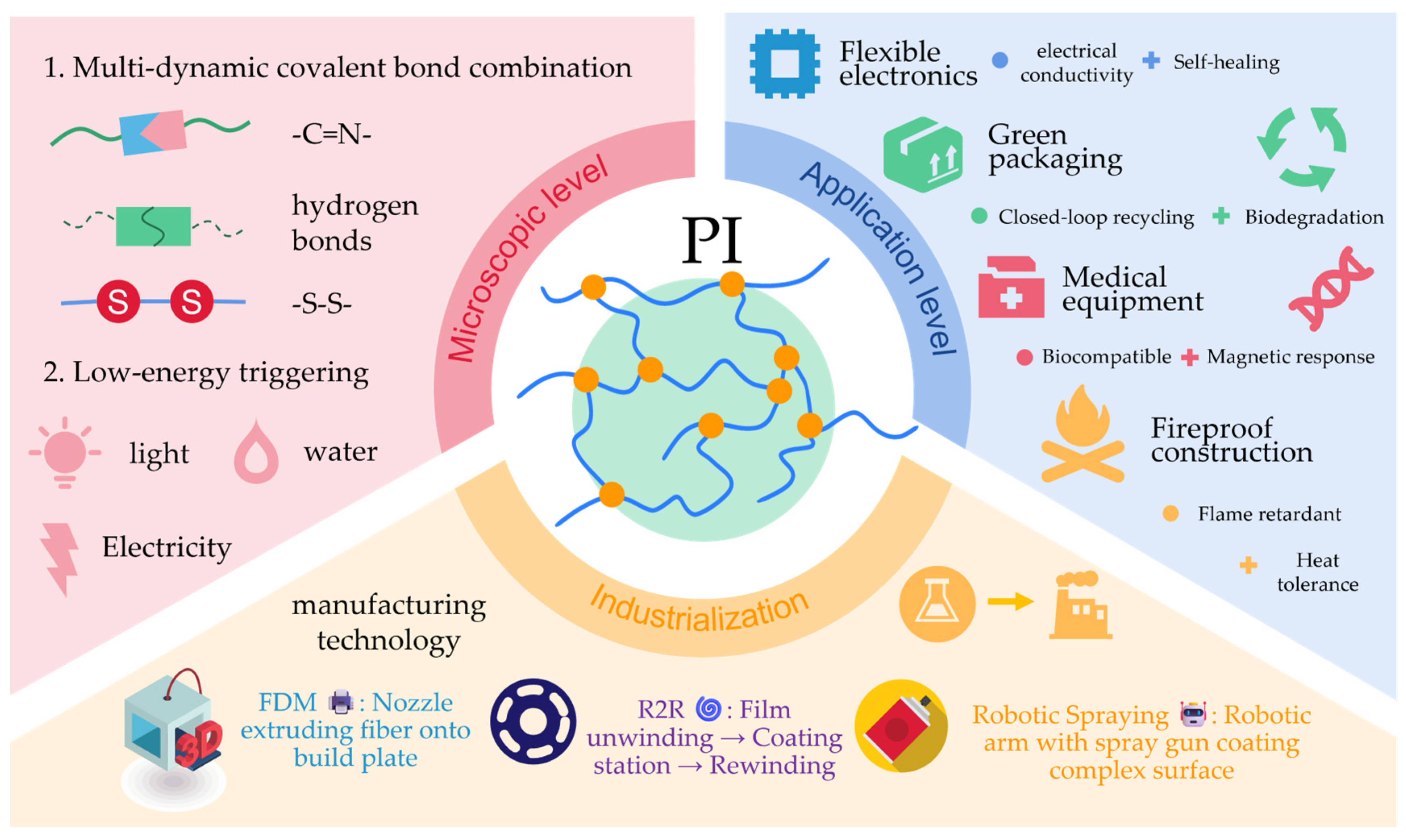
Polyimine-Based Self-Healing Composites: A Review on Dynamic Covalent Thermosets for Sustainable and High-Performance Applications
Abstract
1. Introduction
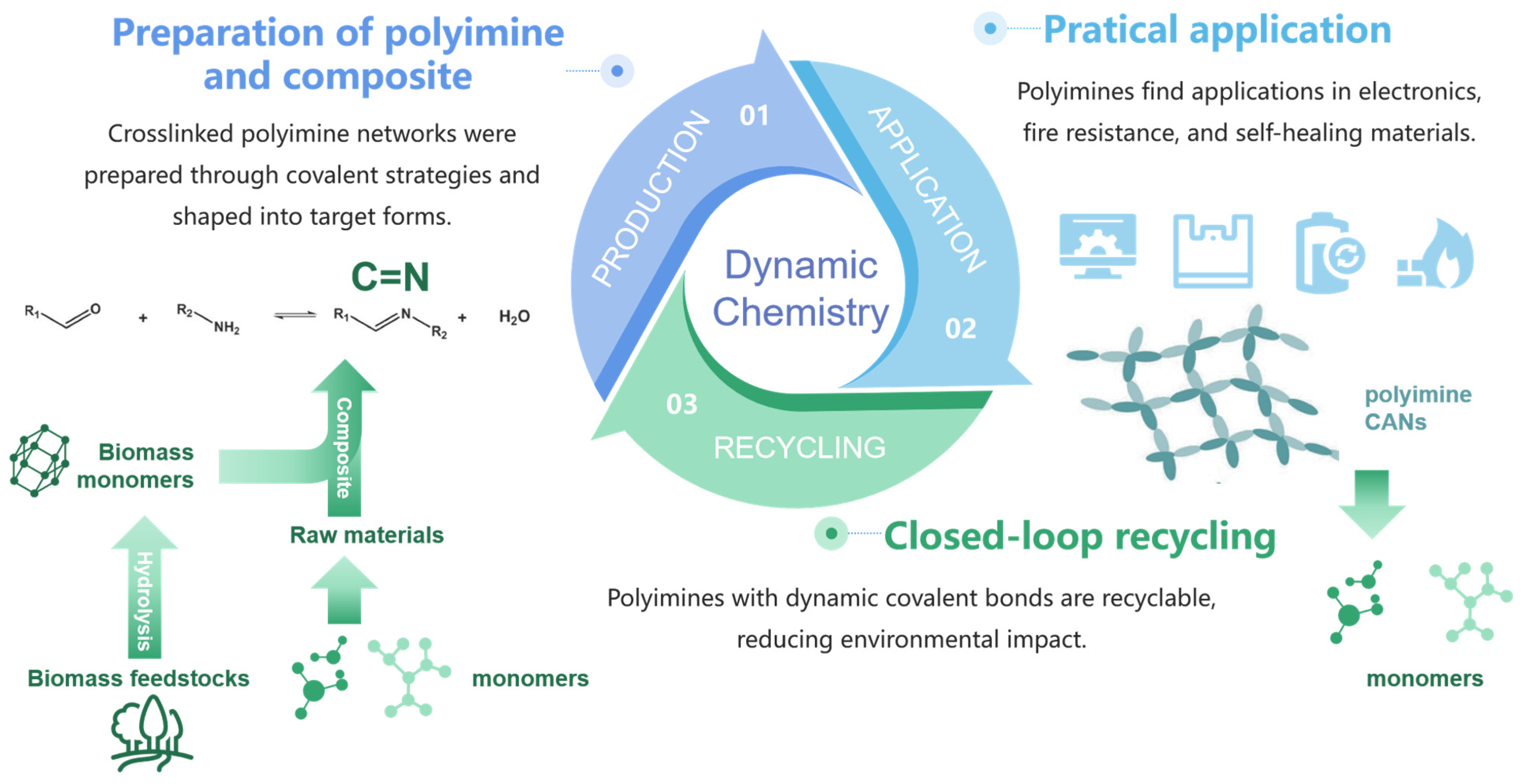
2. Synthesis of Polyimines via Dynamic Chemistry Approaches
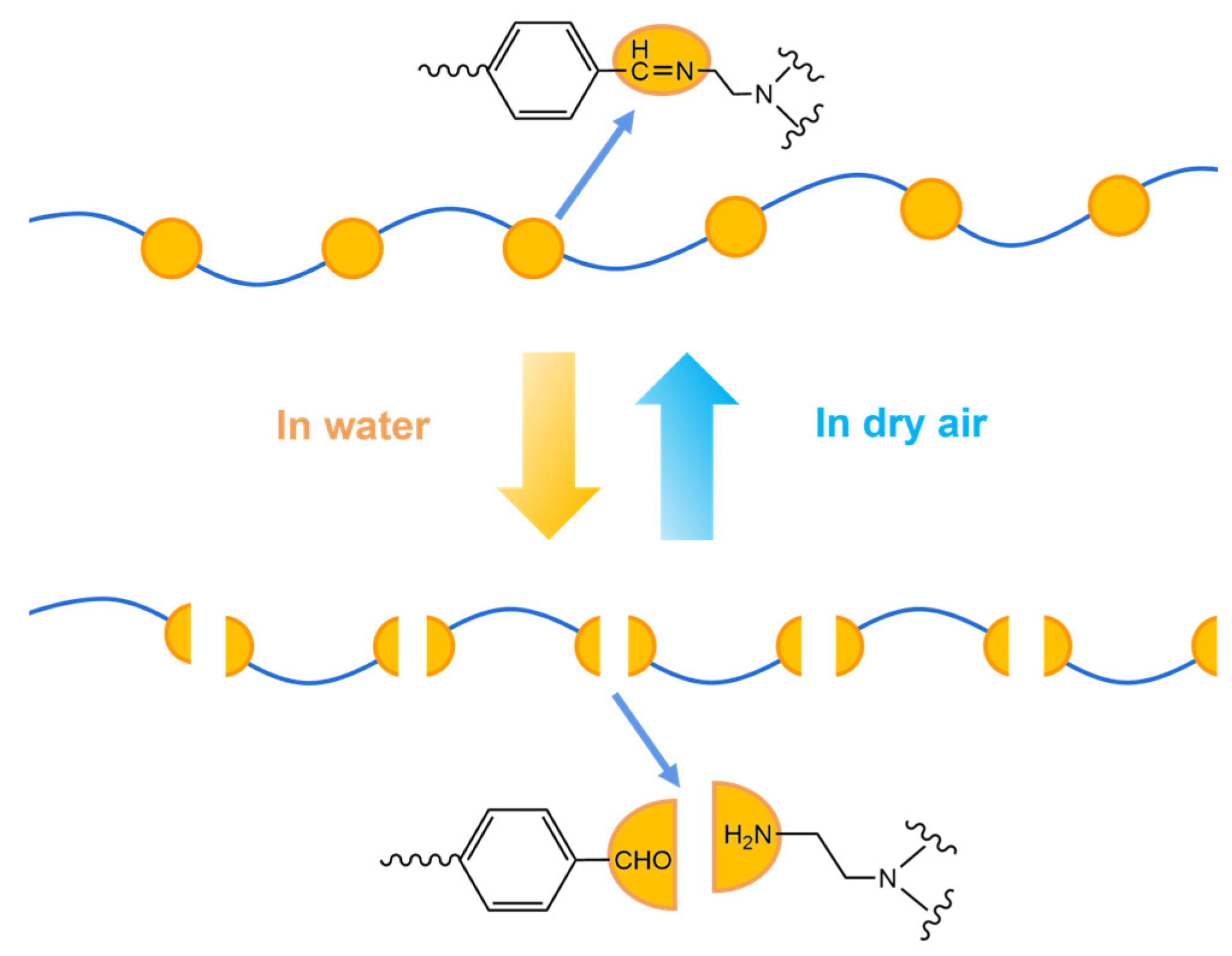
2.1. Dynamic Imine Chemistry
2.2. The Methods for Adapting Polyimine to Different Molding States
2.2.1. Solution Casting
2.2.2. Hot Pressing
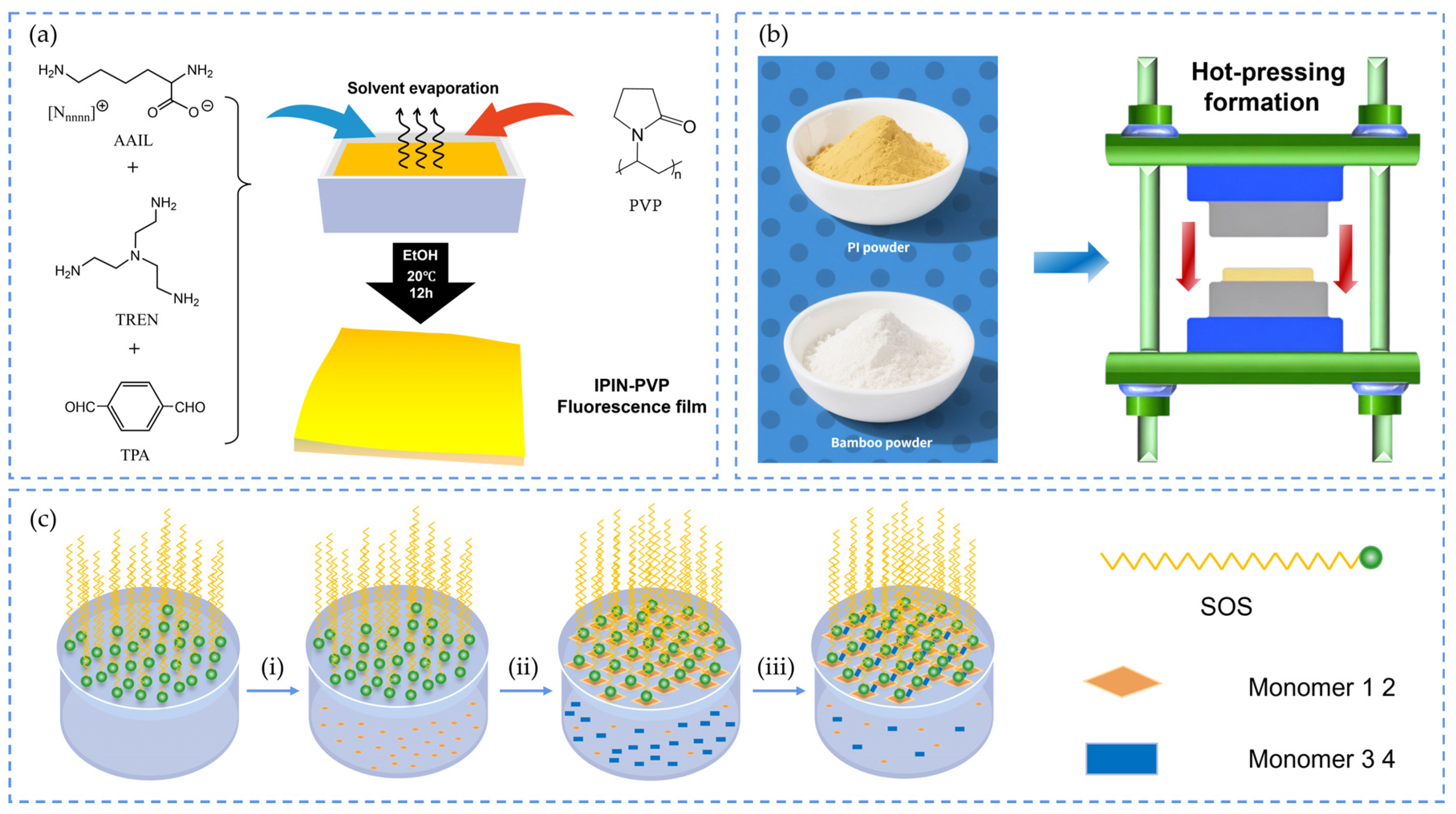
2.2.3. Interfacial Polymerization
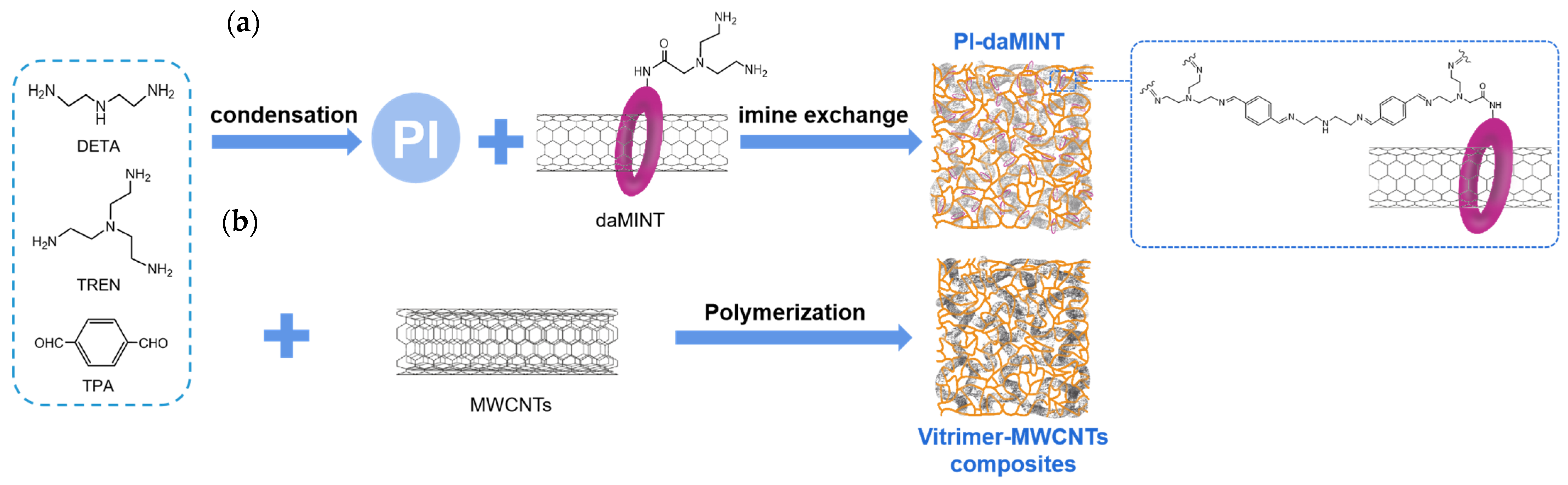
3. Preparation of Polyimine Composites
3.1. Selection of Reinforcements
3.1.1. Organic Reinforcements
3.1.2. Inorganic Reinforcements
3.2. Composite Methods
3.2.1. Physical Blending
3.2.2. Chemical Bonding
3.2.3. Lamination
4. Factors Affecting the Mechanical Properties of the Composites
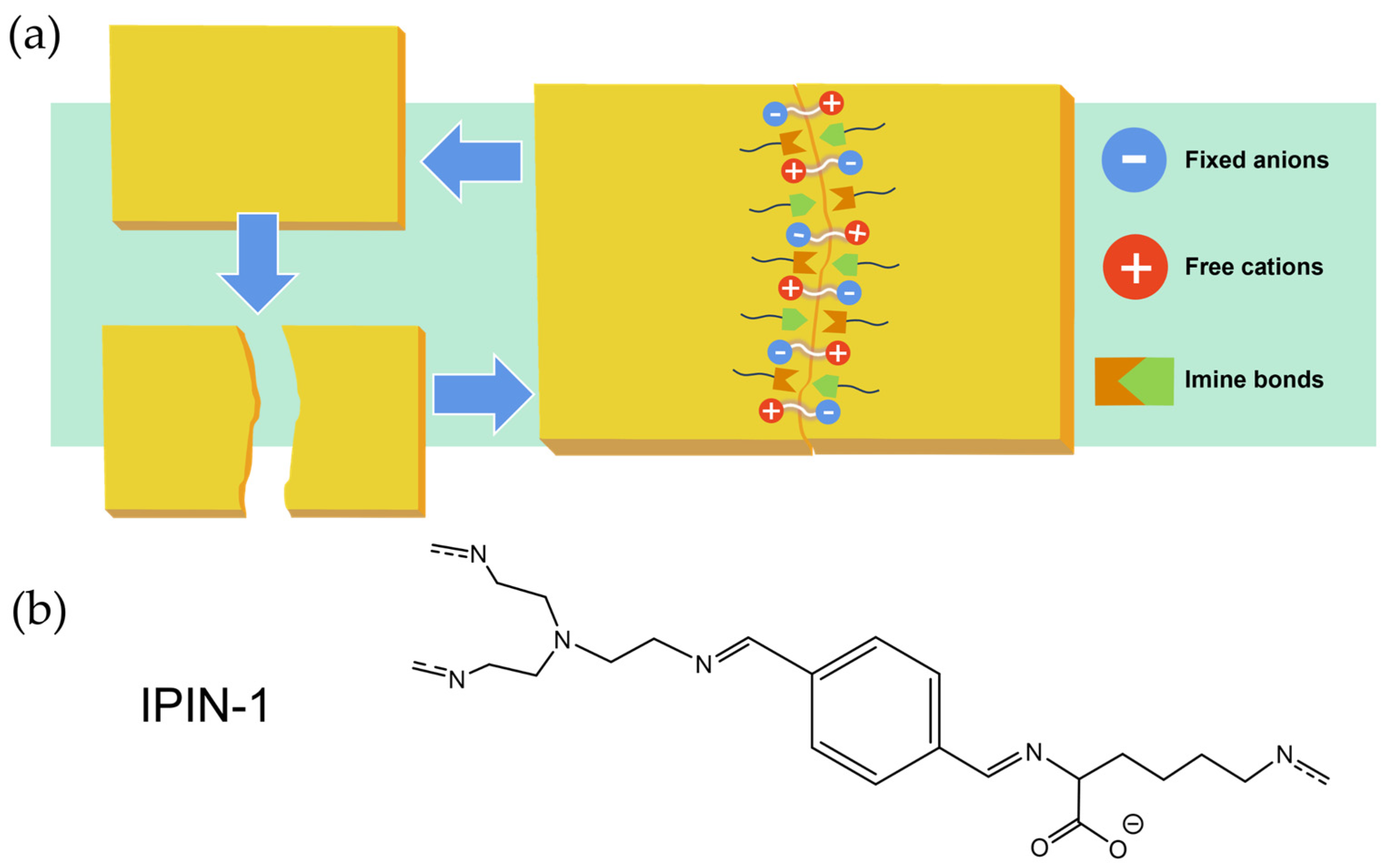
4.1. Correlation Between Dynamic Bonds and Mechanical Properties
4.2. Interfacial Engineering and Multiscale Performance Optimization
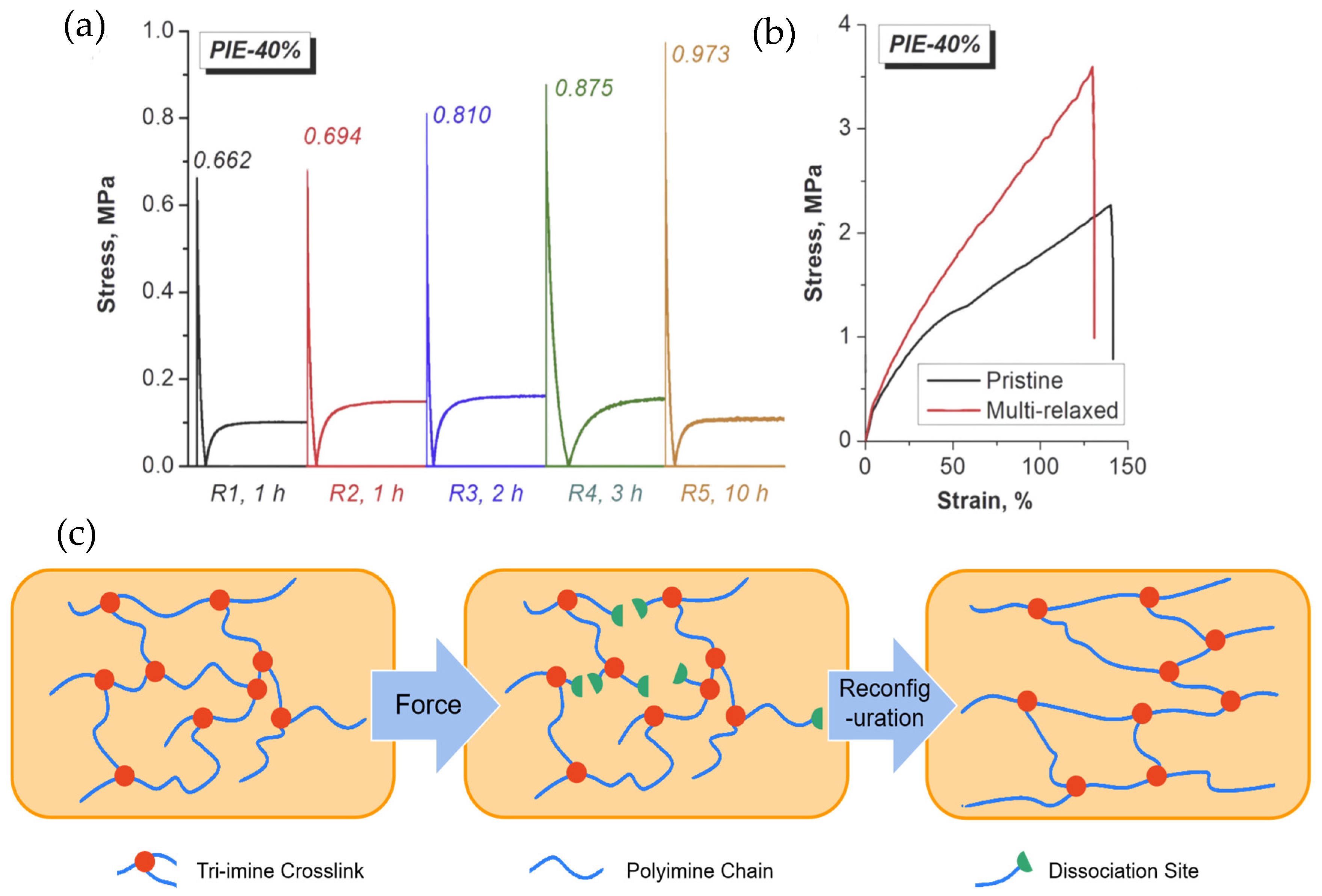
4.3. Impact of Self-Healing Behavior on Mechanical Performance
| Polyimine Materials | Physical Form | Mechanical Property Analysis | Key Findings | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (GPa) | Thermal Stability (Td5%, °C) | Self-Healing Efficiency (%) | Limiting Oxygen Index (LOI, %) | ||||
| IPIN | film | 4.28 | 250 | - | >160 | Self-healing efficiency: 50.04% after 5 min | - | Exhibits good ductility, self-healing ability, and recyclability. Electrochemical sensors fabricated from IPIN-1 demonstrate high response rates and low detection limits for iodine monitoring. | [58] |
| PE0.5-0.2 vitrimer | film | 2.51 | 1158 | - | - | Self-healing at room temperature: 30.0 (6 h), 69.3 (12 h), 96.0 (24 h) | - | The material was synthesized through a simple two-step one-pot process at room temperature, exhibiting outstanding mechanical properties, self-healing capability, degradability, and reprocessability. | [59] |
| CTM-3 | film | 0.56 ± 0.66 | 38.4 ± 0.3 | - | 455.0 | - | 41.6 | A catalyst-free vitrimer featuring enhanced flame retardancy, thermal stability, solvent resistance, mechanical strength, and recyclability, outperforming conventional counterparts in reported studies. | [60] |
| PIM-4 | film | 94.5 ± 2.6 | 6.1 ± 0.9 | 3.5 ± 0.2 | 434 | - | - | Exhibits low water uptake (~0.14–0.15%), excellent mechanical properties minimally affected by absorbed water, chemical resistance, and recyclability, positioning it as a promising alternative to petroleum-based thermosetting resins for harsh environments. | [61] |
| FA-100 | film | 28.47 ± 2.01 | 7.74 ± 1.24 | 0.37 | 161.32 | - | 28.8 | Elevating D-FA content enhances the network’s crosslink density and mechanical properties. Dynamic imine linkages endow the material with reprocessability and acid-degradability. FA-100 demonstrates superior flame resistance, achieving a limiting oxygen index (LOI) of 28.8%. | [62] |
| CO-PIM-75 | film | 62.5 | 12.9 | - | >242 | - | - | High thermal stability. Tensile properties are improved by adjusting the 2,4-ODA/6FAPB ratio, achieving performance comparable to PC. Exhibits good hydrolytic and solvent resistance, with negligible deterioration in mechanical properties after recycling. | [63] |
| Cel-PI | film | 46.3 | 2.2 | 2.9 | - | - | - | Characterized by dynamic network exchange and an amorphous structure, the material achieves excellent thermal processability, mechanical robustness, water/solvent resistance, thermal stability, and recyclability. | [30] |
| PGCS-100 | film | 56.5 | 20.6 | 0.439 | 227.1 | Up to 97.8% | 56.5 | Demonstrates outstanding mechanical properties, high thermal stability, self-healing, welding ability, shape memory, reprocessability, and chemical recyclability. | [42] |
| PI-A1.0/T1.0 | film | 55.2 | 72 | 1.7 | - | - | - | Incorporating hydrogen bond crosslinking significantly enhances mechanical strength and stiffness without sacrificing ductility, offering an effective strategy for strengthening and toughening dynamic covalent thermosets. | [26] |
| 2D Polyimine Films | film | - | 6.5 ± 2.4 | 8.6 ± 2.5 | - | - | - | In situ TEM tensile testing allows nanoscale observation of structural evolution and fracture dynamics. Crack initiation preferentially occurs along (100)/(010) directions, with chemical structure influencing mechanical failure. | [64] |
| CO-PIM-75 | film | 62.5 | 12.9 | - | >242 | - | - | High thermal stability. Tensile properties are improved by adjusting the 2,4-ODA/6FAPB ratio, achieving performance comparable to PC. Exhibits good hydrolytic and solvent resistance, with negligible deterioration in mechanical properties after recycling. | [63] |
| Cel-PI | film | 46.3 | 2.2 | 2.9 | - | - | - | Characterized by dynamic network exchange and an amorphous structure, the material achieves excellent thermal processability, mechanical robustness, water/solvent resistance, thermal stability, and recyclability. | [30] |
| PGCS-100 | film | 56.5 | 20.6 | 0.439 | 227.1 | Up to 97.8% | 56.5 | Demonstrates outstanding mechanical properties, high thermal stability, self-healing, welding ability, shape memory, reprocessability, and chemical recyclability. | [42] |
| PI-A1.0/T1.0 | film | 55.2 | 72 | 1.7 | - | - | - | Incorporating hydrogen bond crosslinking significantly enhances mechanical strength and stiffness without sacrificing ductility, offering an effective strategy for strengthening and toughening dynamic covalent thermosets. | [26] |
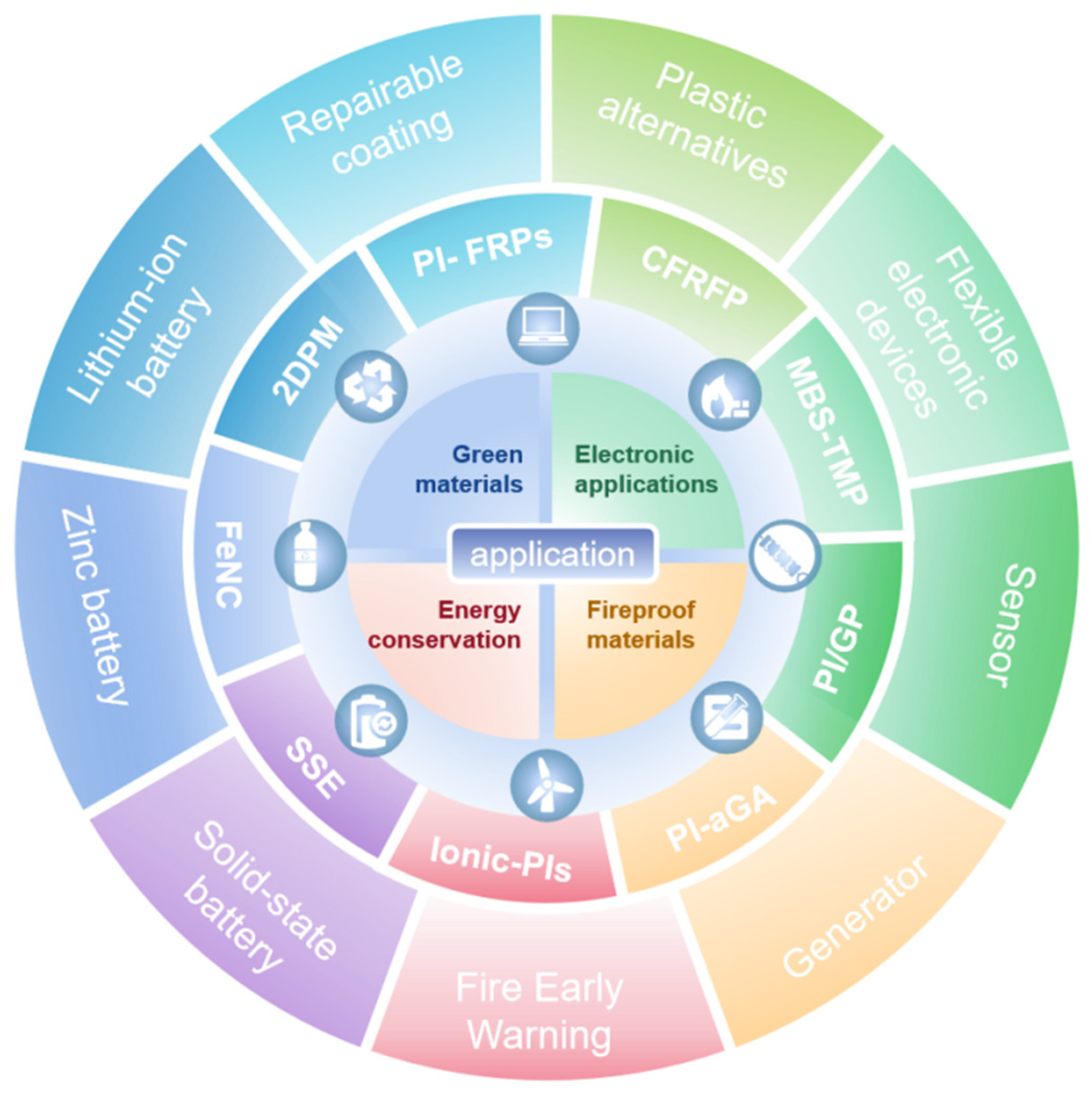
5. Engineering Applications of Polyimine and Composites
5.1. Green Materials
5.2. Electronic Applications
5.3. Energy Storage
5.4. Fireproof Materials
5.5. Other Applications
5.5.1. Flexible Magnetic Soft Robotics Based on Polyimine Composites
5.5.2. Applications of Polyimine Composites in Drug Delivery
5.5.3. Rapid Detection of Volatile Iodine Using Polyimine Composites
6. Conclusions
7. Future Opportunities and Challenges
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PI | Polyimine |
| DCC | Dynamic Covalent Chemistry |
| CANs | Covalent Adaptive Networks |
| SWNTs | Single-Walled Carbon Nanotubes |
| MWCNT | Multi-Walled Carbon Nanotube |
| CNTs | Carbon Nanotubes |
| COF | Covalent Organic Framework |
| CF | Carbon Fiber |
| TENG | Triboelectric Nanogenerator |
| TEGs | Thermoelectric Generators |
| SSEs | Solid-State Electrolytes |
| TFC | Thin-Film Composite |
| MINTs | Mechanically Interlocked Nanotube Derivatives |
| BP/PI | Bamboo Powder/Polyimine Composite |
| PVP | Polyvinylpyrrolidone |
| TREN | Tris(2-aminoethyl)amine |
| TPA | Terephthalaldehyde |
| PGCS | Peach Gum Polysaccharide/Chitosan Composite |
| IPIN | Ionic Polyimine Network |
| ASA | Aerogel-Sol-Aerogel Process |
| TMPNP | TiO2@MXene/P, N-containing Polyimine Nanocomposite |
| CFRPs | Carbon Fiber-Reinforced Composites |
| RY-PI | Ramie Yarn-Reinforced Polyimine Vitrimer Composites |
| PPCs | Paper–Polyimine Composites |
| BNNS/PIH TIM | Boron Nitride Nanosheets/Polyimine Hybrid Thermal Interface Material |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| TGA | Thermogravimetric Analysis |
| DTG | Derivative Thermogravimetric |
| ECL | Electrochemiluminescence |
| Vitrimer | A polymer combining thermoset properties with dynamic covalent adaptability |
References
- Cong, F.; Wang, B.; Li, Y.; Liu, P.; Fu, X.; Liu, X.; Huang, Y.; Hu, Z. Waste-free recycling of multifunctional epoxy resins and carbon fiber composites enabled by dynamic aminal bonds. Compos. Sci. Technol. 2025, 267, 111204. [Google Scholar] [CrossRef]
- Evans, A.M.; Strauss, M.J.; Corcos, A.R.; Hirani, Z.; Ji, W.; Hamachi, L.S.; Aguilar-Enriquez, X.; Chavez, A.D.; Smith, B.J.; Dichtel, W.R. Two-Dimensional Polymers and Polymerizations. Chem. Rev. 2022, 122, 442–564. [Google Scholar] [CrossRef] [PubMed]
- Liguori, A.; Hakkarainen, M. Designed from Biobased Materials for Recycling: Imine-Based Covalent Adaptable Networks. Macromol. Rapid Commun. 2022, 43, 2100816. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Fu, J. Chemical closed-loop recyclable thermosetting polymers based on dynamic covalent bonds. Sci. China-Chem. 2025, 68. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, S.; Ma, Y.; Li, R.; Jin, Y.; Qiu, L.; Zhang, W. Monolithic polyimine vitrimer/graphene aerogel composites. Chin. Chem. Lett. 2023, 34, 107363. [Google Scholar] [CrossRef]
- Xie, D.; Pu, Y.; Bryant, N.D.; Harper, D.P.; Wang, W.; Ragauskas, A.J.; Li, M. Synthesis of Bio-Based Repairable Polyimines with Tailored Properties by Lignin Fractionation. Acs Sustain. Chem. Eng. 2024, 12, 6606–6618. [Google Scholar] [CrossRef]
- Gao, S.; Sun, F.; Brady, A.; Pan, Y.; Erwin, A.; Yang, D.; Tsukruk, V.; Stack, A.G.; Saito, T.; Yang, H.; et al. Ultra-efficient polymer binder for silicon anode in high-capacity lithium-ion batteries. Nano Energy 2020, 73, 104804. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Zhao, K.; Fang, Z.; Qiao, H.; Zhai, L.; Mi, L. Photoactive Covalent Organic Frameworks for Catalyzing Organic Reactions. ChemPlusChem 2022, 87, e202200281. [Google Scholar] [CrossRef]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Fonkui Youmbi, T. Schiff Bases: Synthesis, Characterization and Antimicrobial Properties; University of Johannesburg: Johannesburg, South Africa, 2018. [Google Scholar]
- Taynton, P. Development of Polyimine-Based Dynamic Covalent Network: From Malleable Polymers to High-Performance Composites. Ph.D. Thesis, University of Colorado at Boulder, Boulder, CO, USA, 2016. [Google Scholar]
- Taynton, P.; Yu, K.; Shoemaker, R.K.; Jin, Y.; Qi, H.J.; Zhang, W. Heat- or Water-Driven Malleability in a Highly Recyclable Covalent Network Polymer. Adv. Mater. 2014, 26, 3938–3942. [Google Scholar] [CrossRef]
- Taynton, P.; Ni, H.; Zhu, C.; Yu, K.; Loob, S.; Jin, Y.; Qi, H.J.; Zhang, W. Repairable Woven Carbon Fiber Composites with Full Recyclability Enabled by Malleable Polyimine Networks. Adv. Mater. 2016, 28, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, Q.; Taynton, P.; Zhang, W. Dynamic Covalent Chemistry Approaches Toward Macrocycles, Molecular Cages, and Polymers. Acc. Chem. Res. 2014, 47, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent Adaptable Networks (CANS): A Unique Paradigm in Cross-Linked Polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Hajj, R.; Duval, A.; Dhers, S.; Averous, L. Network Design to Control Polyimine Vitrimer Properties: Physical Versus Chemical Approach. Macromolecules 2020, 53, 3796–3805. [Google Scholar] [CrossRef]
- Zhu, C. Structure-Property Relationship Study of Polyimine-Based Dynamic Covalent Networks and Their Applications Towards Functional Composites. Master’s Thesis, University of Colorado at Boulder, Boulder, CO, USA, 2017. [Google Scholar]
- Yu, P.; Huang, Q.; Wang, Y.; Peng, W.; Jia, Z.; Wang, H.; Ma, J.; Wang, C.; Yan, X. Water-Driven Malleable, Weldable and Eco-Friendly Recyclable Carbon Fiber Reinforced Dynamic Composites. Chin. J. Chem. 2024, 42, 516–522. [Google Scholar] [CrossRef]
- Wang, C.; Eisenreich, F.; Tomovic, Z. Aerogel-To-Sol-To-Aerogel (ASA) Process for Recycling, Repairing, Reprogramming of High-Performance Organic Aerogels. Adv. Funct. Mater. 2024, 34, 2314447. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Wang, Y.-Z.; Ho, K.-S. Cobalt-Based Cathode Catalysts for Oxygen-Reduction Reaction in an Anion Exchange Membrane Fuel Cell. Membranes 2022, 12, 699. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, C.-L.; Dong, L.; Tomovic, Z. Closed-Loop Recyclable High-Performance Aerogels Derived from Polystyrene. Adv. Funct. Mater. 2025, 35, 2420472. [Google Scholar] [CrossRef]
- Huang, T.; Pan, X.; Xi, A.; Chen, W.; Zhang, N.; Zeng, Y. Peach gum-based polyimine networks with water resistant, high strength and recycling performances. Polymer 2024, 315, 127845. [Google Scholar] [CrossRef]
- Lin, L.; Su, Z.; Zhang, H.; Zhou, G.; Zhou, H.; Ren, J.; Wang, X.; Liu, C.; Wang, X. Thermo-processable chitosan-based plastic substitute with self-adaptiveness and closed-loop recyclability. Carbohydr. Polym. 2022, 291, 119479. [Google Scholar] [CrossRef]
- Tiwari, K.; Modak, S.; Sarkar, P.; Ray, S.; Adupa, V.; Reddy, K.A.; Pramanik, S.K.; Das, A.; Karan, S. Interfacial synthesis of large-area ultrathin polyimine nanofilms as molecular separation membrane. Iscience 2022, 25, 104027. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Sahabudeen, H.; Liang, B.; Polozij, M.; Addicoat, M.A.; Gorelik, T.E.; Hambsch, M.; Mundszinger, M.; Park, S.; Lotsch, B.V.; et al. Near-atomic-scale observation of grain boundaries in a layer-stacked two-dimensional polymer. Sci. Adv. 2020, 6, eabb5976. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Zhang, Z.; Zhang, W.; Liu, X. Strengthening and Toughening Dynamic Covalent Thermosets via Hydrogen-Bonded Cross-Links. Macromolecules 2024, 57, 1362–1369. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Zhu, Q.-H.; Guo, S.-J.; Zhang, L.; Yu, C.; Qin, S.; He, L.; Tao, G.-H. Ionic Polyimine-Based Composite Membrane with Inductive and Complexation Synergistic Effects for Sensitive and On-Site Fluorescent Detection of Volatile Iodine. Adv. Mater. 2024, 36, 2311990. [Google Scholar] [CrossRef]
- Tian, T.; Jiang, S.; Lu, B.; Liu, L. Synthesis, degradation Behavior, and recyclability property of novel bio-based Polyimine thermosets. Eur. Polym. J. 2024, 220, 113430. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.; You, C.; Jia, Z.; Huang, Q.; Wang, Y.; Qu, Y.; Dong, X.; Li, R.; Xin, Y.; et al. Reversible Schiff-base chemistry enables thermosetting smart composites with versatile properties. Compos. Commun. 2024, 52, 102153. [Google Scholar] [CrossRef]
- Su, Z.; Cui, L.; Zhang, H.; Xiao, L.; Chi, B.; Xu, H.; Ning, L.; Jia, S.; Wang, X. Robust, waterproof, and degradable cellulose-based polyimine vitrimer for plastic replacement. Chem. Eng. J. 2023, 471, 144501. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, Y.; Zheng, L.; Li, J.; Liang, S.; Liu, Z.; Ren, L. Bio-inspired Polyimine Copolymers: Facial Integration with High Content Variability and Extremal Transitions of Mechanical Properties. J. Bionic Eng. 2017, 14, 119–129. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, M.; Qin, S.; Zheng, Z.; Xu, H.; Ning, L.; Zhang, S.; Jia, S.; Wang, X.; Su, Z. A fully sustainable, flexible, and degradable lignocellulose-based composite film enabled by a bio-based polyimine vitrimer. Int. J. Biol. Macromol. 2025, 307, 141946. [Google Scholar] [CrossRef]
- Cui, L.; Pan, M.; Zhou, Y.; Xu, H.; Ning, L.; Jia, S.; Wang, X.; Su, Z. A strong, biodegradable, and closed-loop recyclable bamboo-based plastic substitute enabled by polyimine covalent adaptable networks. Chem. Eng. J. 2023, 477, 146952. [Google Scholar] [CrossRef]
- Sahabudeen, H.; Qi, H.; Ballabio, M.; Polozij, M.; Olthof, S.; Shivhare, R.; Jing, Y.; Park, S.; Liu, K.; Zhang, T.; et al. Highly Crystalline and Semiconducting Imine-Based Two-Dimensional Polymers Enabled by Interfacial Synthesis. Angew. Chem. Int. Ed. 2020, 59, 6028–6036. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.Y.; Shao, F.; Szczerbinski, J.; McCaffrey, R.; Zenobi, R.; Jin, Y.H.; Schlüter, A.D.; Zhang, W. Synthesis of a Two-Dimensional Covalent Organic Monolayer through Dynamic Imine Chemistry at the Air/Water Interface. Angew. Chem. Int. Ed. 2016, 55, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, X.; Li, W.; Clausner, A.; Conzendorf, S.; Liu, J.; Posseckardt, J.; Jost, B.; Dong, R.; Feng, X.; et al. Patterning damage mechanisms for two-dimensional crystalline polymers and evaluation for a conjugated imine-based polymer. Nanotechnology 2024, 35, 475301. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Chen, T.; Liu, Z.; Li, Y.; Su, H.; Zhu, J.; Zhang, Y.; van der Bruggen, B. Interfacial oligomer splicing toward 3D silicon-centered polyimine nanofilm for rapid molecule/ion differentiation. J. Membr. Sci. 2025, 713, 123354. [Google Scholar] [CrossRef]
- Baig, M.I.; Hardian, R.; Alharthi, F.A.; Fellows, C.M.; Szekely, G. Brine separation with polyamide and polyimine thin film composite nanofiltration membranes obtained from biobased monomers. J. Membr. Sci. 2025, 713, 123324. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Copenhaver, K.; Kelly, P.V.; Senkum, H.; Tekinalp, H.; Gramlich, W.M.; Ozcan, S. Aqueous-Based Polyimine Functionalization of Cellulose Nanofibrils for Effective Drying and Polymer Composite Reinforcement. ACS Appl. Polym. Mater. 2022, 4, 7674–7684. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, Z.; Luo, S.; Jin, Y.; Qiu, L.; Zhang, W. Malleable and Recyclable Conductive MWCNT-Vitrimer Composite for Flexible Electronics. Acs Appl. Nano Mater. 2020, 3, 4845–4850. [Google Scholar] [CrossRef]
- Zhang, S.; Xiong, Z.; Zhang, J.; Zhang, X.; Chen, Y.; Chen, Y. Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites. E-Polymers 2022, 22, 696–704. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, X.; Xi, A.; Chen, W.; Huang, T.; Zeng, Y. Robust, malleable, degradable, self-healable, weldable and recyclable polyimine thermosets from natural peach gum and chitosan. Polym. Chem. 2024, 15, 3287–3299. [Google Scholar] [CrossRef]
- Su, Z.; Huang, S.; Wang, Y.; Ling, H.; Yang, X.; Jin, Y.; Wang, X.; Zhang, W. Robust, high-barrier, and fully recyclable cellulose-based plastic replacement enabled by a dynamic imine polymer. J. Mater. Chem. A 2020, 8, 14082–14090. [Google Scholar] [CrossRef]
- Ding, H.; Wang, J.; Wang, C.; Wu, L.; Zhang, W.; Liu, L.; Lei, Y.; Sun, N.; Zhou, K.; Yu, B. Intermolecular hydrogen bonding enabling mechanically robust, thermally stable, and solvent-resistance bio-based polyimine networks. Compos. Part A Appl. Sci. Manuf. 2025, 196, 109006. [Google Scholar] [CrossRef]
- Isasti, I.; Miranda, S.; Jimenez, D.M.; Parzyszek, S.; Sabanes, N.M.; Pedersen, H.; Perez, E.M. Reinforcement of Polyimine Covalent Adaptable Networks with Mechanically Interlocked Derivatives of SWNTs. Adv. Funct. Mater. 2024, 34, 2408592. [Google Scholar] [CrossRef]
- Zhu, G.; Hou, Y.; Xia, N.; Wang, X.; Zhang, C.; Zheng, J.; Jin, D.; Zhang, L. Fully Recyclable, Healable, Soft, and Stretchable Dynamic Polymers for Magnetic Soft Robots. Adv. Funct. Mater. 2023, 33, 2300888. [Google Scholar] [CrossRef]
- Zhang, S.; Lv, Y.; Li, J.; Liang, S.; Liu, Z. Mechanical enhancement of zirconia reinforced polyimine nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45183. [Google Scholar] [CrossRef]
- Mao, Y.; Shi, S.; Lei, L.; Wang, C.; Wang, D.; Hu, J.; Fu, S. A self-healable and highly flame retardant TiO2@MXene/P, N-containing polyimine nanocomposite for dual-mode fire sensing. Chem. Eng. J. 2024, 479, 147545. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Zhang, L.; Chen, Z.; Qin, R.; Liu, Y.; Jiang, X.; Ye, D.; Liu, Z. Recyclable Carbon Fiber Reinforced Vanillin-Based Polyimine Vitrimers: Degradation and Mechanical Properties Study. Macromol. Mater. Eng. 2022, 307, 2100893. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, S.; Wang, Z.; Zhang, J.; Zhao, W.; He, C.; Chen, Y. Mechanical and Recyclable Properties of Polyimine Enhanced by Biomimetic Modification of Graphene Oxide Sheets/Silicon Carbide Nano-Whiskers. Nanomaterials 2022, 12, 4486. [Google Scholar] [CrossRef]
- Zhu, K.; Li, Z.; Cheng, F.; Wu, C.; Cai, D.; Zhang, Q.; Zhang, H. Preparation of durable superhydrophobic composite coatings with photothermal conversion precisely targeted configuration self-healability and great degradability. Compos. Sci. Technol. 2021, 213, 108926. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Mu, G.-D.; Wang, X.-J.; Zhang, F.; Li, Y.-L.; Lu, D.-J.; Chen, F.-M.; Yang, M.-L.; He, M.-Y.; Liu, T. Fast construction of core-shell structured magnetic covalent organic framework as sorbent for solid-phase extraction of zearalenone and its derivatives prior to their determination by UHPLC-MS/MS. Microchim. Acta 2021, 188, 246. [Google Scholar] [CrossRef]
- Li, P.; Hao, C.; Wang, H.; He, T.; Shu, T.; Li, C.; Yu, L.; Yan, N. Eco-friendly recyclable high performance ramie yarn reinforced polyimine vitrimer composites. Chem. Eng. J. 2023, 457, 141341. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; An, C.; Wu, N.; Wang, X.; Chen, Y. Interface and mechanical properties of combined surface-treated carbon fiber fabrics/polyimine laminates: Experiments and numerical simulations. Polym. Compos. 2025, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Ding, H.; Yu, P.; Jia, Z.; Wang, C.; Wang, H.; He, H.; Xing, W.; Yang, W.; Zhang, P.; et al. Ultrastrong, High Fire Resistance, Repairable, and Recyclable Fluorinated Biobased Polyimine Networks. Acs Mater. Lett. 2024, 6, 3899–3908. [Google Scholar] [CrossRef]
- Li, B.; Zhu, G.; Hao, Y.; Ren, T. Effect of cross-link density on the performance of polyimine/epoxy vitrimers. Smart Mater. Struct. 2024, 33, 025014. [Google Scholar] [CrossRef]
- Lei, Y.; Shan, S.; Lin, Y.; Zhang, A. Network reconfiguration and unusual stress intensification of a dynamic reversible polyimine elastomer. Polymer 2020, 186, 122031. [Google Scholar] [CrossRef]
- Zhang, G.-H.; Zhang, L.; Zhu, Q.-H.; Chen, H.; Yuan, W.-L.; Fu, J.; Wang, S.-L.; He, L.; Tao, G.-H. Self-Healable, Malleable, and Flexible Ionic Polyimine as an Environmental Sensor for Portable Exogenous Pollutant Detection. ACS Mater. Lett. 2022, 4, 136–144. [Google Scholar] [CrossRef]
- Yang, Z.; Li, H.; Mou, X.; Chen, Z.; Lai, X.; Ding, J.; Zeng, X. Functional and Environmental Friendly Polyimine Elastomer Based on the Dynamic Covalent Network for a Flexible Strain Sensor. Macromolecules 2023, 56, 9766–9777. [Google Scholar] [CrossRef]
- Zhang, X.; Eichen, Y.; Miao, Z.; Zhang, S.; Cai, Q.; Liu, W.; Zhao, J.; Wu, Z. Novel Phosphazene-Based flame retardant polyimine vitrimers with Monomer-Recovery and high performances. Chem. Eng. J. 2022, 440, 135806. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, H.; Jia, L.; Lu, X.; Yan, S.; Zhao, J.; Liu, S. Aromatic polyimine covalent adaptable networks with superior water and heat resistances. Eur. Polym. J. 2023, 187, 111912. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Q.; Ma, J.; Liao, Z.; Zhang, J.; Xie, H.; Yang, S.; Xu, C.-A.; Hu, Y.; Yang, Z. Synthesis of bio-based polyimine networks with flame-retardancy, acid-degradablility, and reprocessability. Polymer 2024, 302, 127101. [Google Scholar] [CrossRef]
- Yu, P.; Wang, H.; Li, T.; Wang, G.; Jia, Z.; Dong, X.; Xu, Y.; Ma, Q.; Zhang, D.; Ding, H.; et al. Mechanically Robust, Recyclable, and Self-Healing Polyimine Networks. Adv. Sci. 2023, 10, e2300958. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, X.; Bodesheim, D.; Li, W.; Clausner, A.; Liu, J.; Jost, B.; Dianat, A.; Dong, R.; Feng, X.; et al. Fracture Behavior of a 2D Imine-Based Polymer. Adv. Sci. 2024, 11, e2407017. [Google Scholar] [CrossRef]
- Peng, H.; Li, H.; Guo, E.; Zhai, T. High-Performance Te Nanowires/MoS2/Polyimine Nanocomposite-Based Self-Healable, Recyclable and Screen-Printable Flexible Photodetector for Image Sensing. Adv. Funct. Mater. 2024, 34, 2314743. [Google Scholar] [CrossRef]
- Rajabi-Abhari, A.; Li, P.; Bagheri, M.H.; Khan, A.A.; Hao, C.; Tanguy, N.R.; Ban, D.; Yu, L.; Yan, N. Self-healable, recyclable, and mechanically robust vitrimer composite for high-performance triboelectric nanogenerators and self-powered wireless electronics. Nano Energy 2024, 131, 110306. [Google Scholar] [CrossRef]
- He, X.; Lin, Y.; Ding, Y.; Abdullah, A.M.; Lei, Z.; Han, Y.; Shi, X.; Zhang, W.; Yu, K. Reshapeable, rehealable and recyclable sensor fabricated by direct ink writing of conductive composites based on covalent adaptable network polymers. Int. J. Extrem. Manuf. 2022, 4, 015301. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhong, S.; Chen, J.; Li, Z.; Zhang, C.; Jiang, P.; Huang, X. Soft and Damping Thermal Interface Materials with Honeycomb-Board-Mimetic Filler Network for Electronic Heat Dissipation. Small 2024, 20, e2400115. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Huang, K.; Liu, Y.; Zhu, J. Facile Preparation of Polyimine Vitrimers with Enhanced Creep Resistance and Thermal and Mechanical Properties via Metal Coordination. Macromolecules 2020, 53, 2919–2931. [Google Scholar] [CrossRef]
- Luo, S.; Ma, Y.; Wei, X.; Jin, Y.; Qiu, L.; Zhang, W. Malleable and Recyclable Vitrimer-Graphene Aerogel Composite with High Electrical Conductivity. Acs Appl. Electron. Mater. 2021, 3, 1178–1183. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Deng, S.; Zheng, X.; Zeng, B.; Liu, X.; Xu, Y.; Yuan, C.; Dai, L. Construction of phosphorus-containing polyimine vitrimer film by incorporating ionic liquid BMIM•PF6 for enhanced mechanical, flame-retardant and reprocessable properties. Chem. Eng. J. 2024, 495, 153283. [Google Scholar] [CrossRef]
- Liu, T.; Peng, J.; Liu, J.; Hao, X.; Guo, C.; Ou, R.; Liu, Z.; Wang, Q. Fully recyclable, flame-retardant and high-performance carbon fiber composites based on vanillin-terminated cyclophosphazene polyimine thermosets. Compos. Part B-Eng. 2021, 224, 109188. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, S.; Wang, Z.; Li, C.; Cao, W.; Zhu, Y.; He, C.; Chen, Y. High-Impact Performance and Thermal Properties of Polyimine Nanocomposites Reinforced by Silicon Carbide Nano-Whiskers. Materials 2023, 16, 4587. [Google Scholar] [CrossRef]
- Lyu, M.; Liu, Y.; Yang, X.; Liang, D.; Wang, Y.; Liang, X.; Hu, Y.; Liang, L.; Zhang, C. Vanillin-based liquid crystalline polyimine thermosets and their composites for recyclable thermal management application. Compos. Part B-Eng. 2023, 250, 110462. [Google Scholar] [CrossRef]
- Pomazi, A.; Poor, D.I.; Geier, N.; Toldy, A. Optimising Recycling Processes for Polyimine-Based Vitrimer Carbon Fibre-Reinforced Composites: A Comparative Study on Reinforcement Recovery and Material Properties. Materials 2024, 17, 2372. [Google Scholar] [CrossRef] [PubMed]
- Toldy, A.; Poor, D.I.; Szolnoki, B.; Devecser, B.; Geier, N.; Pomazi, A. Comparative study of flame retardancy in polyimine vitrimers and composites: Evaluating additive and reactive flame retardants acting via gas-, solid-, and combined-phase mechanisms. J. Mater. Sci. Technol. 2024, 196, 101–111. [Google Scholar] [CrossRef]
- Yu, L.; Lei, Z.; Sun, X.; Ding, P.; Wesche, A.; Jin, Y.; Zhang, W.; Long, R. Rapid Fabrication of Fiber-Reinforced Polyimine Composites with Reprocessability, Repairability, and Recyclability. ACS Appl. Polym. Mater. 2021, 3, 5808–5817. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, S.; Pan, M.; Li, M.-C.; Zhang, Y.; Mei, C. Improved processability and high fire safety of wood plastic composites via assembling reversible imine crosslinking network. Chem. Eng. J. 2021, 423, 130295. [Google Scholar] [CrossRef]
- Rahimi, A.; García, J.M. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Si, W.-J.; An, X.-P.; Zeng, J.-B.; Chen, Y.-K.; Wang, Y.-Z. Fully bio-based, highly toughened and heat-resistant poly(L-lactide) ternary blends via dynamic vulcanization with poly(D-lactide) and unsaturated bioelastomer. Sci. China Mater. 2017, 60, 1008–1022. [Google Scholar] [CrossRef]
- Su, Z.; Hu, Y.; Yang, X.; Long, R.; Jin, Y.; Wang, X.; Zhang, W. Production and closed-loop recycling of biomass-based malleable materials. Sci. China Mater. 2020, 63, 2071–2078. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, H.; Yu, P.; He, H.; Huang, Q.; Hong, W.; Liu, C.; Shi, Y.; Wang, J.; Xin, Y.; et al. Soft-Rigid Construction of Mechanically Robust, Thermally Stable, and Self-Healing Polyimine Networks with Strongly Recyclable Adhesion. Small 2024, 20, 2406821. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Li, C.; Guan, H.; Shen, L.; Meng, J.; Guo, K. Sustainable paeonol-derived polyimine-epoxy as a substitute for DGEBA thermoset. React. Funct. Polym. 2024, 194, 105804. [Google Scholar] [CrossRef]
- Wang, R.; Lei, Y.; Ke, L.; Bai, L.; Zheng, Y.; Xu, Z.; Li, T.; Chen, S.; Zhang, D. Construction of self-healing multifunctional hyper-crosslinked hyperbranched polymer @ metal-organic polyhedra (HHMOP) membranes. Eur. Polym. J. 2024, 211, 112966. [Google Scholar] [CrossRef]
- Qi, H.; Wang, Z.; Li, H.; Li, F. Directionally In Situ Self-Assembled Iridium(III)-Polyimine Complex-Encapsulated Metal-Organic Framework Two-Dimensional Nanosheet Electrode To Boost Electrochemiluminescence Sensing. Anal. Chem. 2023, 95, 12024–12031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Shi, C.; Wang, Y.; Wang, Y.; Yu, Y.; Wang, Y.; Deng, Y.; Xiao, J. Recyclable, Healable, and Stretchable High-Power Thermoelectric Generator. Adv. Energy Mater. 2021, 11, 2100920. [Google Scholar] [CrossRef]
- Kuntharin, S.; Harnchana, V.; Klamchuen, A.; Sinthiptharakoon, K.; Thongbai, P.; Amornkitbamrung, V.; Chindaprasirt, P. Boosting the Power Output of a Cement-Based Triboelectric Nanogenerator by Enhancing Dielectric Polarization with Highly Dispersed Carbon Black Nanoparticles toward Large-Scale Energy Harvesting from Human Footsteps. Acs Sustain. Chem. Eng. 2022, 10, 4588–4598. [Google Scholar] [CrossRef]
- Tao, X.; Chen, X.; Wang, Z.L. Design and synthesis of triboelectric polymers for high performance triboelectric nanogenerators. Energy Environ. Sci. 2023, 16, 3654–3678. [Google Scholar] [CrossRef]
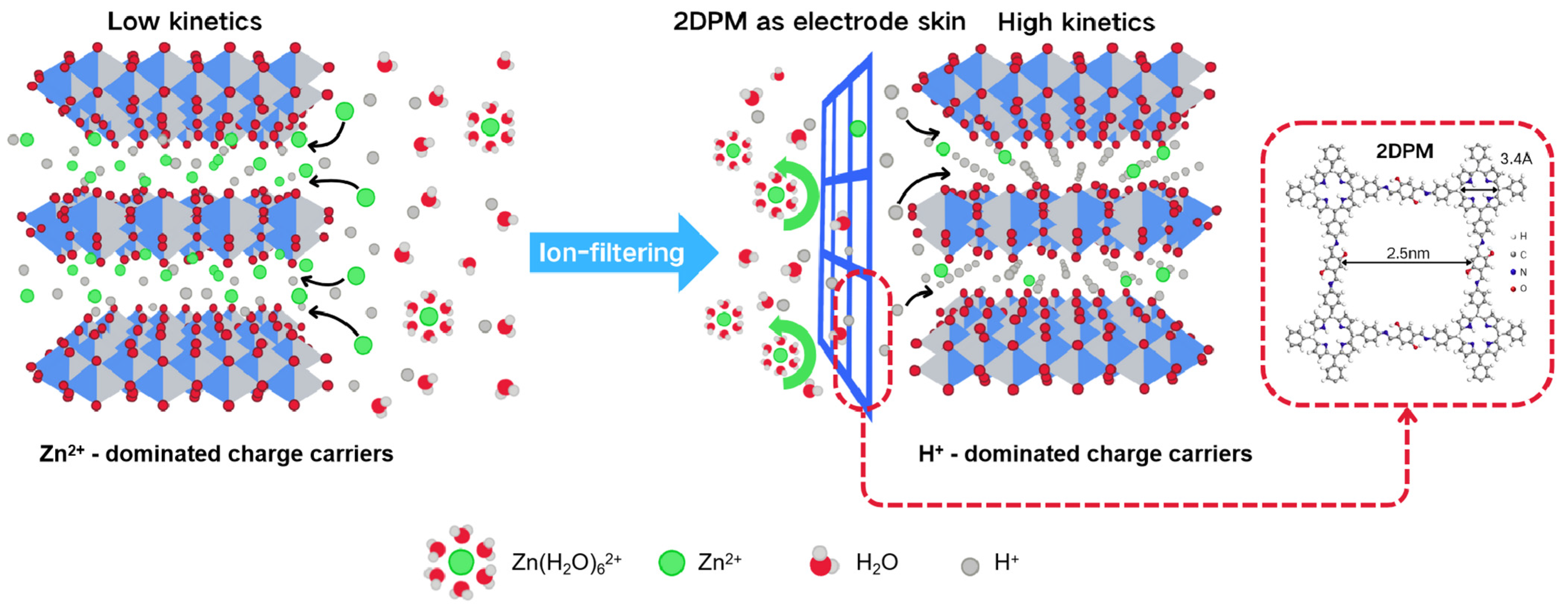
- Guo, Q.; Li, W.; Li, X.; Zhang, J.; Sabaghi, D.; Zhang, J.; Zhang, B.; Li, D.; Du, J.; Chu, X.; et al. Proton-selective coating enables fast-kinetics high-mass-loading cathodes for sustainable zinc batteries. Nat. Commun. 2024, 15, 2139. [Google Scholar] [CrossRef]
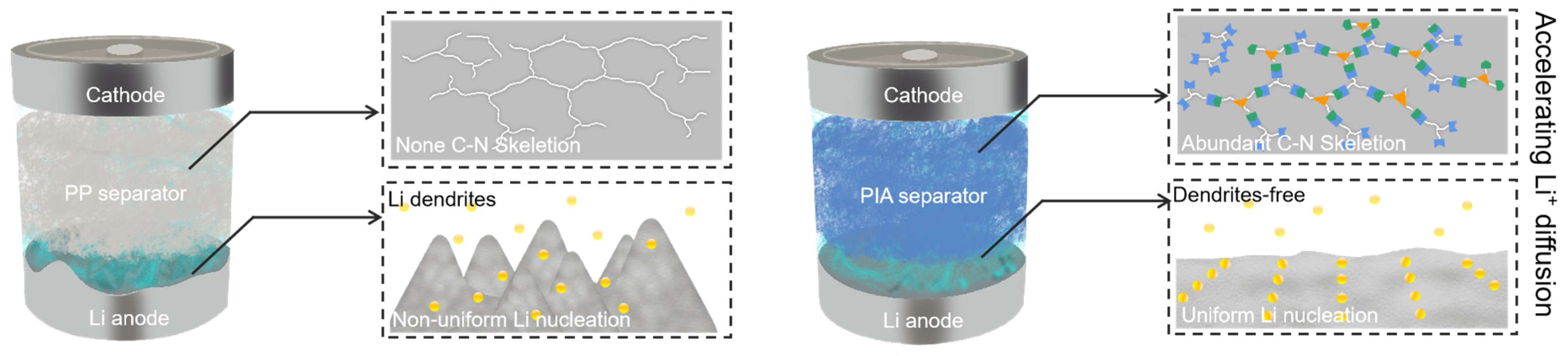
- Ding, L.; Yue, X.; Zhang, X.; Chen, Y.; Liu, J.; Shi, Z.; Wang, Z.; Yan, X.; Liang, Z. A polyimine aerogel separator with electron cloud design to boost Li- ion transport for stable Li metal batteries. Proc. Natl. Acad. Sci. USA 2023, 120, e2314264120. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, J.; Cui, Y.; Fu, R. Precisely tailored morphology of polyimine for simple synthesis of metal sulfide/carbon flower-like superstructures. Carbon 2022, 190, 395–401. [Google Scholar] [CrossRef]
- Song, H.-S.; Rumon, M.M.H.; Rahman Khan, M.M.; Jeong, J.-H. Toward Intelligent Materials with the Promise of Self-Healing Hydrogels in Flexible Devices. Polymers 2025, 17, 542. [Google Scholar] [CrossRef]
- Jiang, C.-S.; Dunlap, N.; Li, Y.; Guthrey, H.; Liu, P.; Lee, S.-H.; Al-Jassim, M.M. Nonuniform Ionic and Electronic Transport of Ceramic and Polymer/Ceramic Hybrid Electrolyte by Nanometer-Scale Operando Imaging for Solid-State Battery. Adv. Energy Mater. 2020, 10, 2000219. [Google Scholar] [CrossRef]
- Long, T.E. Toward Recyclable Thermosets. Science 2014, 344, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Q.; Fang, C.; You, L.; Li, X.; Liu, M.; Dong, X.; Qi, Y.; Wang, B.; Li, W. Dynamic networks for polyimine with disulfide bond to obtain catalyst-free recyclability, multi-degradation and malleability. J. Polym. Res. 2022, 29, 154. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zhu, Y.; Luo, B.; He, J.; Lan, F.; Wu, Y. Rapid synthesis of magnetic polyimine nanospheres at room temperature for enrichment of endogenous C-peptide. Colloid Interface Sci. Commun. 2021, 42, 100390. [Google Scholar] [CrossRef]
- Mokhtari, N.; Taymouri, S.; Mirian, M.; Dinari, M. Covalent triazine-based polyimine framework as a biocompatible pH-dependent sustained-release nanocarrier for sorafenib: An in vitro approach. J. Mol. Liq. 2020, 297, 111898. [Google Scholar] [CrossRef]
- Konar, D.; Jakhar, V.K.; Stewart, K.A.; Lester, D.W.; Veige, A.S.; Sumerlin, B.S. Functionalized Cyclic Polymers and Network Gels. Macromolecules 2024, 57, 1779–1787. [Google Scholar] [CrossRef]
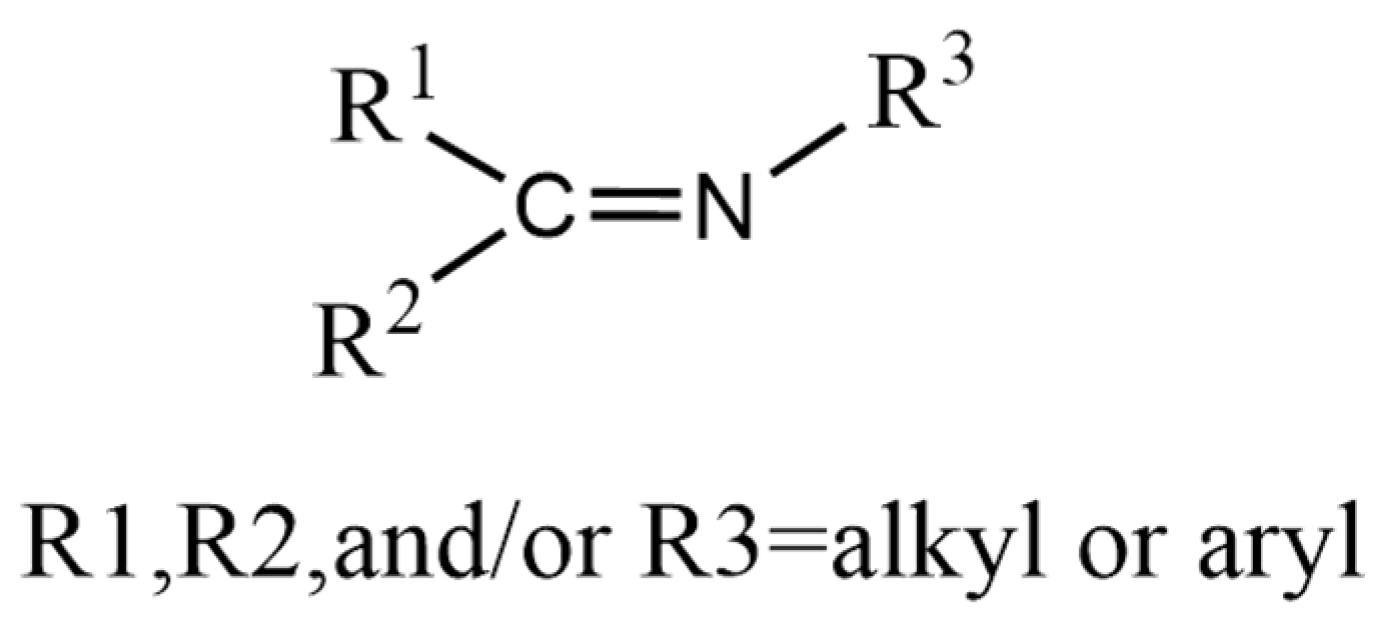


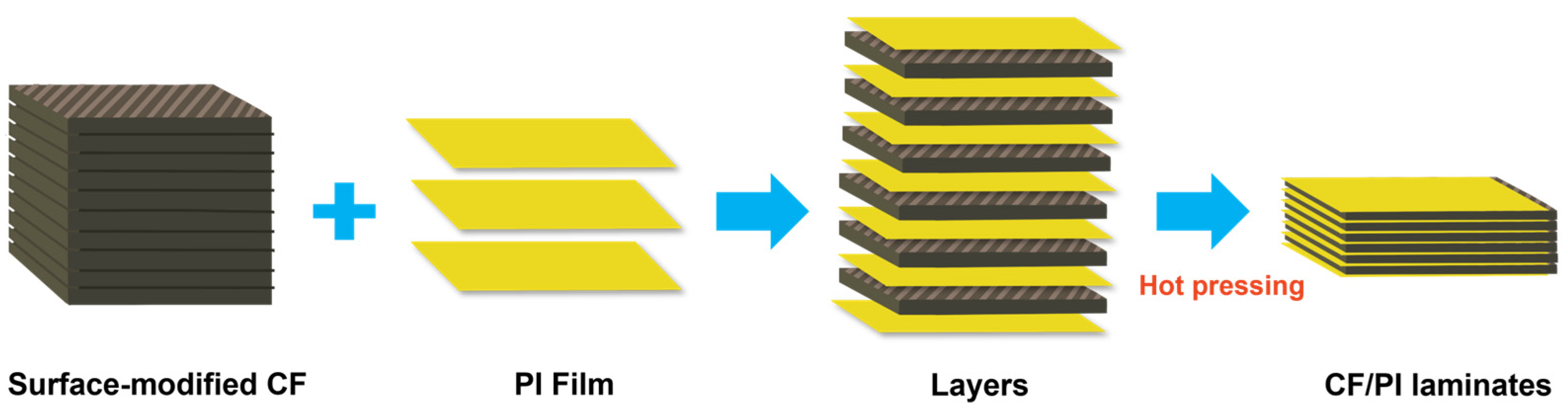

| Method | Process Steps | Advantages | Limitations | Applicable Forms | Ref. |
|---|---|---|---|---|---|
| Solution Casting | Monomer dissolution → Schiff base reaction → solvent evaporation | Uniform filler dispersion, film formation | High energy consumption, solvent recovery challenges | Films, gels, composites | [20,21] |
| Hot Pressing | Powder compaction → heating/pressure → molding | Scalability, simplicity | Particle aggregation, weak interfaces | Bulk materials, multilayers | [22,23] |
| Interfacial Polymerization | Monomer injection → interfacial condensation → film peeling | Ultrathin films, stable interfaces | Harsh reaction conditions, high cost | 2D films, functional coatings | [24,25] |
| Reinforcement | Type | Key Property Improvements | Primary Characteristics Enhanced | Ref. |
|---|---|---|---|---|
| Bamboo Powder (BP) | Organic |
| Toughness and stiffness | [33] |
| Cellulose Nanofibrils (CNFs) | Organic |
| Stiffness | [39] |
| Carbon Nanotubes (CNTs) | Inorganic |
| Conductivity and strength | [40] |
| Graphene Nanoplatelets (GnPs) | Inorganic |
| Strength and flexural properties | [41] |
| Abbreviation of Composite | Reinforcement Phase | Composite Method | Key Performance Parameters | Application Field | Specific Engineering Application Cases | Ref. |
|---|---|---|---|---|---|---|
| MBS-TMP | MoS2, Te nanowires | Fluid-designed solution shearing | Stable photocurrent (retaining 94.3% after 50,000 bending cycles); high carrier mobility | Flexible electronics | Wearable photodetectors (integrated into textiles for imaging and sensing) | [65] |
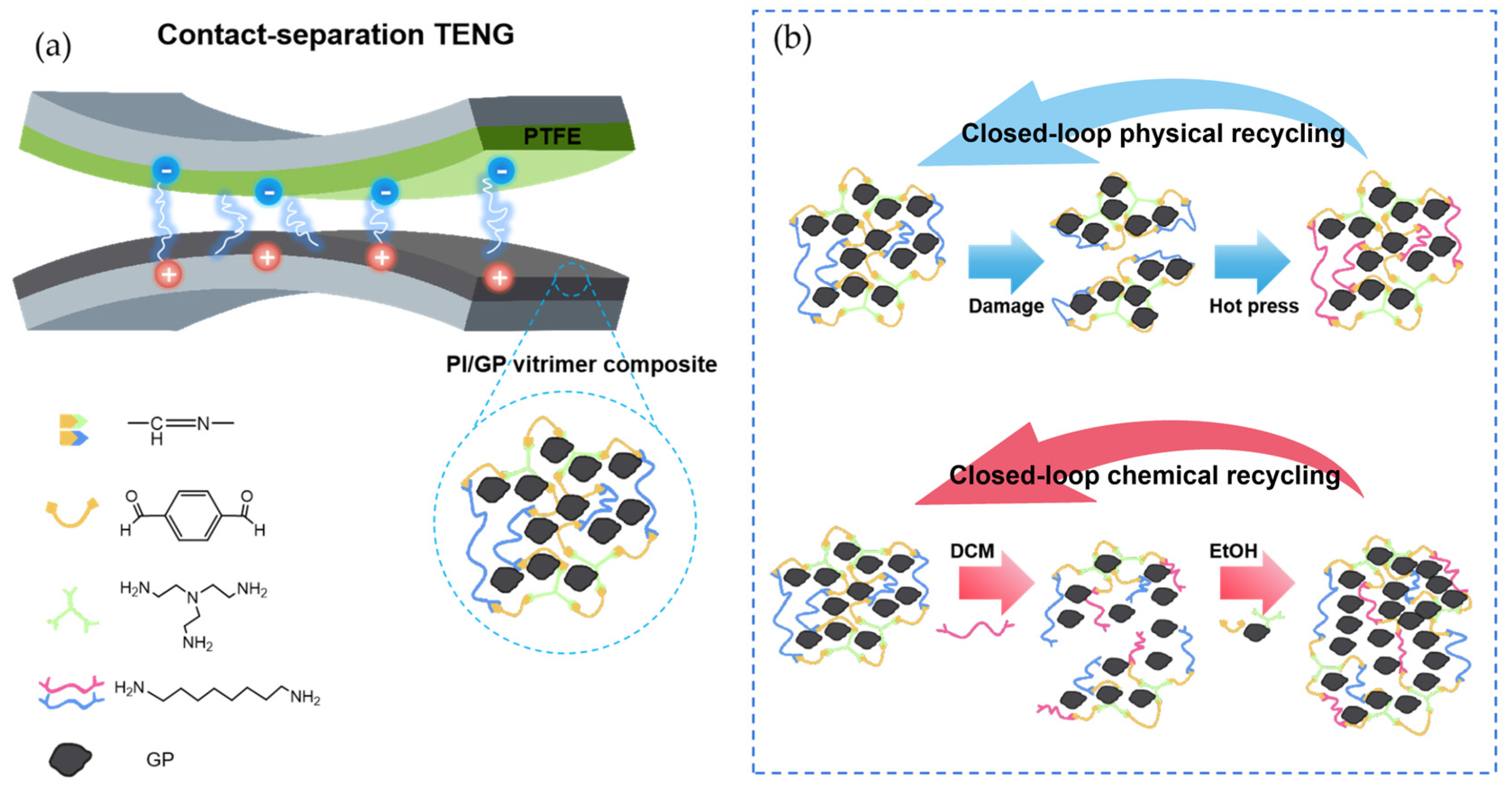
| PI/GP | Graphite–polypropylene | Physical mixing | Self-healing efficiency of 94.6%; output voltage of 1325 V (15 N, 6 Hz) | Energy harvesting | Self-healing triboelectric nanogenerator (TENG) for biomechanical energy collection and wireless transmission | [66] |
| DIW | Multi-walled carbon nanotubes | Physical mixing + partial curing | Elastic modulus of 520 MPa; tunable conductivity | 3D-printed electronic devices | Recyclable 3D-printed sensors (temperature/strain sensors maintaining performance after repair) | [67] |
| BNNS/PIH TIM | Boron nitride nanosheets | Horizontal centrifugal casting | In-plane thermal conductivity of 7.69 W/m·K; low compressive strength (2.16 MPa) | Electronic thermal management | Heat dissipation materials for 5G base stations and automotive IGBT modules (reducing temperature fluctuation under vibration) | [68] |
| PETG/CNF-Imine | Polyimine-coated cellulose nanofibers (CNF-Imine) | Melt blending + hot pressing | Tensile strength increased by 12% (with 30 wt% fibers); strong interfacial bonding | Green materials | Industrial manufacturing requiring high strength and stiffness | [39] |
| PLA/CNF-Imine | Polyimine-coated cellulose nanofibers (CNF-Imine) | Melt blending + hot pressing | Significant stiffness enhancement (up to 30% across fiber range); moderate increases in storage and loss moduli compared to PETG composites | Green materials | Applications requiring stiffness with biodegradable materials | [39] |
| PI-MWCNT | Multi-walled carbon nanotubes | Chemical linking | 97% conductivity recovery; tensile strength of 74 MPa (34% improvement over matrix) | Flexible electronics | Wearable electronic skins (maintaining conductivity after multiple repairs and recycling) | [40] |
| Polyimine-Metal Complex | Metal ions (Cu2+, Mg2+, Fe3+) | One-pot method | Tensile strength: 2.51 MPa; elongation at break: 1158%; self-healing efficiency: 96.0%; thermal stability (Td5%) > 278 °C | Green materials | Reprocessable and recyclable vitrimers with enhanced thermal, mechanical, solvent, and acid resistance, though poor water resistance | [69] |
| PI-aGA | Annealed graphene aerogel | In situ polymerization + hot pressing | Tensile strength of 37 MPa; stable conductivity after repeated reshaping | Flexible electronics | High-conductivity composites for flexible circuits and smart wearable devices | [70] |
| TMPNP | TiO2@MXene | Chemical linking | Limiting oxygen index (LOI) of 32%; resistance variation rate of −95% (at 70 °C) | Fireproof materials | Smart building fireproof coatings (cotton fabrics integrated with wireless fire alarms) | [48] |
| Ionic-PIs | BMIM∙PF6 | Physical mixing | Tensile strength: 51.8 MPa; elastic modulus: 0.84 GPa; toughness: 1.62 MJ/m3; LOI > 27% | Fireproof materials | Material recovery rate exceeding 41.9%; regenerated PI-R membranes retain mechanical strength; enhanced flame retardancy via BMIM∙PF6 sacrificial decomposition | [71] |
| HVP/D230-CF | Carbon fibers | Hot pressing | Tensile strength of 184.4 MPa; LOI of 34.2% | Fireproof materials | Recyclable carbon fiber-reinforced composites for aerospace flame-retardant structures | [72] |
| PI-NdFeB Soft Robot | Magnetic NdFeB microparticles | Physical mixing + solvent evaporation | Tensile strength of 6.3 MPa; elongation at break of 260% | Biomedical applications | Magnetic soft robots designed for minimally invasive surgical procedures and targeted drug delivery applications | [46] |
| CF-PI-daMINT | Mechanically interlocked nanotube derivatives (MINTs) | Planetary ball milling + hot pressing | Tensile strength: 68 ± 9 MPa; Young’s modulus: 3.2 ± 0.2 GPa | Green materials | Efficient reinforcement of PI CANs; superior mechanical performance of PI-daMINT composites due to enhanced SWNT dispersion and load transfer | [45] |
| CFRFP | Carbon fibers | Prepreg compression molding | Tensile stress of 23.7 MPa; water-driven extensibility | Green materials | Weldable/self-healing carbon fiber composites for lightweight automotive parts and recyclable drone structures | [18] |
| PI-SiCw | Silicon carbide nanowhiskers | Hot pressing | Impact strength improved by 154% (with 2% SiCw); flexural strength of 85.55 MPa | Green materials | High-performance electronic packaging materials (high-temperature and impact resistance) | [73] |
| GNPs-P | Graphene nanoplatelets | Hot pressing | Tensile strength of 73.05 MPa (at 0.5 wt%); enhanced thermal conductivity | Electronics | LED heat dissipation substrates (replacing traditional metal heat sinks) | [41] |
| BP/PI | Bamboo powder | Hot pressing | Tensile strength of 45.2 MPa; closed-loop recyclability of 100% | Green materials | Bamboo-based plastic alternatives for eco-friendly packaging and furniture | [33] |
| RY-PI | Ramie yarn | Chemical linking + interlayer compounding | Tensile strength: 144 MPa; Young’s modulus: 0.97 GPa; elongation at break: 25% | Green materials | High-performance and recyclable natural fiber-reinforced plastic composites (NFRPCs) | [53] |
| TD | Graphene nanoplatelets (GnPs) | Hot pressing | Tensile strength: 84 MPa; Young’s modulus: 1.6 GPa; thermal conductivity: 1.8 W m−1 K−1 | Electronics | TDG-sn composites for LED chip heat dissipation and thermal management applications | [74] |
| CFRP | Carbon fibers | Chemical recycling | Tg decreased by 42% in non-woven mat CFRPs; storage modulus (E’) significantly increased; Tg increased by 8% in UD-CFRP | Green materials | Feasibility of recycling fiber-reinforced vitrimer composites, with required process optimizations | [75] |
| VITRIMER TEDAP | Unidirectional carbon fibers | Hot pressing | Tensile strength: 69 MPa; Tg: 192 °C | Fireproof materials | Enhanced thermal stability and flame retardancy compared to epoxy systems; significant pHRR reduction in vitrimer composites | [76] |
| PI-FRPs. | Carbon fibers | Powder compression molding | Young’s modulus increased to ~17.3 GPa at 190 °C (1.25 MPa, 4 min) | Repairable coatings | Low-temperature, mold-free in situ repair of polyimine composites, adapted for curved surface applications | [77] |
| PPCs | Cellulose paper | Hot pressing | Tensile strength: 71 MPa; Young’s modulus: 3.2 GPa | Green materials | Polyimine-filled cellulose papers (PPCs) with excellent mechanical strength, water resistance, gas barrier properties, and recyclability | [43] |
| WPCs | Wood cellulose | Hot pressing | Transition from brittle to ductile fracture; 13% tensile strength improvement in WPC/25pAPP | Fireproof materials | Improved interfacial interaction and mechanical strength due to imine bond network and mechanical interlocking | [78] |
| Polymer | Mechanical Properties | Thermal Properties | Recyclability | |
|---|---|---|---|---|
| Tensile Strength | Tg | Td5% | Recyclable | |
| Epoxy | 55–85 MPa | 105–120 °C | 250–350 °C | × |
| Phenolic Resin | 30–60 MPa | 100–150 °C | 260–350 °C | × |
| Bismaleimide (BMI) | 60–120 MPa | 230–350 °C | 400–500 °C | × |
| Polyimine (PI) | 30–96 MPa | −20–100 °C | 250–400 °C | √ |
| Parameter | Per Tedap | Vitrimer App | Vitrimer Rdp | Vitrimer Tedap |
|---|---|---|---|---|
| pHRR (kW/m2) | 111 | 175 | 290 | 218 |
| Time to pHRR (s) | 110 | 234 | 207 | 260 |
| Parameter | Per Composite | Vitrimer Composite | Vitrimer App Composite | Vitrimer Rdp Composite |
| pHRR (kW/m2) | 351 | 289 | 186 | 152 |
| Time to pHRR (s) | 39 | 140 | 158 | 176 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, S.; Chen, Y. Polyimine-Based Self-Healing Composites: A Review on Dynamic Covalent Thermosets for Sustainable and High-Performance Applications. Polymers 2025, 17, 1607. https://doi.org/10.3390/polym17121607
Wang X, Zhang S, Chen Y. Polyimine-Based Self-Healing Composites: A Review on Dynamic Covalent Thermosets for Sustainable and High-Performance Applications. Polymers. 2025; 17(12):1607. https://doi.org/10.3390/polym17121607
Chicago/Turabian StyleWang, Xiaoxue, Si Zhang, and Yun Chen. 2025. "Polyimine-Based Self-Healing Composites: A Review on Dynamic Covalent Thermosets for Sustainable and High-Performance Applications" Polymers 17, no. 12: 1607. https://doi.org/10.3390/polym17121607
APA StyleWang, X., Zhang, S., & Chen, Y. (2025). Polyimine-Based Self-Healing Composites: A Review on Dynamic Covalent Thermosets for Sustainable and High-Performance Applications. Polymers, 17(12), 1607. https://doi.org/10.3390/polym17121607






