Molecular Design and Role of the Dynamic Hydrogen Bonds and Hydrophobic Interactions in Temperature-Switchable Polymers: From Understanding to Applications
Abstract
1. Introduction
2. Phenomenon of Critical Solution Temperature
3. Molecular Design and Mechanisms of the Temperature-Induced Transition of Homopolymers in Water
3.1. Molecular Design and Mechanism of the Temperature-Induced Transition in Poly(N-alkyl acrylamide)s Based Polymers
3.2. Molecular Design and Mechanism of the Temperature-Induced Transition in Other N-Subtituted Deravatives of Polyacrylamide Based Polymers
3.3. Molecular Design and Mechanism of the Temperature-Induced Transition in Poly(N-alkyl methacrylamide)s Based Polymers
3.4. Molecular Design and Mechanism of the Temperature-Induced Transition in Poly(N-vinylalkylamide) Based Polymers
3.5. Molecular Design and Mechanism of the Temperature-Induced Transition in Lactam-/Pyrrolidone-/Pyrrolidine-Based Polymers
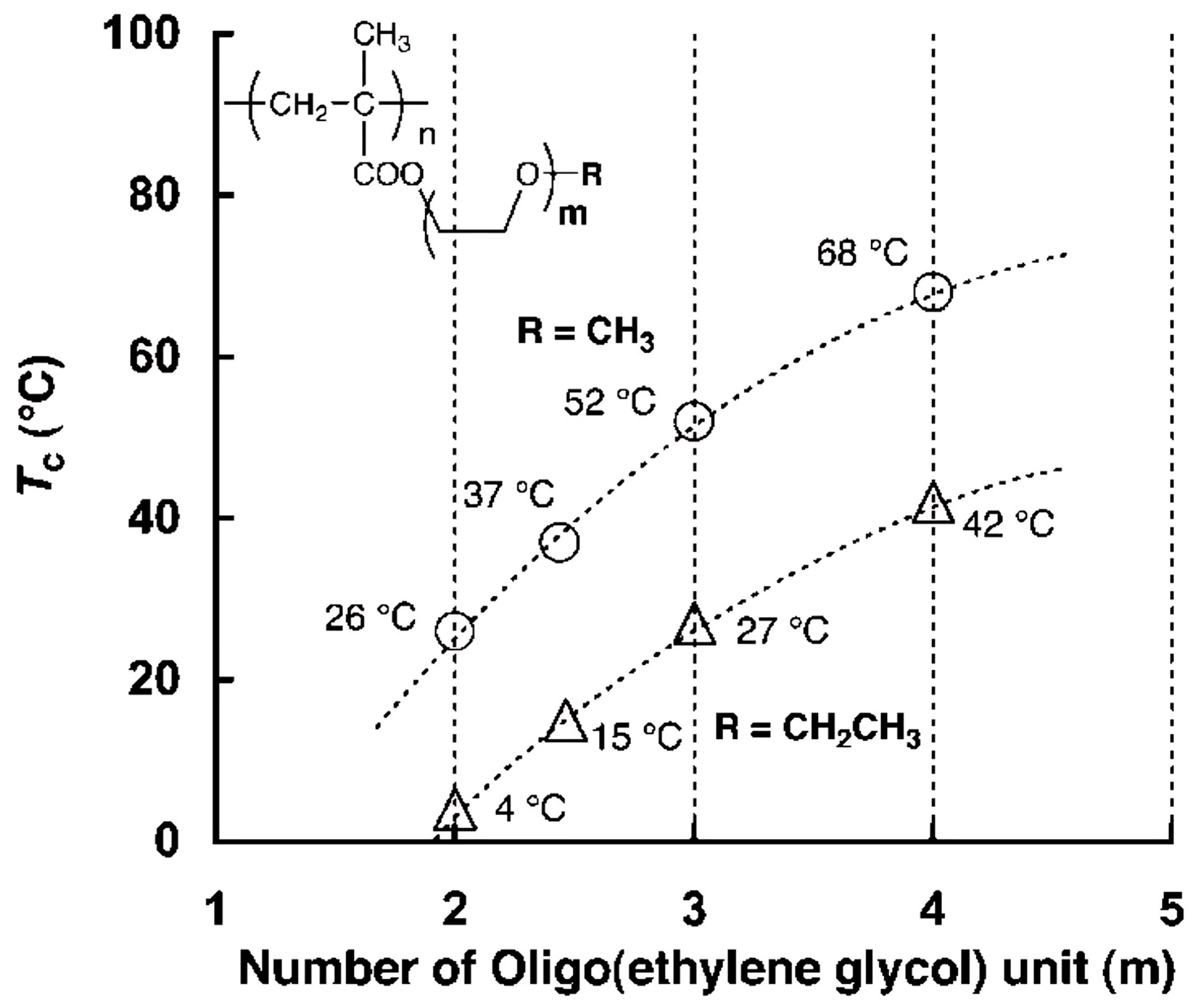
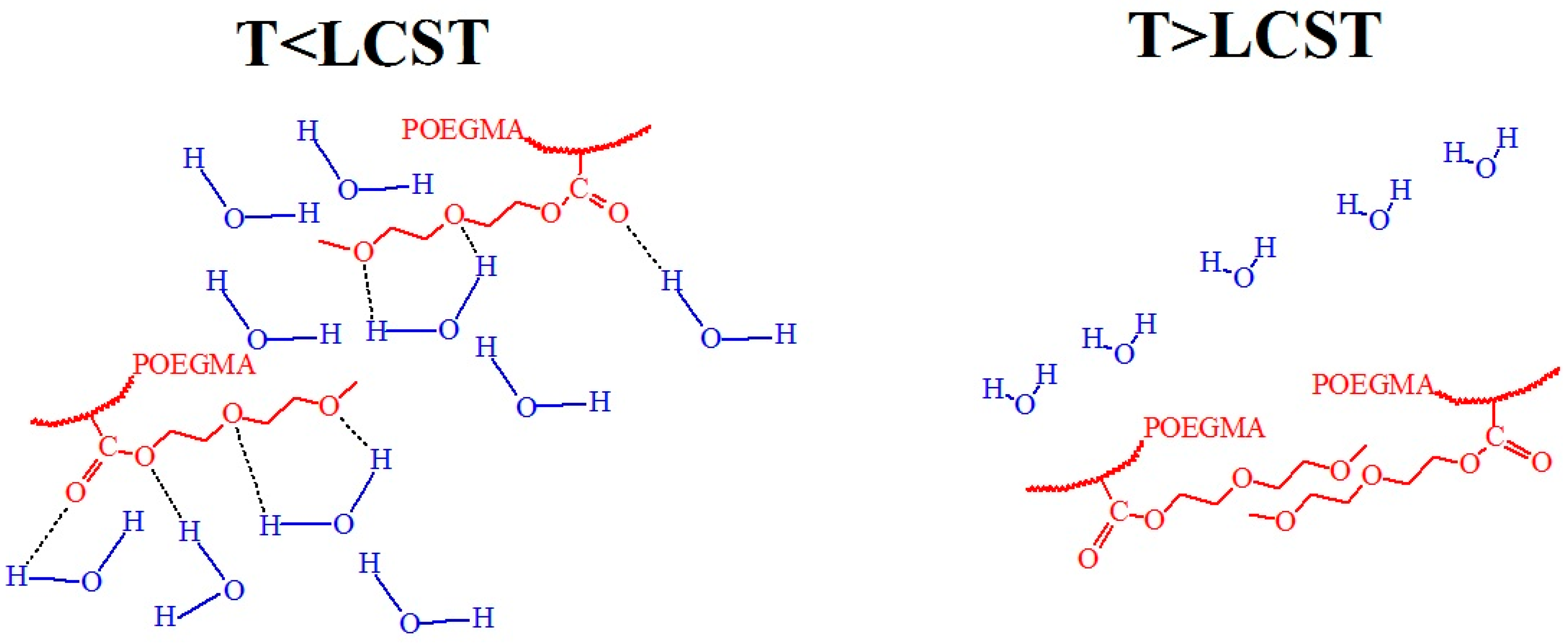
3.6. Molecular Design and Mechanism of the Temperature-Induced Transition in Poly(oligo(ethylene glycol) methacrylate)s—(POEGMA)s
3.7. Molecular Design and Mechanism of the Temperature-Induced Transition in Poly(oligo(ethylene glycol) acrylate)s—(POEGA)s
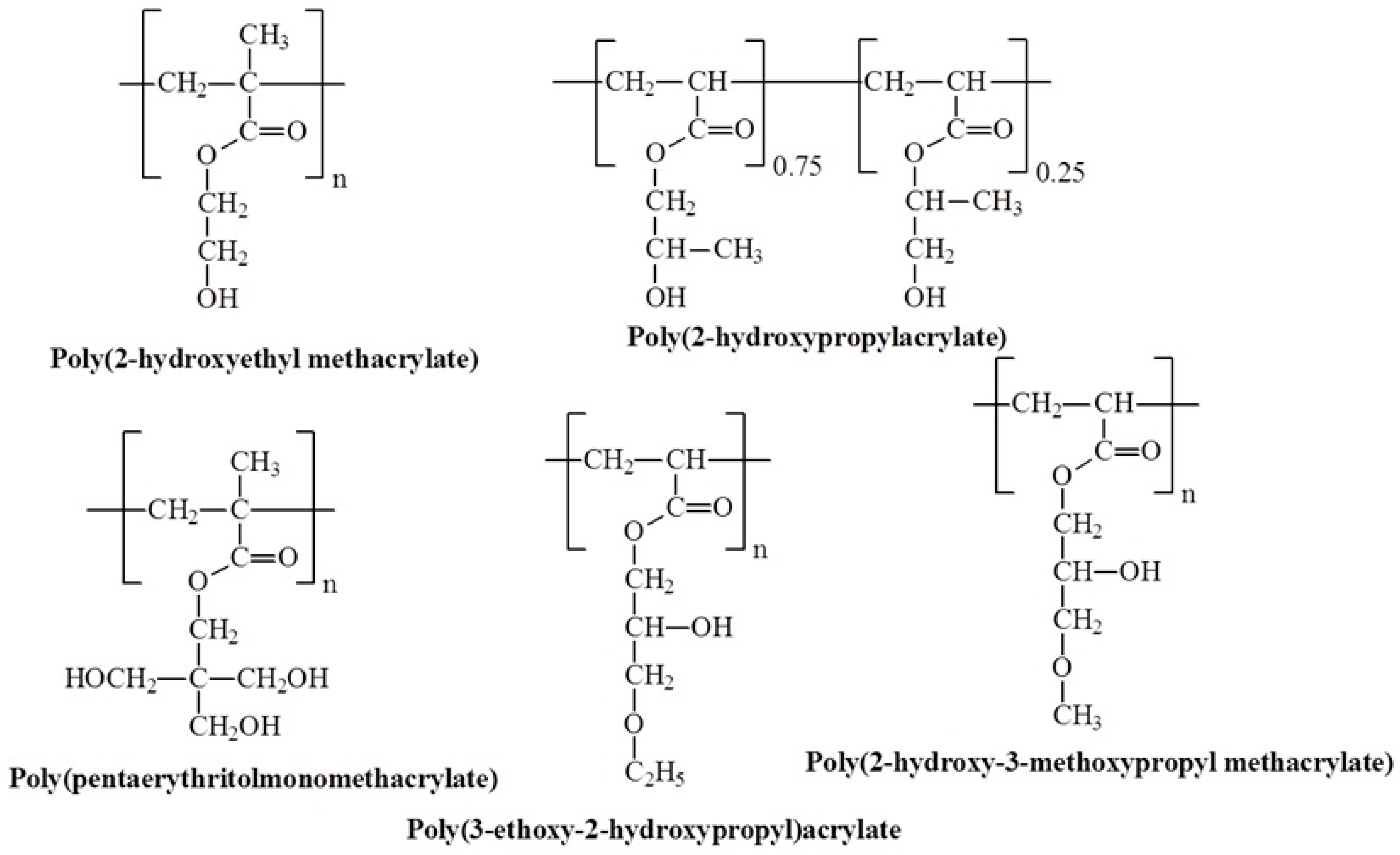
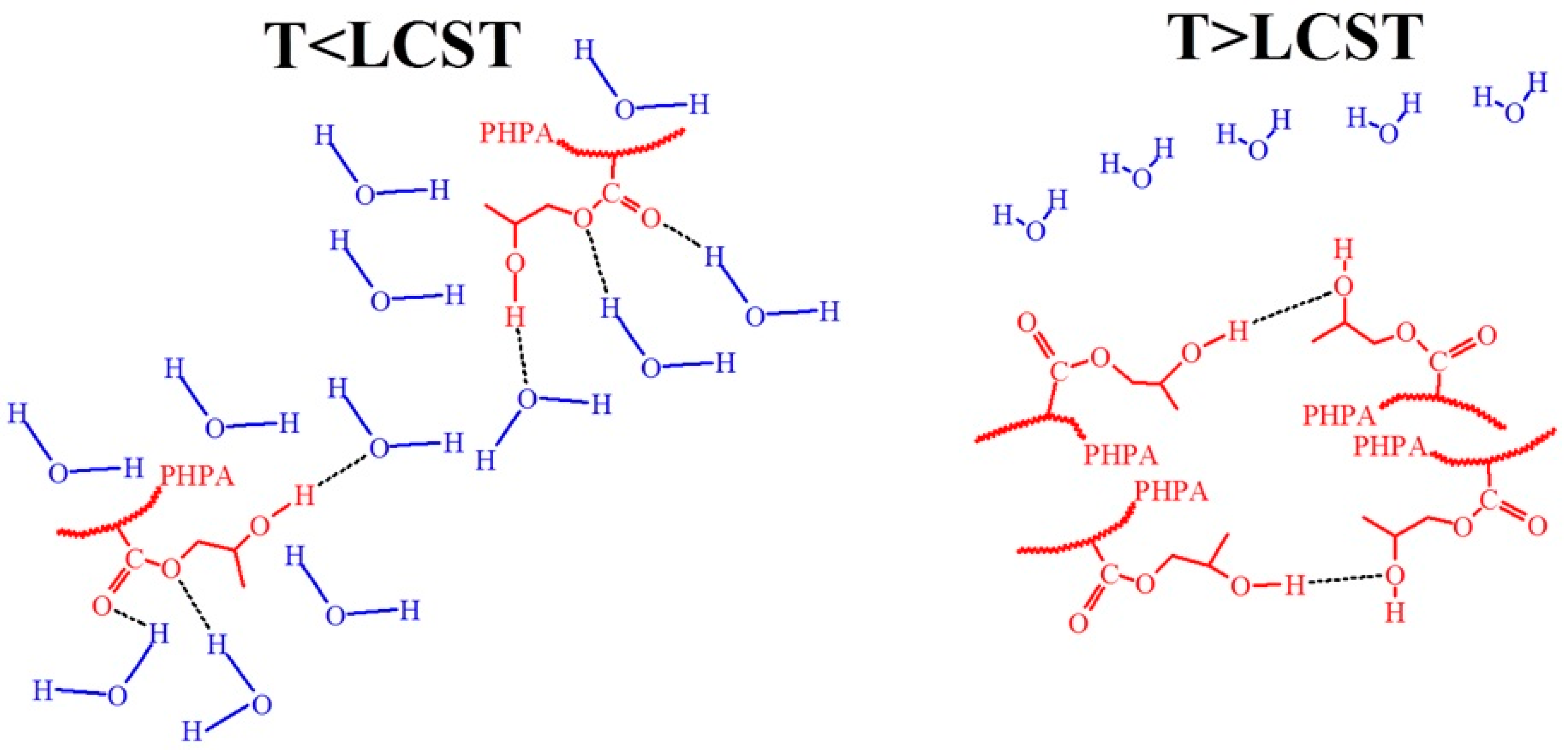
3.8. Molecular Design and Mechanism of the Temperature-Induced Transition in Hydroxyl-Containing Polymers
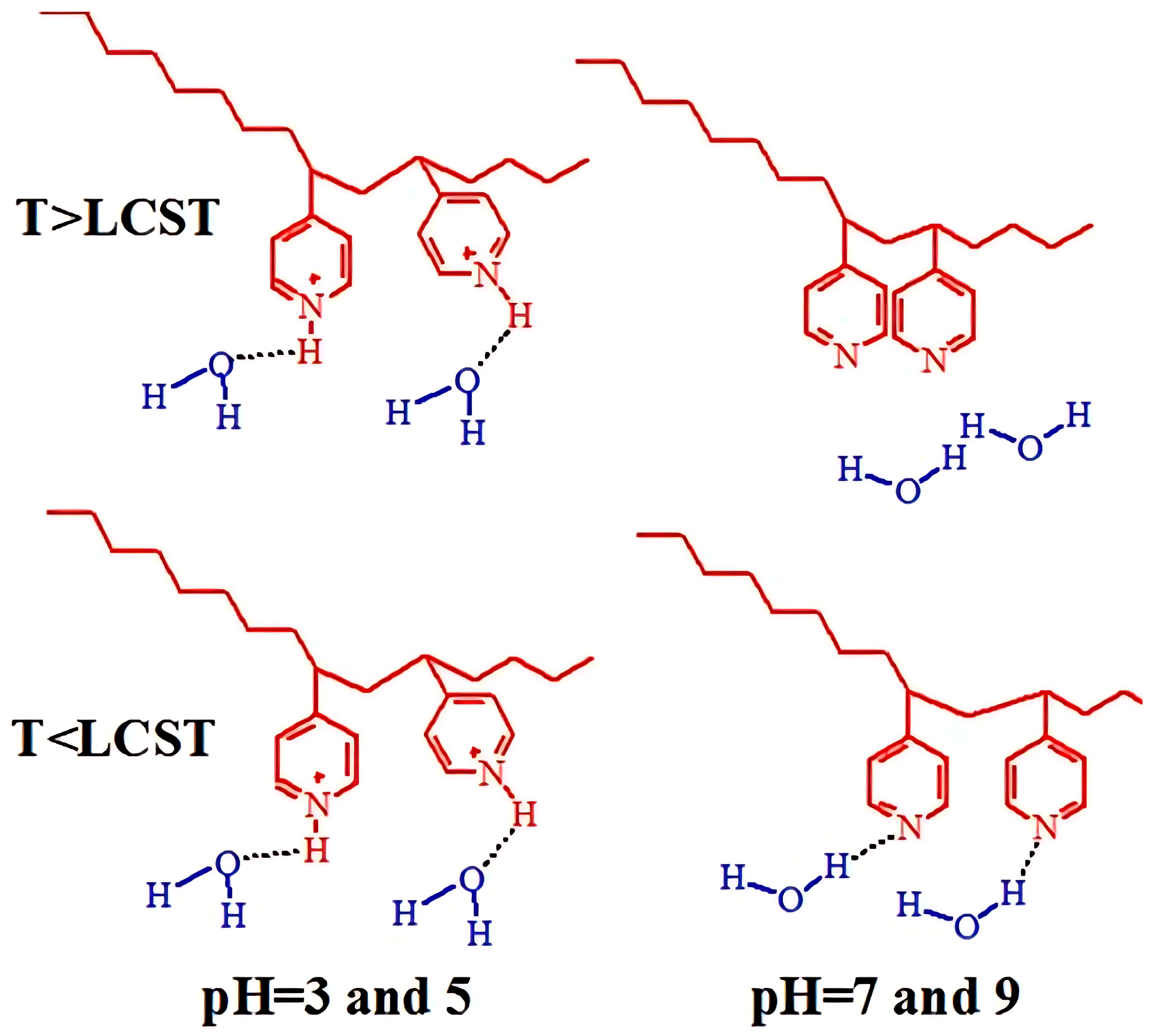
3.9. Molecular Design and Mechanism of the Temperature-Induced Transition in Polyvinylpyridines
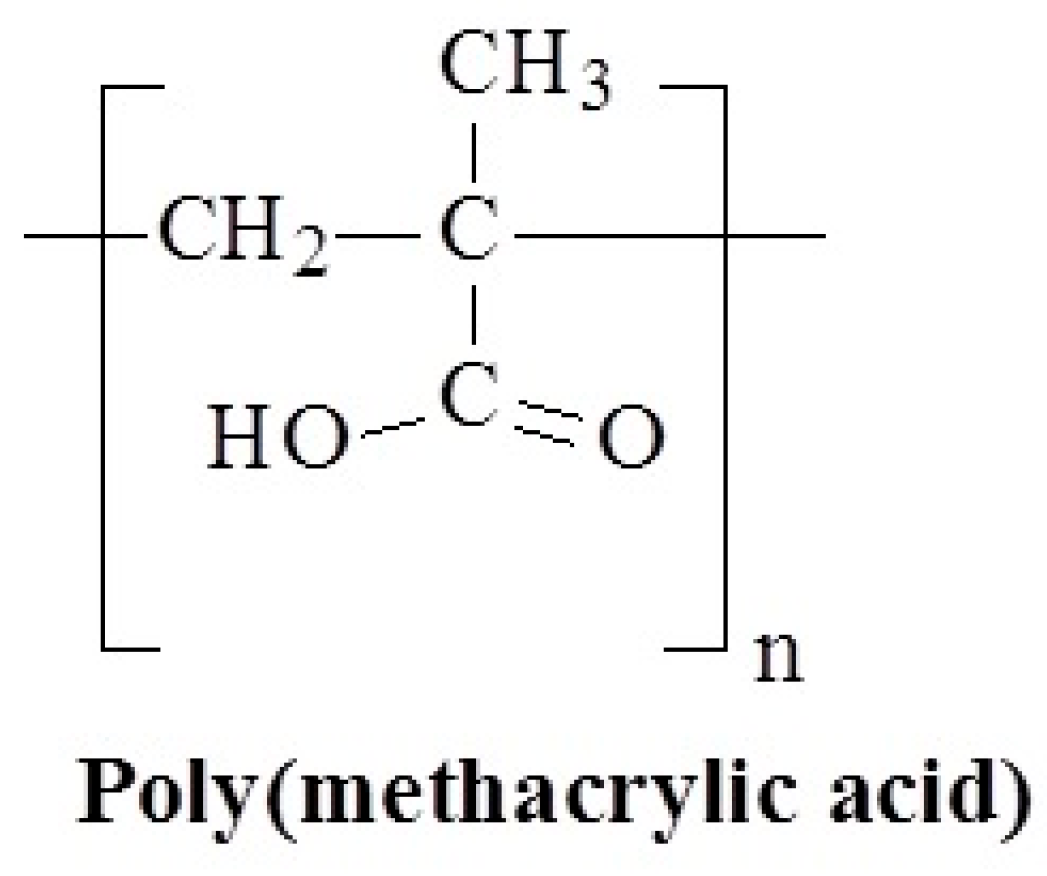
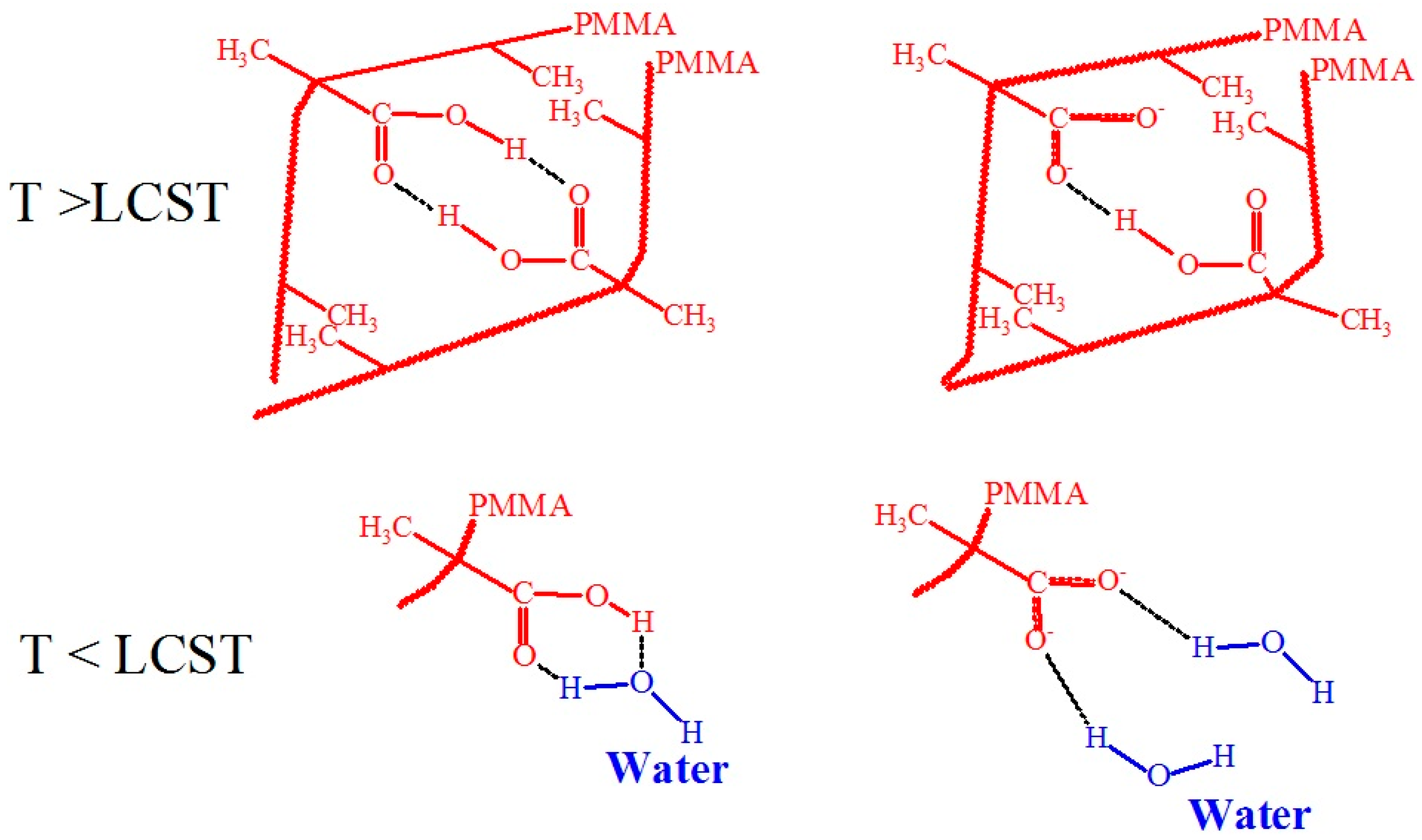
3.10. Molecular Design and Mechanism of the Temperature-Induced Transition in Poly(methacrylic acid)
4. Conclusions
Funding
Conflicts of Interest
References
- Shymborska, Y.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it up: The promise of stimuli-responsive polymer systems in biomedical science. Chem. Rec. 2024, 24, e202300217. [Google Scholar] [CrossRef] [PubMed]
- Aseyev, V.; Belegrinou, S.; Besseling, N.A.M.; Botiz, I.; Stuart, M.A.C.; Hellweg, T.; Junginger, M.; de Keizer, A.; Kita-Tokarczyk, K.; Reiter, G.; et al. Self Organized Nanostructures of Amphiphilic Block Copolymers II; Müller, A.H.E., Borisov, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 29–89. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef]
- Calvino, M.M.; Lazzara, G.; Cavallaro, G.; Milioto, S. Inclusion complexes of triblock L35 copolymer and hydroxyl propyl cyclodextrins: A physico-chemical study. New J. Chem. 2022, 46, 6114–6120. [Google Scholar] [CrossRef]
- Joardder, M.U.H.; Bosunia, M.H.; Hasan, M.M.; Ananno, A.A.; Karim, A. Significance of glass transition temperature of food material in selecting drying condition: An In-Depth Analysis. Food Rev. Int. 2024, 40, 952–973. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Awsiuk, K.; Stetsyshyn, Y.; Raczkowska, J.; Lishchynskyi, O.; Da, P.; Kostruba, A.; Ohar, H.; Shymborska, Y.; Nastyshyn, S.; Budkowski, A. Temperature-controlled orientation of proteins on temperature-responsive grafted polymer brushes: Poly(butyl methacrylate) vs poly(butyl acrylate): Morphology, wetting, and protein adsorption. Biomacromolecules 2019, 20, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Stetsyshyn, Y.; Raczkowska, J.; Lishchynskyi, O.; Awsiuk, K.; Zemla, J.; Dąbczyński, P.; Kostruba, A.; Harhay, K.; Ohar, H.; Orzechowska, B.; et al. Glass transition in temperature-responsive poly (butyl methacrylate) grafted polymer brushes. Impact of thickness and temperature on wetting, morphology, and cell growth. J. Mater. Chem. B 2018, 6, 1613–1621. [Google Scholar] [CrossRef]
- Stegemeyer, H. Liquid Crystals; Baumgartel, H., Franck, E.U., Griinbein, W., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 103–141. [Google Scholar]
- Plate, N.A.; Dobb, M.G.; Finkelmann, H.; Grebowicz, J.; McIntyre, J.E.; Rehage, G.; Shibaev, V.P.; Wunderlich, B. Liquid Crystal Polymers II/III; Gordon, M., Ed.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 173–252. [Google Scholar]
- Stetsyshyn, Y.; Raczkowska, J.; Budkowski, A.; Awsiuk, K.; Kostruba, A.; Nastyshyn, S.; Harhay, K.; Lychkovskyy, E.; Ohar, H.; Nastishin, Y. Cholesterol-based grafted polymer brushes as alignment coating with temperature-tuned anchoring for nematic liquid crystals. Langmuir 2016, 32, 11029–11038. [Google Scholar] [CrossRef] [PubMed]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Lekka, M.; Marzec, M.; Harhay, K.; Ohar, H.; Ostapiv, D.; Sharan, M.; Yaremchuk, I.; et al. Temperature-responsive grafted polymer brushes obtained from renewable sources with potential application as substrates for tissue engineering. Appl. Surf. Sci. 2017, 407, 546–554. [Google Scholar] [CrossRef]
- Folmer-Andersen, J.F.; Lehn, J.M. Thermoresponsive dynamers: Thermally induced, reversible chain elongation of amphiphilic poly (acylhydrazones). J. Am. Chem. Soc. 2011, 133, 10966–10973. [Google Scholar] [CrossRef]
- Roy, N.; Bruchmann, B.; Lehn, J.M. DYNAMERS: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [PubMed]
- Nastyshyn, S.; Stetsyshyn, Y.; Raczkowska, J.; Nastishin, Y.; Melnyk, Y.; Panchenko, Y.; Budkowski, A. Temperature-responsive polymer brush coatings for advanced biomedical applications. Polymers 2022, 14, 4245. [Google Scholar] [CrossRef] [PubMed]
- Stetsyshyn, Y.; Raczkowska, J.; Harhay, K.; Gajos, K.; Melnyk, Y.; Dąbczyński, P.; Shevtsova, T.; Budkowski, A. Temperature-responsive and multi-responsive grafted polymer brushes with transitions based on critical solution temperature: Synthesis, properties, and applications. Colloid Polym. Sci. 2020, 299, 363–383. [Google Scholar] [CrossRef]
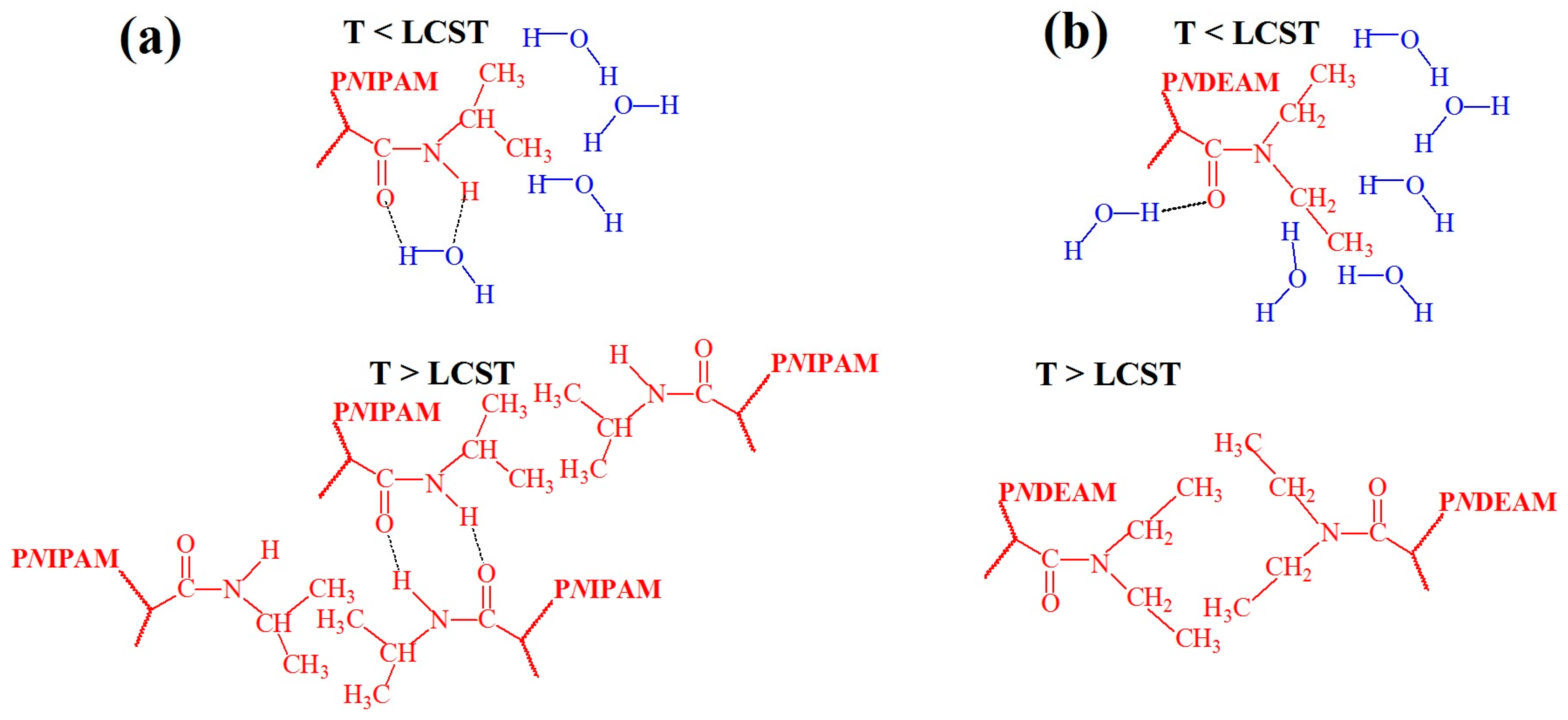
- Heskins, M.; Guillet, J.E. Solution properties of poly (N-isopropylacrylamide). J. Macromol. Sci.—Chem. 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly (N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Silberberg, A.; Eliassaf, J.; Katchalsky, A. Temperature-dependence of light scattering and intrinsic viscosity of hydrogen bonding polymers. J. Polym. Sci. 1957, 23, 259–284. [Google Scholar] [CrossRef]
- Shiomi, T.; Imai, K.; Watanabe, C.; Miya, M. Thermodynamic and conformational properties of partially butyralized poly (vinyl alcohol) in aqueous solution. J. Polym. Sci. Polym. Phys. Ed. 1984, 22, 1305–1312. [Google Scholar] [CrossRef]
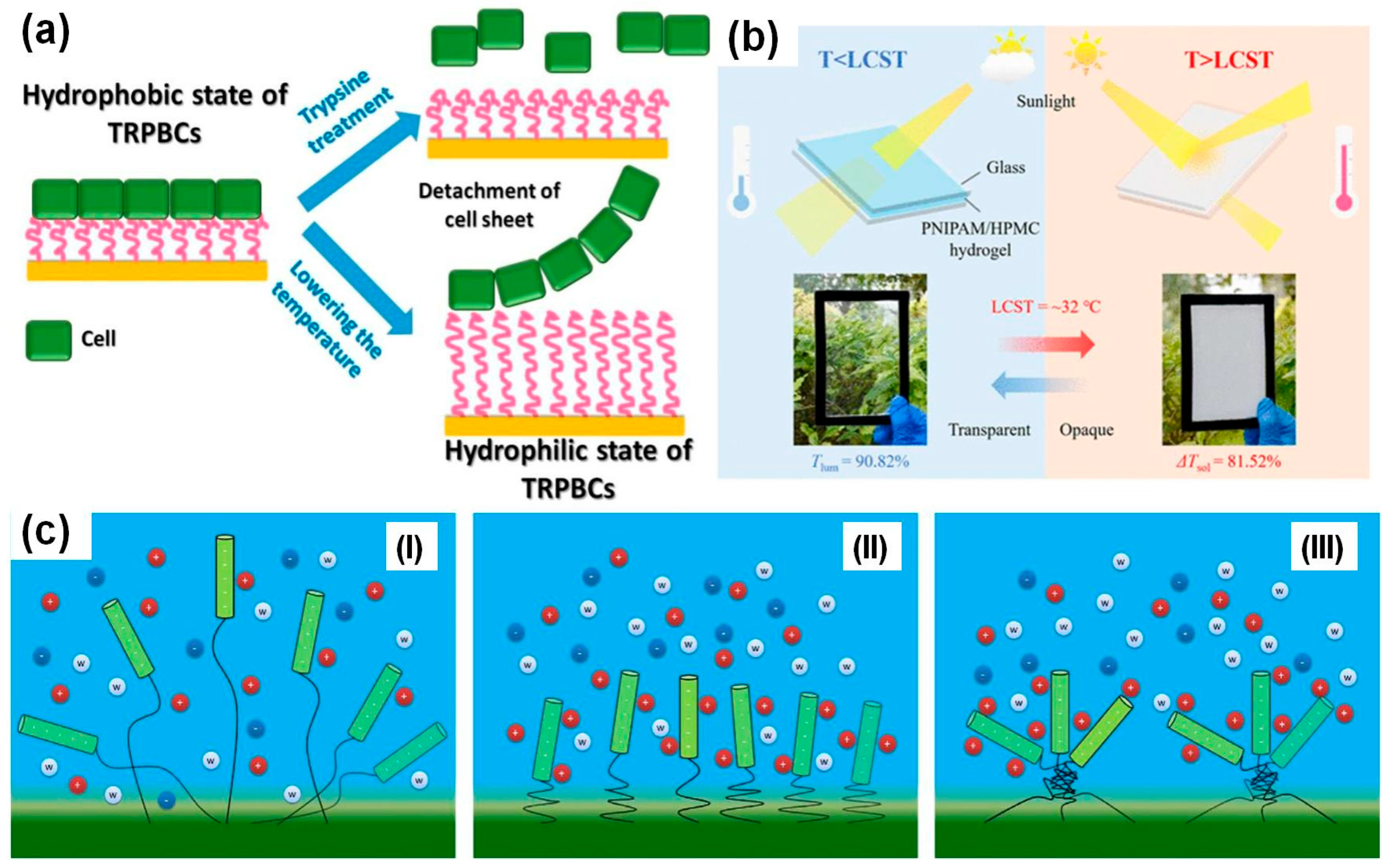
- Kobayashi, J.; Okano, T. Fabrication of a thermoresponsive cell culture dish: A key technology for cell sheet tissue engineering. Sci. Technol. Adv. Mater. 2010, 11, 014111. [Google Scholar] [CrossRef]
- Kano, K.; Yamato, M.; Okano, T. Ectopic transplantation of hepatocyte sheets fabricated with temperature-responsive culture dishes. Hepatol. Res. 2008, 38, 1140–1147. [Google Scholar] [CrossRef]
- Wang, K.; Chen, G.; Weng, S.; Hou, L.; Ye, D.; Jiang, X. Thermo-responsive poly (N-isopropylacrylamide)/hydroxypropylmethyl cellulose hydrogel with high luminous transmittance and solar modulation for smart windows. ACS Appl. Mater. Interfaces 2023, 15, 4385–4397. [Google Scholar] [CrossRef]
- Tang, S.; Sang, X.; Zhu, S.; Ma, L.; Tian, Y.; Luo, J. Fast Optical-Thermal Responsive Intelligent Glass Realized by Hydrated Poly(N-Isopropylacrylamide) Film. Macromol. Mater. Eng. 2021, 306, 2100272. [Google Scholar] [CrossRef]
- Ding, Y.; Duan, Y.; Yang, F.; Xiong, Y.; Guo, S. High-transmittance pNIPAm gel smart windows with lower response temperature and stronger solar regulation. Chem. Eng. J. 2023, 460, 141572. [Google Scholar] [CrossRef]
- Feng, C.; Ding, H.M.; Ren, C.L.; Ma, Y.Q. Designing new strategy for controlling DNA orientation in biosensors. Sci. Rep. 2015, 5, 14415. [Google Scholar] [CrossRef]
- Baddam, V.; Tenhu, H. Thermoresponsive polycations. Polym. Chem. 2023, 14, 3647–3678. [Google Scholar] [CrossRef]
- Flemming, P.; Münch, A.S.; Fery, A.; Uhlmann, P. Constrained thermoresponsive polymers–new insights into fundamentals and applications. Beilstein J. Org. Chem. 2021, 17, 2123–2163. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Avilés-Ramos, N.A.; Ortiz-Acosta, D. A combinatorial approach to studying the effects of N-alkyl groups on poly (N-alkyl and N, N-dialkylacrylamide) Solubility. J. Comb. Chem. 2007, 9, 609–617. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Shymborska, Y.; Stetsyshyn, Y.; Awsiuk, K.; Raczkowska, J.; Bernasik, A.; Janiszewska, N.; Dąbczyński, P.; Kostruba, A.; Budkowski, A. Temperature-and pH-responsive schizophrenic copolymer brush coatings with enhanced temperature response in pure water. ACS Appl. Mater. Interfaces 2023, 15, 8676–8690. [Google Scholar] [CrossRef]
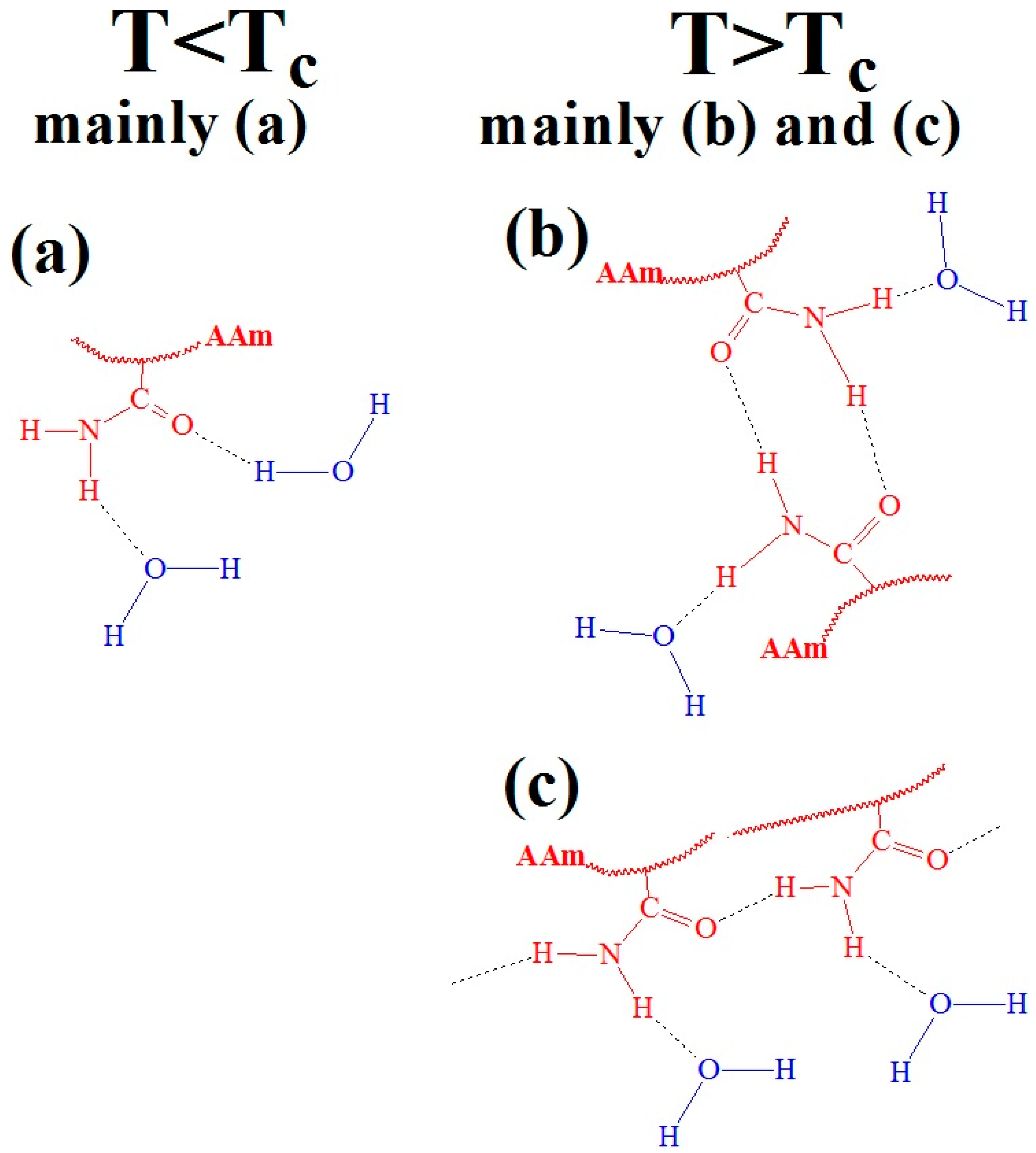
- Neamtu, I.; Chiriac, A.P.; Nita, L.E. Characterization of poly(acrylamide) as temperature-sensitive hydrogel. J. Optoelectron. Adv. Mater. 2006, 8, 1939–1943. [Google Scholar]
- Permyakova, N.M.; Zheltonozhskaya, T.B.; Fedorchuk, S.V.; Zagdanskaya, N.E.; Syromyatnikov, V.G. Temperature effect on hydrogen bonds in triblock copolymers of poly (ethylene oxide) and polyacrylamide. Mol. Cryst. Liq. Cryst. 2007, 468, 53–405. [Google Scholar] [CrossRef]
- Zheltonozhskaya, T.; Partsevskaya, S.; Fedorchuk, S.; Klymchuk, D.; Gomza, Y.; Permyakova, N.; Kunitskaya, L. Micellar nanocontainers based on PAAm-b-PEO-b-PAAm triblock copolymers for poorly soluble drugs. Eur. Polym. J. 2013, 49, 405–418. [Google Scholar] [CrossRef]
- Fedorchuk, S.V.; Zheltonozhskaya, T.B.; Permyakova, N.M.; Gomza, Y.P.; Nessin, S.D.; Klepko, V.V. Structural peculiarities of triblock copolymers containing poly (ethylene oxide) and polyacrylamide. Mol. Cryst. Liq. Cryst. 2008, 497, 268–600. [Google Scholar] [CrossRef]
- Patyukova, E.; Rottreau, T.; Evans, R.; Topham, P.D.; Greenall, M.J. Hydrogen bonding aggregation in acrylamide: Theory and experiment. Macromolecules 2018, 51, 7032–7043. [Google Scholar] [CrossRef]
- Day, J.C.; Robb, I.D. Thermodynamic parameters of polyacrylamides in water. Polymer 1981, 22, 1530–1533. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, X.X.; Luo, J.; Liu, H. Effects of substitution groups on the RAFT polymerization of N-alkylacrylamides in the preparation of thermosensitive block copolymers. Macromolecules 2007, 40, 6481–6488. [Google Scholar] [CrossRef]
- Ito, S. Phase transition of aqueous solution of poly(N-alkylacrylamide) derivatives-effects of side chain structure. Kobunshi Ronbunshu 1989, 46, 437–443. [Google Scholar] [CrossRef]
- Islam, M.R.; Ahiabu, A.; Li, X.; Serpe, M.J. Poly(N-isopropylacrylamide) microgel-based optical devices for sensing and biosensing. Sensors 2014, 14, 8984–8995. [Google Scholar] [CrossRef]
- Shaibie, N.A.; Ramli, N.A.; Faizal, N.D.F.M.; Srichana, T.; Amin, M.C.I.M. Poly(N-isopropylacrylamide)-Based Polymers: Recent Overview for the Development of Temperature-Responsive Drug Delivery and Biomedical Applications. Macromol. Chem. Phys. 2023, 224, 2300157. [Google Scholar] [CrossRef]
- Twaites, B.R.; de las Heras Alarcón, C.; Lavigne, M.; Saulnier, A.; Pennadam, S.S.; Cunliffe, D.; Górecki, D.C.; Alexander, A. Thermoresponsive polymers as gene delivery vectors: Cell viability, DNA transport and transfection studies. J. Control. Release 2005, 108, 472–483. [Google Scholar] [CrossRef]
- Liu, F.; Cui, Y.; Wang, L.; Wang, H.; Yuan, Y.; Pan, J.; Chen, H.; Yuan, L. Temperature-responsive Poly (N-isopropylacrylamide) modified gold nanoparticle–protein conjugates for bioactivity modulation. ACS Appl. Mater. Interfaces 2015, 7, 11547–11554. [Google Scholar] [CrossRef]
- Chen, G.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Feng, L.; Wang, S.; Sun, T.; Song, W.; Jiang, W.; Jiang, L. Dual-responsive surfaces that switch between superhydrophilicity and superhydrophobicity. Adv. Mater. 2006, 18, 432–436. [Google Scholar] [CrossRef]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Change in solvation of poly (N, N-diethylacrylamide) during phase transition in aqueous solutions as observed by IR spectroscopy. Macromolecules 2002, 35, 10172–10177. [Google Scholar] [CrossRef]
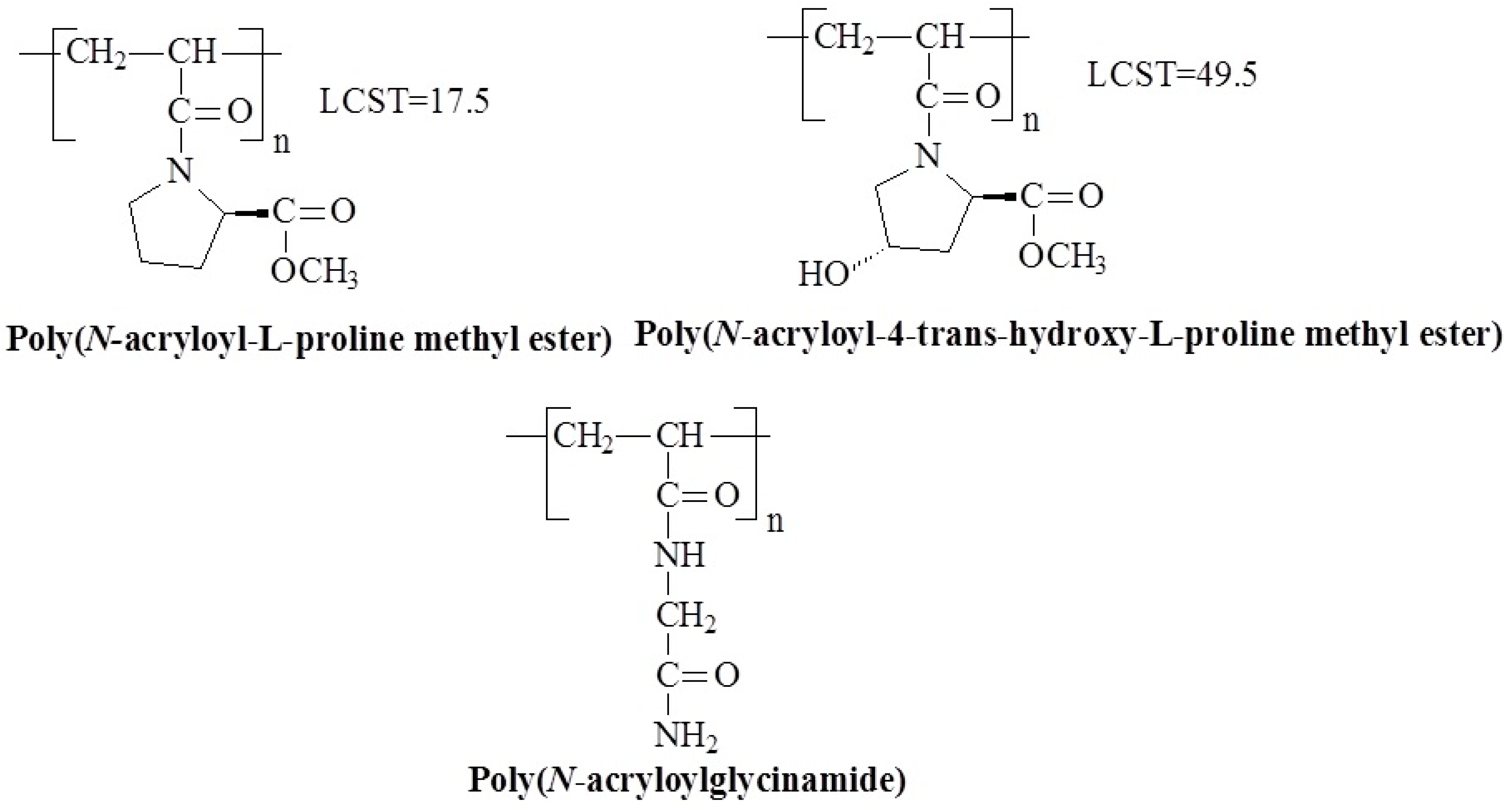
- Yoshida, M.; Omichi, H.; Katakai, R. Light-scattering study of temperature-responsive poly (acryloyl-L-proline methyl ester). Eur. Polym. J. 1992, 28, 1141–1145. [Google Scholar] [CrossRef]
- Mori, H.; Kato, I.; Matsuyama, M.; Endo, T. RAFT polymerization of acrylamides containing proline and hydroxyproline moiety: Controlled synthesis of water-soluble and thermoresponsive polymers. Macromolecules 2008, 41, 5604–5615. [Google Scholar] [CrossRef]
- Carenza, M.; Caliceti, P.; Veronese, F.M.; Martellini, F.; Higa, O.Z.; Yoshida, M.; Katakai, R. Poly(acryloyl-L-proline methyl ester) hydrogels obtained by radiation polymerization for the controlled release of drugs. Radiat. Phys. Chem. 2000, 57, 471–475. [Google Scholar] [CrossRef]
- Martellini, F.; Higa, O.Z.; Takacs, E.; Safranj, A.; Yoshida, M.; Katakai, R.; Carenza, M. Thermally reversible gels based on acryloyl-L-proline methyl ester as drug delivery systems. Radiat. Phys. Chem. 1999, 55, 185–192. [Google Scholar] [CrossRef]
- Yoshida, M.; Asano, M.; Kumakura, M.; Kataki, R.; Mashimo, T.; Yuasa, H.; Yamanaka, H. Thermo-responsive hydrogels based on acryloyl-L-proline methyl ester and their use as long-acting testosterone delivery systems. Drug Des. Deliv. 1991, 7, 159–174. [Google Scholar]
- Hirano, T.; Sugihara, S.; Maeda, Y. Infrared Spectroscopic Study on Hydration and Chiral Interaction of Temperature-Responsive Polymer with l-Proline Moieties. J. Phys. Chem. B 2013, 117, 16356–16362. [Google Scholar] [CrossRef]
- Seuring, J.; Bayer, F.M.; Huber, K.; Agarwal, S. Upper critical solution temperature of poly(N-acryloyl glycinamide) in water: A concealed property. Macromolecules 2012, 45, 374–384. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution: Unexpected Properties from Known Building Blocks. ACS Macro Lett. 2013, 2, 597–600. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, W. Poly(N-acryloyl glycinamide): A fascinating polymer that exhibits a range of properties from UCST to high-strength hydrogels. Chem. Commun. 2018, 54, 10540–10553. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. First example of a universal and cost-effective approach: Polymers with tunable upper critical solution temperature in water and electrolyte solution. Macromolecules 2012, 45, 3910–3918. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Minato, H.; Inui, T.; Saito, I.; Kureha, T.; Shibayama, M.; Suzuki, D. Nanostructure and thermoresponsiveness of poly(N-isopropyl methacrylamide)-based hydrogel microspheres prepared via aqueous free radical precipitation polymerization. RSC Adv. 2021, 11, 13130–13137. [Google Scholar] [CrossRef]
- Kubota, K.; Hamano, K.; Kuwahara, N.; Fujishige, S.; Ando, I. Characterization of poly(N-isopropylmethacrylamide) in water. Polym. J. 1990, 22, 1051–1057. [Google Scholar] [CrossRef]
- Tang, Y.; Ding, Y.; Zhang, G. Role of methyl in the phase transition of poly(N-isopropylmethacrylamide). J. Phys. Chem. B 2008, 112, 8447–8451. [Google Scholar] [CrossRef]
- Djokpé, E.; Vogt, W. N-Isopropylacrylamide and N-Isopropylmethacryl-amide: Cloud Points of Mixtures and Copolymers. Macromol. Chem. Phys. 2001, 202, 750–757. [Google Scholar] [CrossRef]
- Nieuwenhuis, S.; Zhong, Q.; Metwalli, E.; Bießmann, L.; Philipp, M.; Miasnikova, A.; Laschewsky, A.; Papadakis, C.M.; Cubitt, R.; Wang, J.; et al. Hydration and Dehydration Kinetics: Comparison between Poly (N-isopropyl methacrylamide) and Poly (methoxy diethylene glycol acrylate) Films. Langmuir 2019, 35, 7691–7702. [Google Scholar] [CrossRef]
- Suwa, K.; Wada, Y.; Kikunaga, Y.; Morishita, K.; Kishida, A.; Akashi, M. Synthesis and functionalities of poly(N-vinylalkylamide). IV. Synthesis and free radical polymerization of N-vinylisobutyramide and thermosensitive properties of the polymer. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1763–1768. [Google Scholar] [CrossRef]
- Henschel, C.; Schanzenbach, D.; Laschewsky, A.; Ko, C.H.; Papadakis, C.M.; Müller-Buschbaum, P. Thermoresponsive and co-nonsolvency behavior of poly(N-vinyl isobutyramide) and poly(N-isopropyl methacrylamide) as poly(N-isopropyl acrylamide) analogs in aqueous media. Colloid Polym. Sci. 2023, 301, 703–720. [Google Scholar] [CrossRef]
- Le Dû, M.P.; Reitenbach, J.; Kosbahn, D.P.; Spanier, L.V.; Cubitt, R.; Henschel, C.; Müller-Buschbaum, P. Comparison of the Swelling Behavior of Poly(N-Isopropylacrylamide) and Poly(N-Vinylisobutyramide) Thin Films under Water Vapor Exposure. Macromolecules 2025, 58, 1000–1010. [Google Scholar] [CrossRef]
- Suwa, K.; Morishita, K.; Kishida, A.; Akashi, M. Synthesis and functionalities of poly(N-vinylalkylamide). V. Control of a lower critical solution temperature of poly(N-vinylalkylamide). J. Polym. Sci. Part A Polym. Chem. 1997, 35, 3087–3094. [Google Scholar] [CrossRef]
- Tager, A.A.; Safronov, A.P.; Berezyuk, E.A.; Galaev, I.Y. Lower critical solution temperature and hydrophobic hydration in aqueous polymer solutions. Colloid Polym. Sci. 1994, 272, 1234–1239. [Google Scholar] [CrossRef]
- Lau, A.C.; Wu, C. Thermally sensitive and biocompatible poly(N-vinylcaprolactam): Synthesis and characterization of high molar mass linear chains. Macromolecules 1999, 32, 581–584. [Google Scholar] [CrossRef]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Hydration and phase behavior of poly(N-vinylcaprolactam) and poly(N-vinylpyrrolidone) in water. Macromolecules 2001, 35, 217–222. [Google Scholar] [CrossRef]
- Deng, J.; Shi, Y.; Jiang, W.; Peng, Y.; Lu, L.; Cai, Y. Facile synthesis and thermoresponsive behaviors of a well-defined pyrrolidone based hydrophilic polymer. Macromolecules 2008, 41, 3007–3014. [Google Scholar] [CrossRef]
- Lai, H.; Chen, G.; Wu, P.; Li, Z. Thermoresponsive behavior of an LCST-type polymer based on a pyrrolidone structure in aqueous solution. Soft Matter 2012, 8, 2662–2670. [Google Scholar] [CrossRef]
- González, N.; Elvira, C.; Román, J.S. Novel dual-stimuli-responsive polymers derived from ethylpyrrolidine. Macromolecules 2005, 38, 9298–9303. [Google Scholar] [CrossRef]
- Mertoglu, M.; Garnier, S.; Laschewsky, A.; Skrabania, K.; Storsberg, J. Stimuli responsive amphiphilic block copolymers for aqueous media synthesized via reversible addition fragmentation chain transfer polymerisation (RAFT). Polymer 2005, 46, 7726–7740. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo (ethylene glycol)(meth) acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Badi, N.; Lutz, J.F. PEG-based thermogels: Applicability in physiological media. J. Control. Release 2009, 140, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Cai, T.; Chi, C. Thermoresponsive oligo (ethylene glycol)-methacrylate-based polymers and microgels. Soft Matter 2010, 6, 2115–2123. [Google Scholar] [CrossRef]
- Wischerhoff, E.; Badi, N.; Laschewsky, A.; Lutz, J.F. Smart polymer surfaces: Concepts and applications in biosciences. Bioact. Surf. 2011, 240, 1–33. [Google Scholar] [CrossRef]
- Ishizone, T.; Seki, A.; Hagiwara, M.; Han, S.; Yokoyama, H.; Oyane, A.; Carlotti, S. Anionic polymerizations of oligo(ethylene glycol) alkyl ether methacrylates: Effect of side chain length and omega-alkyl group of side chain on cloud point in water. Macromolecules 2008, 41, 2963–2967. [Google Scholar] [CrossRef]
- Tasaki, K. Poly (oxyethylene)−water interactions: A molecular dynamics study. J. Am. Chem. Soc. 1996, 118, 8459–8469. [Google Scholar] [CrossRef]
- Dalgakiran, E.; Tatlipinar, H. The role of hydrophobic hydration in the LCST behaviour of POEGMA 300 by all-atom molecular dynamics simulations. Phys. Chem. Chem. Phys. 2018, 20, 15389–15399. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Fornal, K.; Raczkowska, J.; Zemla, J.; Kostruba, A.; Ohar, H.; Ohar, M.; Donchak, V.; Harhay, K.; Awsiuk, K.; et al. Temperature and pH dual-responsive POEGMA-based coatings for protein adsorption. J. Colloid Interface Sci. 2013, 411, 247–256. [Google Scholar] [CrossRef]
- Zhong, Q.; Metwalli, E.; Kaune, G.; Rawolle, M.; Bivigou-Koumba, A.M.; Laschewsky, A.; Papadakis, C.M.; Cubitt, R.; Mu, P. Switching kinetics of thin thermo-responsive hydrogel films of poly (monomethoxy-diethyleneglycol-acrylate) probed with in situ neutron reflectivity. Soft Matter 2012, 8, 5241–5249. [Google Scholar] [CrossRef]
- Zhong, Q.; Metwalli, E.; Rawolle, M.; Kaune, G.; Bivigou-Koumba, A.M.; Laschewsky, A.; Papadakis, C.M.; Cubitt, R.; Mu, P. Structure and Thermal Response of Thin Thermoresponsive Polystyrene-block-poly (methoxydiethylene glycol acrylate)-block-polystyrene Films. Macromolecules 2013, 46, 4069–4080. [Google Scholar] [CrossRef]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly (oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Zorn, A.M.; Keul, H.; Barner-Kowollik, C.; Moeller, M. Copolymers of 2-hydroxyethylacrylate and 2-methoxyethyl acrylate by nitroxide mediated polymerization: Kinetics, SEC-ESI-MS analysis and thermoresponsive properties. Polym. Chem. 2012, 3, 335–342. [Google Scholar] [CrossRef]
- Steinhauer, W.; Hoogenboom, R.; Keul, H.; Moeller, M. Copolymerization of 2-hydroxyethyl acrylate and 2-methoxyethyl acrylate via RAFT: Kinetics and thermoresponsive properties. Macromolecules 2010, 43, 7041–7047. [Google Scholar] [CrossRef]
- Hua, F.; Jiang, X.; Li, D.; Zhao, B. Well-defined thermosensitive, water-soluble polyacrylates and polystyrenics with short pendant oligo (ethylene glycol) groups synthesized by nitroxide-mediated radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2454–2467. [Google Scholar] [CrossRef]
- Miasnikova, A.; Laschewsky, A. Influencing the phase transition temperature of poly (methoxy diethylene glycol acrylate) by molar mass, end groups, and polymer architecture. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 3313–3323. [Google Scholar] [CrossRef]
- Zhong, Q.; Wang, W.; Adelsberger, J.; Golosova, A.; Koumba, A.M.B.; Laschewsky, A.; Funari, S.S.; Perlich, J.; Roth, S.V.; Papadakis, C.M.; et al. Collapse transition in thin films of poly (methoxydiethylenglycol acrylate). Colloid Polym. Sci. 2011, 289, 569–581. [Google Scholar] [CrossRef]
- Maeda, Y.; Yamauchi, H.; Kubota, T. Confocal micro-raman and infrared spectroscopic study on the phase separation of aqueous poly (2-(2-methoxyethoxy) ethyl (meth) acrylate) solutions. Langmuir 2009, 25, 479–482. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Luzon, M.; Davis, T.P. Design and synthesis of dual thermoresponsive and antifouling hybrid polymer/gold nanoparticles. Macromolecules 2009, 42, 6917–6926. [Google Scholar] [CrossRef]
- Popescu, D.; Hoogenboom, R.; Keul, H.; Moeller, M. Hydroxy functional acrylate and methacrylate monomers prepared via lipase—Catalyzed transacylation reactions. J. Mol. Catal. B Enzym. 2010, 62, 80–89. [Google Scholar] [CrossRef]
- Popescu, D.; Keul, H.; Moeller, M. Highly functional poly(meth)acrylates via cascade reaction. Macromol. Chem. Phys. 2009, 210, 123–139. [Google Scholar] [CrossRef]
- Jiang, X.; Lavender, C.A.; Woodcock, J.W.; Zhao, B. Multiple micellization and dissociation transitions of thermo-and light-sensitive poly (ethylene oxide)-b-poly (ethoxytri (ethylene glycol) acrylate-co-o-nitrobenzyl acrylate) in water. Macromolecules 2008, 41, 2632–2643. [Google Scholar] [CrossRef]
- Dimitrov, P.; Toncheva, N.; Weda, P.; Rangelov, S.; Trzebicka, B.; Dworak, A.; Tsvetanov, C.B. Nano-Templates from Thermoresponsive Poly(ethoxytriethyleneglycol acrylate) for Polymeric Nano-Capsules. Macromol. Symp. 2009, 278, 89–95. [Google Scholar] [CrossRef]
- Weaver, J.V.M.; Bannister, I.; Robinson, K.L.; Bories-Azeau, X.; Armes, S.P.; Smallridge, M.; McKenna, P. Stimulus-responsive water-soluble polymers based on 2-hydroxyethyl methacrylate. Macromolecules 2004, 37, 2395–2403. [Google Scholar] [CrossRef]
- Deng, K.; Tian, H.; Zhang, P.; Zhong, H.; Ren, X.; Wang, H. pH–temperature responsive poly (HPA-Co-AMHS) hydrogel as a potential drug-release carrier. J. Appl. Polym. Sci. 2009, 114, 176–184. [Google Scholar] [CrossRef]
- Taylor, L.D.; Cerankowski, L.D. Preparation of films exhibiting a balanced temperature dependence to permeation by aqueous solutions—A study of lower consolute behavior. J. Polym. Sci. Polym. Chem. Ed. 1975, 13, 2551–2570. [Google Scholar] [CrossRef]
- Eggenhuisen, T.M.; Becer, C.R.; Fijten, M.W.; Eckardt, R.; Hoogenboom, R.; Schubert, U.S. Libraries of statistical hydroxypropyl acrylate containing copolymers with LCST properties prepared by NMP. Macromolecules 2008, 41, 5132–5140. [Google Scholar] [CrossRef]
- Vo, C.D.; Rosselgong, J.; Armes, S.P.; Tirelli, N. Stimulus-responsive polymers based on 2-hydroxypropyl acrylate prepared by RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2032–2043. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Raczkowska, J.; Budkowski, A.; Kostruba, A.; Harhay, K.; Ohar, H.; Awsiuk, K.; Bernasik, A.; Ripak, N.; Zemła, J. Synthesis and postpolymerization modification of thermoresponsive coatings based on pentaerythritol monomethacrylate: Surface analysis, wettability, and protein adsorption. Langmuir 2015, 31, 9675–9683. [Google Scholar] [CrossRef]
- Schweigerdt, A.; Stöbener, D.D.; Schäfer, A.; Kara, S.; Weinhart, M. Impact of Amphiphilicity Balance in Hydroxy-Functional, Isomeric, Thermoresponsive Poly(meth)acrylates. Macromolecules 2023, 56, 8602–8613. [Google Scholar] [CrossRef]
- Inaba, N.; Takasu, K.; Matsuoka, K.; Sada, K. Thermal cleavage of hydrogen bond-induced LCST-type phase separation of PHEMA and related poly(hydroxyalkyl (meth) acrylates) in mixed organic solvents. Polym. Chem. 2024, 15, 2354–2361. [Google Scholar] [CrossRef]
- Chen, F.; Lu, G.; Yuan, H.; Li, R.; Nie, J.; Zhao, Y.; Shu, X.; Zhu, X. Mechanism and regulation of LCST behavior in poly(hydroxypropyl acrylate)-based temperature-sensitive hydrogels. J. Mater. Chem. A 2022, 10, 18235–18247. [Google Scholar] [CrossRef]
- Satoh, M.; Yoda, E.; Hayashi, T.; Komiyama, J. Potentiometric titration of poly(vinylpyridines) and hydrophobic interaction in the counterion binding. Macromolecules 1989, 22, 1808–1812. [Google Scholar] [CrossRef]
- Schlücker, S.; Singh, R.K.; Asthana, B.P.; Popp, J.; Kiefer, W. Hydrogen-bonded pyridine− water complexes studied by density functional theory and Raman spectroscopy. J. Phys. Chem. A 2001, 105, 9983–9989. [Google Scholar] [CrossRef]
- Sicilia, M.C.; Niño, A.; Muñoz-Caro, C. Mechanism of pyridine protonation in water clusters of increasing size. J. Phys. Chem. A 2005, 109, 8341–8347. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Yuen, C.W.; Chan, L. Novel moisture-sensitive shape memory polyurethanes containing pyridine moieties. Polymer 2009, 50, 4424–4428. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Yuen, C.W.; Chan, L. Fourier transform infrared study of supramolecular polyurethane networks containing pyridine moieties for shape memory materials. Polym. Int. 2009, 59, 529–538. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Zemła, J.; Kostruba, A.; Harhay, K.; Marzec, M.; Bernasik, A.; Lishchynskyi, O.; Ohar, H.; et al. Temperature-responsive properties of poly(4-vinylpyridine) coatings: Influence of temperature on the wettability, morphology, and protein adsorption. RSC Adv. 2016, 6, 87469–87477. [Google Scholar] [CrossRef]
- Nastyshyn, S.; Pop-Georgievski, O.; Stetsyshyn, Y.; Budkowski, A.; Raczkowska, J.; Hruby, M.; Lobaz, V. Protein corona of SiO2 nanoparticles with grafted thermoresponsive copolymers: Calorimetric insights on factors affecting entropy vs. enthalpy-driven associations. Appl. Surf. Sci. 2022, 601, 154201. [Google Scholar] [CrossRef]
- Stetsyshyn, Y.; Awsiuk, K.; Kusnezh, V.; Raczkowska, J.; Jany, B.R.; Kostruba, A.; Harhay, K.; Ohar, H.; Lishchynskyi, O.; Shymborska, Y.; et al. Shape-Controlled synthesis of silver nanoparticles in temperature-responsive grafted polymer brushes for optical applications. Appl. Surf. Sci. 2019, 463, 1124–1133. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Brzychczy-Włoch, M.; Gosiewski, T.; Jany, B.; Lishchynskyi, O.; Shymborska, Y.; Nastyshyn, S.; Bernasik, A.; et al. “Command” surfaces with thermo-switchable antibacterial activity. Mater. Sci. Eng. C 2019, 103, 109806. [Google Scholar] [CrossRef]
- Eliassaf, J.; Silberberg, A. The gelation of aqueous solutions of polymethacrylic acid. Polymer 1962, 3, 555–564. [Google Scholar] [CrossRef]
- Robin, C.; Lorthioir, C.; Fall, A.; Ovarlez, G.; Amiel, C.; Le Coeur, C. Unexpected Slow Kinetics of Poly (Methacrylic Acid) Phase Separation in the Semi-Dilute Regime. Polymers 2022, 14, 4708. [Google Scholar] [CrossRef] [PubMed]
- Benetti, E.M.; Reimhult, E.; de Bruin, J.; Zapotoczny, S.; Textor, M.; Vancso, G.J. Poly(methacrylic acid) grafts grown from designer surfaces: The effect of initiator coverage on polymerization kinetics, morphology, and properties. Macromolecules 2009, 42, 1640–1647. [Google Scholar] [CrossRef]
- Cazares-Cortes, E.; Baker, B.C.; Nishimori, K.; Ouchi, M.; Tournilhac, F. Polymethacrylic acid shows thermoresponsivity in an organic solvent. Macromolecules 2019, 52, 5995–6004. [Google Scholar] [CrossRef]
- Sakurai, M.; Imai, T.; Yamashita, F.; Nakamura, K.; Komatsu, T. Temperature dependence of viscosities and potentiometric titration behavior of aqueous poly (acrylic acid) and poly (methacrylic acid) solutions. Polym. J. 1993, 25, 1247–1255. [Google Scholar] [CrossRef]
- Lishchynskyi, O.; Tymetska, S.; Shymborska, Y.; Raczkowska, J.; Awsiuk, K.; Skirtach, A.; Korolko, S.; Chebotar, A.; Budkowski, A.; Stetsyshyn, Y. Temperature-responsive properties of pH-sensitive poly (methacrylic acid) grafted brush coatings with controlled wettability for cell culture. J. Mater. Chem. B 2025, 13, 3618–3632. [Google Scholar] [CrossRef]
- Deng, Y.; Käfer, F.; Chen, T.; Jin, Q.; Ji, J.; Agarwal, S. Let there be light: Polymeric micelles with upper critical solution temperature as light-triggered heat nanogenerators for combating drug-resistant cancer. Small 2018, 14, e1802420. [Google Scholar] [CrossRef]




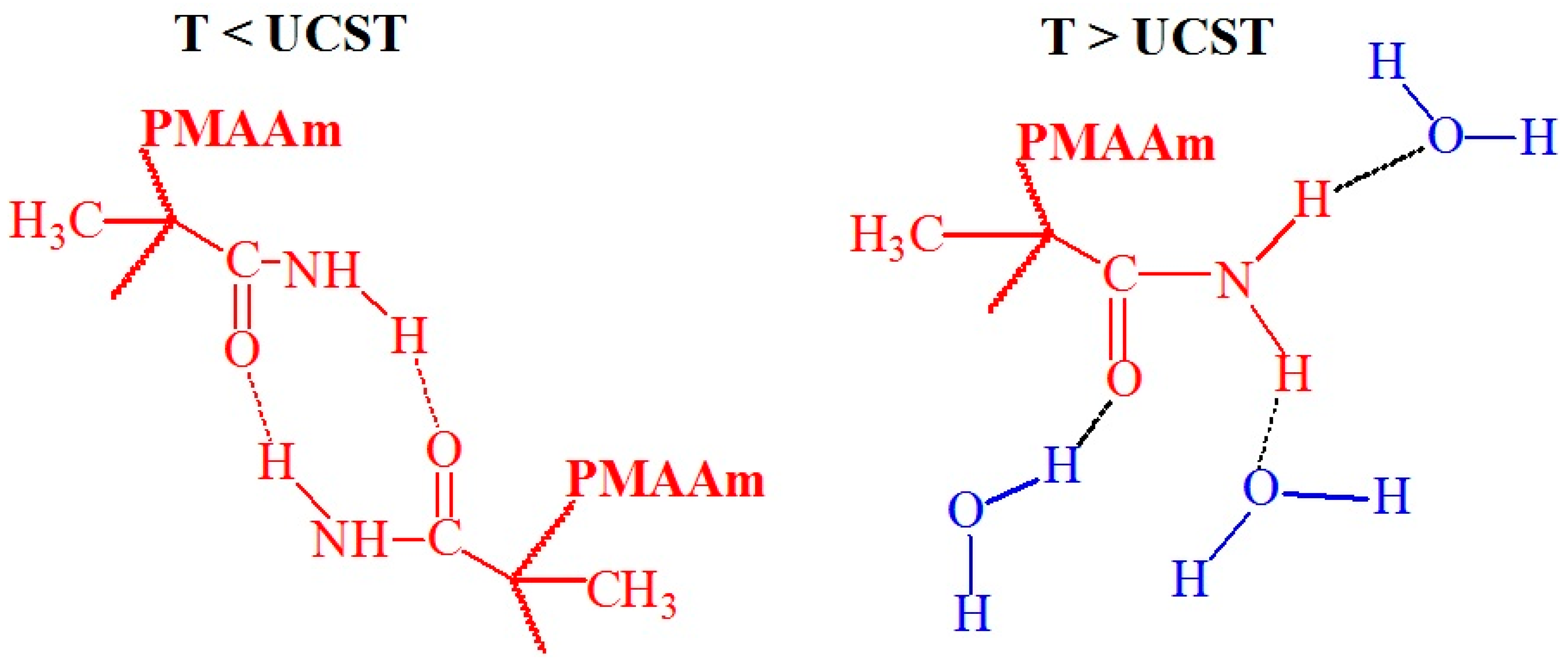

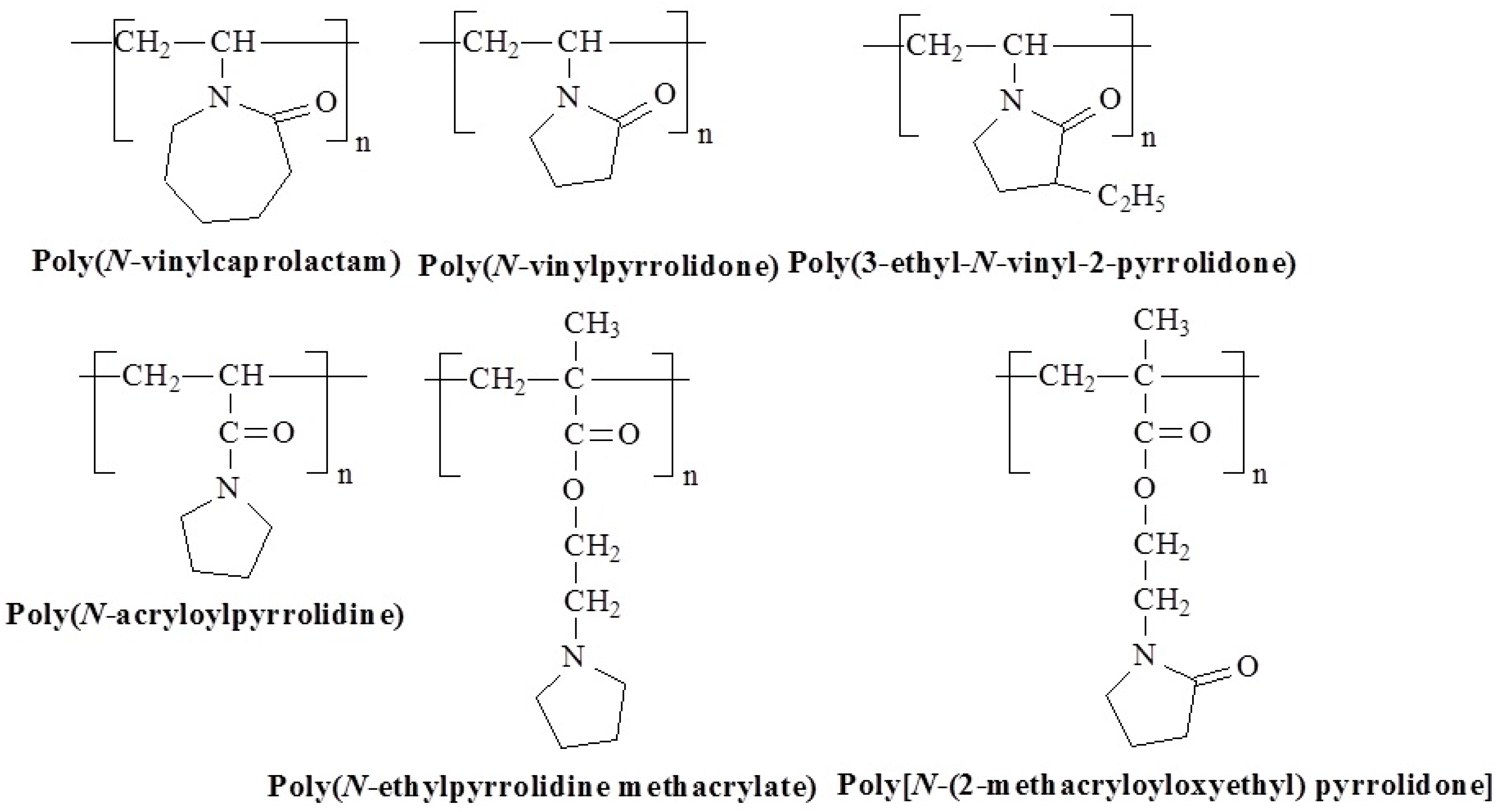
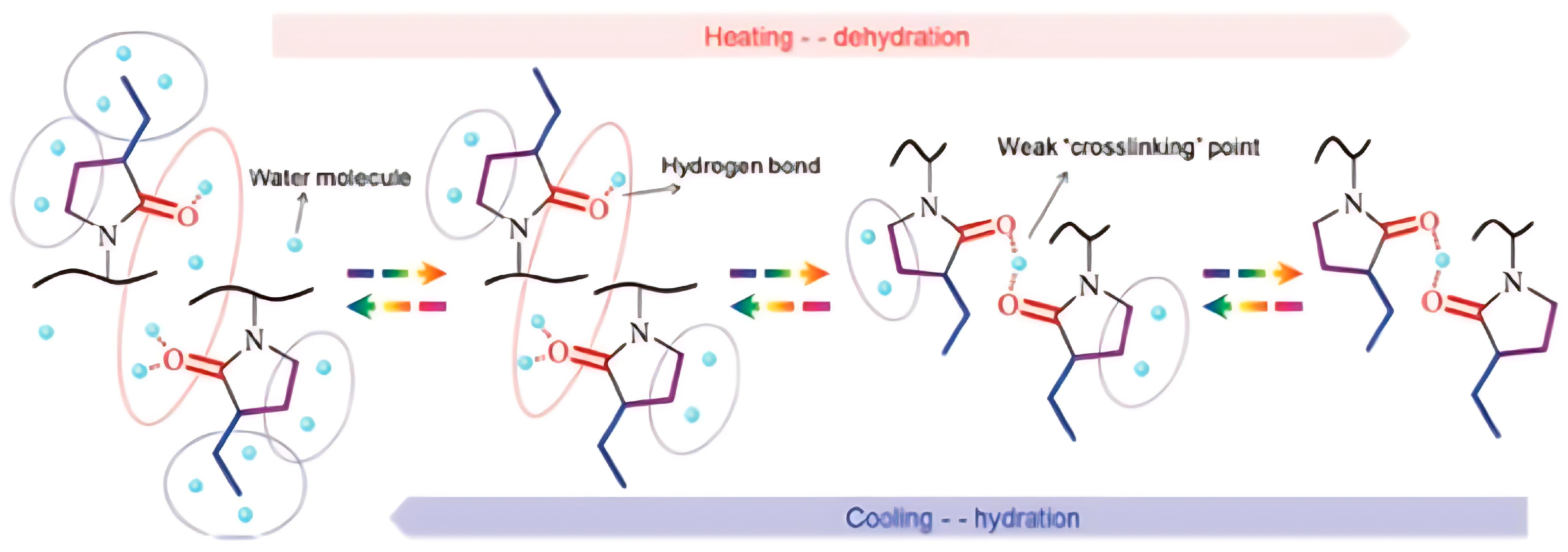
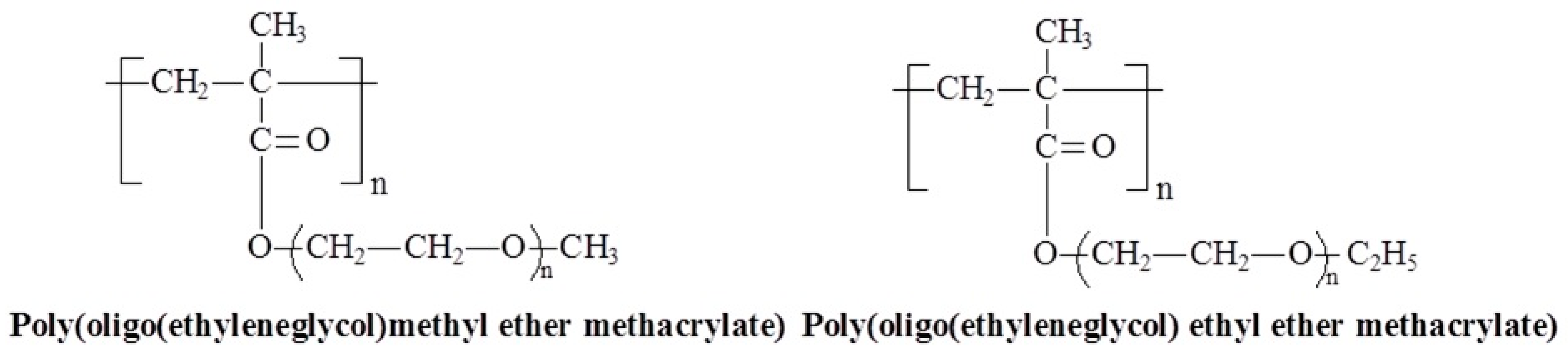


| Polymer Name | Substituted Groups | LCST [°C] | |
|---|---|---|---|
| R1 | R2 | ||
| Poly(N-alkylacrylamide)s | |||
| poly(N-methylacrylamide) | H | Me | soluble |
| poly(N-ethylacrylamide) | H | Et | 50 |
| poly(N-cyclopropylacrylamide) | H | cPr | 45 |
| poly(N-isopropylacrylamide) | H | iPr | 30–36 |
| poly(N-n-propylacrylamide) | H | nPr | 21–25 |
| poly(N-n-butylacrylamide) | H | nBu | insoluble |
| poly(N-isobutylacrylamide) | H | iBu | insoluble |
| poly(N-sec-butylacrylamide) | H | sBu | insoluble |
| poly(N-tert-butylacrylamide) | H | tBu | insoluble |
| Poly(N,N-dialkylacrylamide)s | |||
| poly(N,N-dimethylacrylamide) | Me | Me | soluble |
| poly(N-methyl-N-ethylacrylamide) | Me | Et | 56–70 |
| poly(N-methyl-N-isopropylacrylamide) | Me | iPr | 22 |
| poly(N-methyl-N-n-propylacrylamide) | Me | nPr | 20 |
| poly(N,N-diethylacrylamide) | Et | Et | 32 |
| poly(N-ethyl-N-isopropylacrylamide) | Et | iPr | insoluble |
| poly(N-ethyl-N-n-propylacrylamide) | Et | nPr | insoluble |
| poly(N,N-diisopropylacrylamide) | iPr | iPr | insoluble |
| poly(N,N-dipropylacrylamide) | nPr | nPr | insoluble |
| Polymer Name | Substituted Groups | LCST [°C] | |
|---|---|---|---|
| R1 | R2 | ||
| Poly(N-methylmethacrylamide) | H | Me | soluble |
| Poly(N-ethylmethacrylamide) | H | Et | soluble |
| Poly(N-cyclopropylmethacrylamide) | H | cPr | 59 |
| Poly(N-isopropylmethacrylamide) | H | iPr | 38, 43–45 |
| poly(N-n-propylmethacrylamide) | H | nPr | 28 |
| poly(N-n-butylmethacrylamide) | H | nBu | insoluble |
| poly(N-isobutylmethacrylamide) | H | iBu | insoluble |
| poly(N-sec-butylmethacrylamide) | H | sBu | insoluble |
| poly(N-tert-butylmethacrylamide) | H | tBu | insoluble |
| Polymer Name | R | Number Egus | Abbreviation | LCST [°C] |
|---|---|---|---|---|
| Poly(di(ethylene glycol)methyl ether methacrylate) | Me | 2 | POEG2MEMA | 22, 26 |
| Poly(oligo(ethyleneglycol)3methyl ether methacrylate | Me | 3 | POEG3MEMA | 52 |
| Poly(oligo(ethyleneglycol)4methyl ether methacrylate | Me | 4 | POEG4MEMA | 61, 68 |
| Poly(oligo(ethyleneglycol)8.5methyl ether methacrylate | Me | 8–9 | POEG8.5MEMA | 90 |
| Poly(oligo(ethyleneglycol)22methyl ether methacrylate | Me | 22 | POEG22MEMA | soluble |
| Poly(di(ethylene glycol)ethyl ether methacrylate) | Et | 2 | POEG2EEMA | 4 |
| Poly(oligo(ethyleneglycol)3ethyl ether methacrylate) | Et | 3 | POEG3EEMA | 26 |
| Poly(oligo(ethyleneglycol)4ethyl ether methacrylate | Et | 4 | POEG4EEMA | 42 |
| Polymer Name | R | Number Egus | Abbreviation | LCST [°C] |
|---|---|---|---|---|
| Poly(ethylene glycol)methyl ether acrylate) | Me | 1 | POEG1MEA | <0, 5 |
| Poly(di(ethylene glycol)methyl ether acrylate) | Me | 2 | POEG2MEA | 40 |
| Poly(oligo(ethyleneglycol)3methyl ether acrylate | Me | 3 | POEG3MEA | 70 |
| Poly(oligo(ethyleneglycol)8.5methyl ether acrylate | Me | 8–9 | POEG8.5MEA | 92 |
| Poly(di(ethylene glycol)ethyl ether acrylate) | Et | 2 | POEG2EEA | 10, 16.5 |
| Poly(oligo(ethyleneglycol)3ethyl ether acrylate) | Et | 3 | POEG3EEA | 34, 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stetsyshyn, Y.; Ohar, H.; Budkowski, A.; Lazzara, G. Molecular Design and Role of the Dynamic Hydrogen Bonds and Hydrophobic Interactions in Temperature-Switchable Polymers: From Understanding to Applications. Polymers 2025, 17, 1580. https://doi.org/10.3390/polym17111580
Stetsyshyn Y, Ohar H, Budkowski A, Lazzara G. Molecular Design and Role of the Dynamic Hydrogen Bonds and Hydrophobic Interactions in Temperature-Switchable Polymers: From Understanding to Applications. Polymers. 2025; 17(11):1580. https://doi.org/10.3390/polym17111580
Chicago/Turabian StyleStetsyshyn, Yurij, Halyna Ohar, Andrzej Budkowski, and Giuseppe Lazzara. 2025. "Molecular Design and Role of the Dynamic Hydrogen Bonds and Hydrophobic Interactions in Temperature-Switchable Polymers: From Understanding to Applications" Polymers 17, no. 11: 1580. https://doi.org/10.3390/polym17111580
APA StyleStetsyshyn, Y., Ohar, H., Budkowski, A., & Lazzara, G. (2025). Molecular Design and Role of the Dynamic Hydrogen Bonds and Hydrophobic Interactions in Temperature-Switchable Polymers: From Understanding to Applications. Polymers, 17(11), 1580. https://doi.org/10.3390/polym17111580









