Computational Study of Catalytic Poisoning Mechanisms in Polypropylene Polymerization: The Impact of Dimethylamine and Diethylamine on the Deactivation of Ziegler–Natta Catalysts and Co-Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Study
Determination of Adsorption Energies in Solvent (n-Hexane)
2.2. Experimental Study
2.3. Infrared Spectroscopy
3. Results
3.1. Loss of Productivity in Polypropylene Production
3.2. Structural Characteristics of the Studied Inhibitors
Local Descriptors of the Amines
3.3. Interaction Direct Amine Adsorption on Ziegler–Natta Active Sites
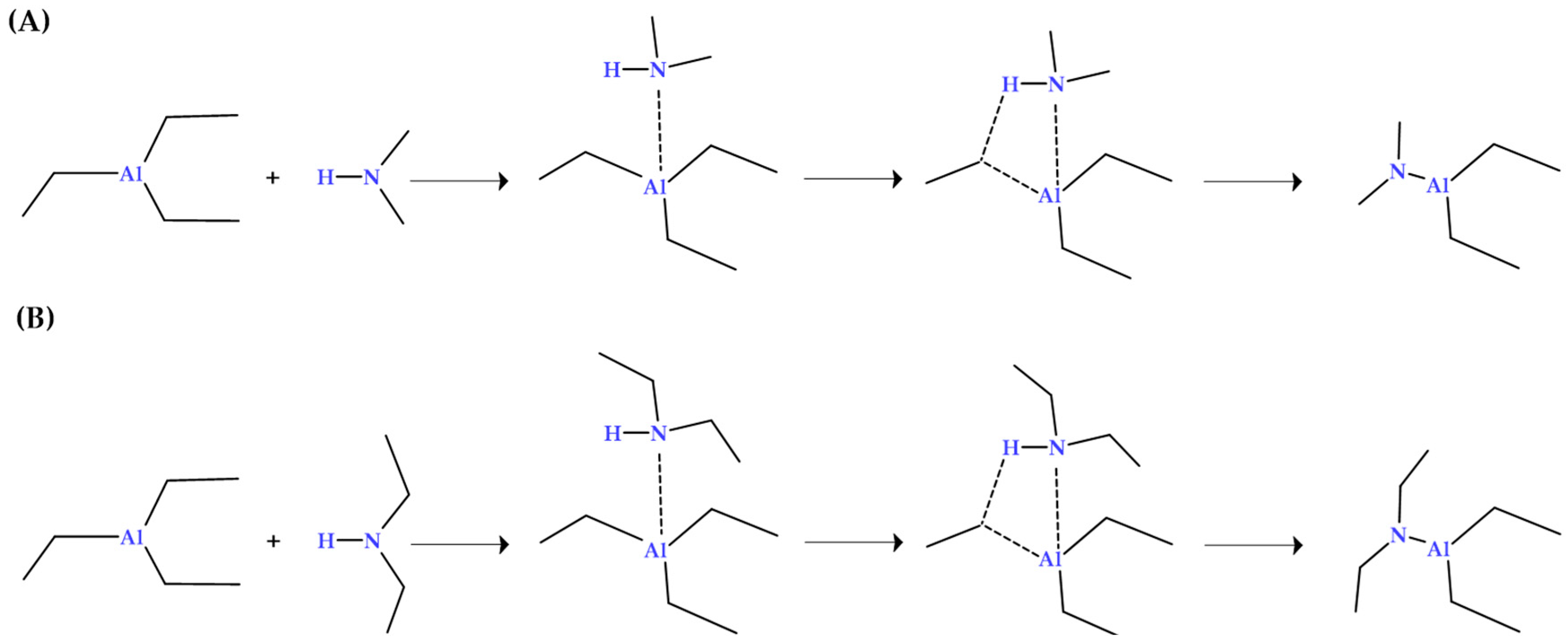
3.4. Mechanism Between Amines and TEAL: Energetic Analysis
Complex Formation Between Toxic Agents and the Co-Catalyst
3.5. Adsorption of the Pre-Formed TEAL–Amine Complex on ZN Catalytic Centers
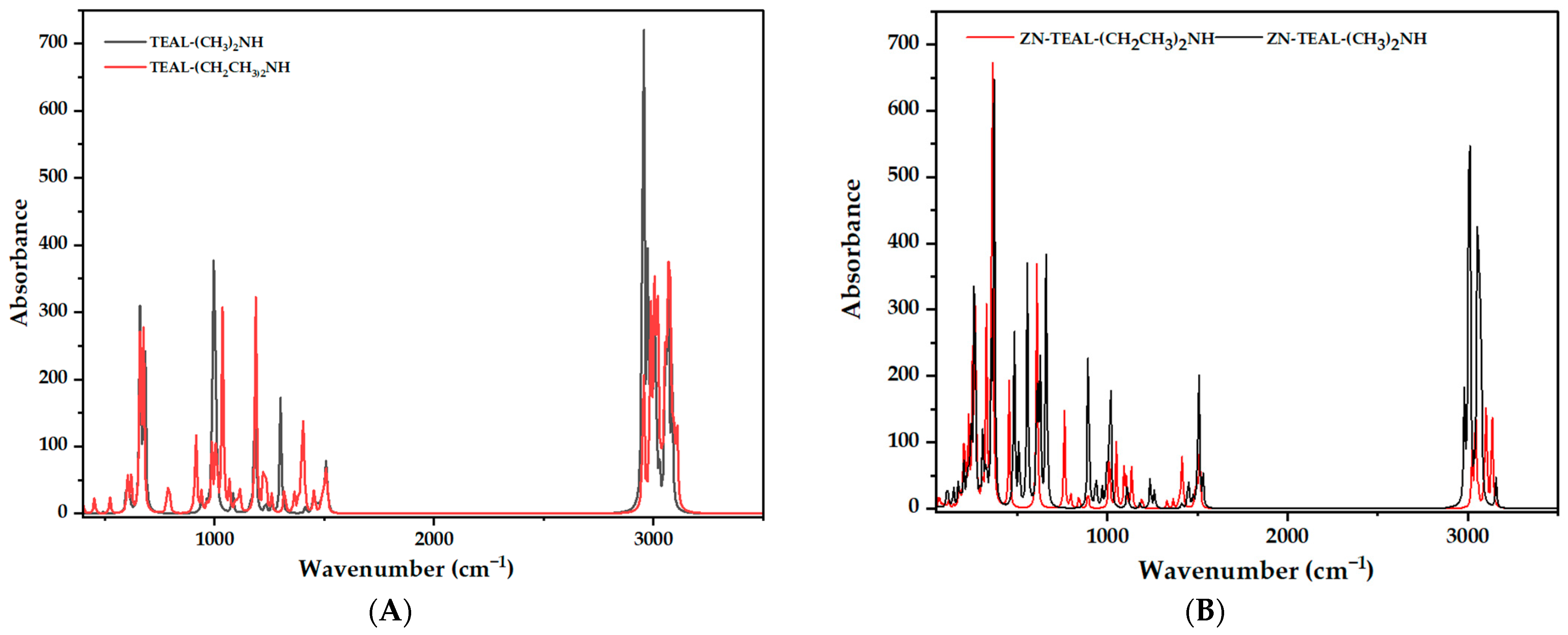
3.6. Spectroscopic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hernández-Fernández, J.; Guerra, Y.; Puello-Polo, E.; Marquez, E. Effects of Different Concentrations of Arsine on the Synthesis and Final Properties of Polypropylene. Polymers 2022, 14, 3123. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Ortega-Toro, R.; Castro-Suarez, J.R. Theoretical–Experimental Study of the Action of Trace Amounts of Formaldehyde, Propionaldehyde, and Butyraldehyde as Inhibitors of the Ziegler–Natta Catalyst and the Synthesis of an Ethylene–Propylene Copolymer. Polymers 2023, 15, 1098. [Google Scholar] [CrossRef]
- Huang, J. Ziegler-Natta catalysts for olefin polymerization: Mechanistic insights from metallocene systems. Prog. Polym. Sci. 1995, 20, 459–526. [Google Scholar] [CrossRef]
- Tangjituabun, K.; Kim, S.Y.; Hiraoka, Y.; Taniike, T.; Terano, M.; Jongsomjit, B.; Praserthdam, P. Effects of various poisoning compounds on the activity and stereospecificity of heterogeneous Ziegler–Natta catalyst. Sci. Technol. Adv. Mater. 2008, 9, 024402. [Google Scholar] [CrossRef]
- Quirk, R.P. Transition metal catalyzed polymerizations: Alkenes and dienes. In Proceedings of the Eleventh Midland Macromolecular Meeting, Midland, MI, USA, 17–21 August 1981; ACS publication: Washington, DC, USA, 1983. [Google Scholar]
- Sacchi, M.C.; Tritto, I.; Locatelli, P. The function of amines in conventional and supported Ziegler-Natta catalysts. Eur. Polym. J. 1988, 24, 137–140. [Google Scholar] [CrossRef]
- Tritto, I.; Sacchi, M.C.; Locatelli, P.; Zannoni, G. Carbon-13 NMR investigation of the interactions between amines and Ziegler-Natta catalysts for.alpha.-olefin polymerization. Macromolecules 1988, 21, 384–387. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Dimethylformamide Impurities as Propylene Polymerization Inhibitor. Polymers 2023, 15, 3806. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Puello-Polo, E.; Márquez, E. Furan as Impurity in Green Ethylene and Its Effects on the Productivity of Random Ethylene–Propylene Copolymer Synthesis and Its Thermal and Mechanical Properties. Polymers 2023, 15, 2264. [Google Scholar] [CrossRef]
- Variation in the Isospecific Active Sites of Internal Donor-Free MgCl2-Supported Ziegler Catalysts: Effect of External Electron Donors-Matsuoka-2001-Macromolecular Rapid Communications-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/1521-3927 (accessed on 20 August 2024).
- Soga, K.; Ohgizawa, M.; Shiono, T.; Lee, D.H. Possibility of mass-transfer resistance in ethylene polymerization with magnesium chloride-supported catalysts. Macromolecules 1991, 24, 1699–1700. [Google Scholar] [CrossRef]
- Kumawat, J.; Gupta, V.K.; Vanka, K. Donor Decomposition by Lewis Acids in Ziegler–Natta Catalyst Systems: A Computational Investigation. Organometallics 2014, 33, 4357–4367. [Google Scholar] [CrossRef]
- Morra, E.; Giamello, E.; Van Doorslaer, S.; Antinucci, G.; D’AMore, M.; Busico, V.; Chiesa, M. Probing the Coordinative Unsaturation and Local Environment of Ti3+ Sites in an Activated High-Yield Ziegler–Natta Catalyst. Angew. Chem. Int. Ed. Engl. 2015, 54, 4857–4860. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.-X.; Yoon, K.-B. Synthesis of polyethylene/exfoliated MoS2 nanocomposites by in situ exfoliation polymerization using Ziegler–Natta catalyst intercalated MoS2. RSC Adv. 2017, 7, 52048–52052. [Google Scholar] [CrossRef]
- Dana, M.; Zohuri, G.H.; Asadi, S. Improvement in volume resistivity and morphology of a blend of polyolefin elastomer with linear low-density polyethylene. Iran. Polym. J. 2019, 28, 587–595. [Google Scholar] [CrossRef]
- Vittoria, A.; Meppelder, A.; Friederichs, N.; Busico, V.; Cipullo, R. Demystifying Ziegler–Natta Catalysts: The Origin of Stereoselectivity. ACS Catal. 2017, 7, 4509–4518. [Google Scholar] [CrossRef]
- Zhao, Z.; Mikenas, T.; Zakharov, V.A.; Nikolaeva, M.; Matsko, M.; Bessudnova, E.; Wu, W. Copolymerization of ethylene with α-olefins over highly active supported ziegler-natta catalyst with vanadium active component. Polyolefins J. 2019, 6, 117–126. [Google Scholar] [CrossRef]
- Kebritchi, A.; Nekoomanesh, M.; Mohammadi, F.; Khonakdar, H.; Wagenknecht, U. Thermal behavior of ethylene/1-octene copolymer fractions at high temperatures: Effect of hexyl branch content. Dir. Open Access J. 2019, 6, 127–138. [Google Scholar] [CrossRef]
- Pasynkiewicz, S. Reactions of organoaluminium compounds with electron donors. Pure Appl. Chem. 1972, 30, 509–522. [Google Scholar] [CrossRef]
- Alkene Polymerization Reactions with Transition Metal Catalysts-Yury Kissin-Google Libros. Available online: https://books.google.com.co/books?hl=es&lr=&id=JjhZiJEY5FsC&oi=fnd&pg=PP1&dq=Y.+V.+Kissin,+Alkene+polymerization+reations+with+transition+metal+catalysts,+Rutgers+%E2%80%93+The+State+University+of+New+Jersey,+USA+2008.&ots=WLjfMW599Z&sig=qrSXtS8KChZBIVkWDrwoBjUYW0Y&redir_esc=y#v=onepage&q&f=false (accessed on 20 August 2024).
- Hoff, R.E.; Mathers, R.T. Handbook of Transition Metal Polymerization Catalysts; John Wiley & Sons: Melbourne, Australia, 2010; p. 575. [Google Scholar]
- Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Evaluation of the Reactivity of Methanol and Hydrogen Sulfide Residues with the Ziegler–Natta Catalyst during Polypropylene Synthesis and Its Effects on Polymer Properties. Polymers 2023, 15, 4061. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Parts per Million of Propanol and Arsine as Responsible for the Poisoning of the Propylene Polymerization Reaction. Polymers 2023, 15, 3619. [Google Scholar] [CrossRef]
- Piovano, A.; Signorile, M.; Braglia, L.; Torelli, P.; Martini, A.; Wada, T.; Takasao, G.; Taniike, T.; Groppo, E. Electronic Properties of Ti Sites in Ziegler–Natta Catalysts. ACS Catal. 2021, 11, 9949–9961. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Mirmohammadi, S.A. A DFT study on the effect of hydrogen in ethylene and propylene polymerization using a Ti-based heterogeneous Ziegler–Natta catalyst. J. Organomet. Chem. 2012, 719, 74–79. [Google Scholar] [CrossRef]
- Correa, A.; Bahri-Laleh, N.; Cavallo, L. How Well Can DFT Reproduce Key Interactions in Ziegler–Natta Systems? Macromol. Chem. Phys. 2013, 214, 1980–1989. [Google Scholar] [CrossRef]
- Bazvand, R.; Bahri-Laleh, N.; Nekoomanesh, M.; Abedini, H. Highly efficient FeCl3 doped Mg(OEt)2/TiCl4-based Ziegler–Natta catalysts for ethylene polymerization. Des. Monomers Polym. 2015, 18, 599–610. [Google Scholar] [CrossRef]
- Nouri-Ahangarani, F.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Karbalaie, M. Synthesis of highly isotactic poly 1-hexene using Fe-doped Mg(OEt)2/TiCl4/ED Ziegler–Natta catalytic system. Des. Monomers Polym. 2016, 19, 394–405. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Hanifpour, A.; Mirmohammadi, S.A.; Poater, A.; Nekoomanesh-Haghighi, M.; Talarico, G.; Cavallo, L. Computational modeling of heterogeneous Ziegler-Natta catalysts for olefins polymerization. Prog. Polym. Sci. 2018, 84, 89–114. [Google Scholar] [CrossRef]
- Guo, X.; Cui, L.; Wang, Y.; Yi, J.; Sun, J.; Liu, Z.; Liu, B. Mechanistic Study on Effect of Electron Donors in Propylene Polymerization Using the Ziegler-Natta Catalyst. J. Phys. Chem. C 2021, 125, 8533–8542. [Google Scholar] [CrossRef]
- Fushimi, M.; Damma, D. The Role of External Donors in Ziegler-Natta Catalysts through Nudged Elastic Band Simulations on Realistic-Scale Models Employing a Universal Neural Network Potential. J. Phys. Chem. C 2024, 128, 6646–6657. [Google Scholar] [CrossRef]
- Benalia, A.; Boukaoud, A.; Amrani, R.; Krid, A. A B3LYP-D3 computational study of electronic, structural and torsional dynamic properties of mono-substituted naphthalenes: The effect of the nature and position of substituent. J. Mol. Model. 2024, 30, 88. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis Meng. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Takasao, G.; Wada, T.; Thakur, A.; Chammingkwan, P.; Terano, M.; Taniike, T. Insight into structural distribution of heterogeneous Ziegler–Natta catalyst from non-empirical structure determination. J. Catal. 2020, 394, 299–306. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1873. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T. Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1934, 1, 104–113. [Google Scholar] [CrossRef]
- Senso, N.; Khaubunsongserm, S.; Jongsomjit, B.; Praserthdam, P. The Influence of Mixed Activators on Ethylene Polymerization and Ethylene/1-Hexene Copolymerization with Silica-Supported Ziegler-Natta Catalyst. Molecules 2010, 15, 9323–9339. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801–3807. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute Hardness: Companion Parameter to Absolute Electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Fallah, M.; Bahri-Laleh, N.; Didehban, K.; Poater, A. Interaction of common cocatalysts in Ziegler–Natta-catalyzed olefin polymerization. Appl. Organomet. Chem. 2020, 34, e5333. [Google Scholar] [CrossRef]
- Yate, L.; Caicedo, J.; Macias, A.H.; Espinoza-Beltrán, F.; Zambrano, G.; Muñoz-Saldaña, J.; Prieto, P. Composition and mechanical properties of AlC, AlN and AlCN thin films obtained by r.f. magnetron sputtering. Surf. Coatings Technol. 2009, 203, 1904–1907. [Google Scholar] [CrossRef]
- Khan, M.; Nowsherwan, G.A.; Shah, A.A.; Riaz, S.; Riaz, M.; Chandio, A.D.; Shah, A.K.; Channa, I.A.; Hussain, S.S.; Ali, R.; et al. A Study of the Structural and Surface Morphology and Photoluminescence of Ni-Doped AlN Thin Films Grown by Co-Sputtering. Nanomaterials 2022, 12, 3919. [Google Scholar] [CrossRef]
- Biswas, M.; Libera, J.A.; Darling, S.B.; Elam, J.W. Polycaprolactone: A Promising Addition to the Sequential Infiltration Synthesis Polymer Family Identified through In Situ Infrared Spectroscopy. ACS Appl. Polym. Mater. 2020, 2, 5501–5510. [Google Scholar] [CrossRef]
- Spagnola, J.C.; Gong, B.; Arvidson, S.A.; Jur, J.S.; Khan, S.A.; Parsons, G.N. Surface and sub-surface reactions during low temperature aluminium oxide atomic layer deposition on fiber-forming polymers. J. Mater. Chem. 2010, 20, 4213–4222. [Google Scholar] [CrossRef]
- Smith, B. How to properly compare spectra, and determining alkane chain length from infrared spectra. Spectroscopy 2015, 30, 40–46. [Google Scholar]
- Shurvell, H.F. Spectra-Structure correlations in the mid-and far-infrared. Handb. Vib. Spectrosc. 2006, 3, 1783–1816. [Google Scholar]



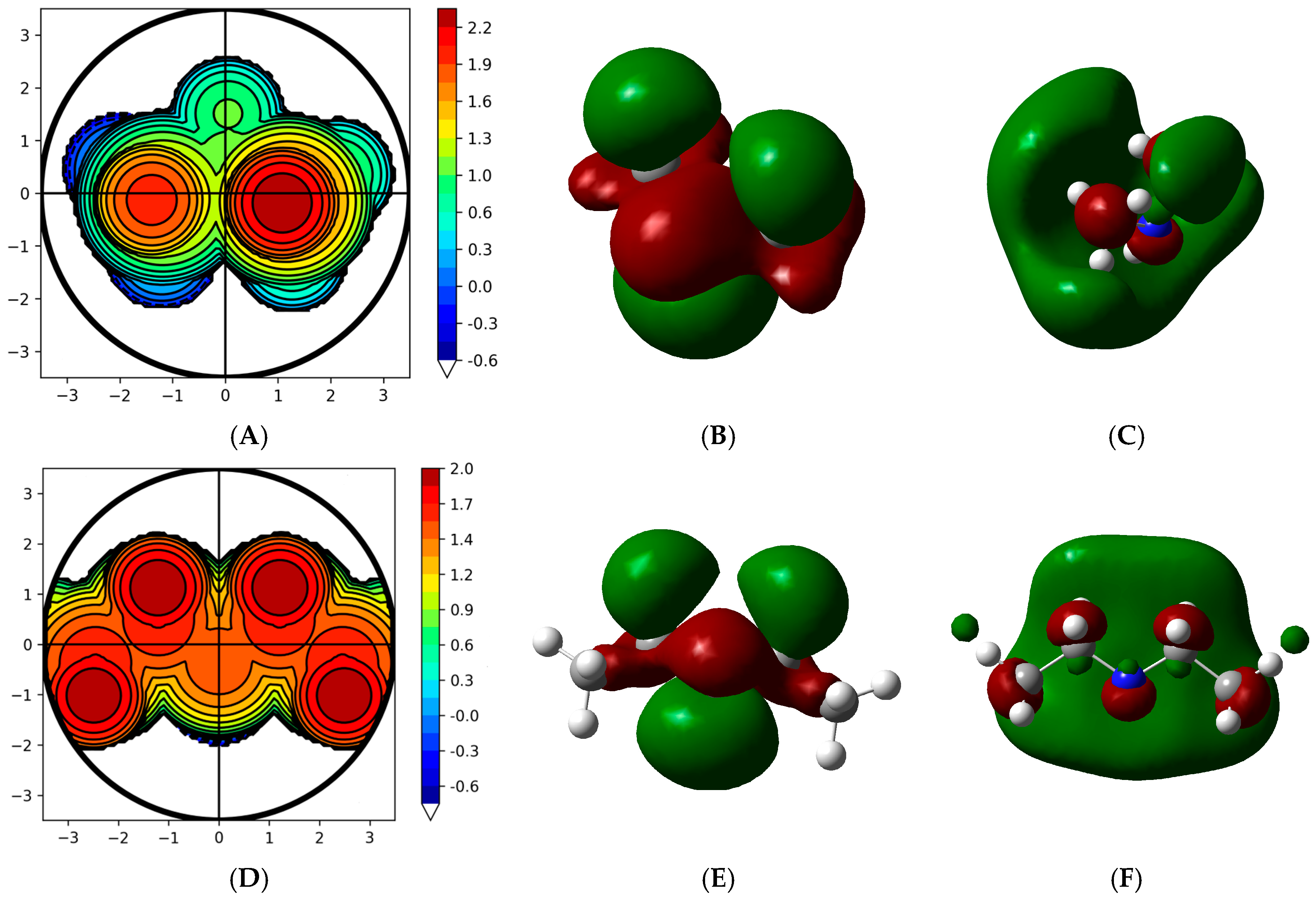




| Materials | PP1 | PP2 | PP3 | PP4 | PP5 | PP6 | PP7 | PP8 |
|---|---|---|---|---|---|---|---|---|
| Catalyst, Kg/h | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Propylene, TM/h | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| TEAl, Kg/h | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Hydrogen, g/h | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Dimethylamine | 0 | 0 | 0 | 0 | 0 | 30 | 60 | 140 |
| Diethylamine | 0 | 40 | 80 | 170 | 0 | 0 | 0 | 0 |
| Selectivity control agent, mol/h | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| T, °C | 70 | 70 | 70 | 70 | 70 | 70 | 70 | 70 |
| Pressure, bar | 27 | 27 | 27 | 27 | 27 | 27 | 27 | 27 |
| Productivity Ziegler–Natta (TM/Kg) | 46 | 43.5 | 41.5 | 36.8 | 46 | 43.4 | 41.8 | 37 |
| % productivity loss | 0 | 5.43 | 9.78 | 20.00 | 0 | 5.65 | 9.13 | 19.57 |
| ΔEHOMO–LUMO (kcal·mol−1) | µ (kcal·mol−1) | η (kcal·mol−1) | Electrophilicity (kcal·mol−1) | Natural Bond Orbital Charge on N | N–C Bond length (Å) | Buried Volume (%VBur) | |
|---|---|---|---|---|---|---|---|
| (CH3)2NH | 136.12 | 73.90 | 136.12 | 20.06 | −0.67260 | 1.457 | 40.2 |
| (C2H5)2NH | 136.39 | 73.64 | 136.39 | 19.87 | −0.67926 | 1.525 | 51.4 |
| Local Descriptors | ||||
|---|---|---|---|---|
| Atom | f+ | f− | f0 |  |
| 1 | 0.0189 | 0.7475 | 0.3832 | |
| 2 | 0.3626 | 0.0260 | 0.1943 | |
| 3 | 0.0357 | 0.0062 | 0.0210 | |
| 4 | 0.0288 | 0.0093 | 0.0190 | |
| 5 | 0.0463 | 0.0825 | 0.0644 | |
| 6 | 0.3625 | 0.0260 | 0.1942 | |
| 7 | 0.0288 | 0.0093 | 0.0190 | |
| 8 | 0.0357 | 0.0062 | 0.0210 | |
| 9 | 0.0463 | 0.0825 | 0.0644 | |
| 10 | 0.0344 | 0.0045 | 0.0195 | |
| Local Descriptors | ||||
|---|---|---|---|---|
| Atom | f+ | f− | f0 | 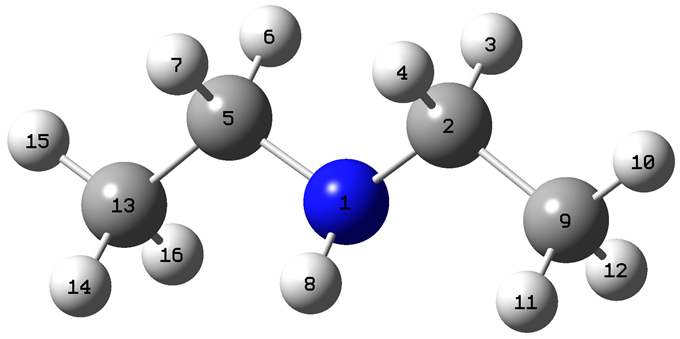 |
| 1 | 0.0160 | 0.6749 | 0.3455 | |
| 2 | 0.1013 | 0.0195 | 0.0604 | |
| 3 | 0.0201 | 0.0224 | 0.0212 | |
| 4 | 0.0368 | 0.0991 | 0.0680 | |
| 5 | 0.1010 | 0.0195 | 0.0602 | |
| 6 | 0.0201 | 0.0223 | 0.0212 | |
| 7 | 0.0369 | 0.0991 | 0.0680 | |
| 8 | 0.0057 | 0.0097 | 0.0077 | |
| 9 | 0.2365 | 0.0097 | 0.1231 | |
| 10 | 0.0157 | 0.0019 | 0.0088 | |
| 11 | 0.0607 | 0.0021 | 0.0314 | |
| 12 | 0.0181 | 0.0030 | 0.0106 | |
| 13 | 0.2364 | 0.0098 | 0.1231 | |
| 14 | 0.0606 | 0.0021 | 0.0314 | |
| 15 | 0.0157 | 0.0019 | 0.0088 | |
| 16 | 0.0182 | 0.0030 | 0.0106 | |
| E (kcal·mol−1) | H (kcal·mol−1) | G (kcal·mol−1) | Ea+ (kcal·mol−1) | Ea− (kcal·mol−1) | ∆G | K | |
|---|---|---|---|---|---|---|---|
| TEAL-(CH3)2NH (R) | −386,200.17 | −386,008.05 | −386,047.27 | 27.11 | −46.08 | −18.97 | 5.79 × 1013 |
| TEAL-(CH3)2NH (TS) | −386,169.11 | −385,980.74 | −386,020.16 | ||||
| TEAL-(CH3)2N (PR) | −386,204.92 | −386,014.50 | −386,066.24 | ||||
| TEAL-(C2H5)2NH (R) | −435,551.70 | −435,322.31 | −435,364.99 | 126.05 | −147.33 | −21.28 | 1.70 × 1015 |
| TEAL-(C2H5)2NH (TS) | −435,423.13 | −435,195.81 | −435,238.94 | ||||
| TEAL-(C2H5)2N (PR) | −435,557.73 | −435,330.11 | −435,386.27 |
| Gas Phase | n-Hexane SMD | |||||||
|---|---|---|---|---|---|---|---|---|
| Structure | E (kcal·mol−1) | H (kcal·mol−1) | G (kcal·mol−1) | Ead (kcal·mol−1) | E (kcal·mol−1) | H (kcal·mol−1) | G (kcal·mol−1) | Ead (kcal·mol−1) |
| ZN | −4,915,906.72 | –4,915,896.61 | –4,915,932.13 | –22.94 | −4,915,957.88 | −4,915,947.78 | −4,915,982.78 | −25.37 |
| TEAL-(CH3)2N | –336,094.15 | –335,953.14 | –335,987.59 | −336,097.67 | −335,956.79 | −335,990.99 | ||
| ZN-(CH3)2N-TEAL | –5,252,023.80 | –5,251,871.47 | –5,251,925.93 | −5,252,080.92 | −5,251,928.06 | −5,251,982.74 | ||
| ZN-(C2H5)2N-TEAL | –5,301,371.90 | –5,301,182.29 | –5,301,239.84 | –18.22 | −5,301,424.39 | −5,301,322.20 | −5,301,366.84 | −14.95 |
| TEAL-(C2H5)2N | –385,446.96 | –385,268.74 | –385,307.63 | −385,451.57 | −385,273.48 | −385,311.98 | ||
| Region (cm−1) | Main Band | Vibrational Assignment | Shift vs. Free Species | Chemical/Structural Significance |
|---|---|---|---|---|
| 3100–2800 (max ≈ 2960) | Set of very intense peaks | Vas and νs. C–H stretches of CH3/CH2 in TEAL (ethyl groups) | Positions essentially unchanged (<5 cm−1); intensity increases owing to the large number of hydrogens | Confirms that the alkyl groups are retained after coordination |
| ≈3260 → 3200 | Moderate peak (often overlapping) | N–H stretch | Downshift of ~30–40 cm−1 relative to free DMA (≈3300 cm−1) | Donation of the N lone pair to Al weakens N–H, lowering its frequency → direct evidence of Al←N coordination |
| 1500–1450 | Medium doublet | CH2 scissoring and asymmetric CH3 deformation | Slight shifts (≤10 cm−1) | Sensitive to minor conformational changes of the ethyl group upon binding to tetracoordinate Al |
| ≈1380 and 1360 | Sharp bands | Symmetric CH3 deformation and N–H “umbrella” | N–H band shows slight intensity decrease | Supports a weaker N–H environment after coordination |
| 1150–1050 (max ≈ 1110) | Medium band | C–N stretch | Shift to higher energy (≈+15 cm−1) vs. free DMA | Higher frequency indicates greater partial π-character in C–N due to back-donation to coordinated N (less σ-density, more π-acceptance) |
| ≈950–850 | Group of peaks | CH3–CH2 rocking/rotation | — | Reflects added rigidity of the ethyl backbone once bound to Al |
| ≈720–640 | New medium band | ν(Al–C)ethyl + CH2 wag components | — | First evidence that ethyl groups remain tetrahedrally bound to Al |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, J.A.H.; Paternina, K.L.O.; Cano-Cuadro, H. Computational Study of Catalytic Poisoning Mechanisms in Polypropylene Polymerization: The Impact of Dimethylamine and Diethylamine on the Deactivation of Ziegler–Natta Catalysts and Co-Catalysts. Polymers 2025, 17, 1834. https://doi.org/10.3390/polym17131834
Fernández JAH, Paternina KLO, Cano-Cuadro H. Computational Study of Catalytic Poisoning Mechanisms in Polypropylene Polymerization: The Impact of Dimethylamine and Diethylamine on the Deactivation of Ziegler–Natta Catalysts and Co-Catalysts. Polymers. 2025; 17(13):1834. https://doi.org/10.3390/polym17131834
Chicago/Turabian StyleFernández, Joaquín Alejandro Hernández, Katherine Liset Ortiz Paternina, and Heidis Cano-Cuadro. 2025. "Computational Study of Catalytic Poisoning Mechanisms in Polypropylene Polymerization: The Impact of Dimethylamine and Diethylamine on the Deactivation of Ziegler–Natta Catalysts and Co-Catalysts" Polymers 17, no. 13: 1834. https://doi.org/10.3390/polym17131834
APA StyleFernández, J. A. H., Paternina, K. L. O., & Cano-Cuadro, H. (2025). Computational Study of Catalytic Poisoning Mechanisms in Polypropylene Polymerization: The Impact of Dimethylamine and Diethylamine on the Deactivation of Ziegler–Natta Catalysts and Co-Catalysts. Polymers, 17(13), 1834. https://doi.org/10.3390/polym17131834








