Advancing Compatibility and Interfacial Interaction Between PEEK and GNPs Through a Strategic Approach Using Pyrene-Functionalized PDMAEMA-b-PMMA Copolymer
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of py-PDMAEMA-b-PMMA
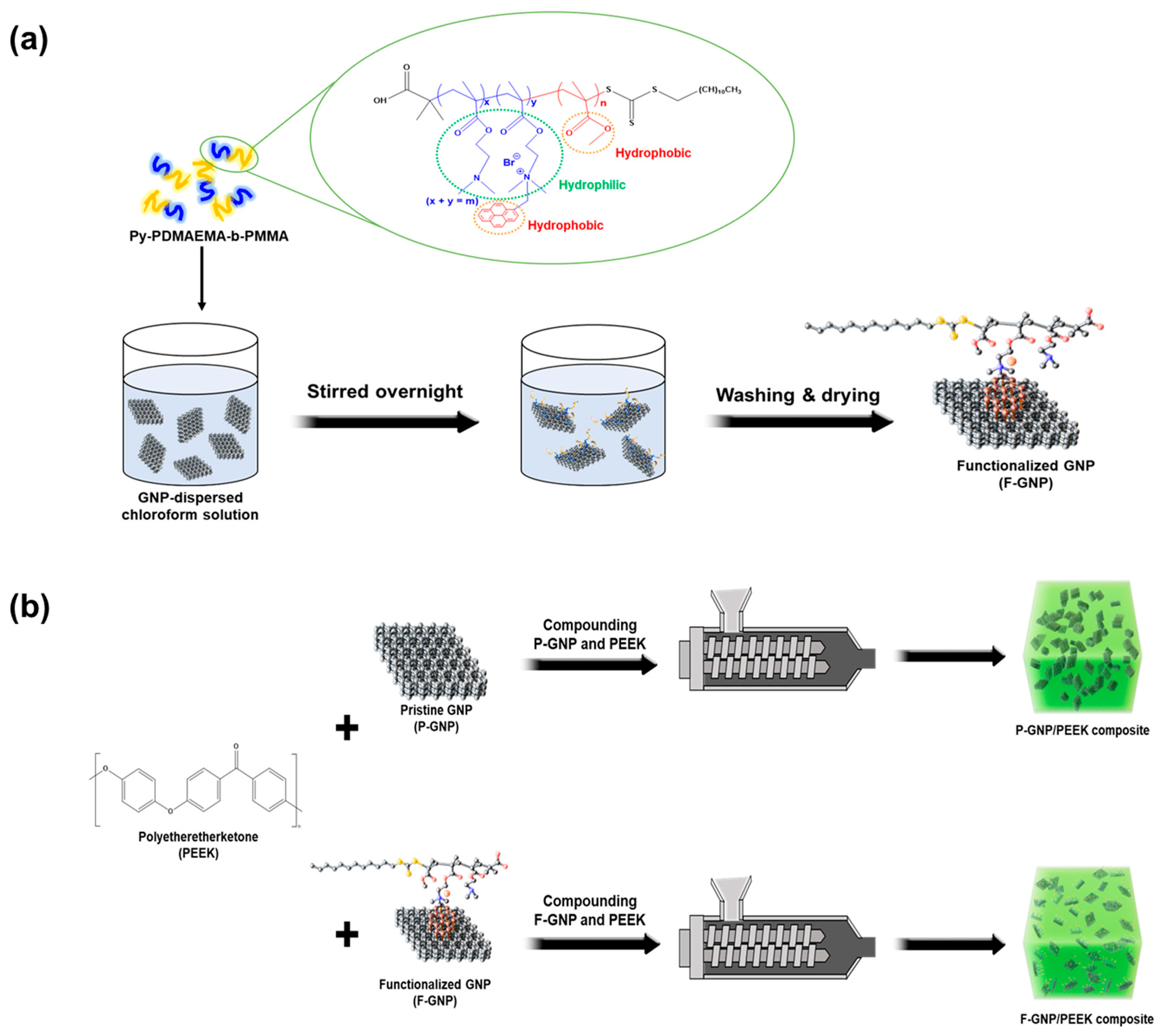
2.3. Surface Treatment of GNPs with py-PDMAEMA-b-PMMA
2.4. Preparation of PEEK/GNP Composites
2.5. Measurements
3. Results and Discussion
3.1. Functionalization of py-PDMAEMA-b-PMMA on GNPs
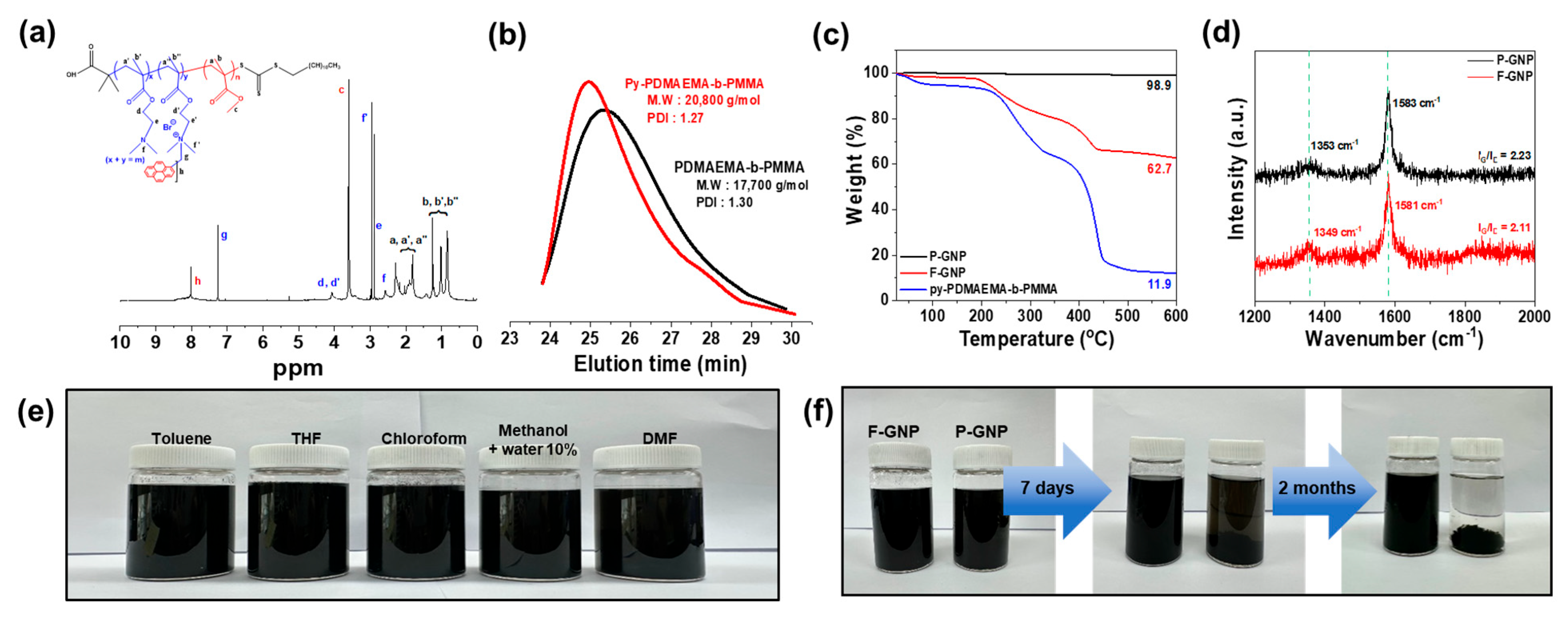
3.2. Characterization of py-PDMAEMA-b-PMMA
3.3. Analysis of py-PDMAEMA-b-PMMA Functionalization Through TEM
3.4. Thermal Behavior and Crystallinity of the P-GNP and F-GNP Composites
3.5. Morphology Analysis of the Composites According to GNP Surface Treatment
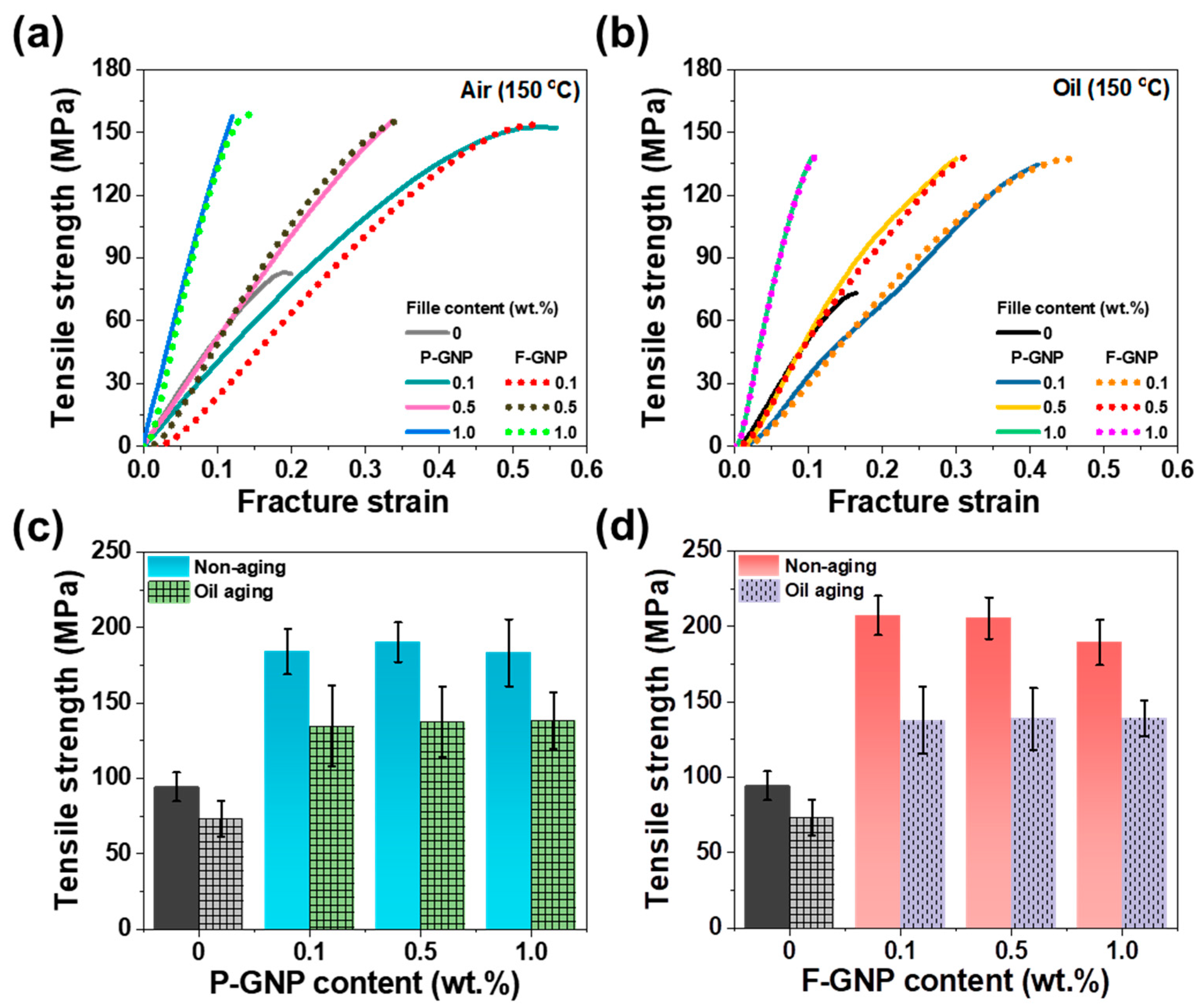
3.6. Mechanical Properties of the GNP Composites
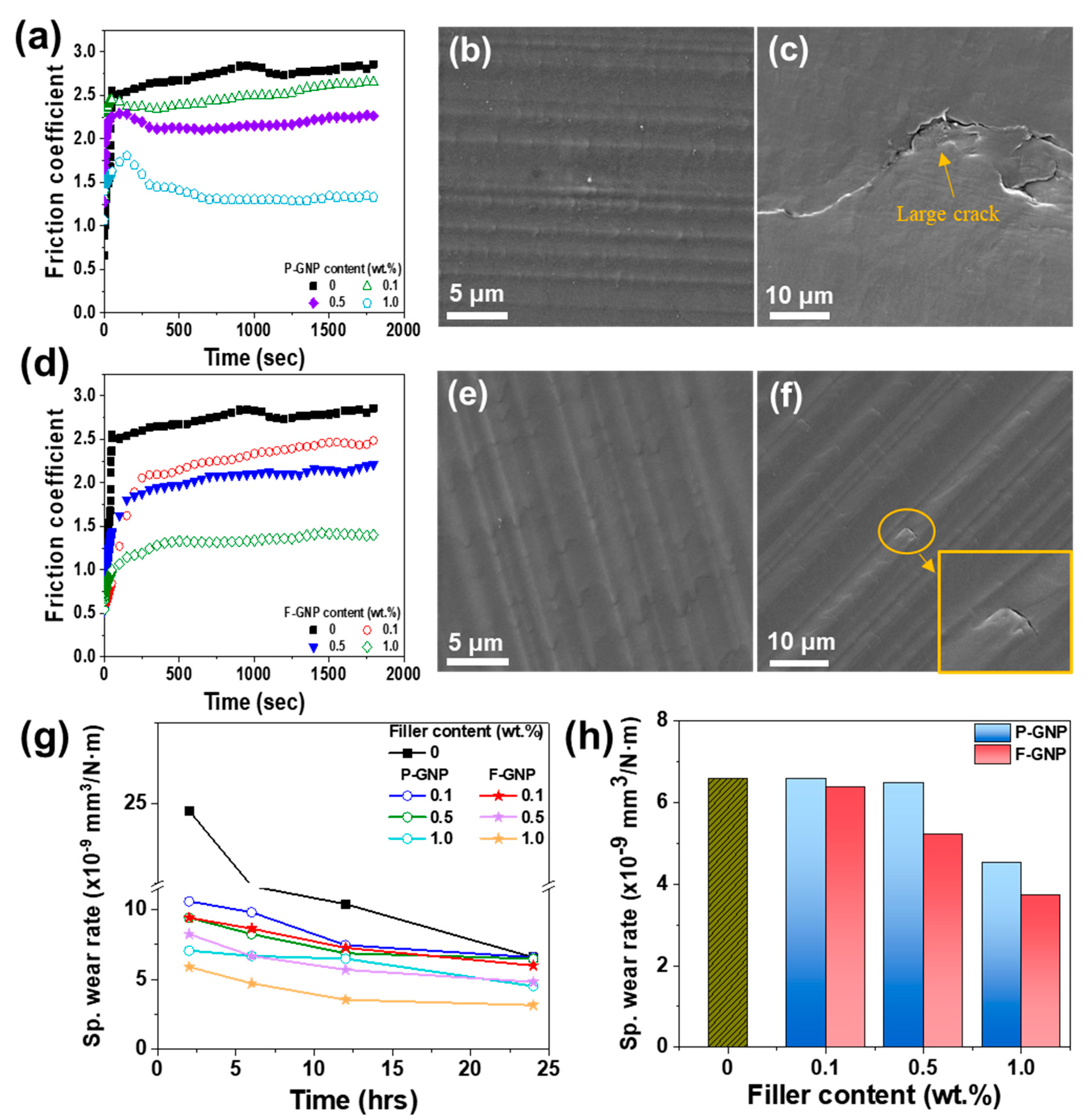
3.7. Tribological Properties of the Composites According to Functionalization
3.8. Thermal Aging Analysis of the P-GNP and F-GNP Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, Z.; Breidt, C.; Chang, L.; Friedrich, K. Wear of PEEK composites related to their mechanical performances. Tribol. Int. 2004, 37, 271–277. [Google Scholar] [CrossRef]
- Skirbutis, G.; Dzingute, A.; Masiliunaite, V.; Sulcaite, G.; Zilinskas, J. A review of PEEK polymer’s properties and its use in prosthodontics. Stomatologija 2017, 19, 19–23. [Google Scholar] [PubMed]
- Hasan, M.M.B.; Cherif, C.; Foisal, A.B.M.; Onggar, T.; Hund, R.D.; Nocke, A. Development of conductive coated polyether ether ketone (PEEK) filament for structural health monitoring of composites. Compos. Sci. Technol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Ma, X.L.; Wen, L.H.; Wang, S.Y.; Xiao, J.Y.; Li, W.H.; Hou, X. Inherent relationship between process parameters, crystallization and mechanical properties of continuous carbon fiber reinforced PEEK composites. Def. Technol. 2003, 24, 269–284. [Google Scholar] [CrossRef]
- Davim, J.P.; Mata, F.; Gaitonde, V.N.; Karnik, S.R. Machinability evaluation in unreinforced and reinforced PEEK composites using response surface models. J. Thermoplast. Compos. 2010, 23, 5–18. [Google Scholar] [CrossRef]
- Leow, C.; Kreider, P.B.; Sommacal, S.; Kluth, P.; Compston, P. Electrical and thermal conductivity in graphene-enhanced carbon-fibre/PEEK: The effect of interlayer loading. Carbon 2023, 215, 118463. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Hou, S.; Xie, J.; Li, Z.; Hu, Q.; Zhou, A.; Liu, X. Effect of morphology and structure of MXene Ti3C2Tx on mechanical, thermal properties of PEEK nanocomposite. Carbon 2024, 228, 119436. [Google Scholar] [CrossRef]
- Burris, D.L.; Sawyer, W.G. Tribological behavior of PEEK components with compositionally graded PEEK/PTFE surfaces. Wear 2007, 262, 220–224. [Google Scholar] [CrossRef]
- Humbe, S.S.; Joshi, G. Polyether ether ketone high-performance composites and blends present trends: A review. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2022; pp. 373–392. [Google Scholar] [CrossRef]
- Gong, R.; Liu, M.; Zhang, H.; Xu, Y. Experimental investigation on frictional behavior and sealing performance of different composites for seal application. Wear 2015, 342–343, 334–339. [Google Scholar] [CrossRef]
- Thongyoug, P.; Tungtrongpairoj, J.; Sooksaen, P.; Doungkeaw, K. Effects of reinforcements on the hardness of composite seal rings. Mater. Today Proc. 2022, 52, 2377–2380. [Google Scholar] [CrossRef]
- Wu, H.; Drzal, L.T. Graphene nanoplatelet paper as a light-weight composite with excellent electrical and thermal conductivity and good gas barrier properties. Carbon 2012, 50, 1135–1145. [Google Scholar] [CrossRef]
- Qin, W.; Vautard, F.; Drzal, L.T.; Yu, J. Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber-matrix interphase. Compos. B Eng. 2015, 69, 335–341. [Google Scholar] [CrossRef]
- Moriche, R.; Jimenez-Suarez, A.; Sanchez, M.; Prolongo, S.G.; Urena, A. Sensitivity, influence of the strain rate and reversibility of GNPs based multiscale composite materials for high sensitive strain sensors. Compos. Sci. Technol. 2018, 155, 100–107. [Google Scholar] [CrossRef]
- Duongthipthewa, A.; Su, Y.; Zhou, L. Electrical conductivity and mechanical property improvement by low-temperature carbon nanotube growth on carbon fiber fabric with nanofiller incorporation. Compos. B Eng. 2020, 182, 107581. [Google Scholar] [CrossRef]
- Sandler, J.; Werner, P.; Shaffer, M.S.P.; Demchuk, V.; Alstadt, V.; Windle, A.H. Carbon-nanofibre-reinforced poly(ether ether ketone) composites. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1033–1039. [Google Scholar] [CrossRef]
- Ata, S.; Hayashi, Y.; Thi, T.B.N.; Tomonoh, S.; Kawauchi, S.; Yamada, T.; Hata, K. Improving thermal durability and mechanical properties of poly(ether ether ketone) with single-walled carbon nanotubes. J. Polym. 2019, 176, 60–65. [Google Scholar] [CrossRef]
- Martinez-Morlanes, M.J.; Castell, P.; Alonso, P.J.; Martinez, M.T.; Puertolas, J.A. Multi-walled carbon nanotubes acting as free radical scavengers in gamma-irradiated ultrahigh molecular weight polyethylene composites. Carbon 2012, 50, 2442–2452. [Google Scholar] [CrossRef]
- Mypati, S.; Sellathurai, A.; Kontopoulou, M.; Docoslis, A.; Barz, D.P.J. High concentration graphene nanoplatelet dispersions in water stabilized by graphene oxide. Carbon 2021, 174, 581–593. [Google Scholar] [CrossRef]
- Trusiano, G.; Matta, S.; Bianchi, M.; Rizzi, L.G.; Frache, A. Evaluation of nanocomposites containing graphene nanoplatelets: Mechanical properties and combustion behavior. Polym. Eng. Sci. 2019, 59, 2062–2071. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Naffakh, M.; Gonzalez-Dominguez, J.M.; Anson, A.; Martinez-Rubi, Y.; Martinez, M.T.; Simard, B.; Gomez, M.A. High performance PEEK/carbon nanotube composites compatibilized with polysulfones-II. Mechanical and electrical properties. Carbon 2010, 48, 3500–3511. [Google Scholar] [CrossRef]
- Wang, R.; Xie, C.; Gou, B.; Xu, H.; Luo, S.; Zhou, J.; Zeng, L. Preparation and properties of carbon-based epoxy nanocomposites: Dynamic mechanical, dielectric, and thermal properties. Polym. Compos. 2020, 41, 4974–7982. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, F.; Dai, J.; Huang, Z. Effect of functionalization of graphene nanoplatelets on the mechanical and thermal properties of silicone rubber composites. Materials 2016, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, M.A.; Yussuf, A.A.; Al-Enezi, S.; Kazemi, R.; Wahit, M.U.; Al-Shammari, T.; Al-Banna, A. Polypropylene/graphene nanocomposites: Effects of GNP loading and compatibilizers on the mechanical and thermal properties. Materials 2019, 12, 3924. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.G.; Tan, J.; Wu, Z.S.; Gentle, I.; Lu, G.Q.; Cheng, H.M. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, M.; Qian, C.; Li, C.; Yang, Y.; Du, X.; Dong, H.; Chen, B. Disordering of graphene nanoplatelet, carbon nanotube and C60 fullerene under shear stress. Carbon 2025, 232, 119802. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Noncovalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [PubMed]
- Krixhnamoorthy, K.; Veerapandian, M.; Kim, G.S.; Kim, S.J. A one step hydrothermal approach for the improved synthesis of graphene nanosheets. Curr. Nanosci. 2012, 8, 934–938. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Xu, D.; Gao, Y.; Sun, Y.; Wang, Z.; Jiang, Z.; Jiang, X.; Zhang, H. A novel graphene nanoplatelets (GNPs) dispersant: Polyaryletherketones with pendent pyrene groups. Macromol. Chem. Phys. 2019, 220, 1800553. [Google Scholar] [CrossRef]
- Hu, K.; Brambilla, L.; Moretti, P.; Bertarelli, C.; Castiglioni, C.; Pappalardo, G.; Sabatino, G. Functional modification of graphene nanoparticles: Covalent grafting of peptides and π bonding for drug loading and delivery. New J. Chem. 2024, 48, 17538–17552. [Google Scholar] [CrossRef]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechancial properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Alsaadi, M.; Hinchy, E.P.; McCarthy, C.T.; Moritz, V.F.; Portela, A.; Devine, D.M. Investigation of thermal, mechanical and shape memory properties of 3D-printed functionally graded nanocomposite materials. Nanomaterials 2023, 13, 2658. [Google Scholar] [CrossRef] [PubMed]
- Yeom, Y.S.; Cho, K.Y.; Seo, H.Y.; Lee, J.S.; Im, D.H.; Nam, C.Y.; Yoon, H.G. Unprecedentedly high thermal conductivity of carbon/epoxy composites derived from parameter optimization studies. Compos. Sci. Technol. 2020, 186, 107915. [Google Scholar] [CrossRef]
- Yaragalla, S.; Zahid, M.; Panda, J.K.; Tsagarakis, N.; Cingolani, R.; Athanassiou, A. Comprehensive enhancement in thermomechanical performance of melt-extruded PEEK filaments by graphene incorporation. Polymers 2021, 13, 1425. [Google Scholar] [CrossRef]
- Cho, K.Y.; Yeom, Y.S.; Seo, H.Y.; Park, Y.H.; Jang, H.N.; Baek, K.Y.; Yoon, H.G. Rational design of multiamphiphilic polymer compatibilizer: Versatile solubility and hybridization of noncovalently functionalized CNT nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 9841–9850. [Google Scholar] [CrossRef]
- Park, J.A.; Cho, K.Y.; Han, C.H.; Nam, A.R.; Kim, J.H.; Lee, S.H.; Choi, J.W. Quaternized amphiphilic block copolymer/graphene oxide and a poly(vinyl alcohol) coating layer on graphene oxide/poly(vinylidene fluoride) electrospun nanofibers for superhydrophilic and antibacterial properties. Sci. Rep. 2019, 9, 383. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhang, J.; Liu, J.; Yang, X.; Zhao, H. Exfoliated graphite oxide decorated by PDMAEMA chains and polymer particles. Langmuir 2009, 25, 11808–11814. [Google Scholar] [CrossRef]
- Alvaredo, A.; Martin, M.I.; Castell, P.; de Villoria, R.G.; Fernandez-Blazquez, J.P. Nonisothermal crystallization behavior of PEEK/graphene nanoplatelets composites from melt and glass states. Polymers 2019, 11, 124. [Google Scholar] [CrossRef]
- Hung, C.Y.; Wang, C.C.; Chen, C.Y. Enhanced the thermal stability and crystallinity of polylactic acid (PLA) by incorporated reactive PS-b-PMMA-b-PGMA and PS-b-PGMA block copolymers as chain extenders. Polymer 2013, 54, 1860–1866. [Google Scholar] [CrossRef]
- Puertolas, J.A.; Castro, M.; Morris, J.A.; Rios, R.; Anson-Casaos, A. Tribological and mechanical properties of graphene nanoplatelet/PEEK composites. Carbon 2019, 141, 107–122. [Google Scholar] [CrossRef]
- Cho, J.H.; Jeon, I.S.; Kim, S.Y.; Lim, S.H.; Jho, J.Y. Improving dispersion and barrier properties of polyketone/graphene nanoplatelet composites via noncovalent functionalization using aminopyrene. ACS Appl. Mater. Interfaces 2017, 9, 27984–27994. [Google Scholar] [CrossRef] [PubMed]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbe, K.; Denape, J.; Chabert, F. A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Naffakh, M.; Gomez, M.A.; Marco, C.; Ellis, G.; Martinez, M.T.; Anson, A.; Gonzalez-Dominguez, J.M.; Martinez-Rubi, Y.; Simard, B. Development and characterization of PEEK/carbon nanotube composites. Carbon 2009, 47, 3079–3090. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Naffakh, M.; Gonzalez-Dominguez, J.M.; Anson, A.; Martinez-Rubi, Y.; Martinez, M.T.; Simard, B.; Gomez, M.A. High performance PEEK/carbon nanotube composites compatibilized with polysulfones-I. Structure and thermal properties. Carbon 2010, 48, 3485–3499. [Google Scholar] [CrossRef]
- Fu, X.; Dong, X.; Yang, G.; Bai, S. Nonisothermal crystallization kinetics of graphene/PA10T composites. Heliyon 2022, 8, e10206. [Google Scholar] [CrossRef]
- Coburn, N.; Douglas, P.; Kaya, D.; Gupta, J.; McNally, T. Isothermal and nonisothermal crystallization kinetics of composites of poly(propylene) and MWCNTs. Adv. Ind. Eng. Polym. Res. 2018, 1, 99–110. [Google Scholar] [CrossRef]
- Cebe, P. Annealing study of poly(etheretherketone). J. Mater. Sci. 1998, 23, 3721–3731. [Google Scholar] [CrossRef]
- Fan, M.; Zhou, S.; Li, Z.; Du, B.; Yu, F.; Yan, H. Effect or crystalline morphology on DC-prestressed breakdown characteristics of PP-based cable insulation. In Proceedings of the 2021 International Conference on Electrical Materials and Power Equipment, Chongqing, China, 11–15 April 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Arzak, A.; Eguiazabal, J.I.; Nazabal, J. Effect of annealing on the properties of poly(ether ether ketone). Polym. Eng. Sci. 1991, 31, 586–591. [Google Scholar] [CrossRef]
- Zhang, K.; Yuan, X.; Li, D.; Du, J.; Wang, B.; Li, T. Mechanical properties of solution-blended graphene nanoplatelets/polyether-ether-ketone nanocomposites. J. Phys. Chem. B 2021, 125, 10597–10609. [Google Scholar] [CrossRef]
- Hussain, M.A.; Shah, S.Z.H.; Megat-Yusoff, P.S.M.; Choudhry, R.S.; Ahmad, F.; Hussnain, S.M. Toughening epoxy resin system using nano-structured block copolymer and graphene nanoplatelets to mitigate matrix microcracks in epoxy nanocomposites: A DoE based framework. Mater. Today Commun. 2025, 43, 111697. [Google Scholar] [CrossRef]
- Gallardo-Lopez, A.; Castillo-Seoane, J.; Munoz-Ferreiro, C.; Lopez-Pernia, C.; Morales-Rodriquez, A.; Poyato, R. Flexure strength and fracture propagation in zirconia ceramic composites with exfoliated graphene nanoplatelets. Ceramics 2020, 3, 78–91. [Google Scholar] [CrossRef]
- Cakir, M.V.; Ozbek, O. Mechanical performance and damage analysis of GNP-reinforced adhesively bonded joints under shear and bending loads. J. Adhesion. 2023, 99, 869–892. [Google Scholar] [CrossRef]
- Durmaz, B.U.; Aytac, A. Enhanced mechanical and thermal properties of graphene nanoplatelets-reinforced polyamide11/poly(lactic acid) nanocomposites. Polym. Eng. Sci. 2023, 63, 105–117. [Google Scholar] [CrossRef]
- Sahm, B.D.; Teixeira, A.B.V.; dos Reis, A.C. Graphene loaded into dental polymers as reinforcement of mechanical properties: A systematic review. Jpn. Dent. Sci. Rev. 2023, 59, 160–166. [Google Scholar] [CrossRef]
- Ejaz, E.; Mubashar, A.; Uddin, E.; Ali, Z.; Arif, N. Effect of functionalised and non-functionalised GNPs addition on strength properties of high viscous epoxy adhesive and lap shear joints. Polym. Test. 2022, 113, 107680. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, S.J.; Jeon, I.Y. Graphene/polymer nanocomposites: Preparation, mechanical properties, and application. Polymers 2022, 14, 4733. [Google Scholar] [CrossRef]
- Kong, D.C.; Yang, M.H.; Zhang, X.S.; Du, Z.C.; Fu, Q.; Gao, X.Q.; Gong, J.W. Control of polymer properties by entanglement: A review. Macromol. Mater. Eng. 2021, 306, 2100536. [Google Scholar] [CrossRef]
- Domun, N.; Hadavinia, H.; Zhang, T.; Liaghat, G.; Vahid, S.; Spacie, C.; Paton, K.R.; Sainsbury, T. Improving the fracture toughness properties of epoxy using graphene nanoplatelets at low filler content. Nanocomposites 2017, 3, 85–96. [Google Scholar] [CrossRef]
- Lee, M.G.; Lee, S.W.; Cho, J.H.; Jho, J.Y. Improving dispersion and mechanical properties of polypropylene/graphene nanoplatelet composites by mixed solvent-assisted melt blending. Macromol. Res. 2020, 28, 1166–1173. [Google Scholar] [CrossRef]
- Carotenuto, G.; Nicola, S.D.; Palomba, M.; Pullini, D.; Horsewell, A.; Hansen, T.W.; Nicolais, L. Mechanical properties of low-density polyethylene filled by graphite nanoplatelets. Nanotechnol. 2012, 23, 485705. [Google Scholar] [CrossRef]
- Mindivan, F. Effect of graphene nanoplatelets (GNPs) on tribological and mechanical behaviors of polyamide 6 (PA6). Tribol. Ind. 2017, 39, 277–282. [Google Scholar] [CrossRef][Green Version]
- Hwang, S.H.; Kim, B.J.; Baek, J.B.; Shin, H.J.; Bae, I.J.; Lee, S.Y.; Park, Y.B. Effects of process parameters and surface treatments of graphene nanoplatelets on the crystallinity and thermomechanical properties of polyamide 6 composite fibers. Compos. B. Eng. 2016, 100, 220–227. [Google Scholar] [CrossRef]
- Achaby, M.E.; Arrakhiz, F.E.; Vaudreuil, S.; Qaiss, A.K.; Bousmina, M.; Fassi-Fehri, O. Mechanical, thermal, and rheological properties of graphene-based polypropylene nanocomposites prepared by melt mixing. Polym. Compos. 2012, 33, 733–744. [Google Scholar] [CrossRef]
- Jin, Z.; Yao, Z.; Sun, Y.; Shen, H. Loading capacity of PEEK blends in terms of wear rate and temperature. Wear 2022, 496, 204306. [Google Scholar] [CrossRef]
- Davim, J.P.; Cardoso, R. Effect of the reinforcement (carbon or glass fibers) on friction and wear behavior of the PEEK against steel surface at long dry sliding. Wear 2009, 266, 795–799. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Nie, J.; Liu, D.; Sui, G. Synergistic effect of graphene nanoplate and carbonized loofah fiber on the electromagnetic shielding effectiveness of PEEK-based composites. Carbon 2019, 143, 154–161. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Yang, W.; Xiao, C.; Wei, M. Effects of different amine-functionalized graphene on the mechanical, thermal, and tribological properties of polyimide nanocomposites synthesized by in situ polymerization. Polymer 2018, 140, 56–72. [Google Scholar] [CrossRef]
- Kim, C.H.; Cho, C.H.; Son, I.T.; Lee, H.M.; Han, J.W.; Kim, J.G.; Lee, J.H. Effect of microscale oil penetration on mechanical and chemical properties of carbon fiber-reinforced epoxy composites. J. Ind. Eng. Chem. 2018, 61, 112–118. [Google Scholar] [CrossRef]
- Hassan, A.E.M.; EiD, A.I.; El-Sheikh, M.; Ali, W.Y. Effect of graphene nanoplatelets and paraffin oil addition on the mechanical and tribological properties of low-density polyethylene nanocomposites. Arab. J. Sci. Eng. 2018, 43, 1435–1443. [Google Scholar] [CrossRef]
- Maria, I.D.S.; Lim, M.W.; Lau, E.V. Multiple roles of graphene nanoparticles (GNP) in microbubble flotation for crude oil recovery from sand. Results Eng. 2021, 11, 100271. [Google Scholar] [CrossRef]
- Wei, X.; Kallio, K.J.; Olsson, R.T.; Hedenqvist, M.S. Performance of glass fiber reinforced polyamide composites exposed to bioethanol fuel at high temperature. NPJ Mater. Degrad. 2022, 6, 69. [Google Scholar] [CrossRef]
- Alvaredo-Atienza, A.; Fernandez-Blazquez, J.P.; Castell, P.; de Villoria, R.G. Production of graphene nanoplate/polyetheretherketone composites by semi-industrial melt-compounding. Heliyon 2020, 6, e03740. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Niasar, M.G. Aging behavior of PEEK, PTFE, and PI insulation materials under thermal oxidative and humid conditions for aerospace applications. J. Appl. Polym. Sci. 2025, 142, e56858. [Google Scholar] [CrossRef]
- McLauchlin, A.R.; Ghita, O.R.; Savage, L. Studies on the reprocessability of poly(ether ether ketone) (PEEK). J. Mater. Process. Technol. 2014, 214, 75–80. [Google Scholar] [CrossRef]
- Bidabadi, B.S.; de Castro, E.M.; Carrola, M.; Koirala, P.; Tehrani, M.; Asadi, A. Engineering the crystalline architecture for enhanced properties in fast-rate processing of Poly(ether ether ketone) (PEEK) nanocomposites. ACS Appl. Eng. Mater. 2024, 2, 2038–2054. [Google Scholar] [CrossRef]




| GNP Content (wt.%) | DSC | XRD | ||
|---|---|---|---|---|
| P-GNP | F-GNP | P-GNP | F-GNP | |
| 0 | 41.01 | 39.99 | ||
| 0.1 | 43.69 | 53.04 | 41.61 | 43.79 |
| 0.5 | 43.17 | 51.34 | 39.98 | 43.22 |
| 1.0 | 41.40 | 46.18 | 39.10 | 40.55 |
| 3.0 | 40.96 | 43.83 | 36.58 | 39.65 |
| GNP Content (wt.%) | Ultimate Tensile Strength (MPa) | Fracture Strain | Shape Parameter (β) | |||
|---|---|---|---|---|---|---|
| P-GNP | F-GNP | P-GNP | F-GNP | P-GNP | F-GNP | |
| 0 | 94.33 (±9.66) | 0.36 | 7.60 | |||
| 0.1 | 184.01 (±14.99) | 207.27 (±13.06) | 0.35 | 0.20 | 5.58 | 5.61 |
| 0.5 | 190.26 (±13.07) | 205.20 (±13.76) | 0.33 | 0.20 | 4.81 | 5.44 |
| 1.0 | 183.13 (±22.31) | 189.31 (±15.02) | 0.15 | 0.18 | 4.71 | 6.90 |
| 3.0 | 182.89 (±19.82) | 186.58 (±20.97) | 0.13 | 0.17 | 5.31 | 5.32 |
| GNP Content (wt.%) | Shape Parameter (β) | |||
|---|---|---|---|---|
| Air Aging | Oil-Immersion Aging | |||
| P-GNP | F-GNP | P-GNP | F-GNP | |
| 0 | 7.19 | 6.24 | ||
| 0.1 | 7.44 | 10.26 | 3.41 | 4.49 |
| 0.5 | 6.03 | 14.22 | 4.08 | 5.29 |
| 1.0 | 5.62 | 25.50 | 5.18 | 9.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, C.-Y.; Im, D.; Lee, J.-H.; Kim, J.; Cho, K.-Y.; Yoon, H.-G. Advancing Compatibility and Interfacial Interaction Between PEEK and GNPs Through a Strategic Approach Using Pyrene-Functionalized PDMAEMA-b-PMMA Copolymer. Polymers 2025, 17, 1599. https://doi.org/10.3390/polym17121599
Nam C-Y, Im D, Lee J-H, Kim J, Cho K-Y, Yoon H-G. Advancing Compatibility and Interfacial Interaction Between PEEK and GNPs Through a Strategic Approach Using Pyrene-Functionalized PDMAEMA-b-PMMA Copolymer. Polymers. 2025; 17(12):1599. https://doi.org/10.3390/polym17121599
Chicago/Turabian StyleNam, Chae-Yun, Dohyun Im, Jun-Hyung Lee, Jinwon Kim, Kie-Yong Cho, and Ho-Gyu Yoon. 2025. "Advancing Compatibility and Interfacial Interaction Between PEEK and GNPs Through a Strategic Approach Using Pyrene-Functionalized PDMAEMA-b-PMMA Copolymer" Polymers 17, no. 12: 1599. https://doi.org/10.3390/polym17121599
APA StyleNam, C.-Y., Im, D., Lee, J.-H., Kim, J., Cho, K.-Y., & Yoon, H.-G. (2025). Advancing Compatibility and Interfacial Interaction Between PEEK and GNPs Through a Strategic Approach Using Pyrene-Functionalized PDMAEMA-b-PMMA Copolymer. Polymers, 17(12), 1599. https://doi.org/10.3390/polym17121599






