Synthesis, Characterization, and Adhesion on Galvanized Steel of Original Thermoset Adhesive Films Based on Aza-Michael Addition Reaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
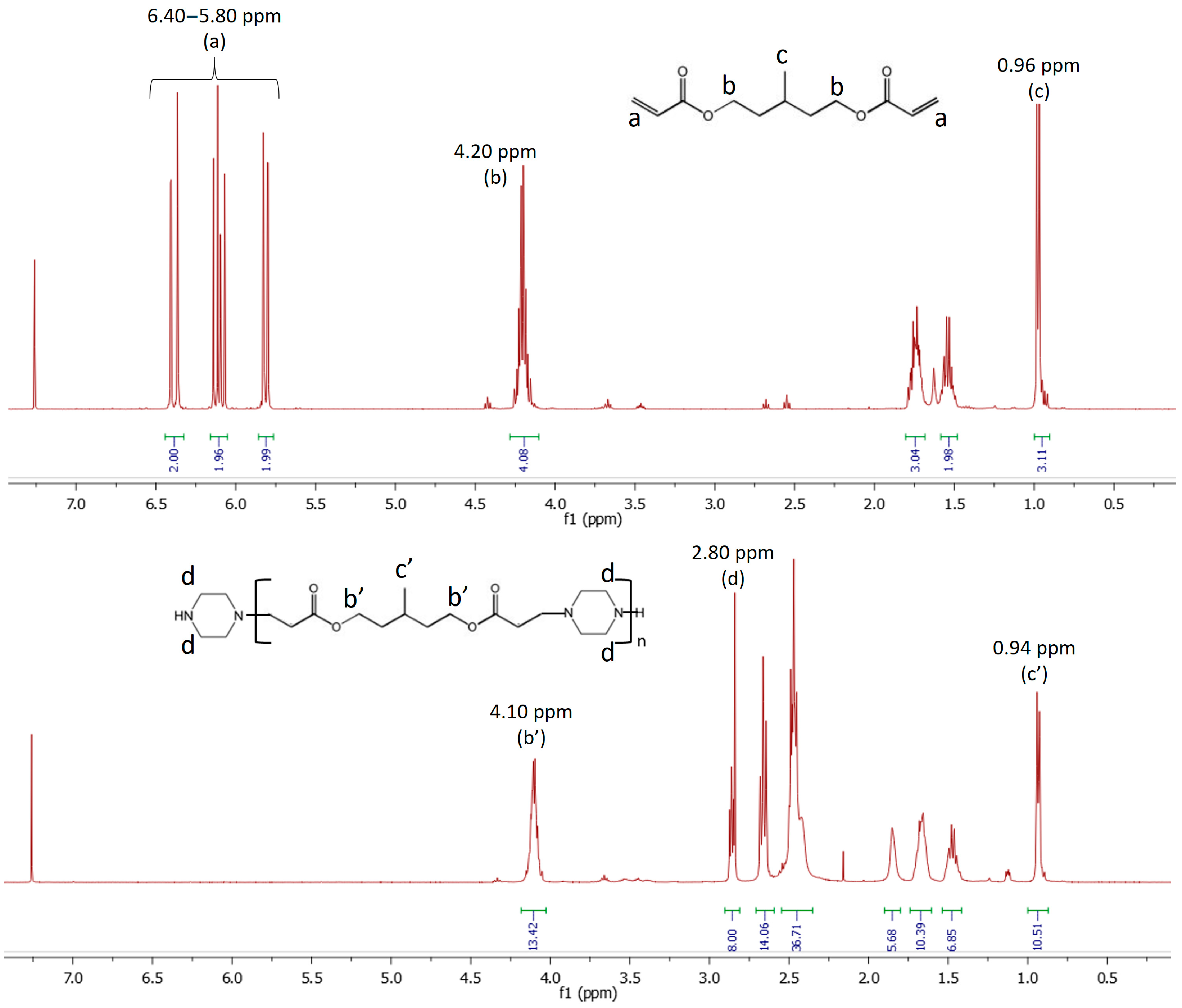
2.2. Synthesis of Linear Difunctional Secondary Amines Prepolymers
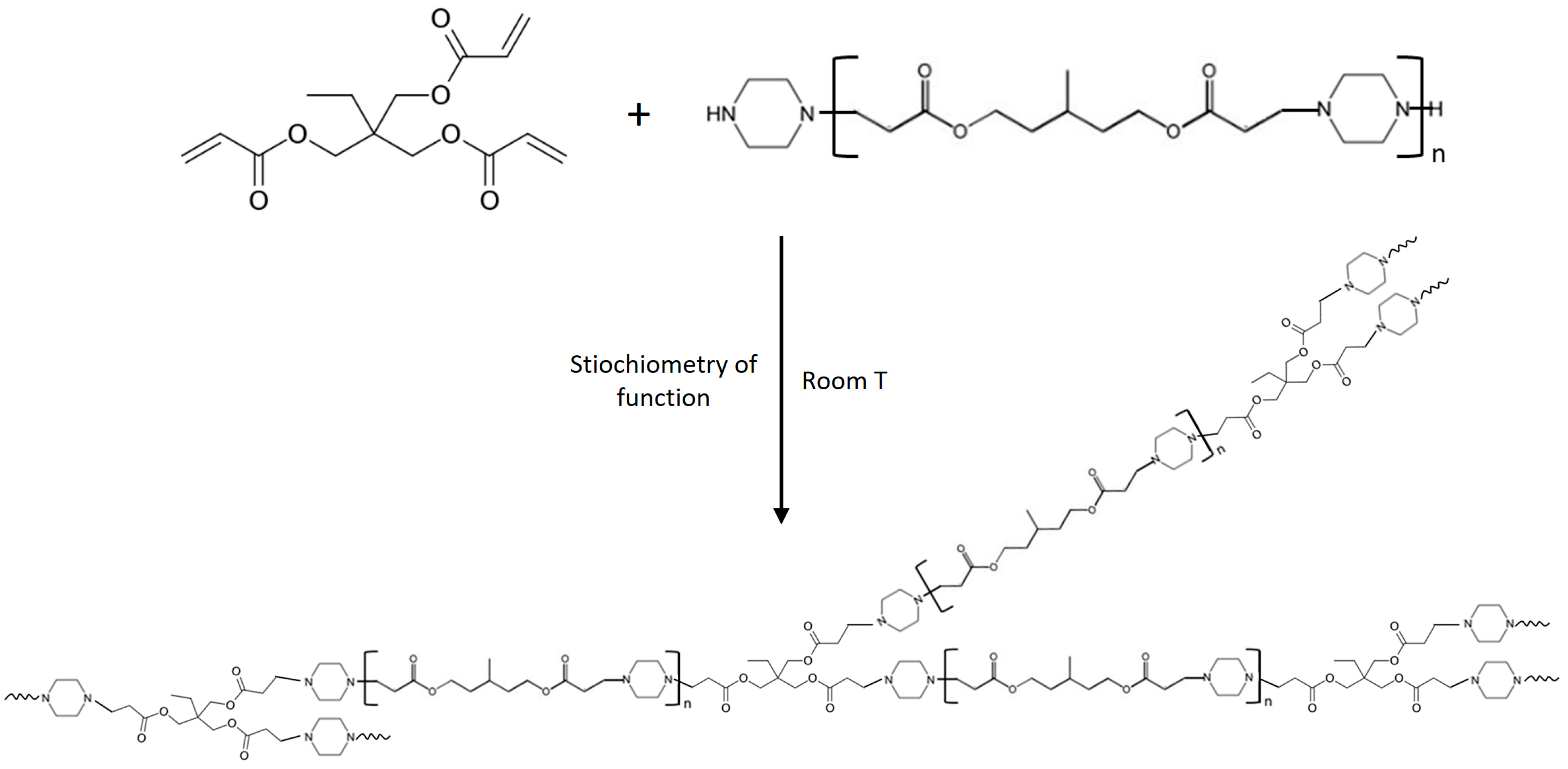
2.3. Adhesive Systems
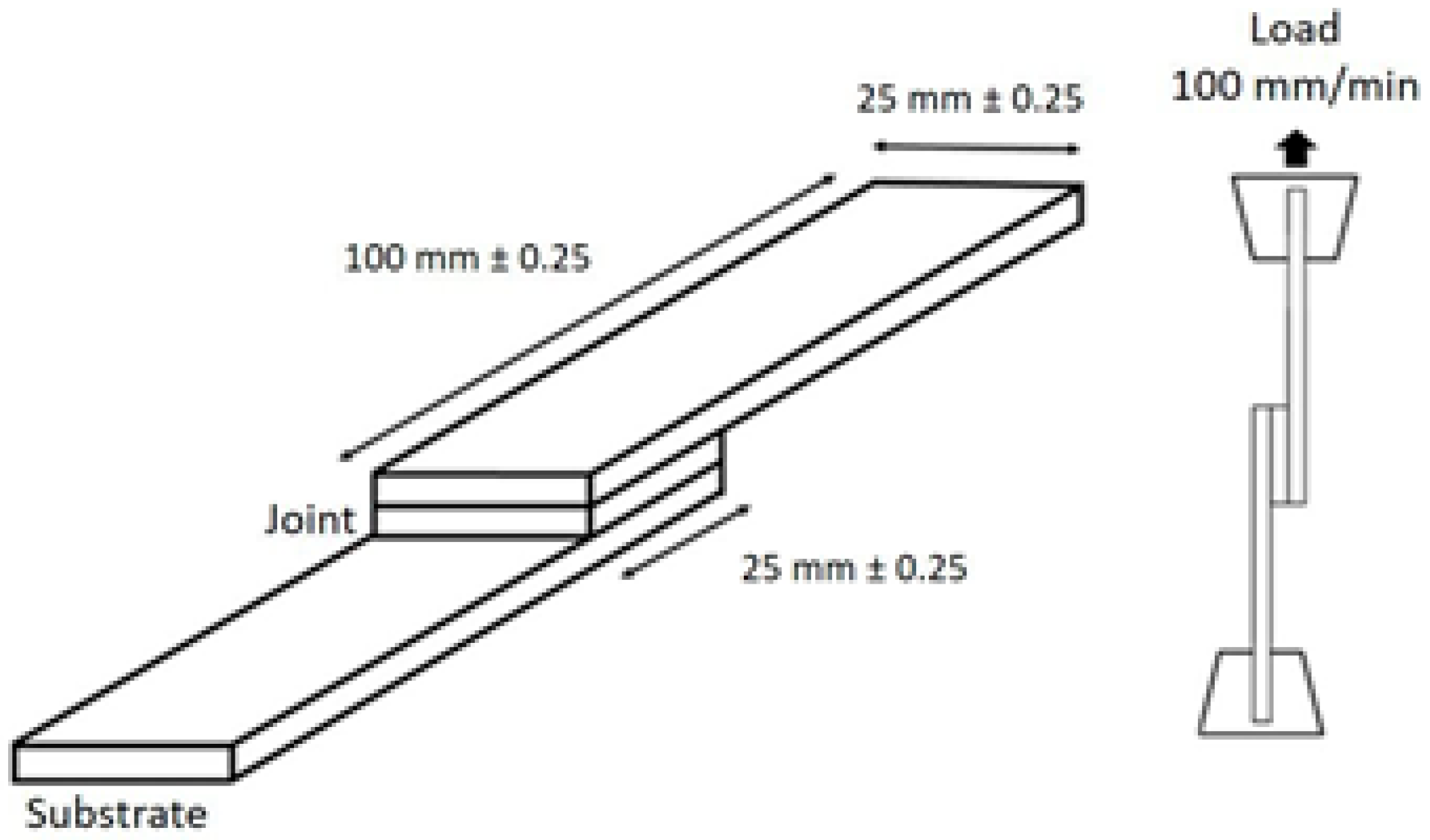
2.4. Methods Ans Apparatus
3. Results
3.1. Amine/Acrylate Aza-Michael Addition Reactivity and Bulk Properties of the Adhesive
3.2. Surface Properties of the Aza-Michael Thermoset Adhesives
4. Discussion
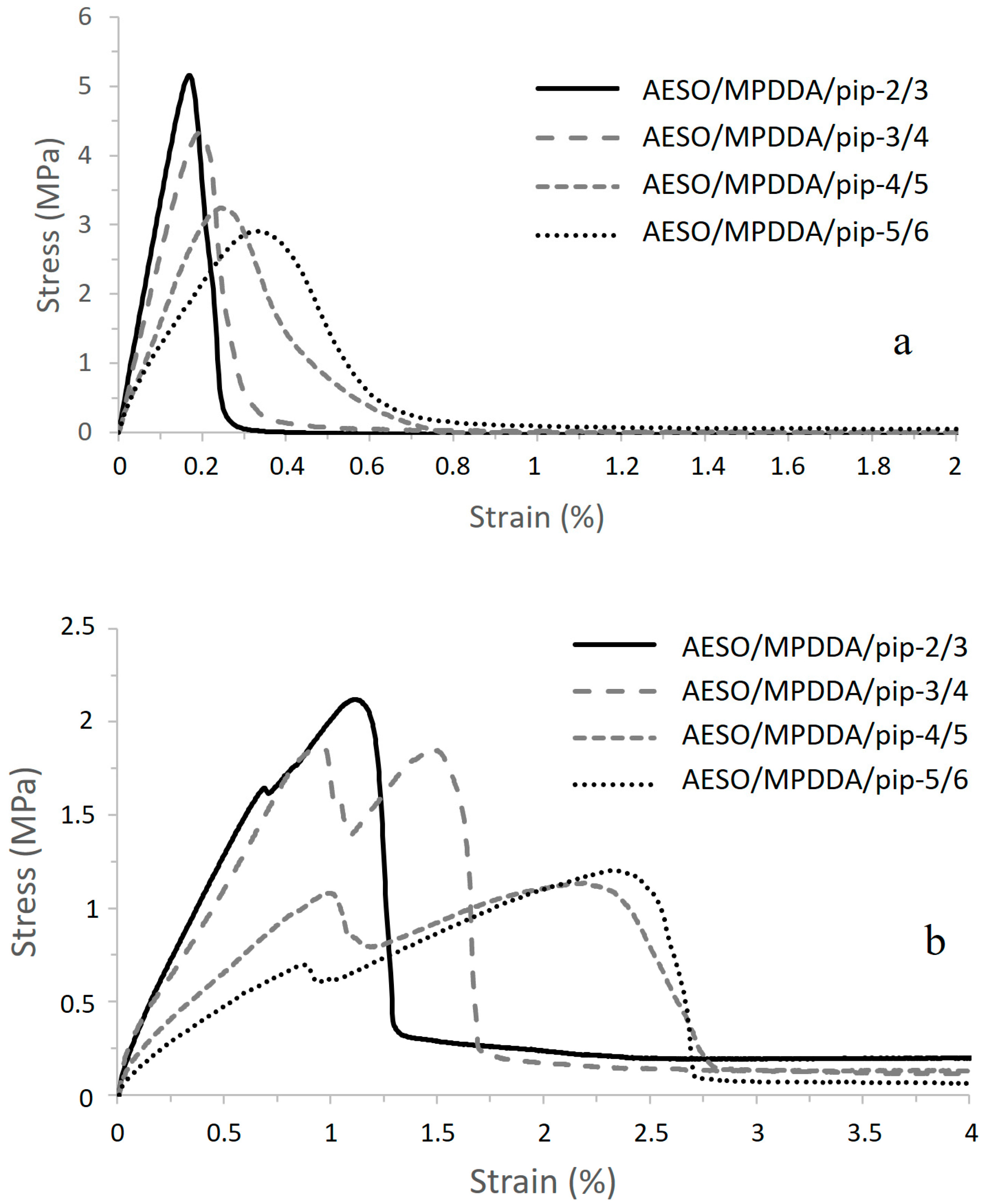
Adhesive Properties of the Aza-Michael Thermoset Adhesives on Galvanized Steel
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PIP | PIPerazine |
| MPDDA | 3-Methyl-1,5-PentaneDiol DiAcrylate |
| AESO | Acrylate Epoxidized Soybean Oil |
| TMPTA | TriMethylolPropane TriAcrylate |
| DTMPTTA | Di(TriMethylolPropane) TeTraAcrylate |
References
- Ecochard, Y.; Auvergne, R.; Boutevin, B.; Caillol, S. Linseed oil-based thermosets by aza-Michael polymerization. Eur. J. Lipid Sci. Technol. 2020, 122, 1–9. [Google Scholar] [CrossRef]
- Fu, H.; Gong, L.; Gong, S. A New Approach Utilizing Aza-Michael Addition for Hydrolysis-Resistance Non-Ionic Waterborne Polyester. Polymers 2022, 14, 2655. [Google Scholar] [CrossRef] [PubMed]
- Konuray, A.O.; Fernández-Francos, X.; Serra, À.; Ramis, X. Sequential curing of amine-acrylate-methacrylate mixtures based on selective aza-Michael addition followed by radical photopolymerization. Eur. Polym. J. 2016, 84, 256–267. [Google Scholar] [CrossRef]
- Genest, A.; Portinha, D.; Fleury, E.; Ganachaud, F. The aza-Michael reaction as an alternative strategy to generate advanced silicon-based (macro)molecules and materials. Prog. Polym. Sci. 2017, 72, 61–110. [Google Scholar] [CrossRef]
- González, G.; Fernández-Francos, X.; Serra, À.; Sangermano, M.; Ramis, X. Environmentally-friendly processing of thermosets by two-stage sequential aza-Michael addition and free-radical polymerization of amine-acrylate mixtures. Polym. Chem. 2015, 6, 6987–6997. [Google Scholar] [CrossRef]
- Kalita, P.; Pegu, C.D.; Dutta, P.; Baruah, P.K. Room temperature solvent free Aza-Michael reactions over nano-cage mesoporous materials. J. Mol. Catal. A Chem. 2014, 394, 145–150. [Google Scholar] [CrossRef]
- Nishibayashi, K.; Kobayashi, Y.; Hayama, N.; Kuramoto, R.; Takemoto, Y. Mechanistic Insight into Asymmetric Hetero-Michael Addition of α, β -Unsaturated Carboxylic Acids Catalyzed by Multifunctional Thioureas. J. Am. Chem. Soc. 2018, 140, 12216–12225. [Google Scholar] [CrossRef]
- Yin, B.; Croutxé-Barghorn, C.; Delaite, C.; Allonas, X. A new synthetic pathway based on one-pot sequential aza-Michael addition and photoCuAAC click reactions. RSC Adv. 2019, 9, 4824–4831. [Google Scholar] [CrossRef]
- Rulev, A.Y. Aza-Michael reaction: Achievements and prospects. Russ. Chem. Rev. 2011, 80, 197–218. [Google Scholar] [CrossRef]
- Fedotova, A. Aromatic and Sterically Hindered Amines in Aza-Michael Reaction: Solvent and High-Pressure Effects. Ph.D. Thesis, Université de Rouen Normandie, Rouen, France, 2018. [Google Scholar]
- Mora, A.S.; Tayouo, R.; Boutevin, B.; David, G.; Caillol, S. A perspective approach on the amine reactivity and the hydrogen bonds effect on epoxy-amine systems. Eur. Polym. J. 2020, 123, 109460–109473. [Google Scholar] [CrossRef]
- Halder, R. New Enantioselective Routes to Nitrogen Containing Compounds: Catalytic Asymmetric Aza-Michael and Aza-Henry Reactions. Ph.D. Thesis, Universidad del Pais Vasco, Bilbao, Spain, 2006. [Google Scholar]
- Peyrton, J.; Avérous, L. Aza-Michael Reaction as a Greener, Safer, and More Sustainable Approach to Biobased Polyurethane Thermosets. ACS Sustain. Chem. Eng. 2021, 9, 4872–4884. [Google Scholar] [CrossRef]
- Baruah, R.; Kumar, A.; Ujjwal, R.R.; Kedia, S.; Ranjan, A.; Ojha, U. Recyclable thermosets based on dynamic amidation and Aza-Michael addition chemistry. Macromolecules 2016, 49, 7814–7824. [Google Scholar] [CrossRef]
- Wodtke, R.; Steinberg, J.; Köckerling, M.; Löser, R.; Mamat, C. NMR-based investigations of acyl-functionalized piperazines concerning their conformational behavior in solution. RSC Adv. 2018, 8, 40921–40933. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Bajpai, M. Synthesis and Characterization of Acrylated Epoxidized Soybean Oil for UV-Cured Coatings. Chem. Chem. Technol. 2011, 5, 317–326. [Google Scholar] [CrossRef]
- ASTM D1002-10; Apparent Shear Strength of Single-Lap-Joint Adhesively Bonded Metal Specimens by Tension Loading (Metal-to-Metal). ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen-Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Martínez-Miranda, M.R.; García-Martínez, V.; Gude, M.R. Gel point determination of a thermoset prepreg by means of rheology. Polym. Test. 2019, 78, 105950–105955. [Google Scholar] [CrossRef]
- Annamalai, M.; Gopinadhan, K.; Han, S.A.; Saha, S.; Park, H.J.; Cho, E.B.; Kumar, B.; Patra, A.; Kim, S.W.; Venkatesan, T. Surface energy and wettability of van der Waals structures—Supplementary Information. Nanoscale 2016, 8, 5764–5770. [Google Scholar] [CrossRef]
- Rulison, C. Two-Component Surface Energy Characterization as a Predictor of Wettability and Dispersability; KRÜSS GmbH: Hamburg, Germany, 2000; Vol. Application, No. 40; pp. 1–22. Available online: https://www.sanyo-si.com/wp-content/uploads/kruss-ar213-en.pdf (accessed on 24 June 2025).
- Packham, D.E. Surface energy, surface topography and adhesion. Int. J. Adhes. Adhes. 2003, 23, 437–448. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Nian, G.; Suo, Z. Strength and toughness of adhesion of soft materials measured in lap shear. J. Mech. Phys. Solids. 2020, 143, 103988. [Google Scholar] [CrossRef]
- Barros, R.B.P.; Campilho, R.D.S.G.; Sánchez-Arce, I.J.; Dionísio, J.M.M.; Madani, K. Evaluation of the cracked lap shear test for mixed-mode fracture toughness estimation of adhesive joints. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2023, 237, 2537–2550. [Google Scholar] [CrossRef]
- Gupta, S.K. Effect of adhesive thickness and adherend material with a surface roughness on lap shear strength of joints in tensile and compressive loading. Adv. Mater. Process. Technol. 2022, 8, 1023–1043. [Google Scholar] [CrossRef]
- Lda Silva, L.F.M.; Rodrigues, T.N.S.S.; Figueiredo, M.A.V.; de Moura, M.F.S.F.; Chousal, J.A.G. Effect of adhesive type and thickness on the lap shear strength. J. Adhes. 2006, 82, 1091–1115. [Google Scholar] [CrossRef]
- Sharpe, L.H. The interphase in adhesion. J. Adhes. 1972, 4, 51–64. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Little, M.S.G.; Watts, J.F. Role of the interphase in the environmental failure of adhesive joints. Acta Mater. 2000, 48, 4543–4553. [Google Scholar] [CrossRef]
- Fan, J.; Karami, J.; Kojouri, A.; Van Hemelrijck, D.; Vassilopoulos, A.; Michaud, V. Investigation of bulk adhesive material and thick adhesive joints for wind turbine applications. In Proceedings of the ECCM 2022—20th European Conference on Composite Materials, Lausanne, Switzerland, 26–30 June 2022; Volume 5, pp. 129–136. [Google Scholar]
- Williamson, D.M.; Hamilton, N.R.; Palmer, S.J.P.; Jardine, A.P.; Leppard, C. Thermodynamic work of adhesion measurements of polymer bonded explosive constituents via the Wilhelmy plate technique and their application to AFM pull-off experiments. J. Phys. Conf. Ser. 2014, 500, 112068. [Google Scholar] [CrossRef]
- Baldan, A. Adhesion phenomena in bonded joints. Int. J. Adhes. Adhes. 2012, 38, 95–116. [Google Scholar] [CrossRef]
- Qu, J. Adhesion and failure of Polymer/metal interfaces in microelectronic packaging. In Handbook of Damage Mechanics; Voyiadjis, G., Ed.; Springer: New York, NY, USA, 2015; pp. 1–30. [Google Scholar] [CrossRef]
- Brandtner-Hafner, M. Mechanical fracture characterization of adhesive interfaces: Introducing a new concept for evaluating adhesive quality. Mater. Test. 2018, 60, 855–861. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Zarrelli, M.; Borriello, A. Fracture Toughening Mechanisms in Epoxy Adhesives. In Adhesives—Applications and Properties; Rudawska, A., Ed.; InTechOpen: London, UK, 2016; pp. 237–269. [Google Scholar] [CrossRef]
- IMiturska-Barańska, I.; Rudawska, A. Influence of Adhesive Compound Viscosity on the Strength Properties of 1.0503 Steel Sheets Adhesive Joints. Adv. Sci. Technol. Res. J. 2022, 16, 196–205. [Google Scholar] [CrossRef]
- Iyengar, Y.; Erickson, D.E. Role of adhesive-substrate compatibility in adhesion. J. Appl. Polym. Sci. 1967, 11, 2311–2324. [Google Scholar] [CrossRef]
- Awada, H.; Noel, O.; Hamieh, T.; Kazzi, Y.; Brogly, M. Contributions of chemical and mechanical surface properties and temperature effect on the adhesion at the nanoscale. Thin Solid Films 2011, 519, 3690–3694. [Google Scholar] [CrossRef]



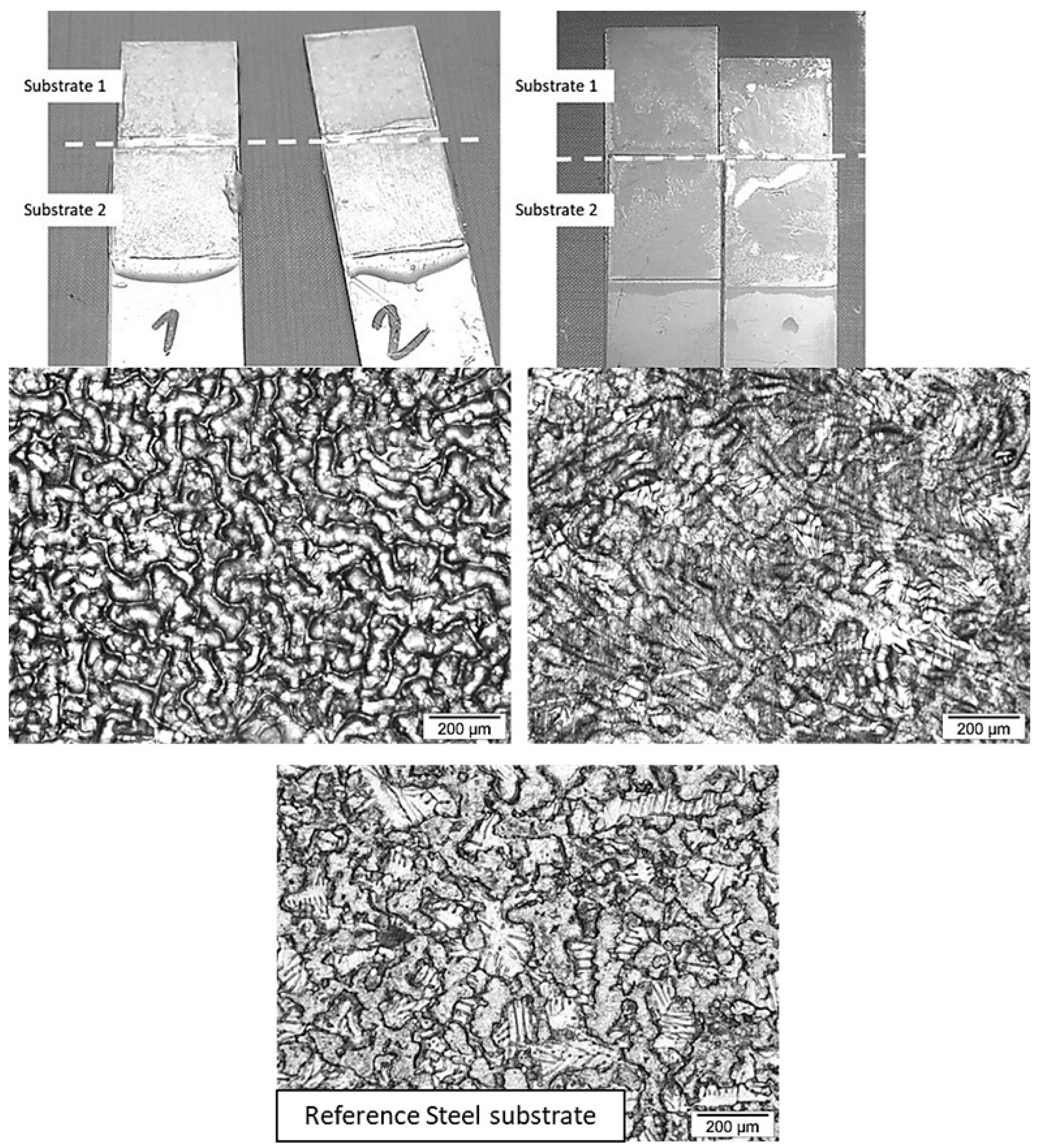

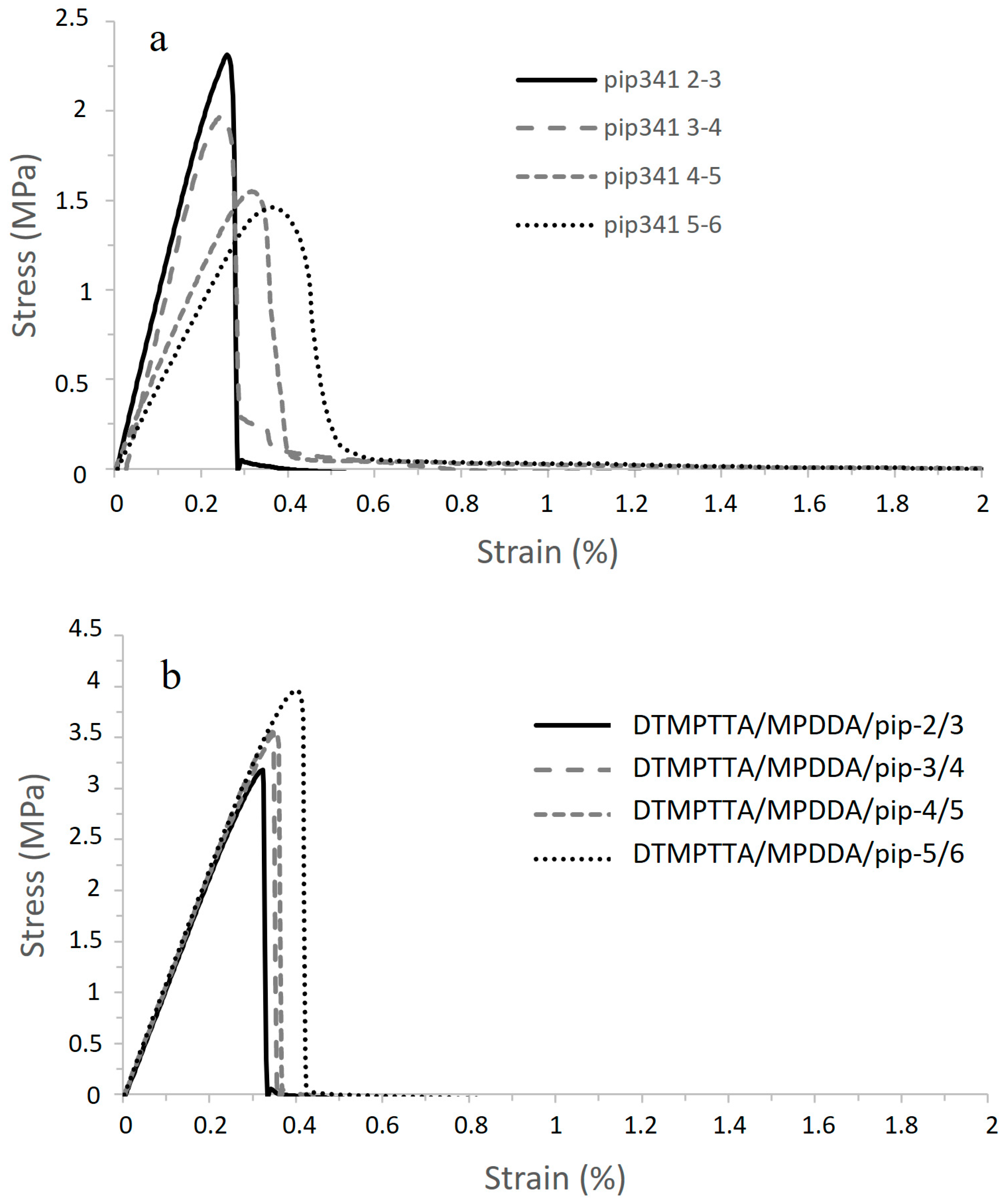
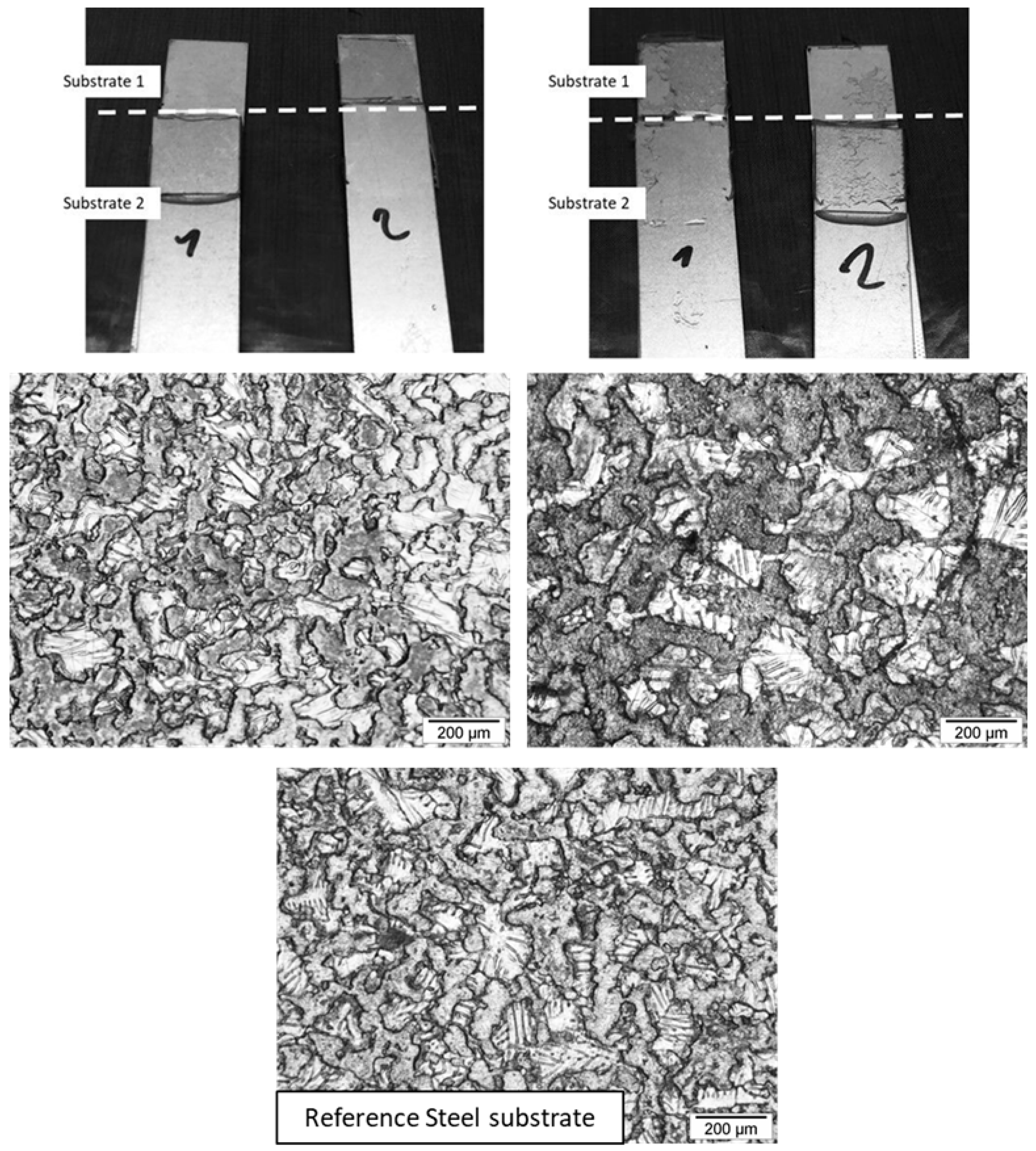


| Acronym | MPDDA/PIP-2/3 | MPDDA/PIP-3/4 | MPDDA/PIP-4/5 | MPDDA/PIP-5/6 | ||||
|---|---|---|---|---|---|---|---|---|
| Acrylate wt% | Amine wt% | Acrylate wt% | Amine wt% | Acrylate wt% | Amine wt% | Acrylate wt% | Amine wt% | |
| AESO | 48.5 | 51.5 | 42.6 | 57.4 | 36.6 | 63.4 | 30.3 | 69.7 |
| TMPTA | 18.6 | 81.4 | 15.2 | 84.8 | 12.2 | 87.8 | 9.5 | 90.5 |
| DTMPTTA | 16.9 | 83.1 | 13.8 | 86.2 | 11.1 | 88.9 | 8.6 | 91.4 |
| Acronym | MPDDA/PIP-2/3 | MPDDA/PIP-3/4 | MPDDA/PIP-4/5 | MPDDA/PIP-5/6 |
|---|---|---|---|---|
| MPDDA/PIP ratio | 2/3 | 3/4 | 4/5 | 5/6 |
| MPDDA wt% | 65 | 68 | 70 | 72 |
| PIP wt% | 35 | 32 | 30 | 28 |
| Predicted DPn | 5 | 7 | 9 | 11 |
| Predicted Mn (g mol−1) | 711 | 1023 | 1336 | 1648 |
| Acronym | MPDDA/PIP-2/3 | MPDDA/PIP-3/4 | MPDDA/PIP-4/5 | MPDDA/PIP-5/6 |
|---|---|---|---|---|
| Predicted Mn (g mol−1) | 711 | 1023 | 1336 | 1648 |
| Calculated Mn (g mol−1) | 867 | 1132 | 1414 | 1882 |
| Repeat units n | 2.75 | 3.35 | 4.25 | 6.75 |
| Tg (°C) | −43 | −41 | −39 | −37 |
| Dynamic viscosity at 25 °C (Pa s) | 8.8 | 24.8 | 28.8 | 34.4 |
| AESO MPDDA/PIP-2/3 | AESO MPDDA/PIP-3/4 | AESO MPDDA/PIP-4/5 | AESO MPDDA/PIP-5/6 | |
|---|---|---|---|---|
| Gel point time (h) | 1.5 | 3 | 4 | 4.5 |
| End of reaction (h) | 2.5 | 5 | 8.5 | >10 |
| η* at gel point (Pa s) | 1420 | 4050 | 5230 | 6070 |
| Crosslinking rate at gel point (s−1) | 7.25 10−3 | 2.04 10−3 | 1.54 10−3 | 1.33 10−3 |
| Tg (°C) | −16 | −20 | −22 | −23 |
| TMPTA MPDDA/PIP-2/3 | TMPTA MPDDA/PIP-3/4 | TMPTA MPDDA/PIP-4/5 | TMPTA MPDDA/PIP-5/6 | |
|---|---|---|---|---|
| Gel point time (h) | 0.916 | 1.333 | 2.5 | 4 |
| End of reaction (h) | 2 | 3.5 | 6 | >10 |
| η* at gel point (Pa s) | 1050 | 2100 | 4730 | 7850 |
| Crosslinking rate at gel point (s−1) | 2.42 × 10−2 | 1.45 × 10−2 | 2.28 × 10−3 | 1.39 × 10−3 |
| Tg (°C) | −15 | −18 | −20 | −22 |
| DTMPTTA MPDDA/PIP-2/3 | DTMPTTA MPDDA/PIP-3/4 | DTMPTTA MPDDA/PIP-4/5 | DTMPTTA MPDDA/PIP-5/6 | |
|---|---|---|---|---|
| Gel point time (h) | 0.666 | 0.916 | 1.666 | 3.25 |
| End of reaction (h) | 2 | 3 | 4.5 | 8 |
| η* at gel point (Pa s) | 890 | 1380 | 2740 | 7120 |
| Crosslinking rate at gel point (s−1) | 5.53 × 10−2 | 2.94 × 10−2 | 7.28 × 10−3 | 2.02 × 10−3 |
| Tg (°C) | −8 | −10 | −13 | −16 |
| Contact Angle (°) | Surface Energy (mJ m−2) | ||||||
|---|---|---|---|---|---|---|---|
| Water | Diiodomethane | 1-Bromo-naphtalen | Glycerol | γsND | γsD | γ | |
| AESO/MPDDA/PIP-2/3 | 58 ± 2 | 40 ± 3 | 42 ± 2 | 39 ± 2 | 17 ± 2 | 32 ± 2 | 49 ± 2 |
| AESO/MPDDA/PIP-3/4 | 60 ± 1 | 38 ± 3 | 42 ± 3 | 40 ± 1 | 15 ± 2 | 33 ± 2 | 47 ± 3 |
| AESO/MPDDA/PIP-4/5 | 65 ± 4 | 40 ± 3 | 39 ± 2 | 44 ± 3 | 12 ± 3 | 34 ± 3 | 46 ± 3 |
| AESO/MPDDA/PIP-5/6 | 67 ± 3 | 42 ± 2 | 28 ± 2 | 50 ± 4 | 10 ± 3 | 36 ± 3 | 46 ± 3 |
| TMPTA/MPDDA/PIP-2/3 | 70 ± 1 | 33 ± 1 | 18 ± 2 | 42 ± 2 | 6 ± 1 | 41 ± 1 | 47 ± 1 |
| TMPTA/MPDDA/PIP-3/4 | 70 ± 2 | 30 ± 2 | 15 ± 3 | 40 ± 1 | 5 ± 2 | 42 ± 2 | 47 ± 2 |
| TMPTA/MPDDA/PIP-4/5 | 78 ± 3 | 28 ± 2 | 12 ± 2 | 45 ± 2 | 4 ± 2 | 44 ± 2 | 48 ± 2 |
| TMPTA/MPDDA/PIP-4/6 | 84 ± 4 | 37 ± 3 | 17 ± 3 | 46 ± 1 | 3 ± 3 | 43 ± 3 | 46 ± 3 |
| DTMPTTA/MPDDA/PIP-2/3 | 60 ± 1 | 28 ± 2 | 25 ± 2 | 41 ± 2 | 13 ± 2 | 39 ± 2 | 52 ± 2 |
| DTMPTTA/MPDDA/PIP-3/4 | 62 ± 3 | 17 ± 3 | 19 ± 2 | 44 ± 3 | 11 ± 3 | 41 ± 3 | 52 ± 3 |
| DTMPTTA/MPDDA/PIP-4/5 | 69 ± 3 | 22 ± 3 | 24 ± 2 | 46 ± 2 | 10 ± 2 | 41 ± 2 | 51 ± 2 |
| DTMPTTA/MPDDA/PIP-5/6 | 76 ± 2 | 26 ± 2 | 20 ± 1 | 52 ± 3 | 9 ± 2 | 42 ± 2 | 51 ± 2 |
| Contact Angle (°) | Surface Energy (mJ m−2) | ||||||
|---|---|---|---|---|---|---|---|
| Water | Diiodomethane | 1-Bromo-naphtalen | Glycerol | γsND | γsD | γ | |
| Galvanized steel | 62 ± 1 | 43 ± 2 | 21 ± 2 | 45 ± 3 | 12 ± 2 | 36 ± 2 | 48 ± 2 |
| Lap-Shear Strength (MPa) | Strain at Break (%) | Shear Modulus (MPa) | Toughness (N mm−1) | |
|---|---|---|---|---|
| AESO/MPDDA/PIP-2/3 | 5.2 ± 0.4 | 0.16 ± 0.04 | 3330 ± 64 | 32.4 ± 0.8 |
| AESO/MPDDA/PIP-3/4 | 4.4 ± 0.2 | 0.20 ± 0.02 | 2620 ± 80 | 31.2 ± 0.5 |
| AESO/MPDDA/PIP-4/5 | 3.2 ± 0.2 | 0.25 ± 0.02 | 2020 ± 11 | 40 ± 3 |
| AESO/MPDDA/PIP-5/6 | 2.8 ± 0.3 | 0.35 ± 0.01 | 1050 ± 25 | 53.2 ± 0.6 |
| Lap-Shear Strength (MPa) | Strain at Break (%) | Shear Modulus (MPa) | Toughness (N mm−1) | |
|---|---|---|---|---|
| AESO/MPDDA/PIP-2/3 | 2.1 ± 0.2 | 1.14 ± 0.04 | 480 ± 5 | 141 ± 4 |
| AESO/MPDDA/PIP-3/4 | 1.8 ± 0.3 | 1.49 ± 0.06 | 400 ± 15 | 173 ± 6 |
| AESO/MPDDA/PIP-4/5 | 1.1 ± 0.2 | 2.16 ± 0.06 | 290 ± 12 | 185 ± 9 |
| AESO/MPDDA/PIP-5/6 | 1.2 ± 0.1 | 2.36 ± 0.04 | 175 ± 5 | 220 ± 10 |
| Lap-Shear Strength (MPa) | Strain at Break (%) | Shear Modulus (MPa) | Toughness (N mm−1) | |
|---|---|---|---|---|
| TMPTA/MPDDA/PIP-2/3 | 2.3 ± 0.2 | 0.26 ± 0.02 | 1015 ± 23 | 29.7 ± 0.8 |
| TMPTA/MPDDA/PIP-3/4 | 2.0 ± 0.3 | 0.25 ± 0.04 | 1050 ± 12 | 30.6 ± 0.6 |
| TMPTA/MPDDA/PIP-4/5 | 1.6 ± 0.2 | 0.33 ± 0.03 | 590 ± 17 | 33.0 ± 0.2 |
| TMPTA/MPDDA/PIP-5/6 | 1.5 ± 0.1 | 0.38 ± 0.02 | 490 ± 16 | 37.3 ± 0.3 |
| Lap-Shear Strength (MPa) | Strain at Break (%) | Shear Modulus (MPa) | Toughness (N mm−1) | |
|---|---|---|---|---|
| DTMPTTA/MPDDA/PIP-2/3 | 3.2 ± 0.2 | 0.32 ± 0.02 | 1090 ± 16 | 46.3 ± 0.2 |
| DTMPTTA/MPDDA/PIP-3/4 | 3.6 ± 0.4 | 0.34 ± 0.03 | 1095 ± 10 | 56.4 ± 0.3 |
| DTMPTTA/MPDDA/PIP-4/5 | 3.6 ± 0.3 | 0.35 ± 0.01 | 1120 ± 28 | 59.5 ± 0.3 |
| DTMPTTA/MPDDA/PIP-5/6 | 4.0 ± 0.3 | 0.41 ± 0.02 | 1135 ± 12 | 74.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavodeau, F.; Brogly, M.; Stumbe, J.-F.; Perrin, R. Synthesis, Characterization, and Adhesion on Galvanized Steel of Original Thermoset Adhesive Films Based on Aza-Michael Addition Reaction. Polymers 2025, 17, 1796. https://doi.org/10.3390/polym17131796
Cavodeau F, Brogly M, Stumbe J-F, Perrin R. Synthesis, Characterization, and Adhesion on Galvanized Steel of Original Thermoset Adhesive Films Based on Aza-Michael Addition Reaction. Polymers. 2025; 17(13):1796. https://doi.org/10.3390/polym17131796
Chicago/Turabian StyleCavodeau, Florian, Maurice Brogly, Jean-François Stumbe, and Rémi Perrin. 2025. "Synthesis, Characterization, and Adhesion on Galvanized Steel of Original Thermoset Adhesive Films Based on Aza-Michael Addition Reaction" Polymers 17, no. 13: 1796. https://doi.org/10.3390/polym17131796
APA StyleCavodeau, F., Brogly, M., Stumbe, J.-F., & Perrin, R. (2025). Synthesis, Characterization, and Adhesion on Galvanized Steel of Original Thermoset Adhesive Films Based on Aza-Michael Addition Reaction. Polymers, 17(13), 1796. https://doi.org/10.3390/polym17131796







