Additive Manufacturing of Thermoset Elastomer–Thermoplastic Composites Using Dual-Extrusion Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Curing Behavior Study
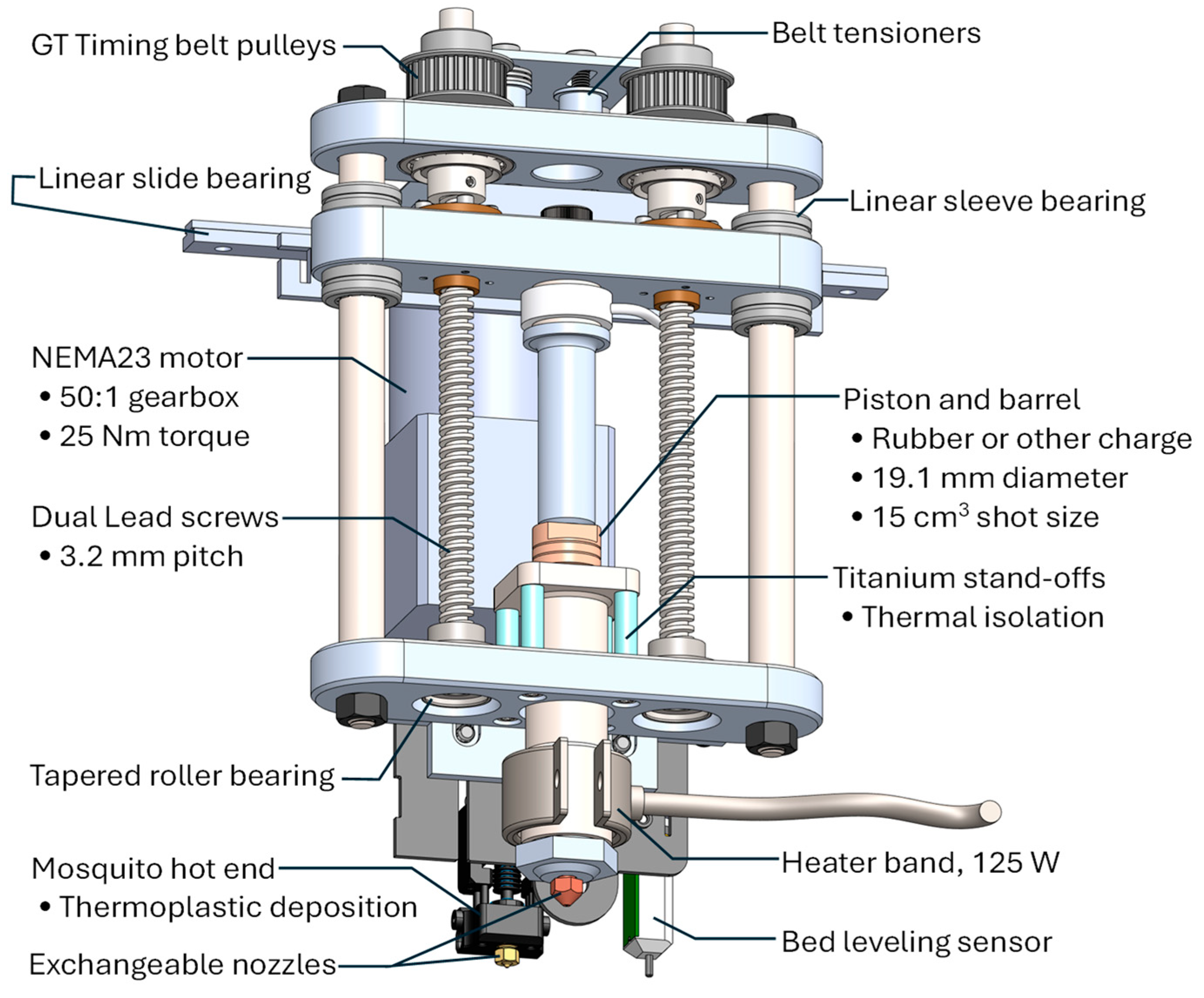
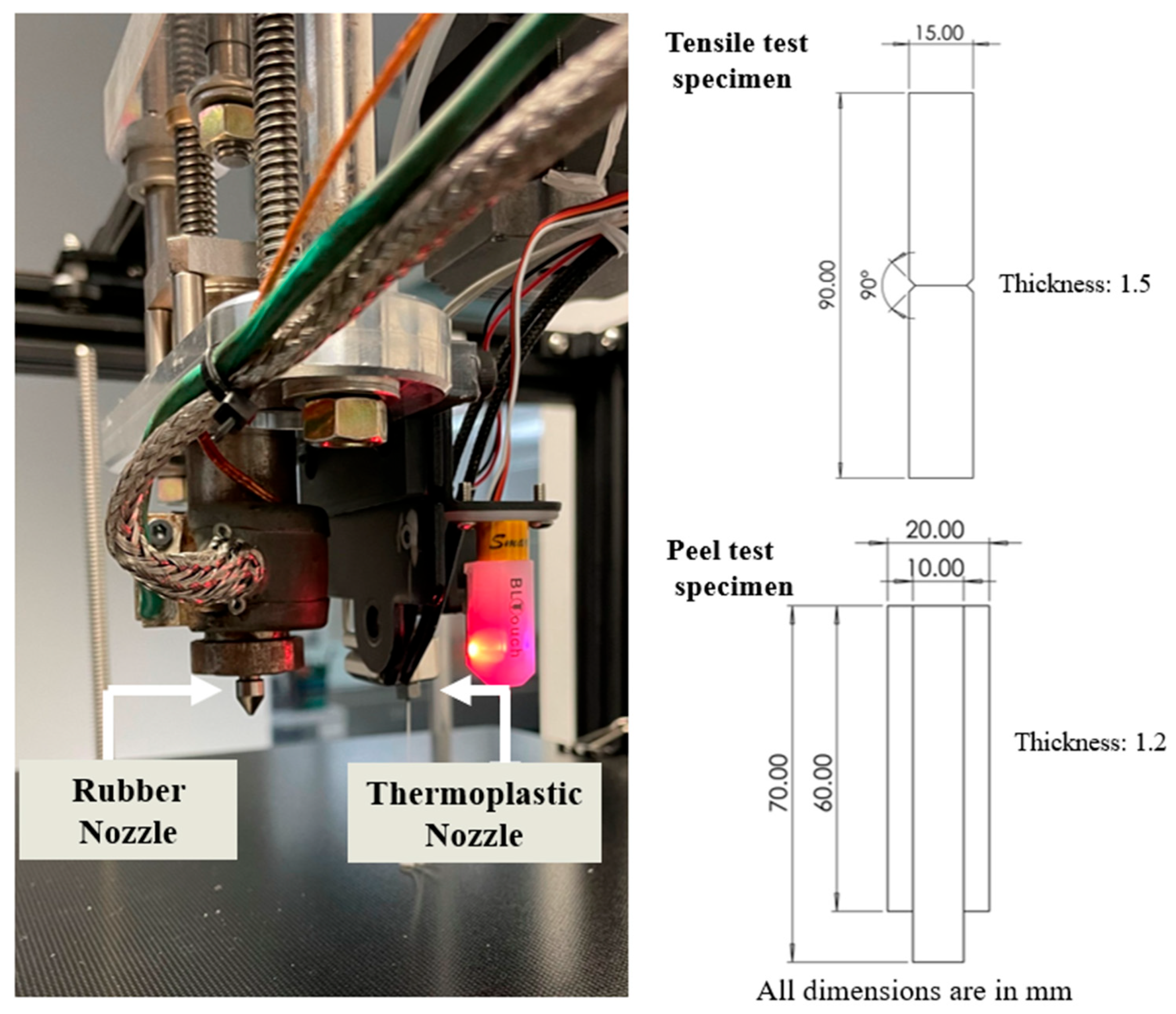
2.3. Multi-Material 3D Printing
2.4. Adhesion Tests
2.5. SEM and Energy-Dispersive X-Ray Spectroscopy
3. Results and Discussion
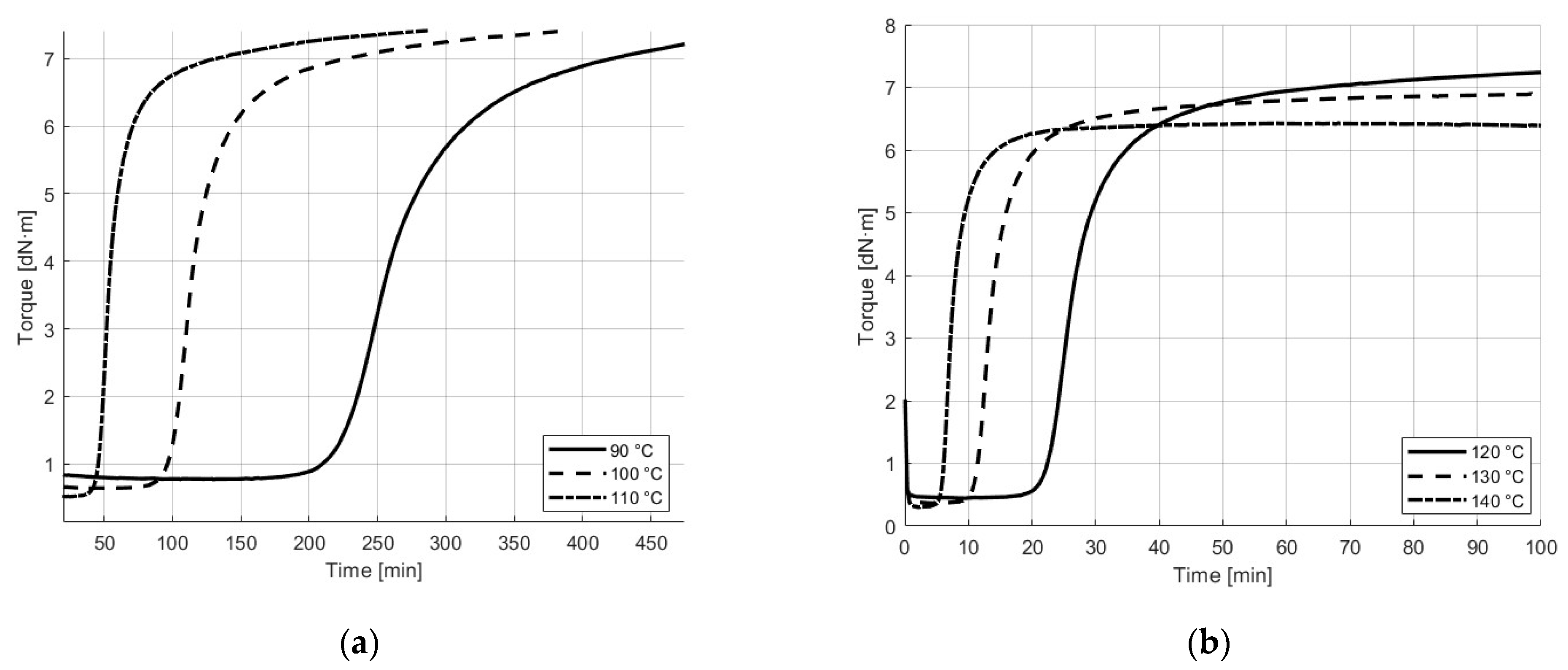
3.1. Rheology and Cure of NBR Compound at Different Printing and Curing Temperatures
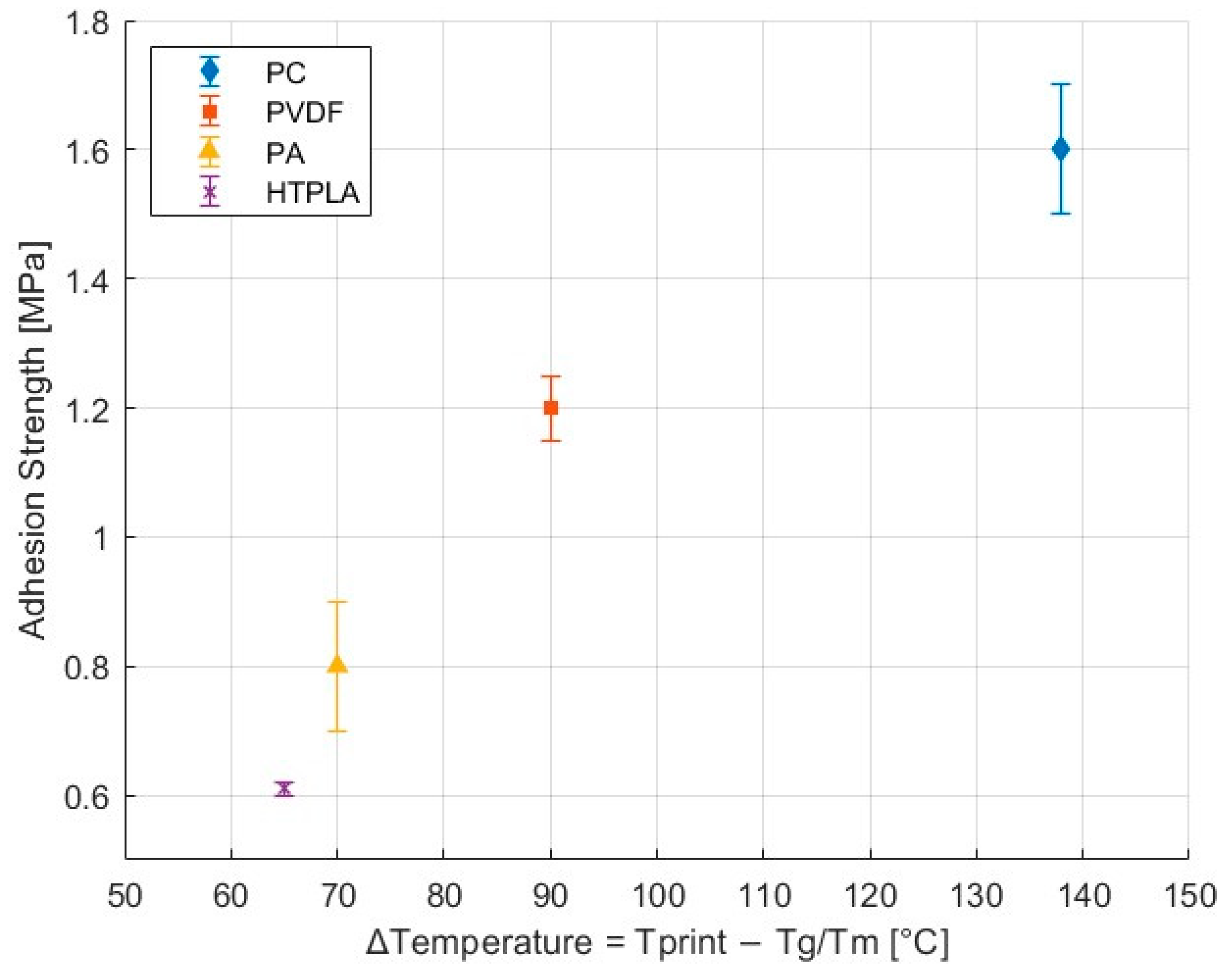
3.2. Tensile Adhesion Test Results
3.2.1. NBR with Thermoplastics
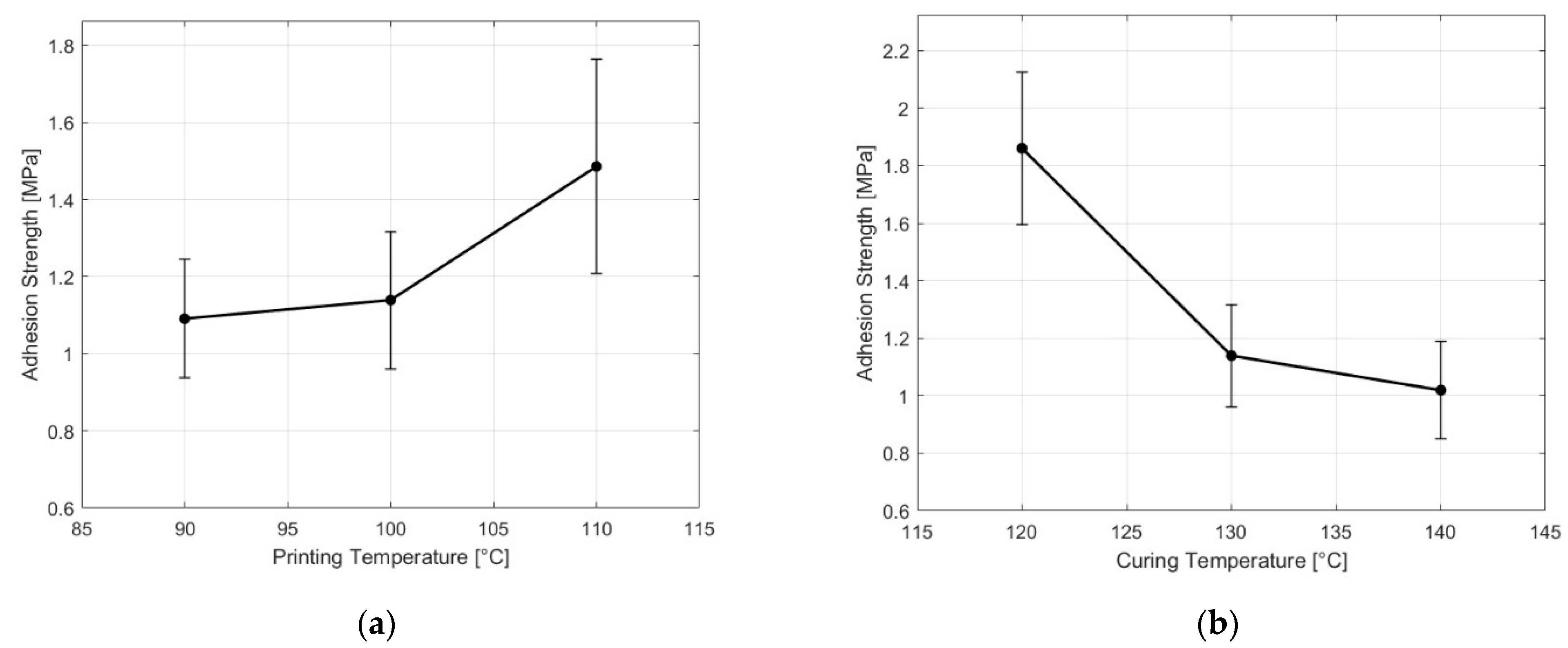
3.2.2. Effect of Processing Parameters on the Adhesion Strength of NBR and PC
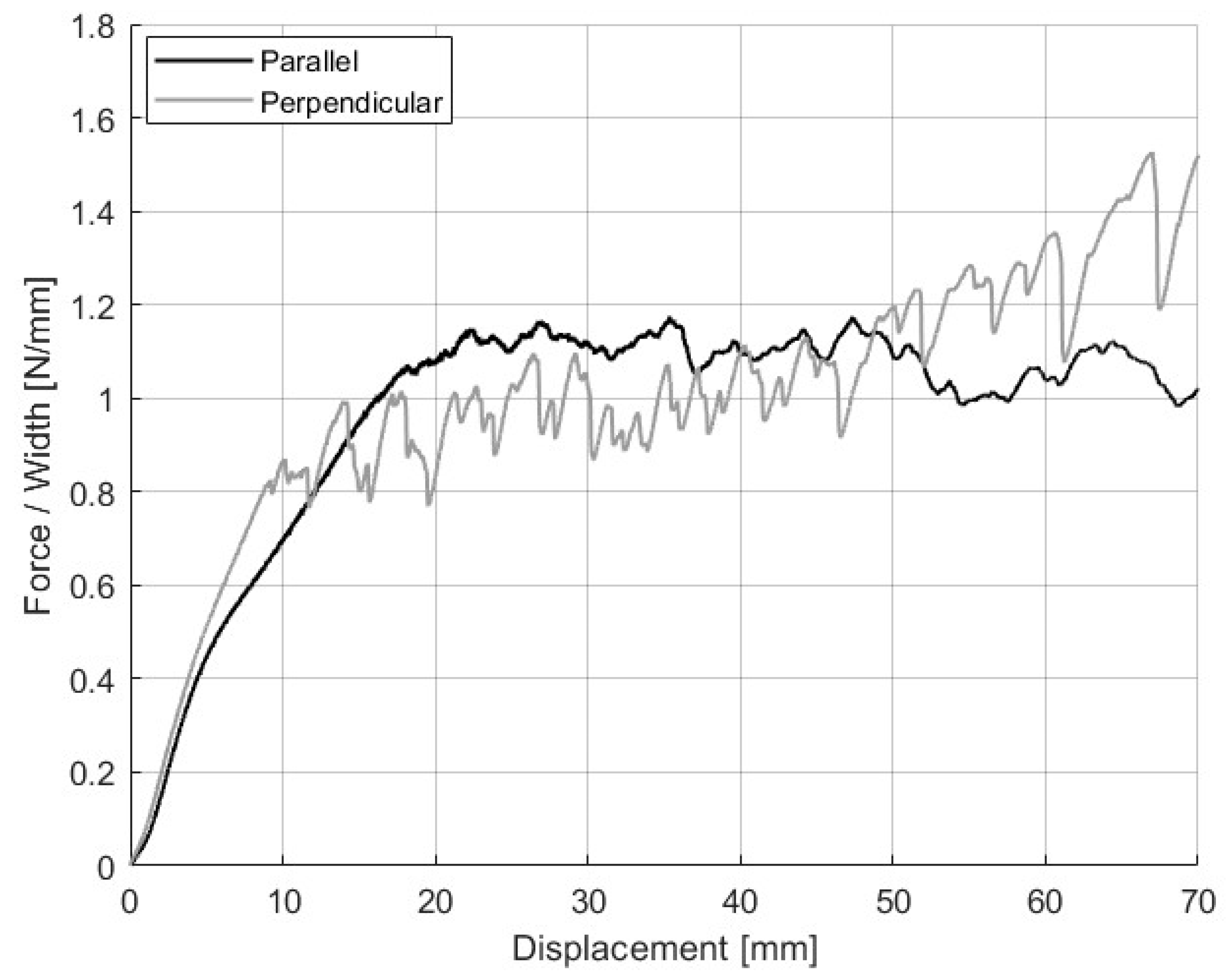
3.3. Raster Orientation Effect on the Adhesion Strength Between NBR and PC
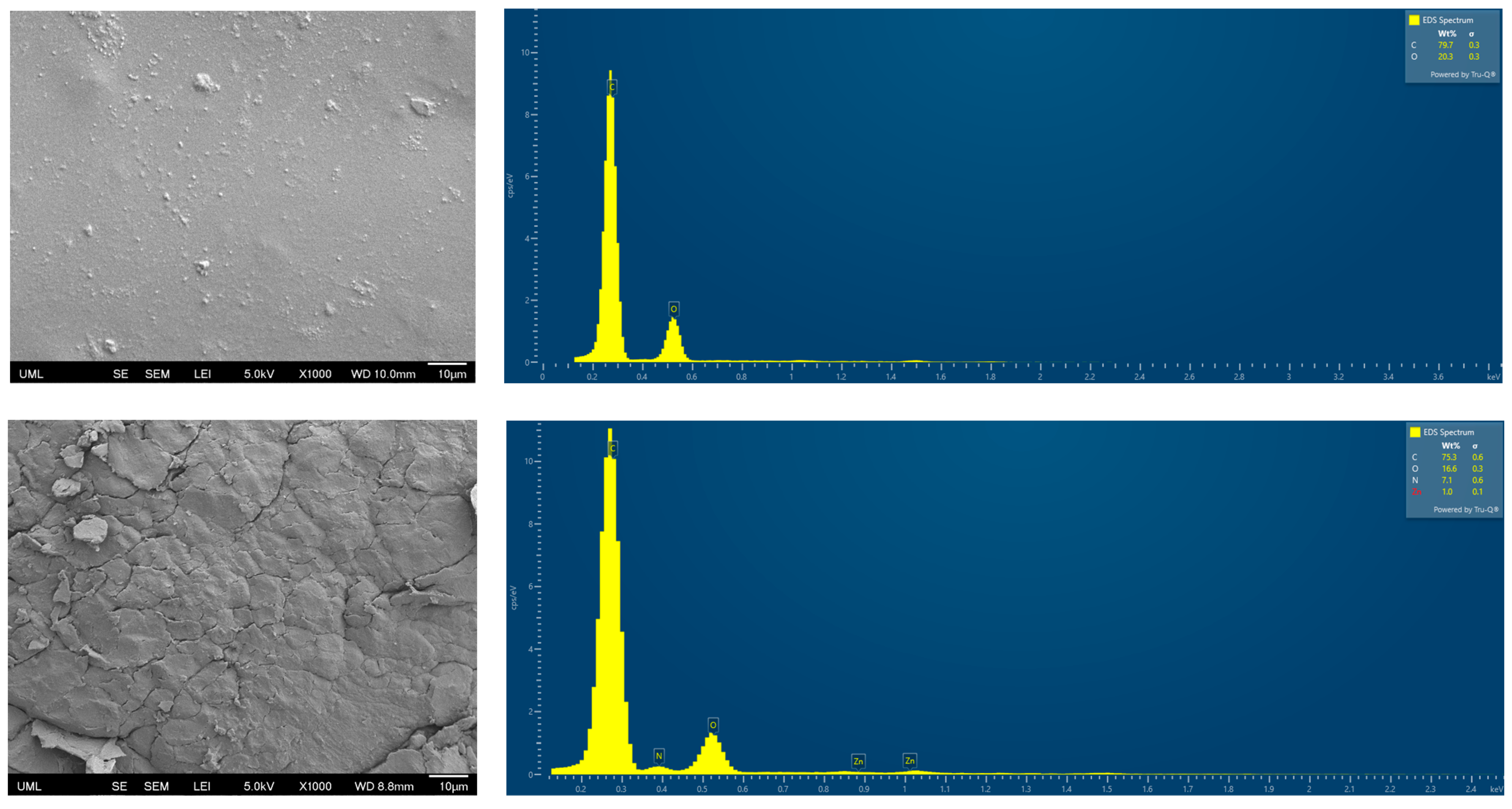
3.4. SEM and Energy-Dispersive X-Ray Spectroscopy (EDS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Herzberger, J.; Sirrine, J.M.; Williams, C.B.; Long, T.E.L. Polymer Design for 3D Printing Elastomers: Recent Advances in Structure, Properties, and Printing. Prog. Polym. Sci. 2019, 97, 101144. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Duran, M.M.; Moro, G.; Zhang, Y.; Islam, A. 3D printing of silicone and polyurethane elastomers for medical device application: A review. Adv. Ind. Manuf. Eng. 2023, 7, 100125. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Fu, J.; He, Y. A Review of 3D Printing Technologies for Soft Polymer Materials. Adv. Funct. Mater. 2020, 30, 2000187. [Google Scholar] [CrossRef]
- Han, D.; Lee, H. Recent advances in multi-material additive manufacturing: Methods and applications. Curr. Opin. Chem. Eng. 2020, 28, 158–166. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Additive Manufacturing Technologies; Springer Nature: Dordrecht, The Netherlands, 2020; pp. 1–675. [Google Scholar] [CrossRef]
- Kumar, N.; Jain, P.K.; Tandon, P.; Pandey, P.M. Additive manufacturing of flexible electrically conductive polymer composites via CNC-assisted fused layer modeling process. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 175. [Google Scholar] [CrossRef]
- Kazmer, D.O.; Kodra, S.; Mubasshir, A.A.; Keaney, E.E.; Mead, J.L. Additive ram material extrusion and diddling of fully compounded thermoset nitrile rubber. Polym. Compos. 2021, 42, 5237–5248. [Google Scholar] [CrossRef]
- Mubasshir, A.A. Additive Manufacturing of Fully Compounded Thermoset Elastomers. Ph.D. Thesis, University of Massachusetts Lowell, Lowell, MA, USA, 2024. [Google Scholar]
- Netto, J.M.J.; Idogava, H.T.; Santos, L.E.F.; Silveira, Z.d.C.; Romio, P.; Alves, J.L. Screw-assisted 3D printing with granulated materials: A systematic review. Int. J. Adv. Manuf. Technol. 2021, 115, 2711–2727. [Google Scholar] [CrossRef]
- Leineweber, S.; Sundermann, L.; Bindszus, L.; Overmeyer, L.; Klie, B.; Wittek, H.; Giese, U. Additive Manufacturing and Vulcanization of Carbon Black-Filled Natural Rubber-Based Components. Rubber Chem. Technol. 2022, 95, 46–57. [Google Scholar] [CrossRef]
- Periyasamy, M.; Mubasshir, A.A.; Kodra, S.; Chandramouli, S.; Campbell, R.; Kazmer, D.O.; Mead, J.L. From Formulation to Application: Effects of Plasticizer on the Printability of Fluoro Elastomer Compounds and Additive Manufacturing of Specialized Seals. Micromachines 2024, 15, 622. [Google Scholar] [CrossRef]
- Verma, A.; Kapil, A.; Klobčar, D.; Sharma, A. A Review on Multiplicity in Multi-Material Additive Manufacturing: Process, Capability, Scale, and Structure. Materials 2023, 16, 5246. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Gokcekaya, O.; Md Masum Billah, K.; Ertugrul, O.; Jiang, J.; Sun, J.; Hussain, S. Multi-material additive manufacturing: A systematic review of design, properties, applications, challenges, and 3D printing of materials and cellular metamaterials. Mater. Des. 2023, 226, 111661. [Google Scholar] [CrossRef]
- Georgopoulou, A.; Vanderborght, B.; Clemens, F. Multi-material 3D Printing of Thermoplastic Elastomers for Development of Soft Robotic Structures with Integrated Sensor Elements. In Proceedings of the Industrializing Additive Manufacturing (AMPA 2020), Zurich, Switzerland, 1–3 September 2020; Springer International Publishing: Cham, Switzerland, 2021; Volume 2, pp. 67–81. [Google Scholar]
- Setter, R.; Wudy, K. Investigation of the bonding behavior between thermosets and thermoplastic elastomers in multi-material additive manufacturing. Polym. Test. 2024, 132, 108366. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Zolfagharian, A.; Bodaghi, M.; Baghani, M. A New Strategy for Achieving Shape Memory Effects in 4D Printed Two-Layer Composite Structures. Polymers 2022, 14, 5446. [Google Scholar] [CrossRef]
- Liu, Q.; Duan, C.; Richter, F.; Shen, W.; Wu, D. Interfacial behavior between thermoplastics and thermosets fabricated by material extrusion-based multi-process additive manufacturing. Addit. Manuf. 2024, 96, 104568. [Google Scholar] [CrossRef]
- Bex, G.J.; Desplentere, F.; De Keyzer, J.; Van Bael, A. Two-component injection moulding of thermoset rubber in combination with thermoplastics by thermally separated mould cavities and rapid heat cycling. Int. J. Adv. Manuf. Technol. 2017, 92, 2599–2607. [Google Scholar] [CrossRef]
- Six, W. Prediction of Interface Strength in 2K Injection Molding of Thermoset Rubbers and Thermoplastics. Ph.D. Thesis, Arenberg Doctoral School, Heverlee, Belgium, 2020. [Google Scholar]
- Sundermann, L.; Klie, B.; Giese, U.; Leineweber, S.; Overmeyer, L. Development, construction and testing of a 3D-printing-system for additive manufacturing of carbon black filled rubber compounds. KGK Kautsch. Gummi Kunstst. 2020, 73, 30–35. [Google Scholar]
- D2084-19a; A Standard Test Method for Rubber Property—Vulcanization Using Oscillating Disk Cure Meter. ASTM International: West Conshohocken, PA, USA, 2019; Volume i, pp. 3–5. [CrossRef]
- D429-14(2023); Standard Test Methods for Rubber Property—Adhesion to Flexible Substrate. ASTM International: West Conshohocken, PA, USA, 1998; Volume 09.01, p. 22. [CrossRef]
- Ignatz-Hoover, F.; To, B.H. Vulcanization. In Rubber Compounding Chemistry and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; Volume 17, pp. 461–522. [Google Scholar] [CrossRef]
- Mark, J.E. Thermoset Elastomers, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323390408. [Google Scholar]
- Rodgers, B.; Waddell, W.H.; Klingensmith, W. Rubber Compounding; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9781482235500. [Google Scholar]
- Khimi, S.R.; Pickering, K.L. A new method to predict optimum cure time of rubber compound using dynamic mechanical analysis. J. Appl. Polym. Sci. 2014, 131, 1–6. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Higgins, J.S.; Lipson, J.E.G.; White, R.P. A simple approach to polymer mixture miscibility. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1009–1025. [Google Scholar] [CrossRef]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9789400760646. [Google Scholar]
- Wu, S. Interfacial Energy, Structure, and Adhesion Between Polymers; Academic Press, Inc.: Cambridge, MA, USA, 1978; Volume 1, ISBN 0125468016. [Google Scholar]
- Helfand, E.; Tagami, Y. Theory of the interface between immiscible polymers. II. J. Chem. Phys. 1972, 56, 3592–3601. [Google Scholar] [CrossRef]
- Helfand, E.; Sapse, A.M. Theory of unsymmetric polymer-polymer interfaces. J. Chem. Phys. 1975, 62, 1327–1331. [Google Scholar] [CrossRef]
- Roe, R.J. Theory of the interface between polymers or polymer solutions. I. Two components system. J. Chem. Phys. 1975, 62, 490–499. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9781420006834. [Google Scholar]
- Su, S. Prediction of the miscibility of pbat/pla blends. Polymers 2021, 13, 2339. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient. In Their Importance in Surface Coating Formulation; Danish Technical Press: Copenhagen, Denmark, 1967; p. 104. [Google Scholar]
- Hansen, C.M. 50 Years with solubility parameters—Past and future. Prog. Org. Coat. 2004, 51, 77–84. [Google Scholar] [CrossRef]
- Stefanis, E.; Panayiotou, C. Prediction of hansen solubility parameters with a new group-contribution method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Liu, S.; Jing, Y.; Tu, J.; Zou, H.; Yong, Z.; Liu, G. Systematic investigation on the swelling behaviors of acrylonitrile-butadiene rubber via solubility parameter and Flory-Huggins interaction parameter. J. Appl. Polym. Sci. 2022, 139, 52172. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, G. Systematic Investigation on the Swelling Response and Oil Resistance of NBR Using the Prediction Models Determined by the Modified Flory–Huggins Interaction Parameter. Polymers 2024, 16, 2696. [Google Scholar] [CrossRef]
- Abbott, S. Chemical Compatibility of Poly(Lactic Acid): A Practical Framework Using Hansen Solubility Parameters. In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; Wiley: Hoboken, NJ, USA, 2010; pp. 83–95. [Google Scholar] [CrossRef]
- Patel, K.G.; Maynard, R.K.; Ferguson, L.S.; Broich, M.L.; Bledsoe, J.C.; Wood, C.C.; Crane, G.H.; Bramhall, J.A.; Rust, J.M.; Williams-Rhaesa, A.; et al. Experimentally Determined Hansen Solubility Parameters of Biobased and Biodegradable Polyesters. ACS Sustain. Chem. Eng. 2024, 12, 2386–2393. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lu, C.; Fu, J.; Huang, Y.; Zheng, Y. Interfacial bonding during multi-material fused deposition modeling (FDM) process due to inter-molecular diffusion. Mater. Des. 2018, 150, 104–112. [Google Scholar] [CrossRef]
- Lo, C.; Laabs, F.C.; Narasimhan, B. Interfacial Adhesion Mechanisms in Incompatible Semicrystalline Polymer Systems. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 2667–2679. [Google Scholar] [CrossRef]
- Islam, A.; Hansen, H.N.; Bondo, M. Experimental investigation of the factors influencing the polymer–polymer bond strength during two-component injection moulding. Int. J. Adv. Manuf. Technol. 2010, 50, 101–111. [Google Scholar] [CrossRef]
- Carella, A.R.; Alonso, C.; Merino, J.C.; Pastor, J.M. Sequential injection molding of thermoplastic polymers. Analysis of processing parameters for optimal bonding conditions. Polym. Eng. Sci. 2002, 42, 2172–2181. [Google Scholar] [CrossRef]
- Candal, M.V.; Gordillo, A.; Santana, O.O.; Sánchez, J.J. Study of the adhesion strength on overmoulded plastic materials using the essential work of interfacial fracture (EWIF) concept. J. Mater. Sci. 2008, 43, 5052–5060. [Google Scholar] [CrossRef]
- Bex, G.; Six, W.; Laing, B.; De Keyzer, J.; Desplentere, F.; Van Bael, A. Effect of process parameters on the adhesion strength in two-component injection molding of thermoset rubbers and thermoplastics. J. Appl. Polym. Sci. 2018, 135, 46495. [Google Scholar] [CrossRef]
- Wu, J.K.; Zheng, K.W.; Nie, X.C.; Ge, H.R.; Wang, Q.Y.; Xu, J.T. Promoters for Improved Adhesion Strength between Addition-Cured Liquid Silicone Rubber and Low-Melting-Point Thermoplastic Polyurethanes. Materials 2022, 15, 991. [Google Scholar] [CrossRef]
- Aradian, A.; Raphaël, E.; De Gennes, P.-G. A Scaling Theory of the Competition between Interdiffusion and Cross-Linking at Polymer Interfaces. Macromolecules 2002, 35, 4036–4043. [Google Scholar] [CrossRef]
- Prager, S.; Tirrell, M. The healing process at polymer-polymer interfaces. J. Chem. Phys. 1981, 75, 5194–5198. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Zhu, W.; Peng, Y.; Ahzi, S.; Chinesta, F. Contributions of interfaces on the mechanical behavior of 3D printed continuous fiber reinforced composites. Constr. Build. Mater. 2022, 340, 127842. [Google Scholar] [CrossRef]
- Hartung, M.; Heim, H.P. UVC Irradiation as a Surface Treatment of Polycarbonate to Generate Adhesion to Liquid Silicone Rubber in an Overmolding Process. Polymers 2024, 16, 1141. [Google Scholar] [CrossRef]
- Adhesion-modified TPE Solutions for Overmolding Strong Bonds for Rigid and Soft-touch Plastics. 2022. Available online: https://www.trinseo.com (accessed on 13 June 2025).
| Material | Brand Name | Heat Deflection Temperature (°C) | Printing Temperature (°C) | Bed Temperature (°C) |
|---|---|---|---|---|
| Polycarbonate (PC) | 3DXMax PC (3DXTECH, Grand Rapids, MI, USA) | 135 | 275–295 | 90 |
| Polyvinylidene Fluoride (PVDF) | FluorX™ PVDF (3DXTECH, Grand Rapids, MI, USA) | 158 | 255–275 | 80 |
| High-Temperature Polylactic Acid (HTPLA) | Proto-pasta HTPLA (Protoplant, Vancouver, WA, USA) | 140 | 200–210 | 60 |
| Polyamide 6 (PA6) | AmideX PA6 Copolymer (3DXTECH, Grand Rapids, MI, USA) | 140 | 270 | 80 |
| Component | Parts per Hundred Rubber (PHR) |
|---|---|
| High molecular weight NBR | 50 |
| Low molecular weight NBR | 50 |
| Carbon Black N330 | 60 |
| Zinc oxide | 4 |
| CBS | 2 |
| Stearic acid | 0.5 |
| Sulfur | 1 |
| Component | Thermoset | Thermoplastic |
|---|---|---|
| Printing speed (mm/s) | 10 | 12 |
| Nozzle size (mm) | 0.8 | 0.4 |
| Layer height (mm) | 0.3 | 0.3 |
| Variable | Printing Temperature (°C) | Curing Temperature (°C) |
|---|---|---|
| Control sample | 100 | 130 |
| Effect of printing temperature | 110 | 130 |
| 90 | 130 | |
| Effect of curing temperature | 100 | 140 |
| 100 | 120 |
| Material | 90 °C | 100 °C | 110 °C | 120 °C | 130 °C | 140 °C |
|---|---|---|---|---|---|---|
| Minimum torque, ML (dN·m) | 0.8 | 0.6 | 0.5 | 0.5 | 0.4 | 0.3 |
| Maximum torque, MH (dN·m) | 7.3 | 7.6 | 7.6 | 7.5 | 6.9 | 6.4 |
| Scorch time, ts2 (min) | 245 | 108 | 51 | 25 | 13 | 7 |
| Cure time, t90 (min) | 359 | 200 | 109 | 50 | 19 | 13 |
| NBR with | Adhesion Strength (MPa) |
|---|---|
| PC | 1.6 ± 0.1 |
| PVDF | 1.2 ± 0.1 |
| PA | 0.8 ± 0.1 |
| HTPLA | 0.6 ± 0.0 |
| Polymer | δD (MPa1/2) | δP (MPa1/2) | δH (MPa1/2) | Ro (MPa1/2) | Ra (MPa1/2) | RED | Adhesion Strength (MPa) |
|---|---|---|---|---|---|---|---|
| NBR 1 | 19.6 | 8.7–9.6 | 6.3–6.7 | - | - | - | |
| PC 2 | 19.1 | 10.9 | 5.1 | 12.1 | 2.29–2.70 | 0.19–0.22 | 1.6 ± 0.1 |
| PVDF 2 | 17 | 12.1 | 10.2 | 4.1 | 6.75–7.34 | 1.65–1.79 | 1.2 ± 0.1 |
| PA 2 | 17 | 3.4 | 10.6 | 5.1 | 8.58–8.98 | 1.68–1.76 | 0.8 ± 0.1 |
| HTPLA 3 | 17.9–18.6 | 9–9.9 | 5.9–6 | 10.7 | 2.14–3.54 | 0.20–0.33 | 0.6 ± 0.0 |
| Sample | Printing Temperature (°C) | Curing Temperature (°C) | Adhesion Strength (MPa) |
|---|---|---|---|
| Control | 100 | 130 | 1.1 ± 0.2 |
| Higher PT | 110 | 130 | 1.5 ± 0.3 |
| Lower PT | 90 | 130 | 1.1 ± 0.2 |
| Higher CT | 100 | 140 | 1.0 ± 0.2 |
| Lower CT | 100 | 120 | 1.9 ± 0.3 |
| Print Direction (°) | Description | Average Force/Width (N/mm) |
|---|---|---|
| 0 | Raster lines parallel to the pull direction | 0.8 ± 0.3 |
| 90 | Raster lines perpendicular to the pull direction | 1.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz Armas, N.; Bhandari, G.; Kodra, S.; Zhang, J.; Kazmer, D.; Mead, J. Additive Manufacturing of Thermoset Elastomer–Thermoplastic Composites Using Dual-Extrusion Printing. Polymers 2025, 17, 1800. https://doi.org/10.3390/polym17131800
Diaz Armas N, Bhandari G, Kodra S, Zhang J, Kazmer D, Mead J. Additive Manufacturing of Thermoset Elastomer–Thermoplastic Composites Using Dual-Extrusion Printing. Polymers. 2025; 17(13):1800. https://doi.org/10.3390/polym17131800
Chicago/Turabian StyleDiaz Armas, Nathalia, Geet Bhandari, Stiven Kodra, Jinde Zhang, David Kazmer, and Joey Mead. 2025. "Additive Manufacturing of Thermoset Elastomer–Thermoplastic Composites Using Dual-Extrusion Printing" Polymers 17, no. 13: 1800. https://doi.org/10.3390/polym17131800
APA StyleDiaz Armas, N., Bhandari, G., Kodra, S., Zhang, J., Kazmer, D., & Mead, J. (2025). Additive Manufacturing of Thermoset Elastomer–Thermoplastic Composites Using Dual-Extrusion Printing. Polymers, 17(13), 1800. https://doi.org/10.3390/polym17131800







