Kinetic and Isotherm Studies of Organic and Inorganic Anions Adsorption from Water by Quaternized Pentablock Copolymeric Film (PTBr)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Water Uptake Measurement
2.3. Characterization Techniques
3. Results and Discussion
3.1. Characterization of Membranes
3.1.1. Morphological Characterization
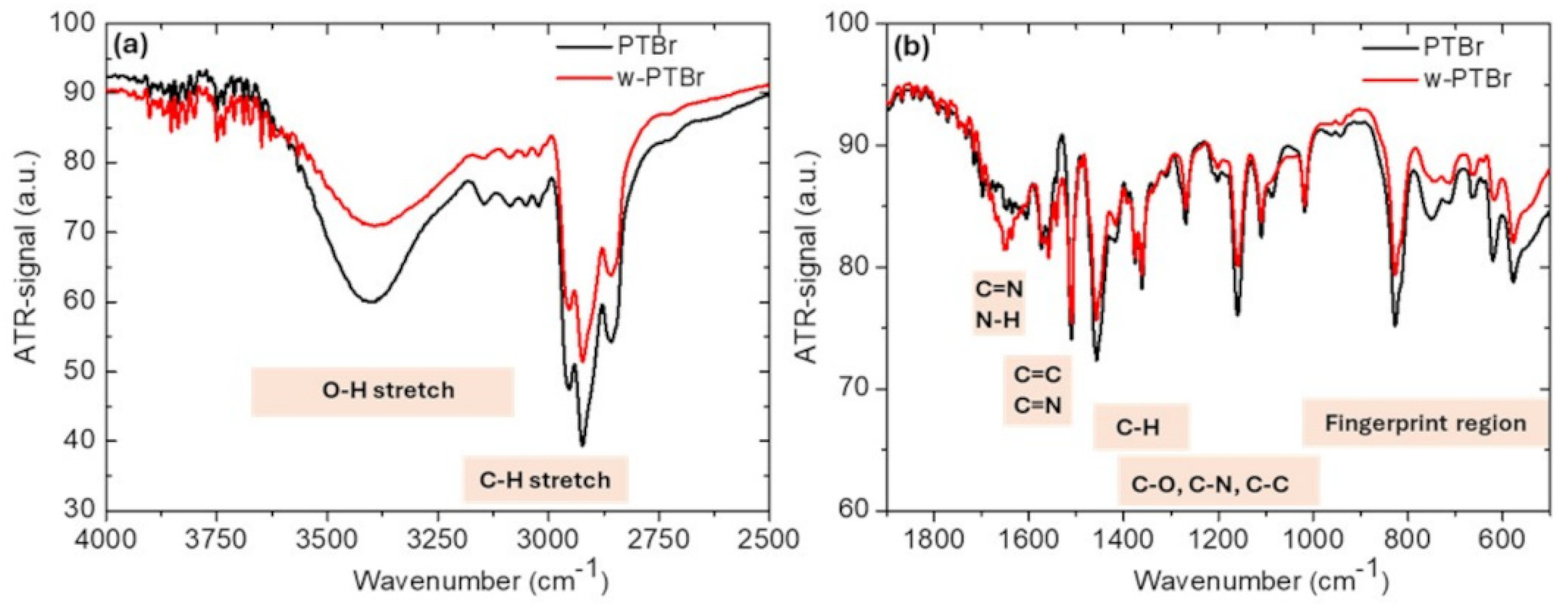
3.1.2. Chemical Characterization: FT-IR and XPS
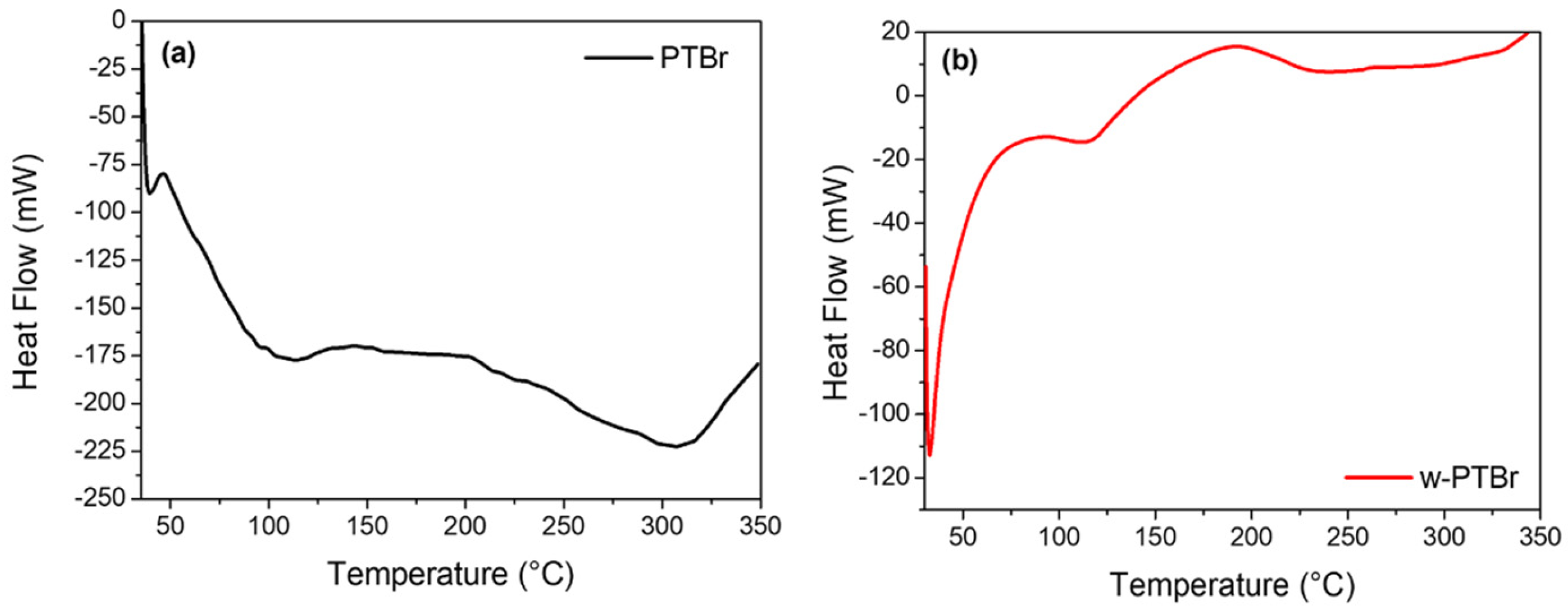
3.1.3. Thermal Analysis
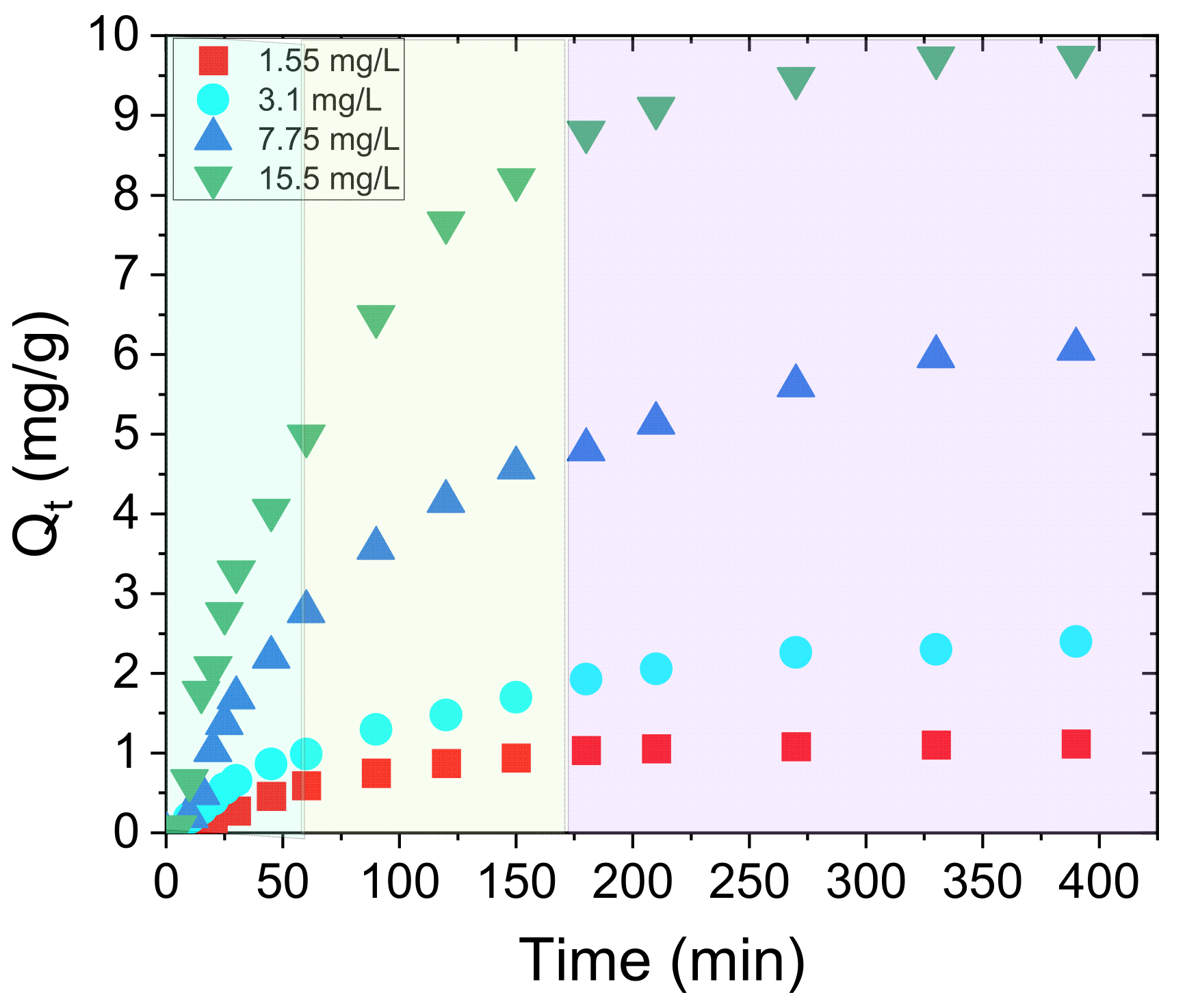
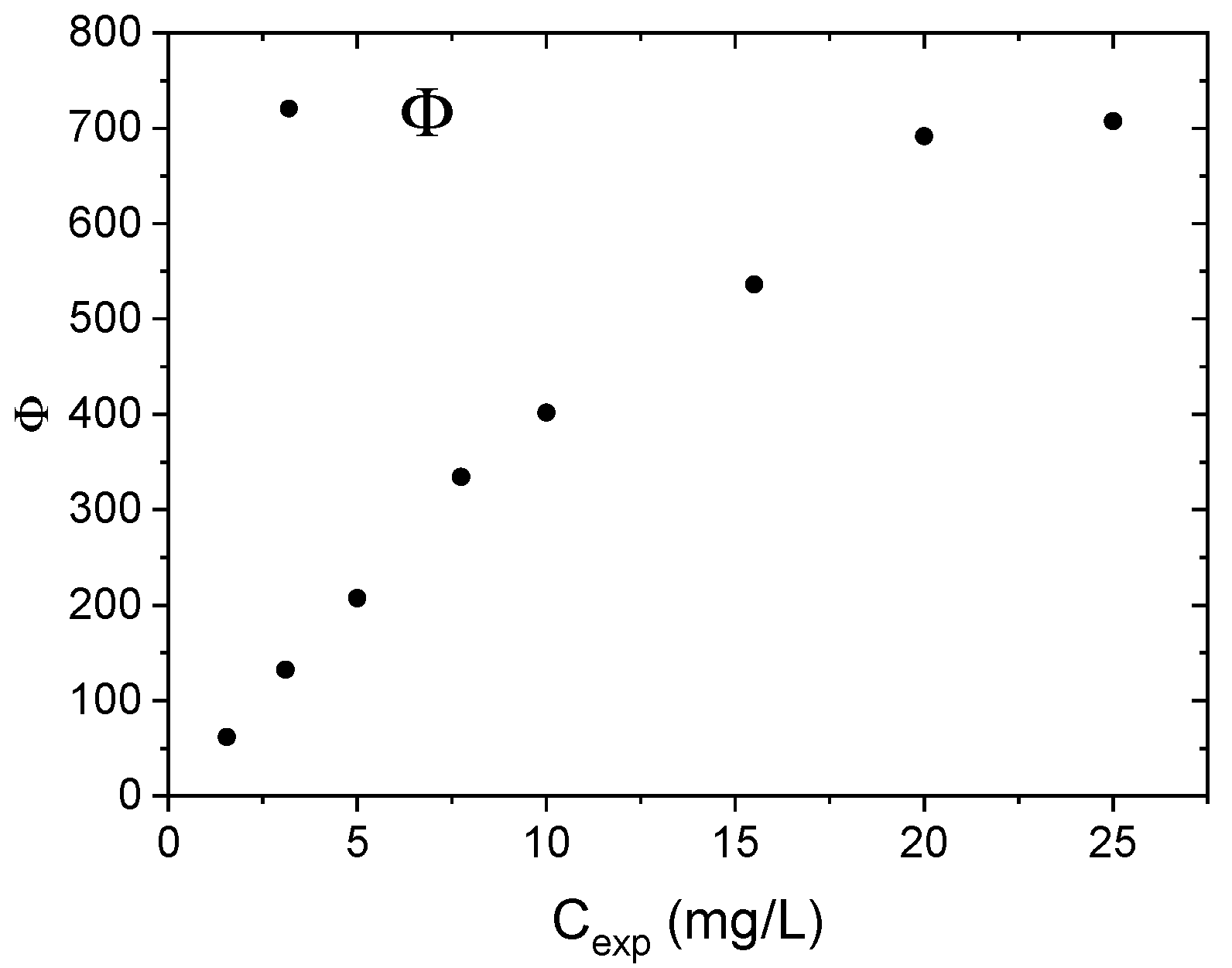
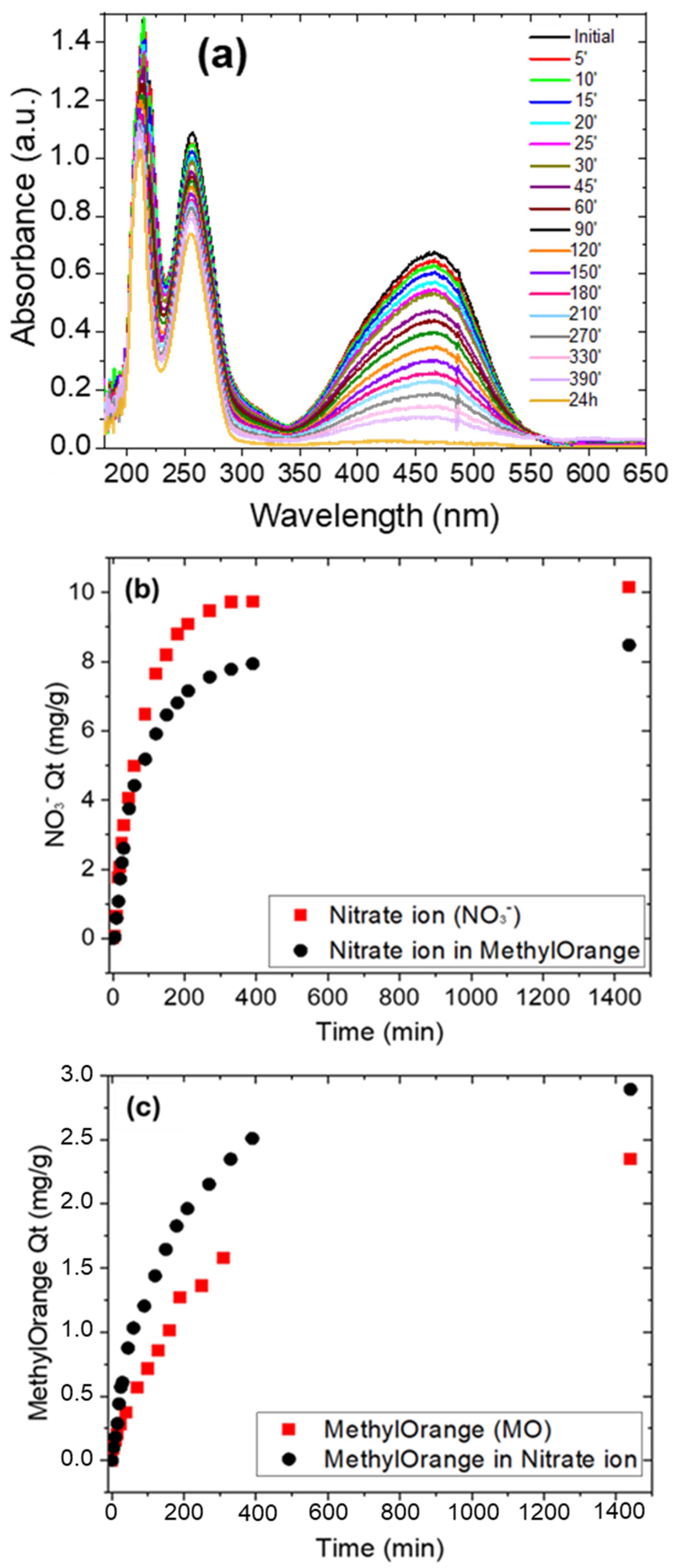
3.2. Adsorption Experiments—Nitrate Ions
3.3. Adsorption Experiments—Organic Pollutants (MO Dye) Removal
3.4. Adsorption Experiments—Mixed Organic and Inorganic Pollutants Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Human Population Growth. Available online: https://www.worldometers.info/world-population (accessed on 6 May 2025).
- Almasoudi, S.; Jamoussi, B. Desalination technologies and their environmental impacts: A review. Sustain. Chem. One World 2024, 1, 100002. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumard, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801. [Google Scholar] [CrossRef]
- Filice, S.; Scuderi, V.; Scalese, S. Sulfonated Pentablock Copolymer (NexarTM) for Water Remediation and Other Applications. Polymers 2024, 16, 2009. [Google Scholar] [CrossRef]
- Singh, B.; Sekon, G.S. Nitrate pollution of groundwater from farm use of nitrogen fertilizers, a review. Agr. Environ. 1979, 4, 207–225. [Google Scholar] [CrossRef]
- Ward, M.H.; Brender, J.D. Drinking Water Nitrate and Health. Encycl. Environ. Health 2011, 15, 167–178. [Google Scholar]
- Løes, A.K.; Eiter, S.; Rittl, T. Calculating the effect of intensive use of urban organic waste on soil concentrations of potentially toxic elements in a peri-urban agriculture context in Norway. Environ. Sci. Eur. 2024, 36, 199. [Google Scholar] [CrossRef]
- Matei, A.; Racoviteanu, G. Review of the technologies for nitrates removal from water intended for human consumption. IOP Conf. Ser. Earth Environ. Sci. 2021, 664, 012024. [Google Scholar] [CrossRef]
- Mohseni-Bandpi, A.; Elliott, D.J.; Zazouli, M.A. Biological nitrate removal processes from drinking water supply—A review. J. Environ. Health Sci. Eng. 2013, 11, 35. [Google Scholar] [CrossRef]
- Degrémont, G. Water Treatment Handbook; Springer: Berlin, Germany, 2007; Volume 6. [Google Scholar]
- Koorngold, E.; Kock, K.; Strathmann, H. Electrodialysis in advanced wastewater treatment. Desalination 1977, 24, 129–139. [Google Scholar] [CrossRef]
- Mackenzie, L.D. Water and Wastewater Engineering: Design Principles and Practice; McGraw-Hill: New York, NY, USA, 2020; Volume 2. [Google Scholar]
- Benkhayaa, S.; M’rabetb, S.; El Harf, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, 03271. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mat. Tod. Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Hell, F.; Lahnsteiner, J.; Frischherz, H.; Baumgartner, G. Experience with full-scale electrodialysis for nitrate and hardness removal. Desalination 1998, 117, 173. [Google Scholar] [CrossRef]
- El Midaoui, A.; Elhannouni, F.; Taky, M.; Chay, L.; Sahli, M.A.M.; Echihabi, L.; Hafsi, M. Optimization of nitrate removal operation from ground water by electrodialysis. Separ. Purif. Technol. 2002, 29, 235–244. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Sun, J.; Yang, H.; Gao, W.; Cao, T.; Zhao, G. Diatomic Pd Cu Metal-Phosphorus Sites for Complete N=N Bond Formation in Photoelectrochemical Nitrate Reduction. Angew. Chem. 2022, 134, 202211373. [Google Scholar] [CrossRef]
- Khademian, M.; Sarrafi, Y.; Tajbakhsh, M.; Hasantabar, V. Quaternary branched polyethylenimine-based nanocomposite for removal of NO3− ions from aqueous solutions. J. Chin. Chem. Soc. 2024, 71, 598–618. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Ongen, A.; Ozcan, H.K.; Aydin, S. Modelling of adsorption kinetic processes -errors, theory and applications. In Advancend Sorption Process Applications; Edebali, S., Ed.; British Library: London, UK, 2019; Chapter 10. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, W. Synthesis of a Novel Polymer Adsorbent and Its Adsorption of Pb (II) and Cu (II) Ions in Water. Processes 2024, 12, 2901. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Chapter 1—Fundamentals of adsorption technology. Interface Sci. Technol. 2021, 33, 1–70. [Google Scholar] [CrossRef]
- Song, H.; Zhou, Y.; Li, A.; Mueller, S. Selective removal of nitrate from water by a macroporous strong basic anion exchange resin. Desalination 2012, 296, 53–60. [Google Scholar] [CrossRef]
- Shen, Z.; Fang, M.; Tang, L.; Shi, J.; Wang, W. Pd/Cu bimetallic nano-catalyst supported on anion exchange resin (A520E) for nitrate removal from water: High property and stability. Environ. Res. 2024, 241, 117616. [Google Scholar] [CrossRef]
- Adam, M.R.; Puteh, M.H.; Othman, D.; Ismail, A.F.; Khongnakorn, W.; Kurniawan, T.A.; Rahman, M.A.; Jaafar, J. Advances in adsorptive membrane technology for water treatment and resource recovery applications: A critical review. J. Environ. Chem. Eng. 2022, 10, 107633. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Libertino, S.; Nicotera, I.; Kosma, V.; Privitera, V.; Scalese, S. Graphene oxide and titania hybrid Nafion membranes for efficient removal of methyl orange dye from water. Carbon 2015, 82, 489–499. [Google Scholar] [CrossRef]
- D’Angelo, D.; Filice, S.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Bi2O3/Nexar® polymer nanocomposite membranes for azo dyes removal by UV–vis or visible light irradiation. Catal. Tod. 2019, 321, 158–163. [Google Scholar] [CrossRef]
- Scalese, S.; Nicotera, I.; D’Angelo, D.; Filice, S.; Libertino, S.; Simari, C.; Dimos, K.; Privitera, V. Cationic and anionic azo-dye removal from water by sulfonated graphene oxide nanosheets in Nafion membranes. New J. Chem. 2016, 40, 3654–3663. [Google Scholar] [CrossRef]
- John, R.; Pal, K.; Jayan, J.S.; Appukuttan, S.; Kuruvilla, J. New emerging review on advances in block copolymer-based water purification membranes. J. Mol. Struct. 2021, 1231, 129926. [Google Scholar] [CrossRef]
- Filice, S.; Mazurkiewicz-Pawlicka, M.; Malolepszy, A.; Stobinski, L.; Kwiatkowski, R.; Boczkowska, A.; Gradon, L.; Scalese, S. Sulfonated Pentablock Copolymer Membranes and Graphene Oxide Addition for Efficient Removal of Metal Ions from Water. Nanomaterials 2020, 10, 1157. [Google Scholar] [CrossRef]
- Filice, S.; D’Angelo, D.; Scarangella, A.; Iannazzo, D.; Compagnini, G.; Scalese, S. Highly effective and reusable sulfonated pentablock copolymer nanocomposites for water purification applications. RSC Adv. 2017, 7, 45521–45534. [Google Scholar] [CrossRef]
- Filice, S.; Scuderi, V.; Zimbone, M.; Libertino, S.; Farina, R.A.; Scalese, S. Sulfonated Pentablock Copolymer with Graphene Oxide for Co2+ Ions Removal: Efficiency, Interaction Mechanisms and Secondary Reaction Products. Coatings 2023, 13, 1715. [Google Scholar] [CrossRef]
- Sciuto, E.L.; Filice, S.; Coniglio, M.A.; Faro, G.; Gradon, L.; Galati, C.; Spinella, N.; Libertino, S.; Scalese, S. Antimicrobial s-PBC Coatings for Innovative Multifunctional Water Filters. Molecules 2020, 25, 5196. [Google Scholar] [CrossRef]
- Yang, J.; Lin, S.; Wang, C.; Xu, X.; Du, P.; Li, Q.; Li, D.; Ma, J.; Wang, G.; Li, M. Highly selective adsorption of nitrates and phosphates in industrial secondary effluent using modified resin and resin recycling. J. Environ. Chem. Eng. 2025, 13, 116412. [Google Scholar] [CrossRef]
- Green, M.D.; Long, T.E. Designing Imidazole-Based Ionic Liquids and Ionic Liquid Monomers for Emerging Technologies. Polym. Rev. 2009, 49, 291–314. [Google Scholar] [CrossRef]
- Hu, F.; Fang, C.; Wang, Z.; Liu, C.; Zhu, B.; Zhu, L. Poly (N-vinyl imidazole) gel composite porous membranes for rapid separation of dyes through permeating adsorption. Sep. Purif. Technol. 2017, 188, 1–10. [Google Scholar] [CrossRef]
- Amininasab, S.M.; Adim, S.; Abdolmaleki, S.; Soleimani, B.; Hassanzadeh, M. Synthesis and Characterization of Chitosan-Based Hydrogels Grafted Polyimidazole as Nitrate Ion Adsorbent from Water and Investigating Biological Properties. J. Polym. Environ. 2025, 33, 1131–1146. [Google Scholar] [CrossRef]
- Geise, G.M.; Freeman, B.D.; Pau, D.R. Characterization of a sulfonated pentablock copolymer for desalination applications. Polymer 2010, 51, 5815–5822. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical Properties of Imidazole-Based Ionic Liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar] [CrossRef]
- Majhi, D.; Dai, J.; Dvinskikh, S.V. Insights into cation–anion hydrogen bonding in mesogenic ionic liquids: An NMR study. Phys. Chem. Chem. Phys. 2022, 24, 23532–23539. [Google Scholar] [CrossRef]
- Szpecht, A.; Zielinski, D.; Galinski, M.; Smiglak, M. Thermal and Electrochemical Properties of Ionic Liquids Bearing Allyl Group with Sulfonate-Based Anions—Application Potential in Epoxy Resin Curing Process. Molecules 2023, 28, 709. [Google Scholar] [CrossRef]
- Awada, H.W.; Gilmana, J.W.; Nydena, M.; Harris, R.H., Jr.; Sutto, T.E.; Callahan, J.; Trulove, P.C.; DeLong, H.C.; Fox, D.M. Thermal degradation studies of alkyl-imidazole salts and their application in nanocomposites. Thermoch. Act. 2004, 409, 3–11. [Google Scholar] [CrossRef]
- Zampino, D.C.; Samperi, F.; Mancuso, M.; Ferreri, T.; Ferreri, L.; Dattilo, S.; Mirabella, E.F.; Carbone, D.C.; Recca, G.; Scamporrino, A.A.; et al. Polymer Blends Based on 1-Hexadecyl-3-methyl Imidazole 1,3-Dimethyl 5-Sulfoisophthalate Ionic Liquid: Thermo Mechanical, Surface Morphology and Antibacterial Properties. Polymers 2023, 15, 970. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Fardis, D.; Ntampou, X.; Tsivintzelis, I.; Panayiotou, C. Thermal Behavior of Poly (vinyl alcohol) in the Form of Physically Crosslinked Film. Polymers 2023, 15, 1843. [Google Scholar] [CrossRef]
- Zhana, Y.; Linb, J.; Zhua, Z. Removal of nitrate from aqueous solution using cetylpyridinium bromide (CPB) modified zeolite as adsorbent. J. Hazard. Mater. 2011, 186, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.S.; Coletti, L.J. In situ ultraviolet spectrophotometry for high resolution and long-term monitoring of nitrate, bromide and bisulfide in the ocean. Deep-Sea Res. Part I 2002, 49, 1291–1305. [Google Scholar] [CrossRef]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of adsorption kinetics including a surface reaction mechanism: A review. Adv. Colloid. Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. Kungl. Sven. Vetensk. Hand. 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of heavy metals from waters by means of natural zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Febrianto, J.; Kosasih, A.N.; Sunarsob, J.; Jua, J.H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mat. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Popuria, S.R.; Vijayaa, Y.; Boddub, V.M.; Abburi, K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009, 100, 194–199. [Google Scholar] [CrossRef]
- Kennedya, L.J.; Vijayab, J.J.; Sekarana, G.; Kayalvizhi, K. Equilibrium, kinetic and thermodynamic studies on the adsorption of m-cresol onto micro- and mesoporous carbon. J. Hazard. Mat. 2007, 149, 134–143. [Google Scholar] [CrossRef]
- Al-Ghoutia, M.A.; Khraishehb, M.A.M.; Ahmadc, M.N.M.; Allen, S. Adsorption behaviour of methylene blue onto Jordanian diatomite: A kinetic study. J. Hazard. Mat. 2009, 165, 589–598. [Google Scholar] [CrossRef]
- Mohan, S.V.; Ramanaiah, S.V.; Rajkumar, B.; Sarma, P.N. Removal of fluoride from aqueous phase by biosorption onto algal biosorbent Spirogyra sp.-IO2: Sorption mechanism elucidation. J. Hazard. Mat. 2007, 141, 465–474. [Google Scholar] [CrossRef]
- Nandi, B.K.; Goswami, A.; Purkait, M.K. Adsorption characteristics of brilliant green dye on kaolin. J. Hazard. Mat. 2009, 161, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S. Halloysite-based hybrids/composites in catalysts. Appl. Clay Sci. 2020, 189, 105537. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solutions. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Largittea, L.; Pasquierb, R. A review of kinetic adsorption models and their application to lead adsorption by activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Gu, T. Surfactant Adsorption at solid-liquid Interfaces. Adv. Colloid Interface Sci. 1991, 37, 1–32. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mat. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A. Arsenic adsorption onto iron oxide-coated cement (IOCC): Regression analysis of equilibrium data with several isotherm models and their optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, Mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, A.D.D. A General Treatment and Classification of the Solute Adsorption Isotherm. J. Col. Interf. Sci. 1974, 47, 3. [Google Scholar] [CrossRef]
- Saraydın, D.; Işıkver, Y.; Karadağ, E. An evaluation on S-type adsorption isotherm in the model of crosslinked polyhydroxamates/oxazine dyes/water interactions. Adsorption 2022, 28, 249–260. [Google Scholar] [CrossRef]
- Hinz, C. Description of sorption data with isotherm equations. Geoderma 2001, 99, 225–243. [Google Scholar] [CrossRef]
- Liyun, Y.; Ping, X.; Maomao, Y.; Hao, B. The characteristics of steel slag and the effect of its application as a soil additive on the removal of nitrate from aqueous solution. Environ. Sci. Pollut. Res. 2017, 24, 4882–4893. [Google Scholar] [CrossRef]
- Phan, T.P.; Nguyen, T.T.; Nguyen, H.N.; Padungthon, S. A Simple Method for Synthesis of Triamine-SiO2 Material toward Aqueous Nitrate Adsorption. Environ. Nat. Res. J. 2019, 17, 59–67. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Shi, X.; Tang, H.; Wang, X.; Wang, S.; Zhang, W.; Lu, J. Preferential Nitrate Removal from Water Using a New Recyclable Polystyrene Adsorbent Functionalized with Triethylamine Groups. Ind. Eng. Chem. Res. 2020, 59, 5194–5201. [Google Scholar] [CrossRef]
- Yang, W.; Shi, X.; Wang, J.; Chen, W.; Zhang, L.; Zhang, W.; Zhang, X.; Lu, J. Fabrication of a Novel Bifunctional Nanocomposite with Improved Selectivity for Simultaneous Nitrate and Phosphate Removal from Water. ACS Appl. Mater. Interf. 2019, 11, 35277–35285. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W.; Ding, X.; Singh, R.P. Adsorption of Nitrate by a Novel Polyacrylic Anion Exchange Resin from Water with Dissolved Organic Matters: Batch and Column Study. Appl. Sci. 2019, 9, 3077. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, W. Polyethylenimine-functionalized polyacrylonitrile anion exchange fiber as a novel adsorbent for rapid removal of nitrate from wastewater. Chemosphere 2020, 258, 127373. [Google Scholar] [CrossRef]

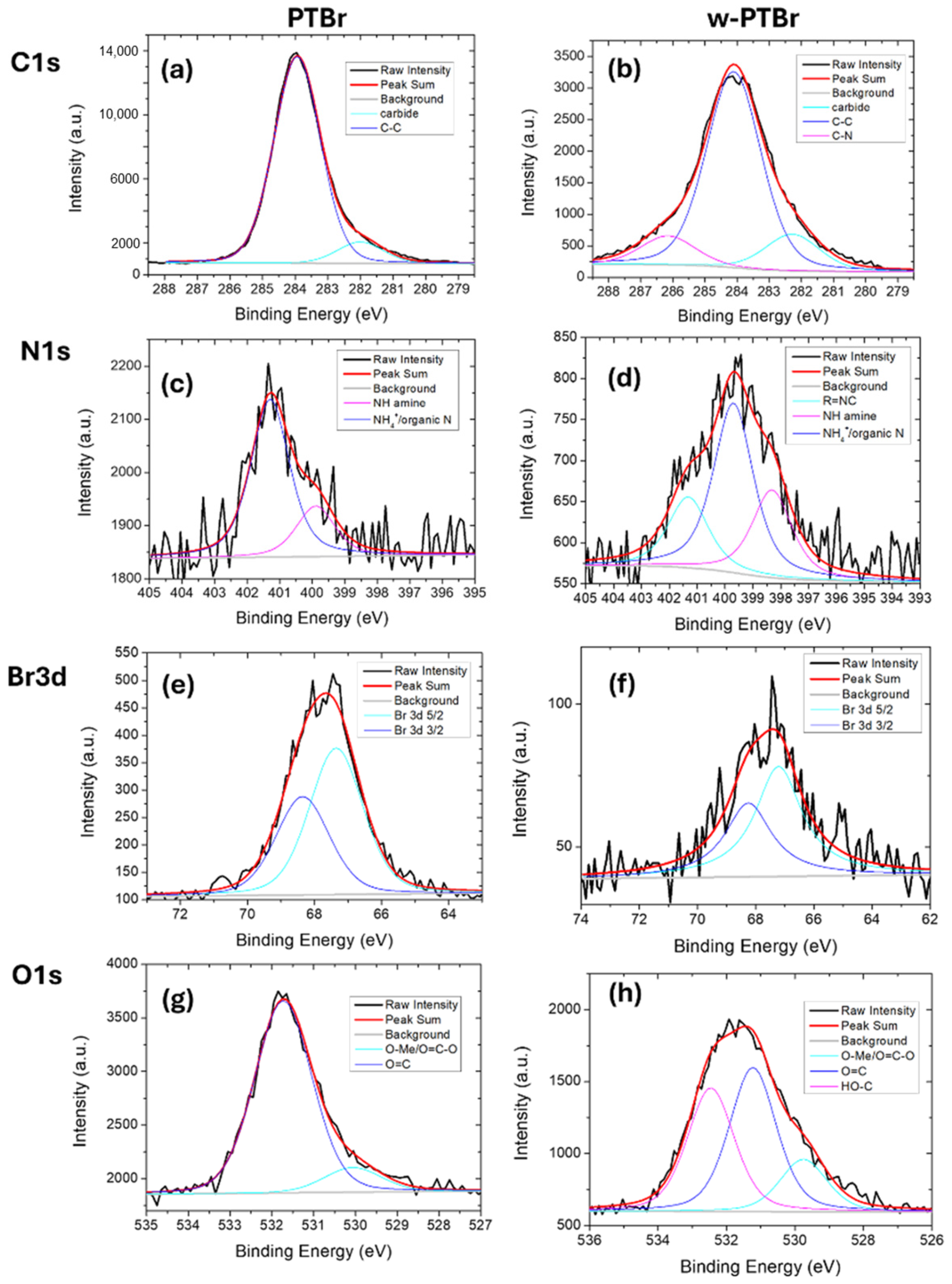



| Peak | Contributions | PTBr | w-PTBr | ||
|---|---|---|---|---|---|
| Peak BE (eV) | (%) | Peak BE (eV) | (%) | ||
| C1s | Carbide / C-H / C vacancies | 282.00 | 9.5 | 282.3 | 14 |
| C-C | 284 | 90.5 | 284.1 | 75 | |
| C-N | -- | -- | 286.1 | 11 | |
| N1s | R=N-C | -- | -- | 398.3 | 26.7 |
| NH (amine) | 399.9 | 24.3 | 399.7 | 51.7 | |
| NH4+ | 401.3 | 75.7 | 401.2 | 21.6 | |
| Br3d | Br 3d5/2 | 67.3 | 59.8 | 67.2 | 59.8 |
| Br 3d3/2 | 68.3 | 40.2 | 68.2 | 40.2 | |
| O1s | O=C-O | 530.1 | 88.6 | 529.7 | 16.4 |
| O=C | 531.7 | 11.4 | 531.2 | 45.0 | |
| HO-C | -- | -- | 532.5 | 38.6 | |
| NO3− Concentration | Qt (NO3− mg/PTBr Moles) | Qt (NO3− mg/Imidazole Moles) |
|---|---|---|
| 1.55 | 76,260 | 125,016 |
| 3.10 | 205,820 | 337,409 |
| 7.75 | 500,200 | 820,000 |
| 15.50 | 832,300 | 1,364,426 |
| NO3− (mg/L) | R2 | k1 (min−1) | Qe (mg/g) | Qe 24h(mg/g) |
|---|---|---|---|---|
| 1.55 | 0.995 | −0.0136 ± 0.0002 | 1.21 | 1.12 |
| 3.10 | 0.992 | −0.0079 ± 0.0002 | 2.46 | 2.51 |
| 7.75 | 0.971 | −0.0104 ± 0.0005 | 6.53 | 6.10 |
| 15.50 | 0.991 | −0.0100 ± 0.0002 | 9.55 | 10.50 |
| PFO | PSO | Interparticle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | k1 (min−1) | Qe (mg/g) | R2 | k2 (g/mg) min−1 | Qe (mg/g) | R2 | K (mg/g) min0.5 | C |
| 0.992 | −0.0035 | 2.29 | 0.782 | 0.0038 | 2.00 | 0.978 | 0.0938 | −0.15 |
| Solutions | Qt (mg/g) | |
|---|---|---|
| NO3− | MO | |
| KNO3 | 10.15 | - |
| MO | - | 2.35 |
| KNO3 + MO | 8.48 | 2.89 |
| 15.50 mg/L NO3− | ||||||||
|---|---|---|---|---|---|---|---|---|
| After 1 h | After 3 h | Up to 330 min | ||||||
| Intraparticle Diffusion | PFO | PSO | ||||||
| R2 | K (mg/g)min0.5 | C | R2 | k2 (min−1) | Qe (mg/g) | R2 | k2 (g/mg)min−1 | Qe (mg/g) |
| 0.996 | 0.8645 | −2.16 | 0.998 | −0.0015 | 9.51 | 0.999 | 0.0016 | 9.35 |
| 3.27 mg/L Methyl Orange | ||||||||
| After 1 h | After 3 h | Up to 330 min | ||||||
| Intraparticle Diffusion | PFO | PFO | ||||||
| R2 | K (mg/g)min0.5 | C | R2 | k1 (min−1) | Qe (mg/g) | R2 | k1 (g/mg)min−1 | Qe (mg/g) |
| 0.972 | 0.0751 | −0.11 | 0.993 | −0.0013 | 2.05 | 0.997 | −0.0007 | 1.84 |
| Adsorbents | Adsorption Capacity (mg/g) | References | |
|---|---|---|---|
| w-PTBr | 10.15 | This work | |
| Steel slag | 2.83 | [68] | |
| Modified SiO2 | 20 | [69] | |
| Polystyrene microspheres modified with trimethylamine functional groups | XAD-4 | 8 | [70] |
| GAC | 3 | ||
| Bifunctional polystyrene microspheres | GAC | 0 | [71] |
| XAD-4 | 3 | ||
| Polyacrylic anion exchange resin | D311 | 28 | [72] |
| AEE-3 | 24 | ||
| Polymer-based anion exchange fibers | PAN-PEI-3C | 31.30 | [73] |
| PAN-PEI-5C | 31.32 | ||
| PAN-PEI-8C | 31.19 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crispi, S.; Filice, S.; Scuderi, V.; Zimbone, M.; Iannazzo, D.; Celesti, C.; Scalese, S. Kinetic and Isotherm Studies of Organic and Inorganic Anions Adsorption from Water by Quaternized Pentablock Copolymeric Film (PTBr). Polymers 2025, 17, 1624. https://doi.org/10.3390/polym17121624
Crispi S, Filice S, Scuderi V, Zimbone M, Iannazzo D, Celesti C, Scalese S. Kinetic and Isotherm Studies of Organic and Inorganic Anions Adsorption from Water by Quaternized Pentablock Copolymeric Film (PTBr). Polymers. 2025; 17(12):1624. https://doi.org/10.3390/polym17121624
Chicago/Turabian StyleCrispi, Simona, Simona Filice, Viviana Scuderi, Massimo Zimbone, Daniela Iannazzo, Consuelo Celesti, and Silvia Scalese. 2025. "Kinetic and Isotherm Studies of Organic and Inorganic Anions Adsorption from Water by Quaternized Pentablock Copolymeric Film (PTBr)" Polymers 17, no. 12: 1624. https://doi.org/10.3390/polym17121624
APA StyleCrispi, S., Filice, S., Scuderi, V., Zimbone, M., Iannazzo, D., Celesti, C., & Scalese, S. (2025). Kinetic and Isotherm Studies of Organic and Inorganic Anions Adsorption from Water by Quaternized Pentablock Copolymeric Film (PTBr). Polymers, 17(12), 1624. https://doi.org/10.3390/polym17121624











