://doi.org/10.3390/polym17131840
Chen Z, Eisen MS. Molecular Engineering of Carboxylated Polysulfone Membranes for Enhancing Salt Rejection. Polymers. 2025; 17(13):1840. https://doi.org/10.3390/polym17131840
Chicago/Turabian StyleChen, Zhuonan, and Moris S. Eisen. 2025. "Molecular Engineering of Carboxylated Polysulfone Membranes for Enhancing Salt Rejection" Polymers 17, no. 13: 1840. https://doi.org/10.3390/polym17131840
APA StyleChen, Z., & Eisen, M. S. (2025). Molecular Engineering of Carboxylated Polysulfone Membranes for Enhancing Salt Rejection. Polymers, 17(13), 1840. https://doi.org/10.3390/polym17131840







