Dual-Layer Natamycin and Boric-Acid-Reinforced PVA/Chitosan by 3D Printing and Electrospinning Method: Characterization and In Vitro Evaluation
Abstract
1. Introduction
2. Experimental
2.1. 3D Printing of Wound Dressing with PVA/Chitosan/Boric Acid Ink
2.2. Coating of 3D Wound Dressings with Natamycin via Electrospinning Method
2.3. Mechanical Test
2.4. Chemical Analysis by FTIR
2.5. DSC Analysis
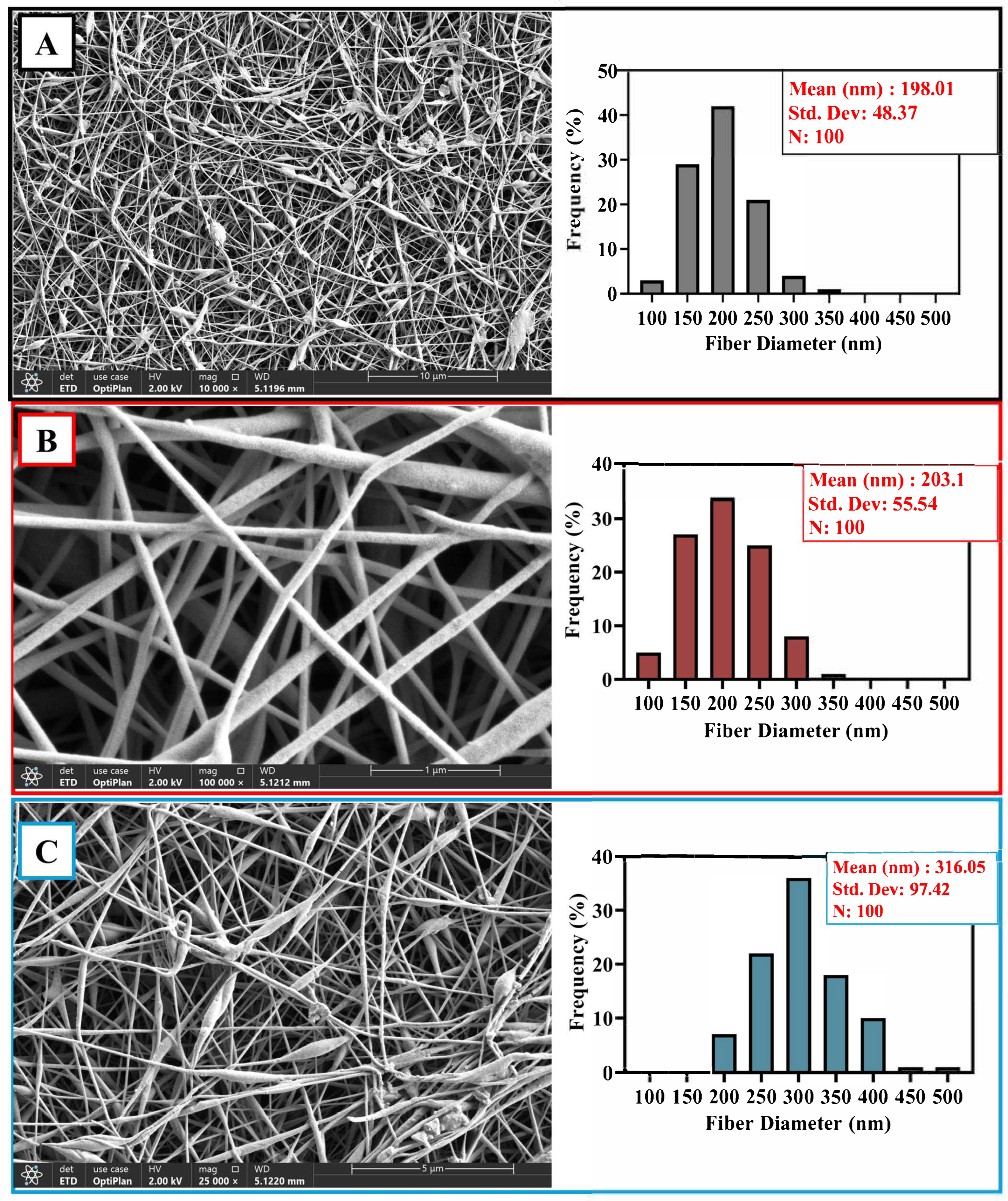
2.6. Morphological Analysis by SEM
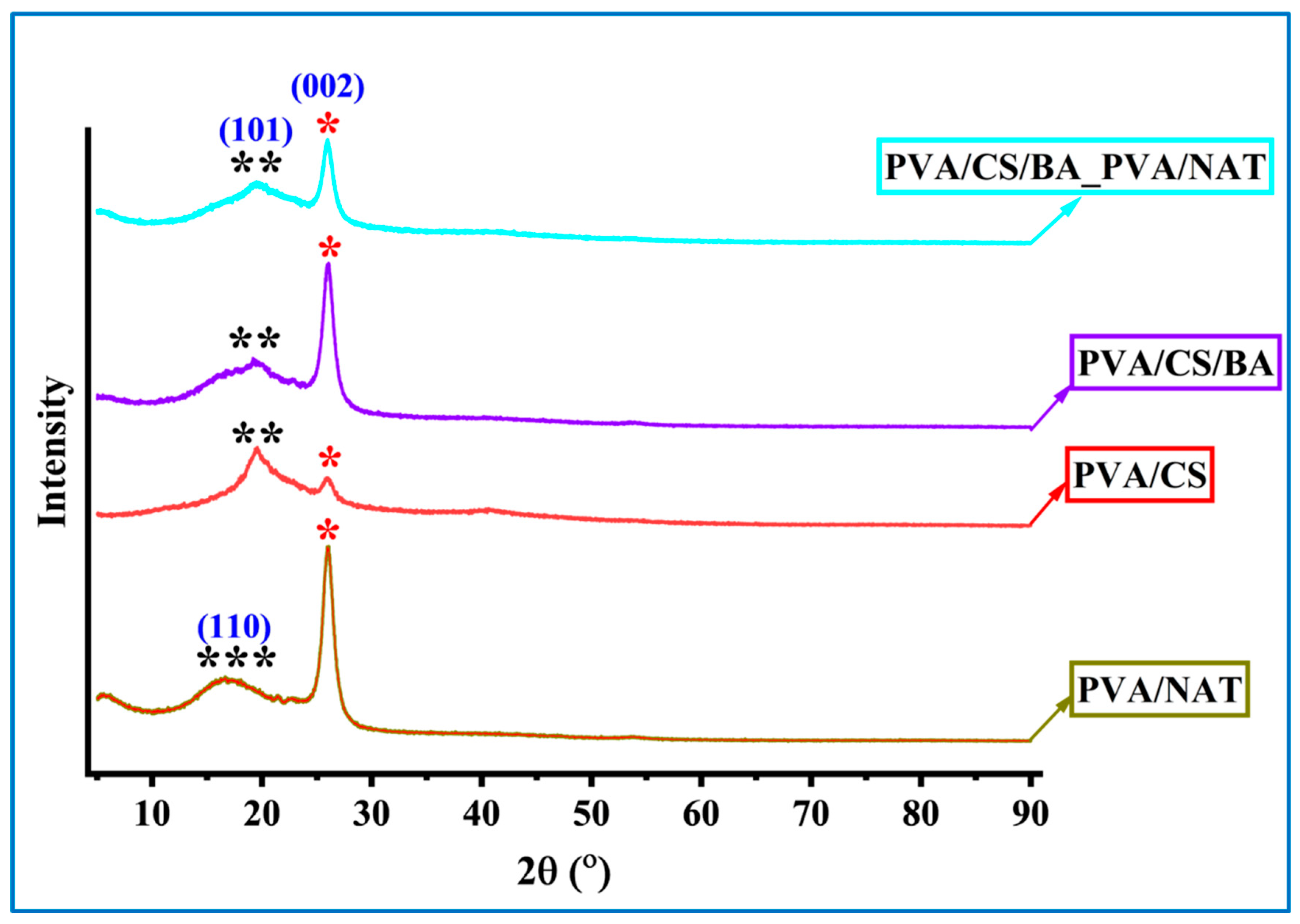
2.7. XRD Analysis
2.8. Evaluation of Natamycin Release
2.9. Antifungal Test
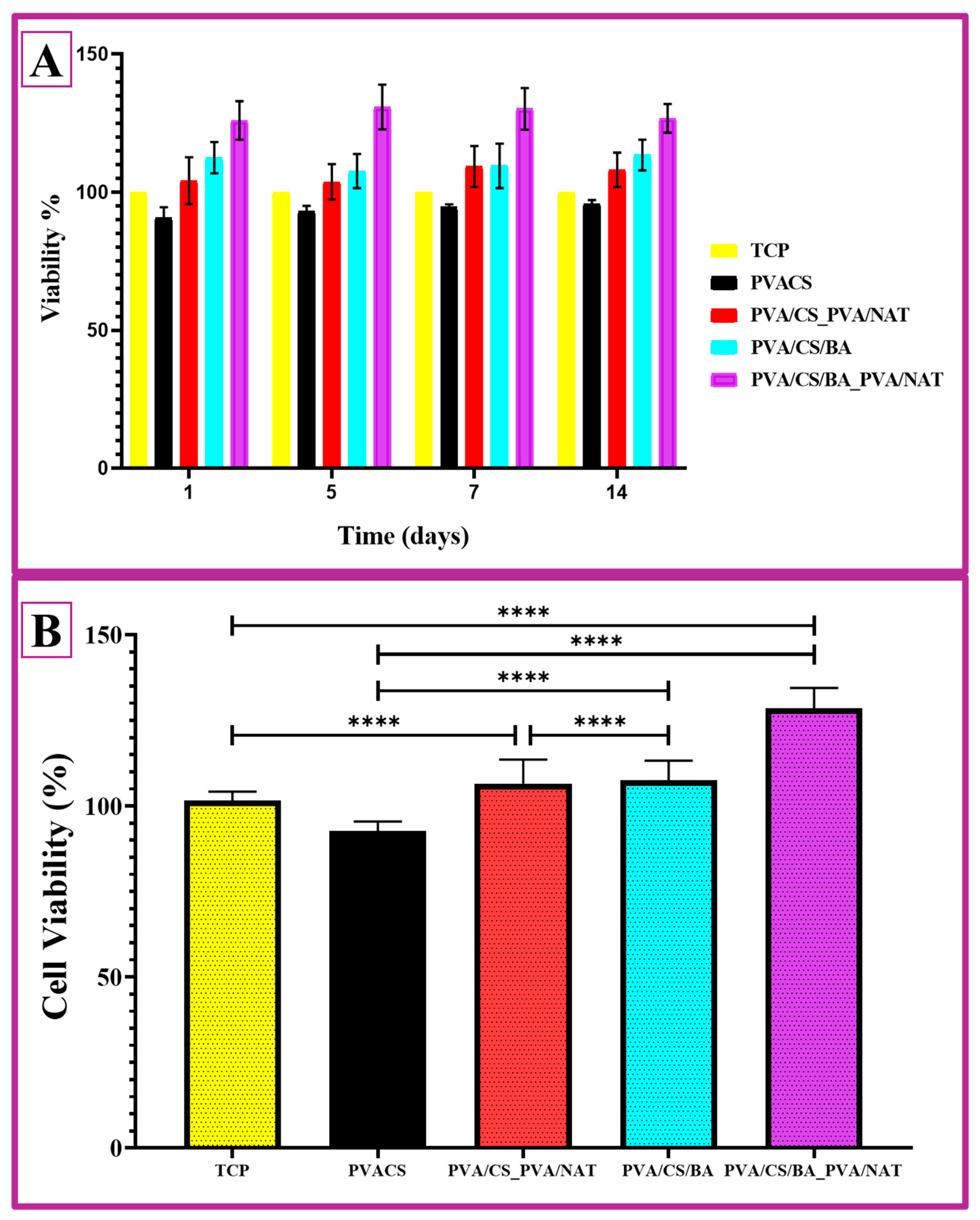
2.10. Cell Viability MTT Test
2.11. Statistical Analysis
3. Result and Discussion
3.1. Mechanical Analysis
3.2. FTIR Analysis of Composite Nanofibers
3.3. DSC
3.4. Morphological Investigation of Composite Nanofibers
3.5. XRD Analysis of the Composite Nanofibers
3.6. Release Kinetics Study
3.7. Antimicrobial Tests
3.8. Cell Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peppas, N.A.; Merrill, E.W. Differential scanning calorimetry of crystallized PVA hydrogels. J. Appl. Polym. Sci. 1976, 20, 1457–1465. [Google Scholar] [CrossRef]
- Arya, A.; Chahar, D.; Bhakuni, K.; Vandana; Kumar, S.; Venkatesu, P. Green Synthesis of Silver Nanoparticles Using Drymaria cordata and Their Biocompatibility with Hemoglobin: A Therapeutic Potential Approach. ACS Appl. Bio Mater. 2024, 7, 977–989. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.S.; Michailovich, K.A.; Subramanian, K.; Sathiamoorthyi, S.; Kandasamy, K.T. Philosophy of selecting ASTM standards for mechanical characterization of polymers and polymer composites. Mater. Plast. 2021, 58, 247–256. [Google Scholar] [CrossRef]
- Ustundag, C.B. Fabrication of porous hydroxyapatite-carbon nanotubes composite. Mater. Lett. 2016, 167, 89–92. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.H.; Yu, J.Y.; Zeng, H.M. Controlling numbers and sizes of beads in electrospun nanofibers. Polym. Int. 2008, 57, 632–636. [Google Scholar] [CrossRef]
- Lin, T.; Wang, X.G. Controlling the morphologies of electrospun nanofibres. In Nanofibers and Nanotechnology in Textiles; Woodhead Publishing: London, UK, 2007; ISBN 9781845691059. [Google Scholar]
- Patra, N.; Barone, A.C.; Salerno, M. Solvent effects on the thermal and mechanical properties of poly(methyl methacrylate) casted from concentrated solutions. Adv. Polym. Technol. 2011, 30, 12–20. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, K.; Li, Z.; Huang, S. Plate-like hydroxyapatite nanoparticles synthesized by the hydrothermal method. J. Phys. Chem. Solids 2009, 70, 243–248. [Google Scholar] [CrossRef]
- Koroleva, M.Y.; Karakatenko, E.Y.; Yurtov, E.V. Synthesis of Hydroxyapatite Nanoparticles by Controlled Precipitation in the Presence of Sodium Dodecyl Sulfate. Colloid J. 2020, 82, 275–283. [Google Scholar] [CrossRef]
- Bzdek, B.R.; Reid, J.P.; Malila, J.; Prisle, N.L. The surface tension of surfactant-containing, finite volume droplets. Proc. Natl. Acad. Sci. USA 2020, 117, 8335–8343. [Google Scholar] [CrossRef] [PubMed]
- Sunita, T.; Sharma, P.; Malviya, R. Influence of Concentration on Surface Tension & Viscosity of Tamarind (Tamarindus Indica) Seed Gum. Ann. Mol. Genet. Med. 2017, 1, 8–12. [Google Scholar] [CrossRef]
- Dinatha, I.K.H.; Diputra, A.H.; Wihadmadyatami, H.; Partini, J.; Yusuf, Y. Nanofibrous electrospun scaffold doped with hydroxyapatite derived from sand lobster shell (Panulirus homarus) for bone tissue engineering. RSC Adv. 2024, 14, 8222–8239. [Google Scholar] [CrossRef]
- Subash, A.; Basanth, A.; Kandasubramanian, B. Biodegradable polyphosphazene–hydroxyapatite composites for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 1093–1111. [Google Scholar] [CrossRef]
- Saad, S.M.I.; Policova, Z.; Acosta, E.J.; Neumann, A.W. Effect of surfactant concentration, compression ratio and compression rate on the surface activity and dynamic properties of a lung surfactant. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.D.; Zhang, S.C.; Huebner, W.; Ownby, P.D.; Gu, H. Effect of the solvent on the particle morphology of spray dried PMMA. J. Mater. Sci. 2001, 36, 3759–3768. [Google Scholar] [CrossRef]
- Duygulu, N.E.; Ciftci, F.; Ustundag, C.B. Electrospun drug blended poly(lactic acid) (PLA) nanofibers and their antimicrobial activities. J. Polym. Res. 2020, 27, 232. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Lin, Z.Y.W.; Wong, K.K.Y.; Lin, M.; Yildirimer, L.; Zhao, X. Electrospun polymeric micro/nanofibrous scaffolds for long-term drug release and their biomedical applications. Drug Discov. Today 2017, 22, 1351–1366. [Google Scholar] [CrossRef] [PubMed]
- Udofia, E.N.; Zhou, W. Microextrusion based 3D printing—A review. In Solid Freeform Fabrication 2018, Proceedings of the 29th Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, SFF 2018, Austin, TX, USA, 13–15 August 2018; The University of Texas at Austin: Austin, TX, USA, 2020; pp. 2033–2060. [Google Scholar]
- Lee, H.R.; Park, J.A.; Kim, S.; Jo, Y.; Kang, D.; Jung, S. 3D microextrusion-inkjet hybrid printing of structured human skin equivalents. Bioprinting 2021, 22, e00143. [Google Scholar] [CrossRef]
- Angarano, M.; Schulz, S.; Fabritius, M.; Vogt, R.; Steinberg, T.; Tomakidi, P.; Friedrich, C.; Mülhaupt, R. Layered gradient nonwovens of in situ crosslinked electrospun collagenous nanofibers used as modular scaffold systems for soft tissue regeneration. Adv. Funct. Mater. 2013, 23, 3277–3285. [Google Scholar] [CrossRef]
- Li, J.L.; Cai, Y.L.; Guo, Y.L.; Fuh, J.Y.H.; Sun, J.; Hong, G.S.; Lam, R.N.; Wong, Y.S.; Wang, W.; Tay, B.Y.; et al. Fabrication of three-dimensional porous scaffolds with controlled filament orientation and large pore size via an improved E-jetting technique. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2014, 102, 651–658. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W. Fabrication of antibacterial chitosan-PVA blended film using electrospray technique for food packaging applications. Int. J. Biol. Macromol. 2018, 107, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun chitosan nanofibers for wound healing application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Loo, H.L.; Goh, B.H.; Lee, L.H.; Chuah, L.H. Application of chitosan-based nanoparticles in skin wound healing. Asian J. Pharm. Sci. 2022, 17, 299–332. [Google Scholar] [CrossRef]
- Innocenti Malini, R.; Lesage, J.; Toncelli, C.; Fortunato, G.; Rossi, R.M.; Spano, F. Crosslinking dextran electrospun nanofibers via borate chemistry: Proof of concept for wound patches. Eur. Polym. J. 2019, 110, 276–282. [Google Scholar] [CrossRef]
- Turhan, E.A.; Akbaba, S.; Tezcaner, A.; Evis, Z. Boron nitride nanofiber/Zn-doped hydroxyapatite/polycaprolactone scaffolds for bone tissue engineering applications. Biomater. Adv. 2023, 148, 213382. [Google Scholar] [CrossRef]
- Kermani, F.; Sadidi, H.; Ahmadabadi, A.; Hoseini, S.J.; Tavousi, S.H.; Rezapanah, A.; Nazarnezhad, S.; Hosseini, S.A.; Mollazadeh, S.; Kargozar, S. Modified Sol–Gel Synthesis of Mesoporous Borate Bioactive Glasses for Potential Use in Wound Healing. Bioengineering 2022, 9, 442. [Google Scholar] [CrossRef]
- Lakshminarayanan, R.; Sridhar, R.; Loh, X.J.; Nandhakumar, M.; Barathi, V.A.; Kalaipriya, M.; Kwan, J.L.; Liu, S.P.; Beuerman, R.W.; Ramakrishna, S. Interaction of gelatin with polyenes modulates antifungal activity and biocompatibility of electrospun fiber mats. Int. J. Nanomed. 2014, 9, 2439–2458. [Google Scholar] [CrossRef]
- Berti, S.; Jagus, R.J.; Flores, S.K. Effect of Rice Bran Addition on Physical Properties of Antimicrobial Biocomposite Films Based on Starch. Food Bioprocess Technol. 2021, 14, 1700–1711. [Google Scholar] [CrossRef]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A natural preservative for food applications—A review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef]
- Bierhalz, A.C.K.; da Silva, M.A.; Braga, M.E.M.; Sousa, H.J.C.; Kieckbusch, T.G. Effect of calcium and/or barium crosslinking on the physical and antimicrobial properties of natamycin-loaded alginate films. LWT Food Sci. Technol. 2014, 57, 494–501. [Google Scholar] [CrossRef]
- Ciftci, F.; Özarslan, A.C.; Evcimen Duygulu, N. Production and comprehensive characterization of PVA/chitosan transdermal composite mats loaded with bioactive curcumin; evaluation of its release kinetics, antioxidant, antimicrobial, and biocompatibility features. J. Appl. Polym. Sci. 2024, 141, e55874. [Google Scholar] [CrossRef]
- Ciftci, F. Release kinetics modelling and in vivo-vitro, shelf-life study of resveratrol added composite transdermal scaffolds. Int. J. Biol. Macromol. 2023, 235, 123769. [Google Scholar] [CrossRef] [PubMed]
- Bhavi, S.M.; Thokchom, B.; Abbigeri, M.B.; Bhat, S.S.; Singh, S.R.; Joshi, P.; Yarajarla, R.B. Green synthesis, characterization, antidiabetic, antioxidant and antibacterial applications of silver nanoparticles from Syzygium caryophyllatum (L.) Alston leaves. Process Biochem. 2024, 145, 89–103. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Modelling natamycin release from alginate/chitosan active films. Int. J. Food Sci. Technol. 2012, 47, 740–746. [Google Scholar] [CrossRef]
- Shirdar, M.R.; Taheri, M.M.; Qi, M.L.; Gohari, S.; Farajpour, N.; Narayanan, S.; Foroozan, T.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Shokuhfar, T. Optimization of the mechanical properties and the cytocompatibility for the pmma nanocomposites reinforced with the hydroxyapatite nanofibers and the magnesium phosphate nanosheets. Materials 2021, 14, 5893. [Google Scholar] [CrossRef]
- Tavukcuoglu, O.; Evcimen Duygulu, N.; Altinbay, A.; Ciftci, F. Green synthesis of silver nanoparticles from Thymus vulgaris and Sambucus nigra extracts in poly (vinyl alcohol) nanofiber matrix: In vitro evaluation. Ind. Crops Prod. 2024, 222, 119825. [Google Scholar] [CrossRef]
- Hong, X.; He, J.; Zou, L.; Wang, Y.; Li, Y.V. Preparation and characterization of high strength and high modulus PVA fiber via dry-wet spinning with cross-linking of boric acid. J. Appl. Polym. Sci. 2021, 138, 51394. [Google Scholar] [CrossRef]
- Uslu, I.; Daştan, H.; Altaş, A.; Yayli, A.; Atakol, O.; Aksu, M.L. Preparation and characterization of PVA/boron polymer produced by an electrospinning technique. e-Polymers 2007, 133, 1–6. [Google Scholar] [CrossRef]
- Başargan, T.; Nasün-Saygili, G. Effect of poly(vinyl alcohol) (PVA) weight ratio on spray-dried apatite–PVA composites. Bioinspired Biomim. Nanobiomater. 2023, 12, 170–177. [Google Scholar] [CrossRef]
- Uslu, İ.; Atakol, O.; Aksu, M.L. Preparation of PVA / Chitosan Doped with Boron Composite Fibers and Their Characterization. Hacet. J. Biol. Chem. 2008, 36, 117–122. [Google Scholar]
- Sun, Y.; Xu, D.; Wang, S. Self-assembly of biomass derivatives into multiple heteroatom-doped 3D-interconnected porous carbon for advanced supercapacitors. Carbon N. Y. 2022, 199, 258–267. [Google Scholar] [CrossRef]
- González-Forte, L.d.S.; Amalvy, J.I.; Bertola, N. Corn starch-based coating enriched with natamycin as an active compound to control mold contamination on semi-hard cheese during ripening. Heliyon 2019, 5, e01957. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Sánchez, L.C.; Vaquiro, H.A.; Solanilla, D.J.F. Physicochemical characterization of films based on xanthan gum. Vitae 2016, 23, S361–S365. [Google Scholar]
- Mouro, C.; Simões, M.; Gouveia, I.C.; Xu, B. Emulsion Electrospun Fiber Mats of PCL/PVA/Chitosan and Eugenol for Wound Dressing Applications. Adv. Polym. Technol. 2019, 2019, 9859506. [Google Scholar] [CrossRef]
- Sheik, S.; Sheik, S.; Nairy, R.; Nagaraja, G.K.; Prabhu, A.; Rekha, P.D.; Prashantha, K. Study on the morphological and biocompatible properties of chitosan grafted silk fibre reinforced PVA films for tissue engineering applications. Int. J. Biol. Macromol. 2018, 116, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Cao, Y.; Rao, J.; Zou, Y.; Zhang, H.; Wu, D.; Chen, K. Application of solution blow spinning to rapidly fabricate natamycin-loaded gelatin/zein/polyurethane antimicrobial nanofibers for food packaging. Food Packag. Shelf Life 2021, 29, 100721. [Google Scholar] [CrossRef]
- Sirisha Nallan Chakravartula, S.; Lourenço, R.V.; Balestra, F.; Quinta Barbosa Bittante, A.M.; Sobral, P.J.d.A.; Dalla Rosa, M. Influence of pitanga (Eugenia uniflora L.) leaf extract and/or natamycin on properties of cassava starch/chitosan active films. Food Packag. Shelf Life 2020, 24, 100498. [Google Scholar] [CrossRef]
- Thi Thu Ha, P.; Xuan Hoa, V.; Kha, T.D.; Dien, N.D.; Thanh, L.D.; Hung, N.Q.; Van Luyen, L. Synthesis and Characterization of Silver Nanoparticles for Antibacterial Application against Bacillus Subtilis and Pseudomonas Aeruginosa. VNU J. Sci. Math.-Phys. 2021, 37, 77–83. [Google Scholar] [CrossRef]
- Ahmed, R.; Tariq, M.; Ali, I.; Asghar, R.; Noorunnisa Khanam, P.; Augustine, R.; Hasan, A. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int. J. Biol. Macromol. 2018, 120, 385–393. [Google Scholar] [CrossRef]
- Vimala, K.; Yallapu, M.M.; Varaprasad, K.; Reddy, N.N.; Ravindra, S.; Naidu, N.S.; Raju, K.M. Fabrication of Curcumin Encapsulated Chitosan-PVA Silver Nanocomposite Films for Improved Antimicrobial Activity. J. Biomater. Nanobiotechnol. 2011, 2, 55–64. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Lin, R.; Cui, S.; Jing, X.; Coseri, S. High mechanical and self-healing carboxymethyl chitosan-hyaluronic acid hybrid hydrogel via multiple dynamic covalent bonds for drug delivery. Eur. Polym. J. 2023, 197, 112342. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Sivakumar, V.; Karthika, K.; Preetha, J.P.; Abirami, T. Design and Evaluation of Polymeric Controlled Release Natamycin Ocular Inserts. Kathmandu Univ. J. Sci. Eng. Technol. 1970, 6, 108–115. [Google Scholar] [CrossRef]
- Verma, P.; Gupta, V.; Manigauha, A. Preparation and Characterization of Natamycin Loaded Bioadhesive inSituOphthalmic Gel for Enhanced Bioavailability. J. Sustain. Mater. Process. Manag. 2022, 2, 41–47. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Schmidt, M.; Tran-Nguyen, D.; Chizek, P. Influence of boric acid on energy metabolism and stress tolerance of Candida albicans. J. Trace Elem. Med. Biol. 2018, 49, 140–145. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, X.; Zhao, L.; Zhang, W.; Zhu, S.; Ma, J. Chitosan Nanoparticles to Enhance the Inhibitory Effect of Natamycin on Candida albicans. J. Nanomater. 2021, 2021, 6644567. [Google Scholar] [CrossRef]
- Delves-Broughton, J. Use of the natural food preservatives, nisin and natamycin, to reduce detrimental thermal impact on product quality. In In-Pack Processed Foods: Improving Quality; Woodhead Publishing: London, UK, 2008; pp. 319–337. ISBN 9781845692469. [Google Scholar]
- Cho, H.L.; Goudar, V.S.; Chan, W.J.; Tseng, F.G. Synthesis of boron-10 enriched chitosan coated PVA/alginate nanoparticles (CHI/ALG-PVA-B NPS) by electrospray technique to treat oral squamous cell carcinoma by boron neutron capture therapy (BNCT). In Proceedings of the 23rd International Conference on Miniaturized Systems for Chemistry and Life Sciences, Basel, Switzerland, 27–31 October 2019; MicroTAS 2019. CBMS: San Diego, CA, USA, 2019; pp. 1590–1591. [Google Scholar]






| E Modulus (MPa) | Ultimate Stress (MPa) | Strain at Break (%) | |
|---|---|---|---|
| PVA/CS/BA | 714.59 ± 12.42 | 47.45 ± 1.26 | 12.57 ± 1.38 |
| PVA/CS/BA_PVA/NAT | 763.04 ± 14.54 | 50.45 ± 2.58 | 11.77 ± 0.49 |
| PVA/CS | 628.36 ± 10.11 | 48.89 ± 2.20 | 13.20 ± 1.22 |
| PVA/CS_PVA/NAT | 653.78 ± 9.94 | 49.30 ± 2.35 | 12.14 ± 1.05 |
| Model | Formula | Parameters | R2 Value | Comment |
|---|---|---|---|---|
| Higuchi | ft = Q = KH t1/2 | kH = 0.00099 | 0.589 | Weak fit—the Higuchi model does not seem to fit the release data. |
| Korsmeyer–Peppas | Mt/M∞ = Krtn + b | k = 3.98 × 10−6, n = 1.84 | 0.997 | Very strong fit—the release can be explained by anomalous diffusion (n > 1). |
| Zero-order | Ct = C0 + K0t | k0 = 0.00018, Q0 = −0.00198 | 0.936 | Good agreement—the release may have occurred at a constant rate. |
| First-order | log Q1 = log Q0 + K1t/2.303 | C0 = 0.00095, k1 = 0.0314 | 0.976 | Good agreement—the release may have occurred with a logarithmically decreasing rate. |
| PVA | PVA/CS | PVA/CS/BA | PVA/CS_ PVA/NAT | PVA/CS/BA_PVA/NAT |
|---|---|---|---|---|
| 0 ± 0 E | 1.05 ± 0.12 D | 1.25 ± 0.08 C | 1.43 ± 0.08 B | 1.64 ± 0.13 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oktay, B.; Ciftci, F.; Erarslan, A.; Ahlatcıoğlu Özerol, E. Dual-Layer Natamycin and Boric-Acid-Reinforced PVA/Chitosan by 3D Printing and Electrospinning Method: Characterization and In Vitro Evaluation. Polymers 2025, 17, 1673. https://doi.org/10.3390/polym17121673
Oktay B, Ciftci F, Erarslan A, Ahlatcıoğlu Özerol E. Dual-Layer Natamycin and Boric-Acid-Reinforced PVA/Chitosan by 3D Printing and Electrospinning Method: Characterization and In Vitro Evaluation. Polymers. 2025; 17(12):1673. https://doi.org/10.3390/polym17121673
Chicago/Turabian StyleOktay, Büsra, Fatih Ciftci, Azime Erarslan, and Esma Ahlatcıoğlu Özerol. 2025. "Dual-Layer Natamycin and Boric-Acid-Reinforced PVA/Chitosan by 3D Printing and Electrospinning Method: Characterization and In Vitro Evaluation" Polymers 17, no. 12: 1673. https://doi.org/10.3390/polym17121673
APA StyleOktay, B., Ciftci, F., Erarslan, A., & Ahlatcıoğlu Özerol, E. (2025). Dual-Layer Natamycin and Boric-Acid-Reinforced PVA/Chitosan by 3D Printing and Electrospinning Method: Characterization and In Vitro Evaluation. Polymers, 17(12), 1673. https://doi.org/10.3390/polym17121673







