Biopolymer/Suture Polymer Interaction: Is It a Key of Bioprosthetic Calcification?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
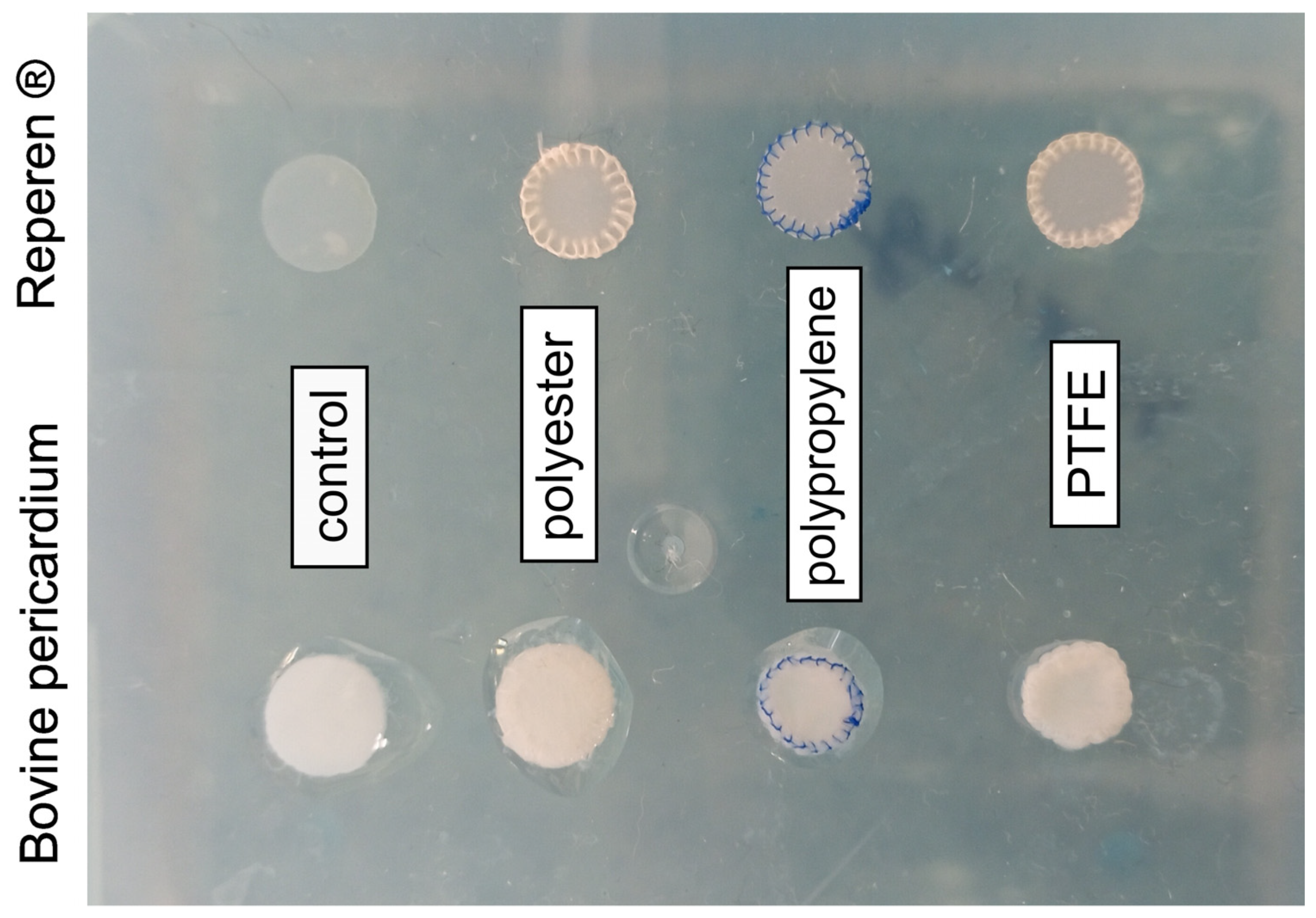
2.2. Subcutaneous Implantation in Rats
2.3. Histological Studies
2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectrometry (EDS) Analysis
2.5. Calcium Content Analysis
2.6. Statistical Analysis
3. Results
3.1. Calcium Content in Biomaterials
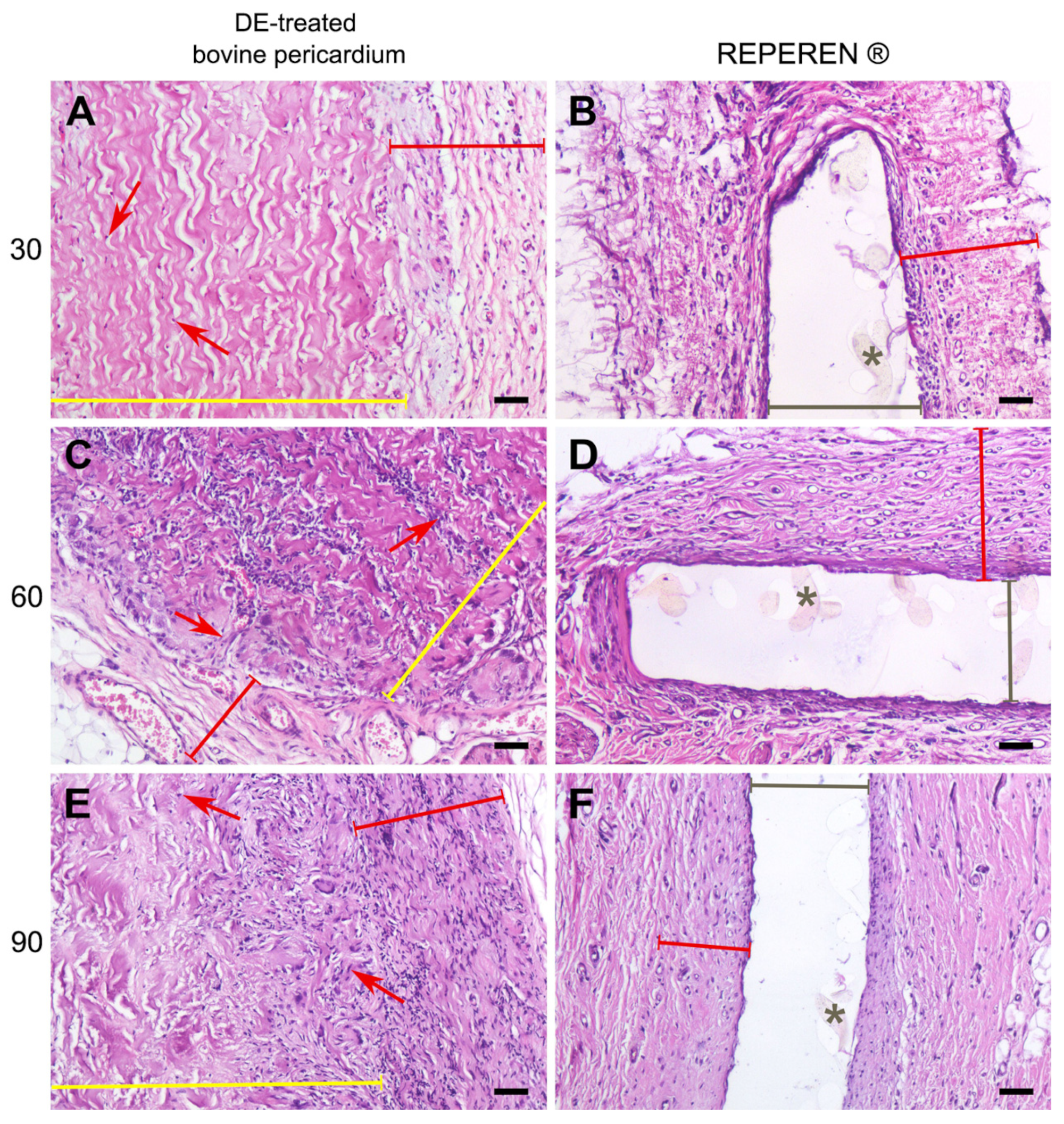
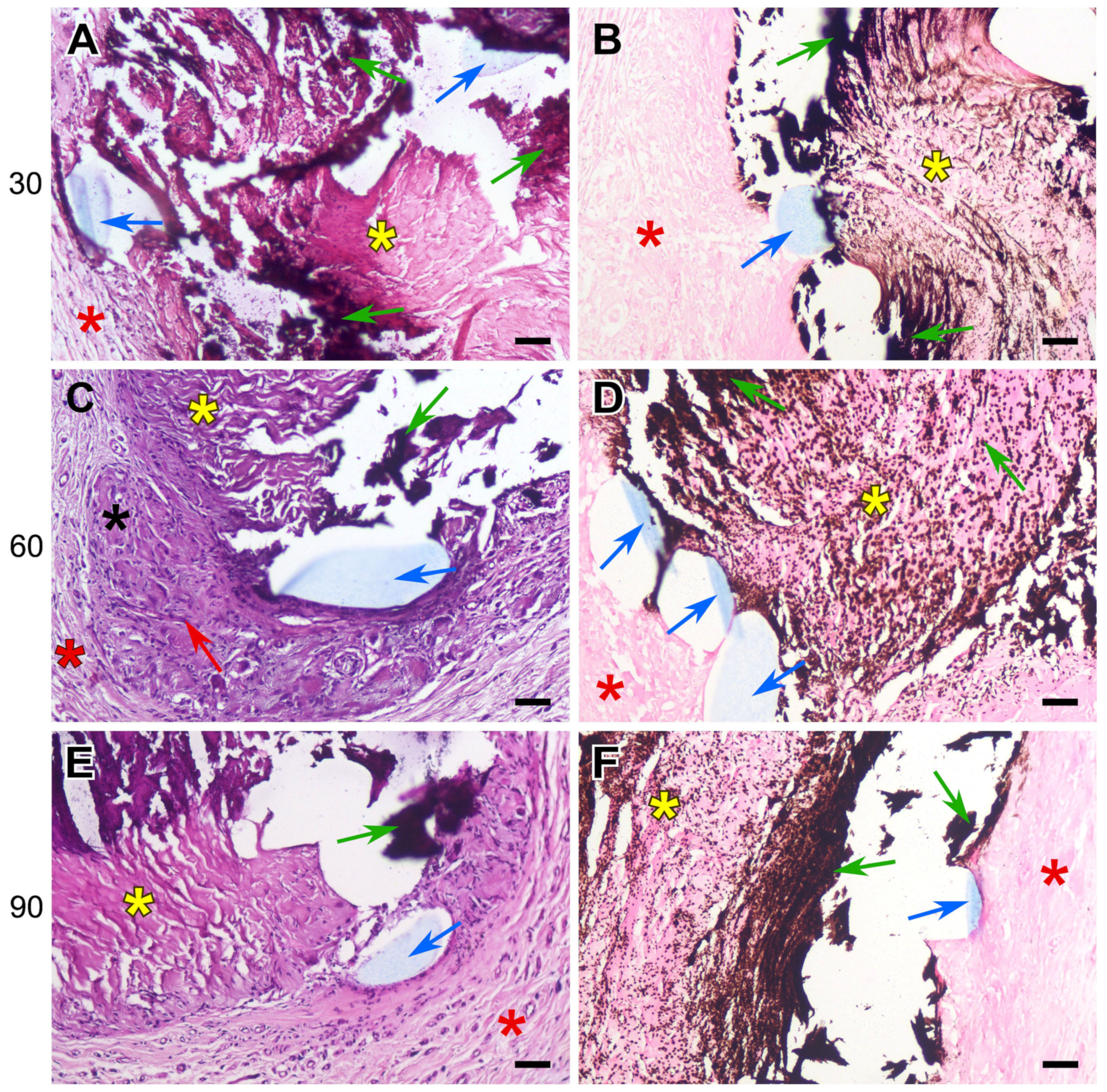
3.2. Histology Results
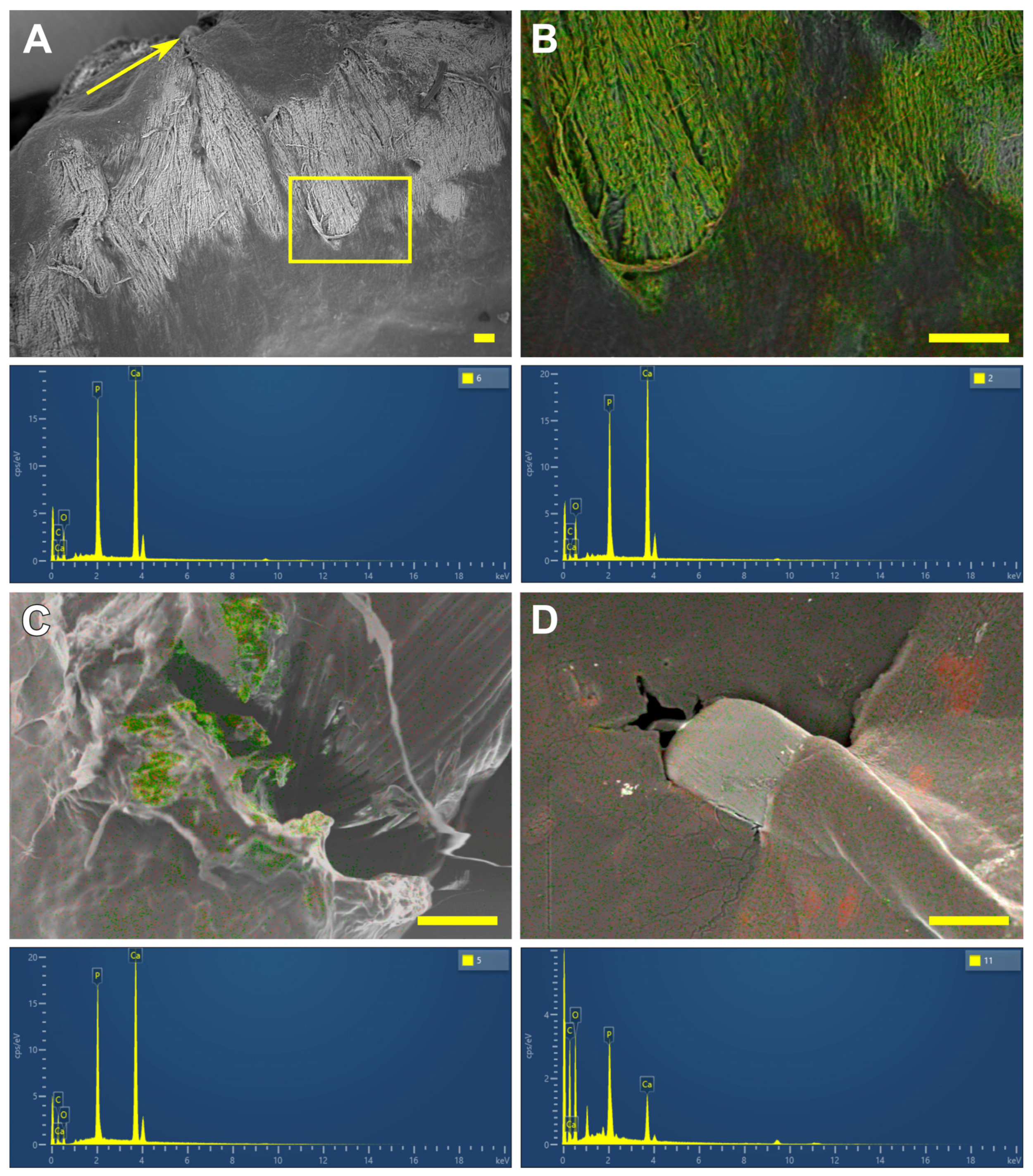
3.3. SEM and EDS Results
4. Discussion
5. Conclusions
- The DE-treated bovine pericardium subcutaneously implanted in rats exhibits a severe FBR without calcification. Any suture material in the implant intensifies the FBR, leading to the lysis and homogenization of collagen near the suture, followed by the calcification of these areas.
- The highest calcium content is found in these collagen implants, sutured with polypropylene. The use of polyester and polytetrafluorethylene allows us to obtain better results.
- Compared with xenogeneic collagen, the non-porous film made of the synthetic polymer REPEREN shows s very weak FBR and no calcification in both the control and sutured samples.
- Manufacturing cardiovascular collagenous bioprostheses with polyester suture material can be recommended; prostheses from REPEREN can be sutured with any of the three materials studied.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | bovine pericardium |
| FBR | foreign body reaction |
| FBGCs | foreign body giant cells |
| DE | ethylene glycol diglycidyl ether |
| PE | polyester |
| PP | polypropylene |
| PTFE | polytetrafluorethylene |
| SEM | scanning electron microscopy |
| EDS | energy dispersive spectrometry |
| Ca | calcium |
| P | phosphorus |
References
- Schoen, F.J.; Levy, R.J. Bioprosthetic Heart Valve Calcification: Clinicopathologic Correlations, Mechanisms, and Prevention. In Cardiovascular Calcification and Bone Mineralization; Aikawa, E., Hutcheson, J.D., Eds.; Humana Press: Totowa, NJ, USA; Springer Nature: Cham, Switzerland, 2020; pp. 183–218. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, Y.; Yim, W.Y.; Wang, S.; Xu, L.; Shi, J.; Qiao, W.; Dong, N. Mechanisms and Drug Therapies of Bioprosthetic Heart Valve Calcification. Front. Pharmacol. 2022, 13, 909801. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, A.B.; Chiu, P.; Baiocchi, M.; Lingala, B.; Patrick, W.L.; Fischbein, M.P.; Woo, Y.J. Mechanical or biologic prostheses for aortic-valve and mitral-valve replacement. N. Engl. J. Med. 2017, 377, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.R.; Pibarot, P. Prosthetic valves: Part 1–selection. e-J. Cardiol. Pract. 2021, 20. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-20/prosthetic-heart-valves-part-1-selection (accessed on 25 February 2025).
- Tod, T.J.; Dove, J.S. The association of bound aldehyde content with bioprosthetic 812 tissue calcification. J. Mater. Sci. Mater. Med. 2016, 27, 8. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of Bioprosthetic Heart Valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef]
- Guo, G.; Jin, L.; Jin, W.; Chen, L.; Lei, Y.; Wang, Y. Radical polymerization-crosslinking method for improving extracellular matrix stability in bioprosthetic heart valves with reduced potential for calcification and inflammatory response. Acta Biomater. 2018, 82, 44–55. [Google Scholar] [CrossRef]
- Guo, G.; Jin, L.; Wu, B.; He, H.; Yang, F.; Xu, L.; Lei, Y.; Wang, Y. A method for simultaneously crosslinking and functionalizing extracellular matrix-based biomaterials as bioprosthetic heart valves with enhanced endothelialization and reduced inflammation. Acta Biomater. 2021, 119, 89–100. [Google Scholar] [CrossRef]
- Melder, R.J.; Naso, F.; Nicotra, F.; Russo, L.; Vesely, I.; Tuladhar, S.R.; Calafiore, A.M.; Zilla, P.; Alessandro Gandaglia, A.; Korossis, S. Preventing extrinsic mechanisms of bioprosthetic degeneration using polyphenols. Eur. J. Cardiothorac. Surg. 2023, 63, ezac583. [Google Scholar] [CrossRef]
- Shibata, K.; Maeda, S.; Kashiyama, N.; Nakatsuji, H.; Ryugo, M.; Tsutsumi, Y.; Monta, O. Long term valve performance of St Jude Medical Epic porcine bioprosthesis in aortic position. J. Artif. Organs 2024, 27, 131–137. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, Y.; Wu, Y.; Wang, J.; Chen, M.; Feng, Y.; Chen, S.; Zhou, D.; Ge, J. Safety and effectiveness of the SAPIEN 3 transcatheter heart valve in the treatment of severe aortic stenosis: Early clinical outcomes of a multicenter study in China. Chin. J. Clin. Thorac. Cardiovasc. Surgery. 2022, 12, 553–559. [Google Scholar]
- neocor.ru. Available online: https://en.neocor.ru/quality-management (accessed on 25 February 2025).
- Nishi, C.; Nakajima, N.; Ikada, Y. In vitro evaluation of cytotoxicity of diepoxy compounds used for biomaterial modification. J. Biomed. Mater. Res. 1995, 29, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Noishiki, Y.; Miyata, T. Polyepoxy compound fixation. In Encyclopedia of Biomaterials and Biomedical Engineering; Wnek, G., Bowlin, G., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; Volume 1, pp. 1264–1273. [Google Scholar]
- Connolly, J.M.; Alferiev, I.; Clark-Grue, J.N.; Eidelman, N.; Sacks., M.; Palmatory, E.; Kronsteiner, A.; Defelice, S.; Xu, J.; Ohri, R.; et al. Triglycidylamine crosslinking of porcine aortic valve cusps or bovine pericardium results in improved biocompatibility, biomechanics, and calcification resistance: Chemical and biological mechanisms. Am. J. Pathol. 2005, 166, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.M.; Bakay, M.A.; Alferiev, I.S.; Gorman, R.G.; Gorman, J.H., 3rd; Kruth, H.S.; Ashworth, P.E.; Kutty, J.K.; Schoen, F.J.; Bianco, R.W.; et al. Triglycidyl amine crosslinking combined with ethanol inhibits bioprosthetic heart valve calcification. Ann. Thorac. Surg. 2011, 92, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, I.Y.; Karpova, E.V.; Oparina, L.A.; Poveschenko, O.V.; Surovtseva, M.A.; Titov, A.T.; Ksenofontov, A.L.; Vasilieva, M.B.; Kuznetsova, E.V.; Bogachev-Prokophiev, A.V.; et al. Cross-linking method using pentaepoxide for improving bovine and porcine bioprosthetic pericardia: A multiparametric assessment study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111473. [Google Scholar] [CrossRef]
- Zhuravleva, I.Y.; Karpova, E.V.; Dokuchaeva, A.A.; Titov, A.T.; Timchenko, T.P.; Vasilieva, M.B. Calcification of Various Bioprosthetic Materials in Rats: Is It Really Different? Int. J. Mol. Sci. 2023, 24, 7274. [Google Scholar] [CrossRef]
- Barbarash, O.; Rutkovskaya, N.; Hryachkova, O.; Gruzdeva, O.; Ponasenko, A.; Kondyukova, N.; Odarenko, Y.; Barbarash, L. Impact of recipient-related factors on structural dysfunction of xenoaortic bioprosthetic heart valves. Patient Prefer. Adherence 2015, 9, 389–399. [Google Scholar]
- Astapov, D.A.; Karaskov, A.M.; Semenova, E.I.; Demidov, D.P. The mithral valve replacement with biological prostheses: Early and long-term results. Khirurgiia 2013, 9, 18–23. (In Russian) [Google Scholar]
- Barbarash, L.; Rutkovskaya, N.; Barbarash, O.; Odarenko, Y.; Stasev, A.; Uchasova, E. Prosthetic heart valve selection in women of childbearing age with acquired heart disease: A case report. J. Med. Case Rep. 2016, 10, 51. [Google Scholar] [CrossRef]
- Nichay, N.R.; Zhuravleva, I.Y.; Kulyabin, Y.Y.; Timchenko, T.P.; Voitov, A.V.; Kuznetsova, E.V.; Soynov, I.A.; Zubritskiy, A.V.; Bogachev-Prokophiev, A.V.; Karaskov, A.M. In search of the best xenogeneic material for a paediatric conduit: An analysis of clinical data. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 34–41. [Google Scholar] [CrossRef]
- Zhuravleva, I.Y.; Nichay, N.R.; Kulyabin, Y.Y.; Timchenko, T.P.; Korobeinikov, A.A.; Polienko, Y.F.; Shatskaya, S.S.; Kuznetsova, E.V.; Voitov, A.V.; Bogachev-Prokophiev, A.V.; et al. In search of the best xenogeneic material for a paediatric conduit: An experimental study. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 738–744. [Google Scholar] [CrossRef]
- Singla, A.; Lee, C.H. Effect of elastin on the calcification rate of collagen-elastin matrix systems. J. Biomed. Mater. Res. 2002, 60, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Rucker, R.B. Calcium Binding to Elastin Protein-Metal Interactions, Advances. In Experimental Medicine and Biology; Friedman, M., Ed.; Springer: Boston, MA, USA, 1974; pp. 185–209. [Google Scholar] [CrossRef]
- Karaskov, A.; Bogachev-Prokophiev, A.; Sharifulin, R.; Zheleznev, S.; Demin, I.; Pivkin, A.; Zhuravleva, I. Right Ventricular Outflow Tract Replacement with Xenografts in Ross Patients Older Than 60 Years. Ann. Thorac. Surg. 2016, 101, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Karaskov, A.; Sharifulin, R.; Zheleznev, S.; Demin, I.; Lenko, E.; Bogachev-Prokophiev, A. Results of the Ross procedure in adults: A single-centre experience of 741 operations. Eur. J. Cardiothorac. Surg. 2016, 49, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Nichay, N.R.; Zhuravleva, I.Y.; Kulyabin, Y.Y.; Zubritskiy, A.V.; Voitov, A.V.; Soynov, I.A.; Gorbatykh, A.V.; Bogachev-Prokophiev, A.V.; Karaskov, A.M. Diepoxy- Versus Glutaraldehyde-Treated Xenografts: Outcomes of Right Ventricular Outflow Tract Reconstruction in Children. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 56–64. [Google Scholar] [CrossRef]
- Sharifulin, R.; Bogachev-Prokophiev, A.; Demin, I.; Afanasyev, A.; Ovcharov, M.; Pivkin, A.; Sapegin, A.; Zhuravleva, I.; Karaskov, A. Allografts and xenografts for right ventricular outflow tract reconstruction in Ross patients. Eur. J. Cardiothorac. Surg. 2021, 59, 162–169. [Google Scholar] [CrossRef]
- Zhuravleva, I.Y. Biocidal Composition for Aseptic Storage of Preserved Prosthetic Material from Tissues of Animal Origin. Patent RU2580621C1, 10 April 2016. Available online: https://patents.google.com/patent/RU2580621C1/en (accessed on 25 February 2025).
- Zhuravleva, I.Y.; Dokuchaeva, A.A.; Vaver, A.A.; Kreiker, L.V.; Mochalova, A.B.; Chepeleva, E.V.; Surovtseva, M.A.; Kolodin, A.N.; Kuznetsova, E.V.; Grek, R.I. A Novel Polymer Film to Develop Heart Valve Prostheses. Polymers 2024, 16, 3373. [Google Scholar] [CrossRef]
- Schoen, F.J.; Levy, R.J.; Nelson, A.C.; Bernhard, W.F.; Nashef, A.; Hawley, M. Onset and progression of experimental bioprosthetic heart valve calcification. Lab. Investig. 1985, 52, 523–532. [Google Scholar]
- Kastellorizios, M.; Tipnis, N.; Burgess, D.J. Foreign Body Reaction to Subcutaneous Implants. Adv. Exp. Med. Biol. 2015, 865, 93–108. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Kim, H.J.; Zheng, C.; Harkins, L.; Tao, W.; Deschenes, E. Foreign body response to synthetic polymer biomaterials and the role of adaptive immunity. Biomed. Mater. 2022, 17, 022007. [Google Scholar] [CrossRef]
- Milhomem, A.C.; Gomes, R.S.; Tomé, F.D.; Dos Santos Arruda, F.; Franco, P.I.R.; da Costa, E.L.; Pereira, J.X.; Vinaud, M.C.; de Souza Lino Júnior, R. Polymethylmethacrylate Microspheres are Immunologically Inert in Mouse Tissues. Aesthetic Plast. Surg. 2023, 47, 2813–2822. [Google Scholar] [CrossRef]
- Borchiellini, P.; Rames, A.; Roubertie, F.; L’Heureux, N.; Kawecki, F. Development and characterization of biological sutures made of cell-assembled extracellular matrix. Biofabrication 2023, 15, 045018. [Google Scholar] [CrossRef] [PubMed]
- Gersak, B. Presence of calcium in the vessel walls after end-to-end arterial anastomoses with polydioxanone and polypropylene sutures in growing dogs. J. Thorac. Cardiovasc. Surg. 1993, 106, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Stacey-Clear, A.; McCarthy, K.A.; Hall, D.A.; Pile-Spellman, E.R.; Mrose, H.E.; White, G.; Cardenosa, G.; Sawicka, J.; Mahoney, E.; Kopans, D.B. Calcified suture material in the breast after radiation therapy. Radiology 1992, 183, 207–208. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, D.C.; Fomina, A.; Uiterwijk, M.; Hooijmans, C.R.; Akiva, A.; Kluin, J.; Bouten, C.V.C.; Smits, A.I.P.M. Calcification in Pulmonary Heart Valve Tissue Engineering: A Systematic Review and Meta-Analysis of Large-Animal Studies. JACC Basic. Transl. Sci. 2023, 8, 572–591. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.; Cahalane, R.; Hengst, R.; Akyildiz, A.; Farrell, E.; Gijsen, F.; Aikawa, E.; van der Heiden, K.; Wissing, T. The interplay of collagen, macrophages, and microcalcification in atherosclerotic plaque cap rupture mechanics. Basic. Res. Cardiol. 2024, 119, 193–213. [Google Scholar] [CrossRef]
- Han, X.; Gao, C.; Lu, W.; Yan, J.; Xu, H.; Guo, Z.; Qin, W.; Lu, N.; Gao, J.; Zhu, W.; et al. Macrophage-Derived Extracellular DNA Initiates Heterotopic Ossification. Inflammation 2023, 46, 2225–2240. [Google Scholar] [CrossRef]
- Jo, J.; Acharya, M.; KC, P.B.; Maharjan, A.; Lee, D.; Gautam, R.; Kwon, J.T.; Kim, K.; Kim, C.; Heo, Y.; et al. Immunodysregulatory potentials of polyethylene or polytetrafluorethylene microplastics to mice subacutely exposed via intragastric intubation. Toxicol. Res. 2023, 39, 419–427. [Google Scholar] [CrossRef]
- Fischer, F.C.; Ludtke, S.; Thackray, C.; Pickard, H.M.; Haque, F.; Dassuncao, C.; Endo, S.; Schaider, L.; Sunderland, E.M. Binding of Per- and Polyfluoroalkyl Substances (PFAS) to Serum Proteins: Implications for Toxicokinetics in Humans. Environ. Sci. Technol. 2024, 58, 1055–1063. [Google Scholar] [CrossRef]
- Meneguzzi, A.; Fava, C.; Castelli, M.; Minuz, P. Exposure to Perfluoroalkyl Chemicals and Cardiovascular Disease: Experimental and Epidemiological Evidence. Front. Endocrinol. 2021, 12, 706352. [Google Scholar] [CrossRef]
- Lee, S.; Kang, K.-K.; Sung, S.-E.; Choi, J.-H.; Sung, M.; Seong, K.-Y.; Lee, J.; Kang, S.; Yang, S.Y.; Lee, S.; et al. In Vivo Toxicity and Pharmacokinetics of Polytetrafluoroethylene Microplastics in ICR Mice. Polymers 2022, 14, 2220. [Google Scholar] [CrossRef]





| Material Combinations | Localization | Structure (Min and Max Ca/P Ratio) | Ca/P Ratio | |
|---|---|---|---|---|
| Sample Material | Suture Material | |||
| 30 days | ||||
| DE-treated bovine pericardium | none (control) | - | - | - |
| polyester | near the ligature | “mosaic” (from 0.17 to 1.68) | 0.93 (0.58; 1.26) | |
| polypropylene | near the ligature | “mosaic” (from 0.32 to 9.42) | 1.30 (1.20; 1.48) | |
| PTFE | near the ligature | “mosaic” (from 0.29 to 11.41) | 1.57 (1.41; 2.73) | |
| 60 days | ||||
| DE-treated bovine pericardium | none (control) | - | - | - |
| polyester | inward deposit growth | “mosaic” (from 0.24 to 13.49) | 1.48 (1.30; 2.77) | |
| polypropylene | inward deposit growth | “mosaic” (from 0.74 to 3.39) | 1.37 (1.23; 1.50) | |
| PTFE | in width and depth of the implant | homogenous | 1.70 (1.62; 1.82) | |
| 90 days | ||||
| DE-treated bovine pericardium | none (control) | - | - | - |
| polyester | in deep layers | homogenous | 1.69 (1.62; 1.72) | |
| polypropylene | in deep layers | “mosaic” (from 0.98 to 4.14) | 1.40 (1.31; 1.55) | |
| PTFE | in deep layers | homogenous | 1.68 (1.65; 1.69) | |
| REPEREN® | none (control) | - | - | - |
| polyester | connective tissue capsule | P predominance | 0.60 (0.51; 0.67) | |
| polypropylene | connective tissue capsule | P predominance | 0.21 (0.14; 0.35) | |
| PTFE | connective tissue capsule | P predominance | 0.61 (0.49; 0.63) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuravleva, I.Y.; Dokuchaeva, A.A.; Vaver, A.A.; Kreiker, L.V.; Kuznetsova, E.V.; Grek, R.I. Biopolymer/Suture Polymer Interaction: Is It a Key of Bioprosthetic Calcification? Polymers 2025, 17, 1576. https://doi.org/10.3390/polym17111576
Zhuravleva IY, Dokuchaeva AA, Vaver AA, Kreiker LV, Kuznetsova EV, Grek RI. Biopolymer/Suture Polymer Interaction: Is It a Key of Bioprosthetic Calcification? Polymers. 2025; 17(11):1576. https://doi.org/10.3390/polym17111576
Chicago/Turabian StyleZhuravleva, Irina Yu., Anna A. Dokuchaeva, Andrey A. Vaver, Ludmila V. Kreiker, Elena V. Kuznetsova, and Rostislav I. Grek. 2025. "Biopolymer/Suture Polymer Interaction: Is It a Key of Bioprosthetic Calcification?" Polymers 17, no. 11: 1576. https://doi.org/10.3390/polym17111576
APA StyleZhuravleva, I. Y., Dokuchaeva, A. A., Vaver, A. A., Kreiker, L. V., Kuznetsova, E. V., & Grek, R. I. (2025). Biopolymer/Suture Polymer Interaction: Is It a Key of Bioprosthetic Calcification? Polymers, 17(11), 1576. https://doi.org/10.3390/polym17111576






