Abstract
Fluorinated polyimide (FPI), renowned for its exceptional low-dielectric properties, colorless transparency, high-temperature resistance, and flexibility, has emerged as an ideal material for addressing challenges in 5G/6G high-frequency signal transmission and flexible electronic substrates. Nevertheless, the structure–property relationship between molecular architectures and the dielectric characteristics of FPI films remains insufficiently understood, necessitating urgent elucidation of the underlying mechanisms. In this study, a diamine monomer containing bis-amide bonds, 4-amino-N-{4-[(4-aminobenzoyl)amino]phenyl}benzamide (PABA), was synthesized. Subsequently, six FPI films (FPAIs, FPEIs, and FPEsIs) with distinct structural features were prepared through homopolymerization of PABA and five other diamines (containing amide bonds, ether, and ester groups) with fluorinated dianhydride (6FDA). Systematic characterization of thermal, mechanical, optical, and dielectric properties revealed that these films exhibit excellent thermal stability (Tg: 296–388 °C), mechanical strength (σ: 152.5–248.1 MPa, E: 2.1–3.4 GPa), and optical transparency (T550 nm: 82–86%). Notably, they demonstrated a low dielectric constant (Dk as low as 2.8) and dielectric loss (Df down to 0.002) under both low- and high-frequency electric fields. Furthermore, molecular dynamics simulations and quantum chemical were employed to calculate critical physical parameters and HOMO–LUMO energy levels of the six FPIs. This computational analysis provides deeper insights into the structure–performance correlations governing dielectric behavior and optical transparency in FPIs. The findings establish valuable theoretical guidance for designing advanced PI films with tailored dielectric properties and high transparency.
1. Introduction
With the rapid advancement of artificial intelligence, the Internet of Things (IoT) [1], and 5G/6G communication technologies [2,3,4], the demand for interlayer insulation materials in electronic devices continues to grow. Simultaneously, as large-scale integrated circuits evolve toward miniaturization, high integration, and flexibility, there is an increasing need to enhance signal transmission speed [5,6,7]. Due to its outstanding comprehensive properties, including excellent thermal stability, chemical stability, and mechanical performance, as well as its favorable structural design and formability, PI has found widespread application in fields such as electrical insulation, microelectronics, flexible substrates, and aerospace [8,9,10]. Conventional PI films typically exhibit a dielectric constant (Dk) of approximately 3.5 and a dielectric loss (Df) of around 0.01 [11,12,13]. However, the demands of high integration and high-speed signal transmission often lead to increased operating temperatures and greater signal losses, thereby affecting device performance and longevity [14,15]. To ensure fast and stable signal transmission, insulation materials must not only possess good mechanical and thermal properties but also exhibit low Dk and Df values [16,17,18]. Consequently, the design and synthesis of next-generation low-dielectric PI materials have become a key research focus in microelectronics and materials science.
Fluorine (F) modification is an effective approach to lowering the Dk of PI [19,20,21]. The strong electron-withdrawing ability of F (with the highest electronegativity of 4.0) allows it to capture electrons and exhibit weak polarization tendencies. Incorporating F into the PI molecular structure effectively reduces molecular polarization [22]. Additionally, F disrupts the structural regularity of polymer chains and increases the free volume, further reducing the number of polarizable molecular units per unit volume [23]. Moreover, F incorporation significantly lowers the water absorption of the polymer, thereby enhancing dielectric stability. Typically, F is introduced into the PI structure via two primary substituents: F and trifluoromethyl (CF3), leading to the synthesis of FPI. Ando et al. reported a strategy involving the substitution of benzene ring hydrogen atoms with F, leading to a series of FPIs with Dk values as low as 2.73 at 10 GHz (23 ± 1 °C, 30% RH) [24]. Scola et al. synthesized FPI films by introducing perfluorinated benzene side groups into dianhydrides, achieving a Dk as low as 2.01 at 1 kHz (40–60% RH) [25]. Comparatively, most FPIs incorporate F into the PI backbone via the “CF3” functional group. Huang et al. developed a novel perfluorocyclobutyl-phenyl-ether-based PI, which exhibited exceptional moisture resistance and maintained ultra-low-dielectric stability (Dk < 2.5) even under prolonged high-humidity conditions [26]. Li et al. synthesized three FPIs via the homopolymerization of 6FDA with three different CF3-substituted diamines, all of which demonstrated ultra-low Dk values (~1.84 at 104 Hz) [27]. Zhang et al. introduced a rigid non-planar fluorenyl diamine with a CF3-phenyl group into the PI main chain, reducing molecular chain polarization and increasing free volume, yielding an FPI film with an ultra-low Dk (~1.93) [28]. Chen et al. and our research group investigated the correlation between F content (F%) and Dk in PI, revealing a strong correlation coefficient of 0.98 [29]. Overall, fluorination is an effective strategy for reducing Dk; however, it is noteworthy that an optimal F% range of 25–37% is critical, as excessive F incorporation beyond this range has minimal impact on further Dk reduction.
The Df in polymers primarily originates from polarization loss and conduction loss. Polarization loss occurs during the polarization and depolarization processes of polymer dielectrics. At high external electric field frequencies, the internal polarization process of the dielectric cannot synchronize with the field variations, resulting in hysteresis effects and polarization loss. Conduction loss, on the other hand, is induced by leakage currents and can be classified into bulk-limited conduction and electrode-limited conduction. In the low-frequency range, dipoles within the material have sufficient response time, making conduction loss dominant and largely independent of frequency. However, under high-frequency electric fields, the dipole response time shortens, leading to a significant increase in polarization loss. At this stage, the Df is jointly determined by conduction and polarization losses and increases with frequency. Liu et al. incorporated a rigid aromatic ester dianhydride monomer (TAHQ, terephthalate-based dianhydride) into the PI backbone and optimized the imidization process to induce liquid crystalline structures, ultimately obtaining liquid crystalline PI films with extremely low Df values (0.00365 at 1 MHz, 0.00184 at 10 GHz) [30]. Our research group has previously reported the introduction of ether and ester groups into PI structures to significantly reduce the Df by modulating polymer orientation, crystallinity, and the rigidity–flexibility balance [31,32,33]. While FPI exhibits substantial advantages in reducing Dk, its Df behavior differs, showing a weaker correlation with F% but a stronger dependence on other structural factors. To meet the requirements of high-speed data transmission in 5G/6G high-frequency communications, a more comprehensive understanding of the structure–property relationships governing Df and Dk in FPI films is urgently needed.
In this study, we synthesized a diamine monomer, 4-amino-N-{4-[(4-aminobenzoyl)amino]phenyl}benzamide (PABA), and subsequently homopolymerized it with six different diamines containing amide, ester, or ether linkages using 4,4′-(hexafluoroisopropylidene)phthalic anhydride (6FDA) as the dianhydride monomer. This process resulted in the fabrication of six FPIs (FPAIs, FPEIs, and FPEsIs) with distinct structural characteristics. A comprehensive characterization of these films demonstrated excellent thermal, mechanical, and optical properties. Furthermore, we systematically investigated their dielectric properties, including Dk and Df, under low- and high-frequency conditions and various environmental settings. Finally, molecular dynamics (MD) simulations and quantum chemical calculation were employed to elucidate the structure–property relationships between different functional groups and the dielectric and optical performance of these FPIs. The findings of this study provide valuable insights for the rational design and synthesis of low-Dk, low-Df, and highly transparent PI materials.
2. Materials and Methods
2.1. Materials
4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA), 4,4′-diaminobenzanilide (DABA), 4,4′-oxydianiline (ODA), p-phenylenediamine (PDA) 1,4-bis(4-aminophenoxy)benzene (TPE), p-aminophenyl p-aminobenzoate (APAB), [4-(4-aminobenzoyl)oxyphenyl] 4-aminobenzoate (ABHQ), and bis(4-aminophenyl) terephthalate (BATP) were purchased from Tianjin Zhongtai Chemical Technology Co., Ltd. (Tianjin, China) with purity > 99.5%. N, N-dimethylformamide (DMF), and N, N-Dimethylacetamide (DMAc) was obtained from J&K Scientific Ltd. (Beijing, China) (purity 99.8%, water content < 50 ppm). Deuterated dimethyl sulfoxide (DMSO-d6), tetrahydrofuran (THF), pyridine (99.5%), palladium on carbon (Pd/C, 10%), 4-nitrobenzoyl chloride (99.8%), anhydrous ethanol (EtOH), and petroleum ether (PE) were sourced from Adamas Reagent Co., Ltd. (Shanghai, China). All reagents were used as received without further purification.
2.2. Characterization
The characterization of nuclear magnetic resonance hydrogen spectra (1H NMR) was conducted using a Bruker AVANCE III HD 600 MHz spectrometer (Ettlingen, Germany). A sample of 20–40 mg was weighed into a tube, dissolved in 0.5 mL of deuterated solvent (DMSO-d6), and subsequently analyzed. The Fourier transform infrared (FT-IR) spectra of the PI films were obtained using a Thermo Fisher IS-5 spectrometer (Waltham, WA, USA) in transmission mode. The samples were directly placed on a barium fluoride (BaF2) substrate and measured under ambient dry conditions within a spectral range of 4000–500 cm−1, with 16 scans per measurement. The molecular weight of the poly(amic acid) (PAA) precursor of PI was determined using a TOSOH HLC-8320 gel permeation chromatography (GPC) system (Tokyo, Japan). Polystyrene (PS) was used as the calibration standard, and chromatography-grade DMAc containing lithium bromide (0.03 M) and phosphoric acid (0.03 M) was used as the eluent. The glass transition temperature (Tg) of the films was measured using a TA Instruments Q800 dynamic mechanical analyzer (DMA) (New Castle, DE, USA). The films were cut into rectangular strips (3.5 mm in width) using a cutter, and the test was conducted in dynamic tensile mode at a heating rate of 5 °C/min over a temperature range of 30–400 °C, with a frequency of 1 Hz under a nitrogen atmosphere. The coefficient of thermal expansion (CTE) of the films was determined using a TA Instruments Q400 thermomechanical analyzer (TMA) (USA). In tensile mode, the sample was first heated to 250 °C (below its Tg) to eliminate thermal history. The measurement was then performed under a nitrogen atmosphere at a heating rate of 5 °C/min over a temperature range of 25–400 °C, with a fixed load of 0.005 N. The thermal stability of the films was analyzed using a PerkinElmer Pyris Discovery 550 thermogravimetric analyzer (TGA) (Shelton, CT, USA). Approximately 10 mg of each sample was tested under a nitrogen atmosphere. Initially, the sample was heated at a rate of 20 °C/min to 110 °C and held for 20 min to remove moisture. The temperature was then lowered to 50 °C before a second heating cycle was performed at a rate of 10 °C/min within a temperature range of 50–800 °C. The mechanical properties of the films were evaluated using a Zhuhai Sansi Taijie electronic universal testing machine (Zhuhai, China). The tensile properties were measured according to the ASTM standard, with a tensile rate of 5 mm/min. The films were cut into rectangular strips (10 mm in width) before testing. At least six specimens were tested for each sample, and the average value was reported as the final result. The high-frequency dielectric properties of the films were measured at 25 °C and 40% relative humidity using a Keysight PNA N5227B vector network analyzer (Santa Rosa, CA, USA). The split cylindrical resonator method was employed, utilizing a resonant cavity fixture (Keysight 85072A) with the TE011 resonant mode to determine the Dk and Df at frequencies of 10, 40, and 60 GHz. The low-frequency dielectric properties of the films were assessed at 25 °C and 40% relative humidity (RH). The film thickness was first measured using a micrometer with a precision of 0.001 mm, followed by silver sputtering on both sides as a pre-treatment. Subsequently, the Dk and Df were measured over a frequency range of 102–106 Hz using an Agilent E4980A precision LCR meter (Santa Clara, CA, USA). The optical transmittance and cutoff wavelength of the films were determined using a Shimadzu UV-2600 spectrophotometer (Kyoto, Japan) in transmission mode. The films were directly affixed to the detection window for measurement over a wavelength range of 200–800 nm, with a film thickness of 30–50 μm. The colorimetric values of the PI films were measured using a PerkinElmer Lambda 950 UV/Vis/NIR spectrophotometer (USA). The CIELAB and Yellowness Index (YI) values were obtained under a standard CIE D65 light source with a 10° observer angle. During moisture uptake evaluations, FPI films preconditioned at 80 °C for two hours were submerged in 25 °C deionized water for 48 h. Following immersion, surface moisture was removed from the retrieved specimens using absorbent paper, after which their weights were promptly recorded. The percentage of water absorption (A) was determined through the equation: , where m1 and m2 represent the initial and post-hydration mass of the films, respectively.
2.3. Monomer Synthesis
Synthesis of N, N′-(1,4-phenylene)bis(4-nitrobenzamide) (PNBA). Under a nitrogen atmosphere, 4-nitrobenzoyl chloride was dissolved in anhydrous THF (48 mL) in a 250 mL three-necked round-bottom flask. In a separate single-necked flask, p-PDA (3.3 g, 30.4 mmol) and pyridine (8.4 mL) were dissolved in THF (54 mL). Using a constant-pressure dropping funnel, the PDA and pyridine solution was slowly added dropwise to the 4-nitrobenzoyl chloride solution at 0 °C (Scheme 1). The reaction mixture was then stirred at room temperature for 24 h, and the reaction progress was monitored using thin-layer chromatography (TLC). Upon completion, 150 mL of deionized water was added to the reaction mixture, and the resulting solid was collected by filtration, yielding the crude dinitro compound. The crude product was washed three times with THF and subsequently dried under vacuum at 100 °C for 12 h to obtain the pure dinitro compound (14.7 g, 89% yield). 1H NMR (600 MHz, DMSO-d6): δ 10.61 (s, 2H, N-H), 8.38 (d, J = 1.2 Hz, 4H, Ar-H), 8.20 (d, J = 0.6 Hz, 4H, Ar-H), 7.80 (d, J = 0.6 Hz, 4H, Ar-H) ppm.
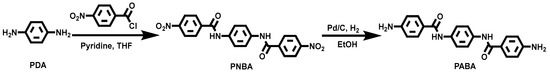
Scheme 1.
Synthetic route of PABA.
Synthesis of 4-Amino-N-{4-[(4-aminobenzoyl)amino]phenyl}benzamide (PABA). The dinitro compound (10.0 g, 18.4 mmol) was dissolved in DMF (100 mL), and 0.5 g of Pd/C (10 wt.%) was added as a catalyst (Scheme 1). The mixture was stirred under a hydrogen atmosphere for 12 h, with the reaction progress monitored by TLC. Upon completion, the Pd/C catalyst was removed by filtration from the solution. The filtrate was poured into an excess amount of water, resulting in the precipitation of a white solid. The precipitate was collected by filtration, recrystallized from a mixed solvent of PE and THF (1/10), and dried under vacuum to obtain PABA (7.5 g, 84% yield). 1H NMR (600 MHz, DMSO-d6) δ 9.73 (s, 2H, N-H), 7.72 (d, J = 1.2Hz, 4H, Ar-H), 7.67 (s, 4H, Ar-H), 6.60 (d, J = 0.6Hz, 4H, Ar-H), 5.75 (s, 4H, NH2) ppm.
2.4. Preparation of Polyimide Films
All six types of PI films were prepared using a two-step thermal imidization method. A 100 mL three-necked flask and a polytetrafluoroethylene (PTFE) stirring paddle were placed in a forced-air oven at 80 °C for approximately 10 min. After removal, they were transferred to a mechanical stirring apparatus, connected to a nitrogen gas inlet, and purged with nitrogen. At room temperature, DABA (0.9084 g, 4.0 mmol) was added to the flask, followed by the addition of 10 mL of DMAc. The mixture was stirred until the diamine was completely dissolved. Subsequently, dianhydride 6FDA (2.1867 g, 4.0 mmol) was added, along with an additional 2 mL of DMAc, and the reaction was stirred continuously for 12 h. The resulting product was a pale yellow, transparent, and viscous PAA solution. The PAA solution was then placed in a vacuum oven at room temperature for degassing for more than 10 h in preparation for the next step.
The PI films were fabricated using a solution-casting method. Under a controlled cleanroom environment with humidity maintained below 35%, a clean and dry glass plate was placed onto an automatic film coater. The degassed PAA solution was evenly poured onto the glass plate, and the coating knife was adjusted to a height of 500 μm. The film was cast at a coating speed of 5 mm/min, ensuring uniform deposition of the polymer solution onto the glass substrate. The coated glass plate was then transferred to a vacuum oven, where it was subjected to stepwise solvent removal at 80 °C, 100 °C, and 120 °C for 1 h at each temperature. Subsequently, the film was transferred to a high-temperature oven under a nitrogen atmosphere and thermally imidized at 150 °C, 200 °C, 250 °C, and 300 °C, each for 1 h, with a heating rate of 5 °C/min. After the thermal treatment, the oven was allowed to cool to below 50 °C. The glass plate containing the FPI film was then immersed in hot water to facilitate film detachment. The detached film was rinsed thoroughly with ultra-pure water and dried in a forced-air oven at 80 °C for 1 h. The final FPI films, with a thickness of approximately 40–50 μm and nearly transparent appearance, were sealed in self-locking plastic bags for further characterization.
3. Results and Discussion
3.1. Monomer Syntheses
The chemical structures of PNBA and PABA were confirmed by 1H NMR spectroscopy. Figure 1a,b present the 1H NMR spectra of PNBA and PABA, respectively. In Figure 1a, the singlet signal at 10.61 ppm corresponds to the proton signal of the amide bond in the PNBA structure (labeled as c). The three pairs of doublet signals appearing in the range of 8.39–7.79 ppm are attributed to the aromatic protons of PNBA, corresponding to positions a, b, and d. Similarly, in Figure 1b, the singlet peak at 9.73 ppm is assigned to the proton signal of the amide bond in the diamine structure of PABA (labeled as d). The three sets of signals in the range of 7.73–6.59 ppm correspond to the aromatic protons at positions c, e, and b in PABA, while the peak at 5.75 ppm is attributed to the protons of the two amino groups. These results confirm the successful synthesis of the intermediate PNBA and the diamine PABA, both with high yield and purity.
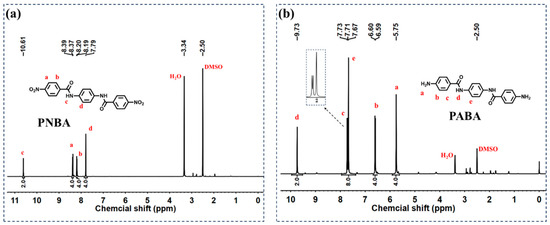
Figure 1.
(a) 1H NMR spectra of PNBA; (b) 1H NMR spectra of PABA.
3.2. Characterization of Polyimides
A series of FPI films were prepared via thermal imidization by homopolymerizing six diamine with 6FDA, as illustrated in Scheme 2. The molecular weights of the precursor PAA were determined by GPC, and the results are summarized in Table 1. The weight-averaged molecular weight (Mw) ranged from 9.2 × 104 to 15.2 × 104 g/mol, with a polydispersity index (PDI) of 1.9–2.4, indicating that all polymers possessed sufficiently high molecular weights and good molecular weight distributions, enabling the formation of tough FPI films.
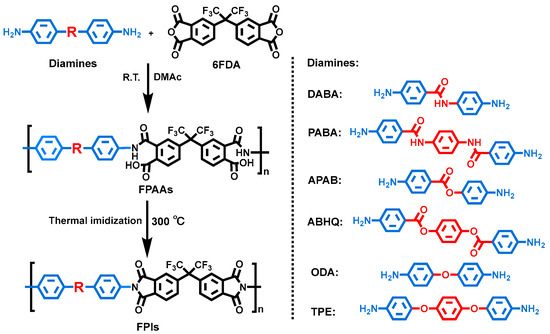
Scheme 2.
General synthesis procedure for FPIs.

Table 1.
Physical parameters of the FPIs.
The chemical structures of the PI films were characterized by FT-IR spectroscopy, and the corresponding infrared spectra are shown in Figure 2. The FT-IR spectra of DABA-6FDA and PABA-6FDA exhibit a broad absorption band in the range of 3241–3552 cm−1, attributed to the N-H stretching vibration of the amide bonds. The characteristic peaks at 1784 cm−1, 1718 cm−1, and 721 cm−1 correspond to the asymmetric stretching, symmetric stretching, and bending vibrations of the C=O groups in the imide rings, respectively. Additionally, the peaks around 1658 cm−1 in the spectra of DABA-6FDA and PABA-6FDA are assigned to the C=O stretching vibration of amide bonds. The absorption band near 1374 cm−1 corresponds to the C-N stretching vibration of the imide rings. Furthermore, the peaks at 1545 cm−1 and 1318 cm−1 are attributed to the coupled in-plane bending (II band) and out-of-plane bending (III band) vibrations of the C-N and N-H bonds in the amide groups. For APAB-6FDA and ABHQ-6FDA, the peaks at 1296 cm−1 and 1065 cm−1 correspond to the asymmetric stretching vibration of the C-O-C ether groups and the single-bond C-O stretching vibration, respectively. Similarly, for ODA-6FDA and TPE-6FDA, the absorption peaks at 1295 cm−1 and 1089 cm−1 are assigned to the asymmetric and symmetric stretching vibrations of the C-O-C ether groups, respectively. Apart from PABA-6FDA and DABA-6FDA, which inherently contain amide bonds, no residual amide bond peaks were observed around 1650 cm−1 in the spectra of the other polymers, indicating the complete imidization of the polymers. These results confirm the successful synthesis of the target FPI films with fully imidized structures.
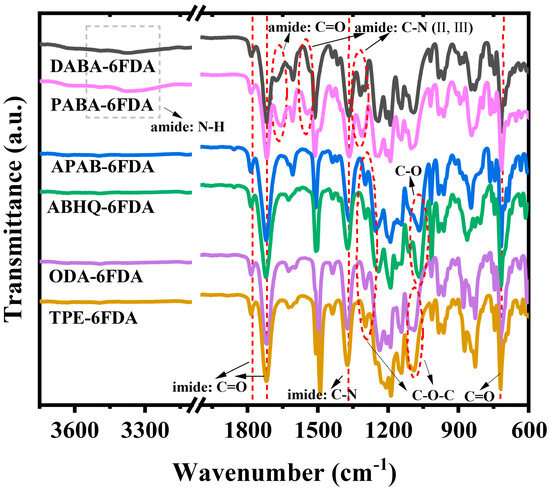
Figure 2.
FT-IR spectra of the FPI films.
3.3. Solubility and Water Absorption of FPIs
The solubility properties of polymers are critical for their processability and potential applications. The solubility data for the six synthesized FPIs with different functional groups are summarized in Table S1. The FPEsIs (ABHQ-6FDA and APAB-6FDA) exhibited poor solubility, which can be attributed to the fact that ester groups act as mesogenic units [34].The presence of ester groups increases the crystallinity of the polymer, leading to more ordered molecular chain packing, which prevents solvent molecules from penetrating between the polymer chains. In contrast, the FPAIs (DABA-6FDA and PABA-6FDA) and FPEIs (ODA-6FDA and TPE-6FDA) exhibited good solubility in both highly polar and less polar solvents. This improved solubility can be attributed to the presence of the hexafluoroisopropylidene (-C(CF3)2-) unit, a bulky and twisted structural moiety, which effectively disrupts the close molecular packing of the FPI chains. This disruption results in lower crystallinity and enhanced solvent affinity, thereby significantly improving the solubility of these FPIs.
PIs typically exhibit high water absorption, primarily due to the presence of highly polar imide rings in their backbone. Therefore, understanding water absorption behavior is crucial for evaluating its impact on the dielectric properties of PIs. The water absorption of the six FPI films was measured, and the results are summarized in Table 1 and Figure 3a. The incorporation of ester groups, which are low-polarity and hydrophobic, significantly reduces the water absorption of FPI films. As a result, the FPEsIs (APAB-6FDA and ABHQ-6FDA) exhibited much lower water absorption compared to the other four FPIs without ester groups, with values consistently below 1%. Additionally, APAB-6FDA showed higher water absorption than ABHQ-6FDA, suggesting that a higher ester content leads to lower water uptake. Conversely, the FPAIs (DABA-6FDA and PABA-6FDA) and FPEIs (ODA-6FDA and TPE-6FDA) exhibited higher water absorption due to their hydrophilic nature, which allows them to form hydrogen bonds with water molecules. Moreover, in terms of hydrophilicity, amide bonds are more hydrophilic than ether linkages. Consequently, the FPAIs exhibited higher water absorption than the FPEIs.
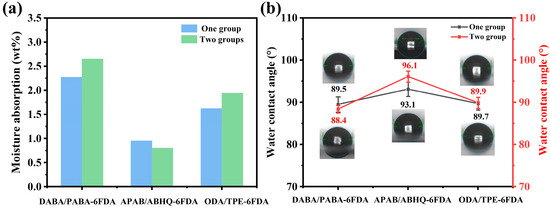
Figure 3.
(a) Comparison of FPI films’ water absorption; (b) water CA for FPI films.
The wettability of polymer films is another critical factor influencing their hydrophilicity and is closely related to dielectric performance. The wettability of the six FPI films was evaluated by measuring their static water contact angles (CA), as shown in Figure 3b and listed in Table 1. The results indicate that the PEsIs exhibited a CA greater than 90°, whereas the FPEIs and FPAIs showed a CA below 90°. Specifically, the water CA of the APAB-6FDA and ABHQ-6FDA films were 93.1° and 96.1°, respectively, demonstrating that an increase in ester content leads to a larger CA. On the other hand, the water CA of the PABA-6FDA and DABA-6FDA films were 88.4° and 89.5°, while those of ODA-6FDA and TPE-6FDA were 89.7° and 89.9°, indicating that the FPAIs exhibited higher wettability than the FPEIs. In summary, incorporating ester groups into the PI backbone effectively reduces the film’s wettability, which in turn helps lower the Dk of FPI films.
3.4. Dielectric Properties
The Dk and Df of six FPI films with different functional groups were measured at 10, 40, and 60 GHz under conditions of 25 °C and 40% RH, with the results summarized in Table 2. The FPAIs (DABA-6FDA and PABA-6FDA) exhibited relatively high Dk values, approximately 3.2 and 3.7, respectively, with Df values of 0.009 and 0.012. A comparison between DABA-6FDA and PABA-6FDA showed that as the amide content increased, both Dk and Df increased significantly. According to the Clausius–Mossotti equation, , ε is the Dk, N is the dipole density, α is the polarizability, and ε0 is the permittivity of free space (a constant) [35]. An increase in the amide content directly enhances both N and α, leading to a higher Dk. To further investigate the mechanism underlying the influence of different functional groups on the dielectric properties of PIs, molecular simulations were performed on the six FPI molecular chains, and the relevant physical parameters were obtained (the computational simulation process is provided in the supporting materials), as listed in Table 3. The molar polarizability per unit volume (α/V) of the FPAIs was 0.863 and 0.925, which was higher than that of the FPEIs and FPEsIs. The amide group (-NHCO-) is a highly polar moiety with a large dipole moment (4.323 D for 6FDA-DABA and 4.610 D for 6FDA-PABA). Therefore, the FPAI films exhibit higher Dk and Df values than both the FPEI and FPEsI films. Moreover, since the Dk of water is as high as approximately 80 under low-frequency electric fields and decreases slightly at higher frequencies, and the Df of water increases from about 0.01 at low frequencies to approximately 0.484 at high frequencies [14,36,37,38], the water absorption of polymers has a significant impact on their Dk and Df. Accordingly, the high-dielectric properties observed in the FPAI films are consistent with the previously reported water absorption characterization results. The FPEsIs exhibited an ultra-low Dk of approximately 3.0 and low Df values of ≈0.003 and ≈0.002, respectively. A comparison between APAB-6FDA and ABHQ-6FDA revealed that as the ester content increased, the Dk values remained nearly unchanged, whereas the Df values showed a decreasing trend. This can be attributed to the ester groups, which are highly oriented and crystalline structural units. Incorporating them into the FPI backbone enhances the polymer’s molecular orientation and crystallinity, thereby restricting dipole moments and molecular chain mobility, which effectively reduces Df [4,30,34,39,40]. Meanwhile, the increased ester content significantly enhanced the conformational rigidity of the polymer chains, as indicated by Kuhn segment parameter (Afr) and radius of gyration (Rg) values. The rigid polymer backbone restricted local molecular motions, such as vibrations and rotations, thereby reducing the ability of dipoles to reorient under high-frequency electric fields. This constrained dipole motion led to lower polarization relaxation energy dissipation, ultimately manifesting as a reduction in Df. Additionally, the increase in ester content markedly enhanced intermolecular interactions, as reflected by an increase in the cohesive energy density (CED). Stronger intermolecular forces not only stabilized the linear conformation of polymer chains but also restricted segmental motion, thereby reducing dipole mobility. This “locking effect” diminished the contribution of relaxation polarization at high frequencies, further lowering the Df [33]. For the FPEIs (ODA-6FDA and TPE-6FDA), the Dk values were approximately 2.9 and 3.3, while the Df values were 0.006 and 0.002, respectively. Similar to ester groups, increasing the ether content led to a rise in Dk, whereas Df decreased significantly. However, ether linkages serve as flexible bridging units, facilitating molecular orientation under high-frequency electric fields [31,32], thereby effectively reducing Df.

Table 2.
Dielectric properties of FPI films at high frequency.

Table 3.
Physical parameters obtained from MD simulations of FPIs.
As the frequency increased, all the FPI films exhibited a slight decline in both Dk and Df, which aligns with theoretical models of polarization saturation and constrained molecular motion [41,42]. At frequencies above 10 GHz, the rapid oscillation of the electric field allows only the fastest polarization mechanisms (such as electronic and atomic polarization) to keep pace with field variations. In contrast, orientation polarization (dipole rotation) is significantly suppressed due to inertia, reducing its contribution. At high frequencies, the polarizability (α) of electronic and atomic polarization tends to stabilize, while the free volume of the material may increase due to restricted polymer chain motion, leading to a reduction in Dk. At very high frequencies (e.g., 60 GHz), dipoles fail to complete full reorientation due to inertia, resulting in lower energy dissipation. At this stage, Df is predominantly governed by the slight lag in electronic and atomic polarization, both of which exhibit minimal energy loss. Overall, FPAIs demonstrate potential for high-dielectric applications, while FPEsIs and FPEIs are more suitable for 5G/6G communication and other low-dielectric applications.
Given the varying sensitivity of FPIs with different molecular structures to environmental humidity, it is essential to investigate their dielectric behavior under different humidity conditions. In this study, the effects of humidity (20%, 40%, and 80% RH) on the Dk and Df of six FPI films with different functional groups were measured at 25 °C under a 10 GHz high-frequency electric field. The results are presented in Figure 4. A comparison of FPAIs, FPEsIs, and FPEIs revealed that the FPAIs exhibited the highest Dk and Df values, as well as the greatest sensitivity to humidity. This behavior is attributed to the hydrophilic nature of amide groups, which readily form hydrogen bonds with water molecules in the environment, making the material highly susceptible to moisture absorption. Furthermore, the greater the number of amide groups, the higher the humidity sensitivity. Notably, the FPEsIs displayed exceptional stability in Dk and Df across different humidity conditions. This stability is primarily attributed to the liquid crystalline nature of ester groups, which promotes molecular chain orientation and enhances local crystallinity, effectively hindering the penetration of water molecules into the PI matrix. In contrast, the PEIs exhibited slightly higher sensitivity to humidity than the FPEsIs, which is likely due to the weak hydrophilicity of ether groups. Overall, the humidity sensitivity of the investigated FPIs follows the trend FPAIs > FPEIs > FPEsIs, which is consistent with the trend observed in their water absorption properties.
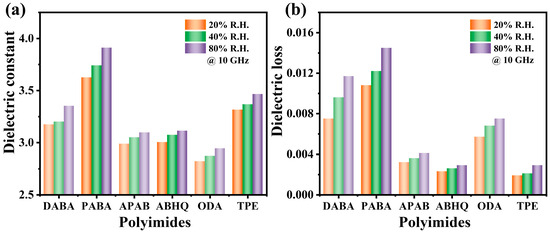
Figure 4.
(a) RH dependence of Dk and (b) Df for the six FPIs at 10 GHz.
Investigating the dielectric properties of PI films at low frequencies is equally important. To further explore this aspect, the dielectric performance of the synthesized FPI films was measured over a low-frequency range (103–106 Hz), and their dielectric spectra at different frequencies are presented in Figure 5. For all FPI films, the Dk decreases with increasing frequency. In the low-frequency region, dipolar orientation polarization plays a dominant role in the dielectric response. The polar functional groups on the polymer backbone have sufficient time to realign with the alternating electric field, resulting in high polarization ability and a relatively high dielectric constant. Among these, FPAIs exhibit the highest Dk values due to their strong polarity. As the frequency increases, the dipoles struggle to follow the rapid changes in the electric field, leading to a decrease in effective polarizability and consequently a reduction in Dk. The Df values, on the other hand, exhibit fluctuations with increasing frequency. However, the overall trend shows an initial slight increase followed by stabilization. This behavior is primarily attributed to the large dipole moments of highly polar functional groups (such as amide and ester groups), which require a longer response time to align with the electric field, leading to increased energy dissipation and a slight rise in Df at lower frequencies [43]. In summary, regardless of the applied frequency, the incorporation of ester groups into the FPI backbone effectively reduces both Dk and Df values, while the introduction of ether groups decreases Df but slightly increases Dk.
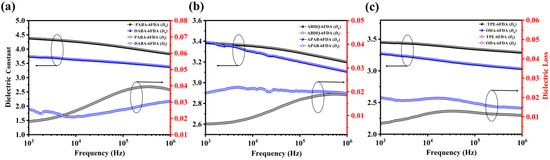
Figure 5.
(a–c) Dielectric spectra of the FPI films.
3.5. Optical Properties
FPI films, particularly those containing CF3 groups, generally exhibit excellent colorless transparency, making them widely applicable in optoelectronic devices. The optical properties of the six FPI films were evaluated using UV–Vis spectrophotometry and a Yellowness Index (YI) meter, with the results summarized in Figure 6 and Table 4. The FPEsIs demonstrated the most outstanding optical performance, with a cut-off wavelength (λcut-off) of 339 nm, and T450 nm and T550 nm values reaching 80% and 86%, respectively, while the YI was as low as 4.74. A comparison between APAB-6FDA and ABHQ-6FDA revealed that an increased ester group content in the FPI backbone enhances the colorless transparency of the films. This is attributed to the poor planarity of the ester group (Figure S1), which induces distortions in the polymer backbone, disrupts conjugation, and reduces electron cloud migration between the diamine and dianhydride moieties, thereby minimizing light absorption. Additionally, the LUMO–HOMO gap of the PEsIs was found to be larger than that of the other FPI structures (Figure 6c), with APAB-6FDA at 3.543 eV and ABHQ-6FDA at 3.749 eV. As the ester content increased, the widening of the bandgap led to a blue shift in the absorption edge, further reducing absorption in the visible region. For the FPAIs and FPEIs, relatively good colorless transparency was observed, with λcut-off values ranging from 372 to 387 nm, T450 nm and T550 nm in the ranges of 59–68% and 82–86%, respectively, and YI values between 8.71 and 9.56. However, as the content of amide and ether groups increased, the optical properties of the corresponding FPI films deteriorated. This is mainly due to hydrogen bonding interactions between the N–H in amide groups and adjacent C=O groups, which enhance interchain interactions and promote the formation of charge transfer complexes (CTCs). Furthermore, the electron-donating nature of the amide group facilitates charge transfer between diamine and dianhydride units, leading to a reduced LUMO–HOMO gap (DABA-6FDA: 3.180 eV; PABA-6FDA: 2.737 eV) and consequently a decline in optical performance. It is also noteworthy that the flexibility of ether linkages causes looser molecular packing and increased free volume within the polymer. However, the -C(CF3)2- group in 6FDA is a strong electron-withdrawing moiety, which can suppress CTC effects. When more ether linkages are introduced, their electron-donating effect may counteract the electron-withdrawing nature of F atoms, ultimately leading to a reduction in the bandgap (ODA-6FDA: 3.327 eV; TPE-6FDA: 3.243 eV).
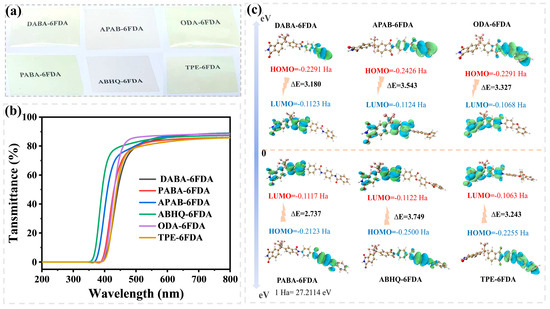
Figure 6.
(a) Photographs of FPI films; (b) UV–Vis spectra of FPI films; (c) HOMO–LUMO gap energies of FPIs.

Table 4.
The optical parameters of the FPI films.
3.6. Thermal and Mechanical Properties
The Tg, thermal decomposition temperature, and thermal dimensional stability of the six FPI films were evaluated using DMA, TGA, and TMA, respectively. The corresponding data are summarized in Table 5. Figure 7a presents the Tan Delta curves obtained from DMA, where the peak values correspond to the Tg of the films. The Tg values of all the FPI films ranged from 296 °C to 388 °C. Among these materials, the FPAI films exhibited the highest Tg values compared to the FPEsIs and FPEIs. Specifically, PABA-6FDA and DABA-6FDA exhibited Tg values of 383 °C and 388 °C, respectively. This enhancement is primarily attributed to the introduction of hydrogen bonding in the amide groups, which strengthens intermolecular interactions and thus increases the Tg of the FPI films. This is supported by the results of polymer simulation calculations (Table 3), which show that the FPAIs possess higher values of Rg (39.85 and 49.04 Å), Afr (2.71 and 2.84 Å), and CED (413.8 and 460.4 J/cm3) compared to the FPEsIs and FPEIs, indicating greater chain rigidity and stronger intermolecular interactions. In contrast, the FPEIs exhibited lower Tg values, with ODA-6FDA and TPE-6FDA showing Tg values of 320 °C and 296 °C, respectively. This reduction is due to the high flexibility of ether linkages, which reduces the rigidity of the polymer backbone, leading to a lower Tg for the FPEIs. For the FPEsIs, the Tg values of APAB-6FDA and ABHQ-6FDA were recorded as 337 °C and 344 °C, respectively. Additionally, due to the inherent rigidity of ester groups, the gradual elevation in ester group content induces a moderate enhancement in the Tg of FPI films.

Table 5.
Thermal and mechanical performance of FPI films.
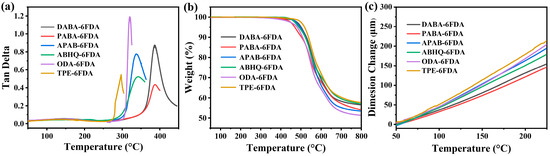
Figure 7.
(a) DMA curve of FPI films; (b) TGA curve of FPI films; (c) TMA curve of FPI films.
The thermal stability of the films was evaluated using TGA, and the corresponding TGA curves of the six FPI films are presented in Figure 7b, with detailed data summarized in Table 5. Compared to conventional aromatic FPIs, these FPI films exhibit relatively lower thermal stability due to the presence of a higher proportion of single-bonded functional groups, such as -C(CF3)2-, ester, amide, and ether groups. The measured Td5% and Td10% values ranged from 467 °C to 516 °C and 495 °C to 539 °C, respectively. However, given the inherently high thermal stability of FPI materials, these FPI films still possess sufficient thermal resistance to meet the requirements of 5G communication and optoelectronic applications. Additionally, at 800 °C, the residual mass fraction of these FPI films ranged from 50.2% to 54.2%, demonstrating their high char yield and excellent thermal stability. The dimensional stability of the six FPI films was further analyzed via TMA, and the TMA curves are displayed in Figure 7c, with the CTE values listed in Table 5. The FPAIs exhibited the lowest CTE, with DABA-6FDA and PABA-6FDA showing a CTE of 14.7 and 11.2 ppm/K, respectively. This reduction in CTE is attributed to hydrogen bonding interactions between amide groups, which enhance intermolecular interactions and thus improve dimensional stability. In contrast, the FPEIs (ODA-6FDA and TPE-6FDA) exhibited the highest CTE, at 49.5 and 52.5 ppm/K, respectively. This is primarily due to the flexibility of ether linkages and their bent conformation, which reduces the rigidity of the polymer backbone. The CTE values of the FPEsIs (APAB-6FDA and ABHQ-6FDA) were 37.5 and 32.3 ppm/K, respectively, which can be attributed to the molecular architecture of ester groups exhibiting inherent rigidity and aggregation orientation. These structural characteristics endow FPEsIs with superior thermal dimensional stability compared to FPEIs. Furthermore, the incremental incorporation of ester functionalities was observed to induce a further reduction in the CTE of FPIs.
To further investigate their mechanical performance, six FPI films containing different functional groups were evaluated using a universal testing machine, with the results summarized in Table 5. Both the FPEsIs and FPEIs demonstrated good mechanical properties, with ultimate tensile strengths of 149.8–167.2 MPa and 152.5–161.6 MPa, Young’s moduli of 2.5–2.6 GPa and 2.1–2.3 GPa, and elongations at break of 9.5–11.3% and 12.5–14.3%, respectively. In contrast, the FPAIs exhibited significantly superior mechanical properties, owing to hydrogen bonding interactions that enhance intermolecular forces within the PI matrix. These films achieved remarkably higher ultimate tensile strengths of 219.4–248.1 MPa, Young’s moduli of 3.1–3.4 GPa, and elongations at break of 16.7–18.6%. Overall, the incorporation of amide groups into the PI backbone is more effective in enhancing mechanical performance than that of ether or ester groups.
4. Conclusions
This study first synthesized a diamine monomer (PABA) and then selected five commercial diamines for homopolymerization with 6FDA dianhydride via thermal imidization, producing six FPI films (FPAIs, FPEsIs, and FPEIs) containing distinct functional groups. The systematic characterization of the dielectric, optical, thermal, and mechanical properties revealed that the FPAIs exhibited the highest Dk and Df across both high- and low-frequency ranges. In contrast, the FPEsIs and FPEIs demonstrated ultra-low-dielectric performance, particularly achieving a Df as low as ~0.002 at high frequencies, with Dk values ranging from 2.8 to 3.3, alongside excellent dielectric humidity stability, underscoring their significant application potential in 5G/6G communication systems. Regarding optical performance, the FPEsI films displayed optimal colorless transparency, with a T450 nm of 80% and a YI of 4.74, whereas the FPAIs and FPEIs exhibited slightly inferior optical properties. In terms of thermal and mechanical performance, the FPAIs demonstrated superior thermomechanical stability due to hydrogen bonding interactions from amide linkages, featuring a higher Tg (388 °C), lower CTE (11.2 ppm/K), and enhanced σ (248.1 MPa), E (3.4 GPa), and ε (18.6%). The comparative analysis between the FPEsIs and FPEIs indicated that the FPEsIs possessed a lower CTE, higher E, and reduced ε. Finally, molecular dynamics simulations and quantum chemical calculation were employed to comprehensively investigate the mechanistic influence of functional group structures on the dielectric and optical properties of the FPI films, providing essential theoretical guidance for the rational design and application of FPIs in high-frequency communication and optoelectronic device fields.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym17111505/s1, References [44,45,46,47,48,49,50] are cited in the supplementary materials. Table S1: Solubility of FPI powders; Figure S1: The dihedral angle of FPIs. The Supporting Information is available free of charge.
Author Contributions
W.X.: methodology, writing—original draft, formal analysis, and data curation. X.H.: methodology and data curation. Y.Z.: formal analysis. L.J.: funding acquisition. W.Y.: writing—review and editing. Q.L.: supervision. P.X.: conceptualization and investigation, writing—original draft, supervision, formal analysis validation, funding acquisition, and final editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported financially by the National Natural Science Foundation of China (NSFC 52403010), Ningbo City “Science and Technology Innovation Yongjiang 2035” Science and Technology Innovation Ecosystem Incubation Project (2024Z037), and Startup funds provided by the Ningbo University of Technology (24KQ106).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ali Khan, M.U.; Raad, R.; Tubbal, F.; Theoharis, P.I.; Liu, S.; Foroughi, J. Bending Analysis of Polymer-Based Flexible Antennas for Wearable, General IoT Applications: A Review. Polymers 2021, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, G.; Zhou, Y.; Liu, G.; Wang, J.; Han, S. Progress in low dielectric polyimide film—A review. Prog. Org. Coat. 2022, 172, 107103. [Google Scholar] [CrossRef]
- Dong, X.; Wan, B.; Zha, J.W. Versatile Landscape of Low-k Polyimide: Theories, Synthesis, Synergistic Properties, and Industrial Integration. Chem. Rev. 2024, 124, 7674–7711. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Lu, Q. Polyimide films with ultralow dielectric loss for 5G applications: Influence and mechanism of ester groups in molecular chains. Eur. Polym. J. 2023, 200, 112544. [Google Scholar] [CrossRef]
- Xiao, X.; Qi, H.; Tao, Y.; Kikkawa, T. Study on the interfacial adhesion property of low-k thin film by the surface acoustic waves with cohesive zone model. Appl. Surf. Sci. 2016, 388, 448–454. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, X.; Ren, T.; Saeed, H.A.M.; Wang, Q.; Cui, X.; Huai, K.; Huang, S.; Xia, Y.; Fu, K.; et al. Research progress of low dielectric constant polymer materials. J. Polym. Eng. 2022, 42, 677–687. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Cheng, W.; Zou, J.; Zhao, D. Progress on Polymer Composites with Low Dielectric Constant and Low Dielectric Loss for High-Frequency Signal Transmission. Front. Mater. 2021, 8, 774843. [Google Scholar] [CrossRef]
- Xiao, P.; He, X.; Zheng, F.; Lu, Q. Soluble polyimides with ultralow dielectric constant and dielectric loss and high colorless transparency based on spirobisindane-bis (aryl ester) diamines. Eur. Polym. J. 2024, 221, 113580. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Min, Y. Reducing the Permittivity of Polyimides for Better Use in Communication Devices. Polymers 2023, 15, 1256. [Google Scholar] [CrossRef]
- Lian, M.; Zhao, F.; Liu, J.; Tong, F.; Meng, L.; Yang, Y.; Zheng, F. The Pivotal Role of Benzimidazole in Improving the Thermal and Dielectric Performance of Upilex-Type Polyimide. Polymers 2023, 15, 2343. [Google Scholar] [CrossRef]
- Xiao, P.; He, X.; Lu, Q. Exceptionally High-Temperature-Resistant Kapton-Type Polyimides with Tg > 520 °C: Synthesis via Incorporation of Spirobis(indene)-bis(benzoxazole)-Containing Diamines. Polymers 2025, 17, 832. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sawada, R.; Yanagimoto, S.; Yanagimoto, Y.; Ando, S. Frequency-dependent dielectric properties of aromatic polyimides in the 25–330 GHz range. Appl. Phys. Lett. 2024, 124, 232903. [Google Scholar] [CrossRef]
- Watanabe, K.; Kaneko, M.; Zhong, X.; Takada, K.; Kaneko, T.; Kawai, M.; Mitsumata, T. Effect of Water Absorption on Electric Properties of Temperature-Resistant Polymers. Polymers 2024, 16, 521. [Google Scholar] [CrossRef]
- Sawada, R.; Ando, S. Polarization Analysis and Humidity Dependence of Dielectric Properties of Aromatic and Semialicyclic Polyimides Measured at 10 GHz. J. Phys. Chem. C 2024, 128, 6979–6990. [Google Scholar] [CrossRef]
- Hatton, B.D.; Landskron, K.; Hunks, W.J.; Bennett, M.R.; Shukaris, D.; Perovic, D.D.; Ozin, G.A. Materials chemistry for low-k materials. Mater. Today 2006, 9, 22–31. [Google Scholar] [CrossRef]
- Volksen, W.; Miller, R.D.; Dubois, G. Low Dielectric Constant Materials. Chem. Rev. 2010, 110, 56–110. [Google Scholar] [CrossRef]
- Deng, B.; Chen, K.; Bei, R.; Tian, W.; Liang, L.; Liu, Q.; Li, C.; Huang, H.; Kang, X.; Liu, Q.; et al. Fluorine-Free Thermoplastic High-Frequency Low Dielectric Poly(ether imide)s for Flexible Copper Clad Laminates. ACS Appl. Polym. Mater. 2025, 7, 4239–4250. [Google Scholar] [CrossRef]
- Hasegawa, M.; Fukuda, T.; Ishii, J. Poly(ester imide)s with Low Linear Coefficients of Thermal Expansion and Low Water Uptake (VII): A Strategy to Achieve Ultra-Low Dissipation Factors at 10 GHz. Polymers 2024, 16, 653. [Google Scholar] [CrossRef]
- Xiao, P.; He, X.; Zheng, F.; Lu, Q. Super-heat resistant, transparent and low dielectric polyimides based on spirocyclic bisbenzoxazole diamines with Tg > 450 °C. Polym. Chem. 2022, 13, 3660–3669. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated high-performance polymers: Poly(arylene ether)s and aromatic polyimides containing trifluoromethyl groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Wu, X.; Cai, J.; Cheng, Y. Synthesis and characterization of high fluorine-containing polyimides with low-dielectric constant. J. Appl. Polym. Sci. 2022, 139, 51972. [Google Scholar] [CrossRef]
- He, X.; Zhang, S.; Zhou, Y.; Zheng, F.; Lu, Q. The “fluorine impact” on dielectric constant of polyimides: A molecular simulation study. Polymer 2022, 254, 125073. [Google Scholar] [CrossRef]
- Nagella, S.R.; Ha, C.-S. Structural Designs of Transparent Polyimide Films with Low Dielectric Properties and Low Water Absorption: A Review. Nanomaterials 2023, 13, 2090. [Google Scholar] [CrossRef]
- Watanabe, Y.; Shibasaki, Y.; Ando, S.; Ueda, M. Synthesis and Characterization of Novel Low-k Polyimides from Aromatic Dianhydrides and Aromatic Diamine Containing Phenylene Ether and Perfluorobiphenyl Units. Polym. J. 2006, 38, 79–84. [Google Scholar] [CrossRef]
- Simone, C.D.; Vaccaro, E.; Scola, D.A. The Synthesis and Characterization of Highly Fluorinated Aromatic Polyimides. J. Fluorine Chem. 2019, 224, 100–112. [Google Scholar] [CrossRef]
- Peng, W.; Lei, H.; Qiu, L.; Bao, F.; Huang, M. Perfluorocyclobutyl-containing transparent polyimides with low dielectric constant and low dielectric loss. Polym. Chem. 2022, 13, 3949–3955. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Y.; Yang, Y.; Chen, J.; Li, C.; Liu, H.; Liu, W.; Li, X.; Zhang, T.; Xue, R.; et al. The influence of diamine structure on low dielectric constant and comprehensive properties of fluorinated polyimide films. Eur. Polym. J. 2025, 222, 113614. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Qu, L.; Zou, B.; Chen, Z.; Zhang, Y.; Liu, S.; Chi, Z.; Chen, X.; Xu, J. Exceptionally thermostable and soluble aromatic polyimides with special characteristics: Intrinsic ultralow dielectric constant, static random access memory behaviors, transparency and fluorescence. Mater. Chem. Front. 2017, 1, 326–337. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Lin, Y.-C.; Chen, Y.-C.; Wu, P.-H.; Ando, S.; Ueda, M.; Chen, W.-C. Correlating the Molecular Structure of Polyimides with the Dielectric Constant and Dissipation Factor at a High Frequency of 10 GHz. ACS Appl. Polym. Mater. 2021, 3, 362–371. [Google Scholar] [CrossRef]
- Yin, Q.; Qin, Y.; Lv, J.; Wang, X.; Luo, L.; Liu, X. Reducing Intermolecular Friction Work: Preparation of Polyimide Films with Ultralow Dielectric Loss from MHz to THz Frequency. Ind. Eng. Chem. Res. 2022, 61, 17894–17903. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Lu, Q. The structure design of poly(ester imide)s with low dielectric loss and high mechanical properties. J. Mater. Chem. C 2025, 13, 1388–1394. [Google Scholar] [CrossRef]
- Zhang, C.; He, X.; Lu, Q. High-frequency low-dielectric-loss in linear-backbone-structured polyimides with ester groups and ether bonds. Commun. Mater. 2024, 5, 55. [Google Scholar] [CrossRef]
- He, X.; Zhang, S.; Zhang, C.; Xiao, P.; Zheng, F.; Lu, Q. Decoding high-frequency dielectric loss of Poly(ester imide)s: Molecular simulation and experiment validation. Polymer 2024, 308, 127337. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Z.; Pan, Q.; Liu, S.; Zhao, J. Effect of Fluorinated Substituents on Solubility and Dielectric Properties of the Liquid Crystalline Poly(ester imides). ACS Appl. Polym. Mater. 2023, 5, 141–151. [Google Scholar] [CrossRef]
- Zhu, T.; Yu, Q.; Zheng, W.; Bei, R.; Wang, W.; Wu, M.; Liu, S.; Chi, Z.; Zhang, Y.; Xu, J. Intrinsic high-k–low-loss dielectric polyimides containing ortho-position aromatic nitrile moieties: Reconsideration on Clausius–Mossotti equation. Polym. Chem. 2021, 12, 2481–2489. [Google Scholar] [CrossRef]
- Bei, R.; Chen, K.; Liu, Q.; He, Y.; Li, C.; Huang, H.; Guo, Q.; Chi, Z.; Xu, J.; Chen, Z.; et al. Relationship among the Water Adsorption, Polymer Structure, and High-Frequency Dissipation Factor: Precise Analysis of Water Adsorption of Low-Dielectric Constant Polyimide Films. Macromolecules 2024, 57, 2142–2153. [Google Scholar] [CrossRef]
- Bei, R.; Chen, K.; He, Y.; Li, C.; Chi, Z.; Liu, S.; Xu, J.; Zhang, Y. A systematic study of the relationship between the high-frequency dielectric dissipation factor and water adsorption of polyimide films. J. Mater. Chem. C 2023, 11, 10274–10281. [Google Scholar] [CrossRef]
- Tchangai, T.; Segui, Y.; Doukkali, K. Water sorption in polyamide–imide films and its effect on dielectric loss. J. Appl. Polym. Sci. 1989, 38, 305–312. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Z.; Zhang, X.; Pan, Q.; Liu, S.; Cao, B.; Zhao, J. Soluble Liquid Crystalline Poly(ester imide)s with High Glass Transition Temperatures and Improved Dielectric Properties. ACS Appl. Polym. Mater. 2022, 4, 4234–4243. [Google Scholar] [CrossRef]
- Sapich, B.; Stumpe, J.; Kricheldorf, H.R.; Fritz, A.; Schönhals, A. Synthesis, Dielectric, and Photochemical Study of Liquid Crystalline Main Chain Poly(ester imide)s Containing Cinnamoyl Moieties. Macromolecules 2001, 34, 5694–5701. [Google Scholar] [CrossRef]
- Zhu, L. Exploring Strategies for High Dielectric Constant and Low Loss Polymer Dielectrics. J. Phys. Chem. Lett. 2014, 5, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Prateek; Thakur, V.K.; Gupta, R.K. Recent Progress on Ferroelectric Polymer-Based Nanocomposites for High Energy Density Capacitors: Synthesis, Dielectric Properties, and Future Aspects. Chem. Rev. 2016, 116, 4260–4317. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Y.; Li, J.; Fu, M.; Zou, G.; Ando, S.; Zhuang, Y. Tröger’s Base (TB)-Based Polyimides as Promising Heat-Insulating and Low-K Dielectric Materials. Macromolecules 2023, 56, 2164–2174. [Google Scholar] [CrossRef]
- Eftekhari, A.; Amin, J.S.; Zendehboudi, S. A molecular dynamics approach to investigate effect of pressure on asphaltene self-aggregation. J. Mol. Liq. 2023, 376, 121347. [Google Scholar] [CrossRef]
- Chen, F.; Liu, F.; Du, X. Molecular dynamics simulation of crosslinking process and mechanical properties of epoxy under the accelerator. J. Appl. Polym. Sci. 2023, 140, e53302. [Google Scholar] [CrossRef]
- Hamciuc, C.; Ronova, I.A.; Hamciuc, E.; Bruma, M. The effect of the rotation hindrance on physical properties of some heterocyclic polyamides containing pendent imide groups. Die Angew. Makromol. Chem. 1998, 254, 67–74. [Google Scholar] [CrossRef]
- Salahshoori, I.; Mohseni, A.; Namayandeh Jorabchi, M.; Ghasemi, S.; Afshar, M.; Wohlrab, S. Study of modified PVDF membranes with high-capacity adsorption features using Quantum mechanics, Monte Carlo, and Molecular Dynamics Simulations. J. Mol. Liq. 2023, 375, 121286. [Google Scholar] [CrossRef]
- Wakita, J.; Sekino, H.; Sakai, K.; Urano, Y.; Ando, S. Molecular Design, Synthesis, and Properties of Highly Fluorescent Polyimides. J. Phys. Chem. B 2009, 113, 15212–15224. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Semichem Inc.: Shawnee, KS, USA, 2016.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).