Low-Power NIR-Triggered Photothermal Inactivation of Pseudomonas aeruginosa with Polypyrrole Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bacterial Strains and Growth Conditions
2.3. Methods
2.3.1. PPy-NPs Synthesis
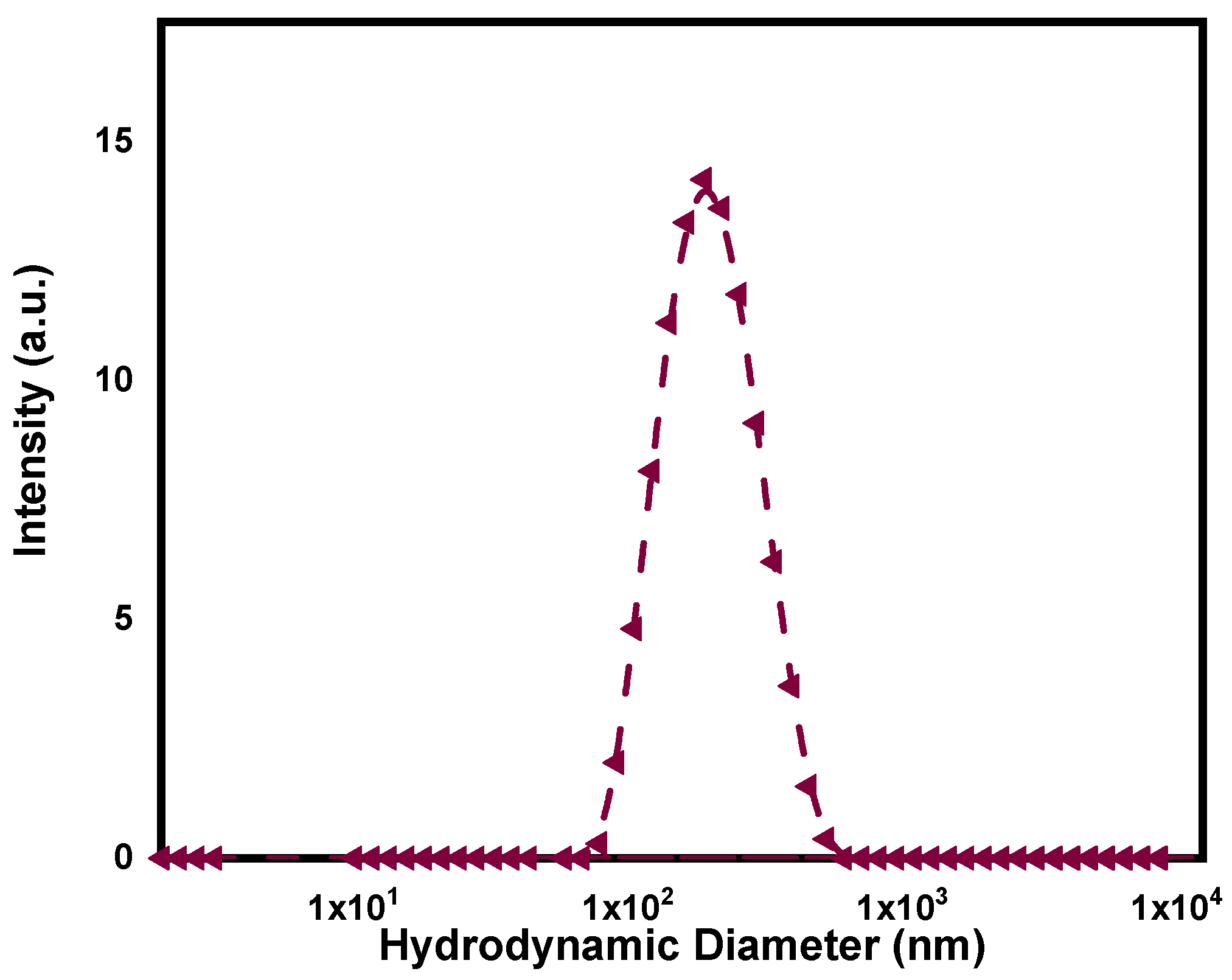
2.3.2. DLS and Z-Potential
2.3.3. Electronic Microscopy (SEM and TEM)
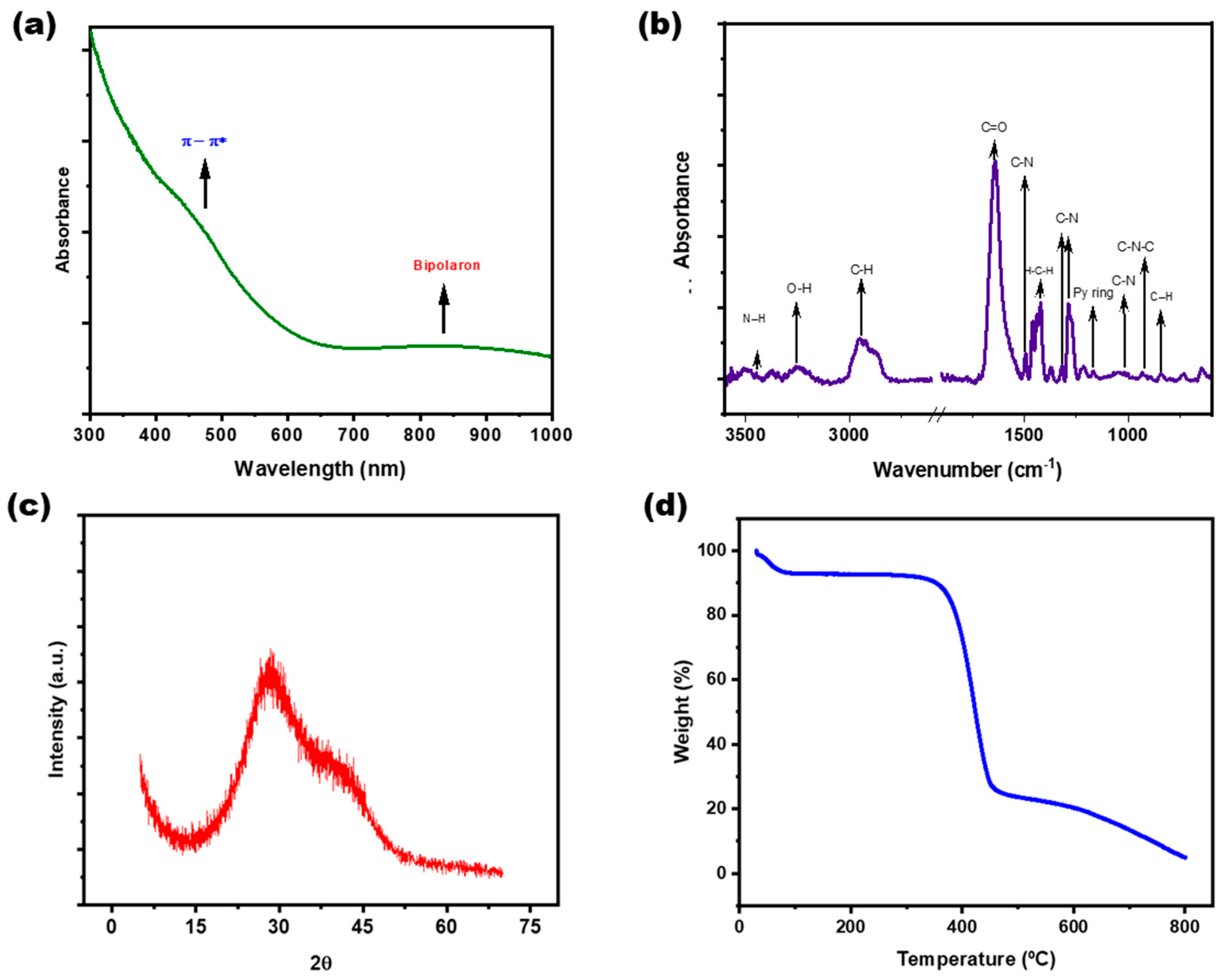
2.3.4. Spectroscopic Characterization (UV–Visible and FTIR)
2.3.5. X-Ray Diffraction (XRD)
2.3.6. Thermogravimetric Analysis (TGA)
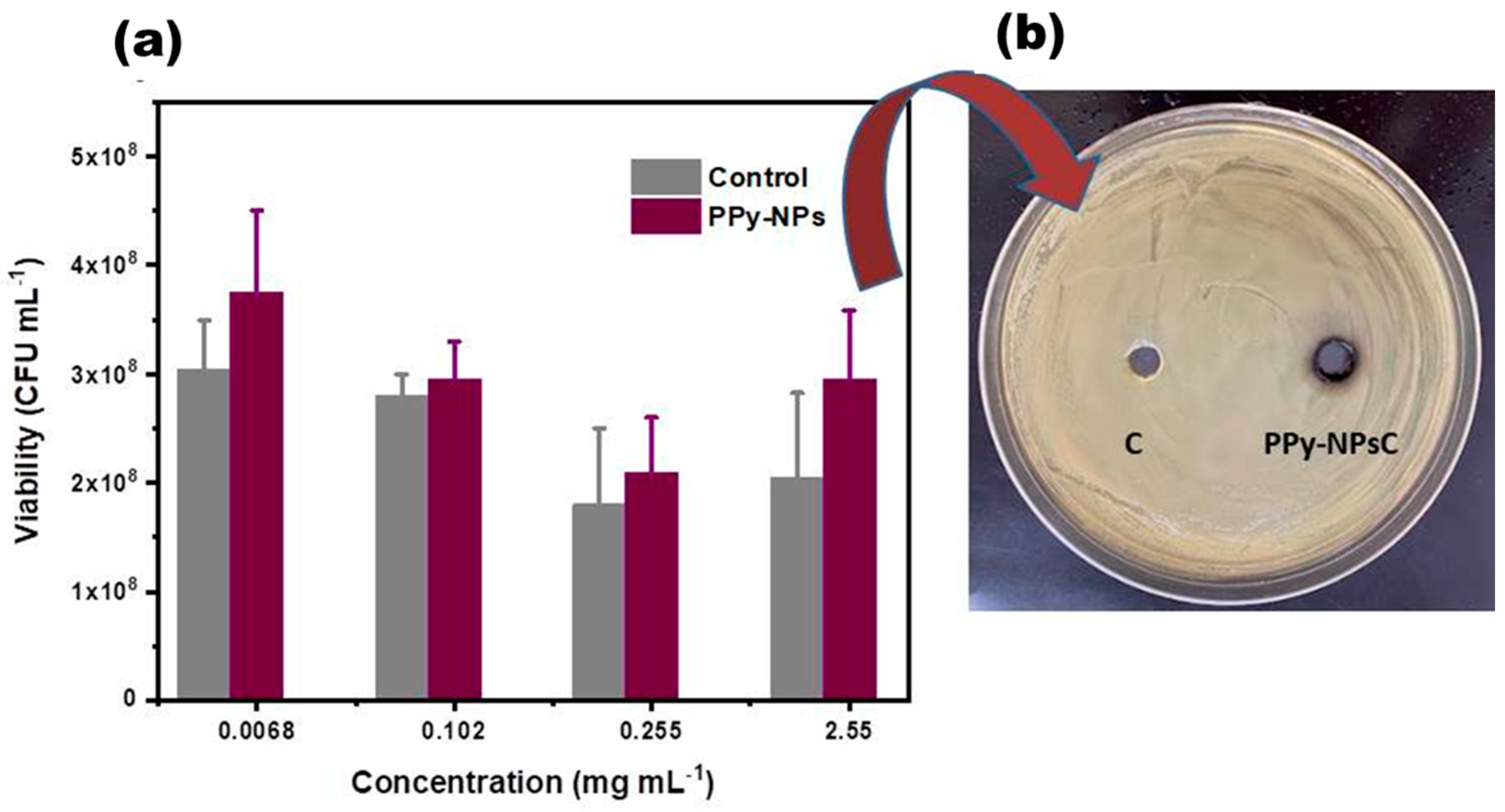
2.3.7. Evaluation of P. aeruginosa Viability at Different Concentrations of PPy-NPs
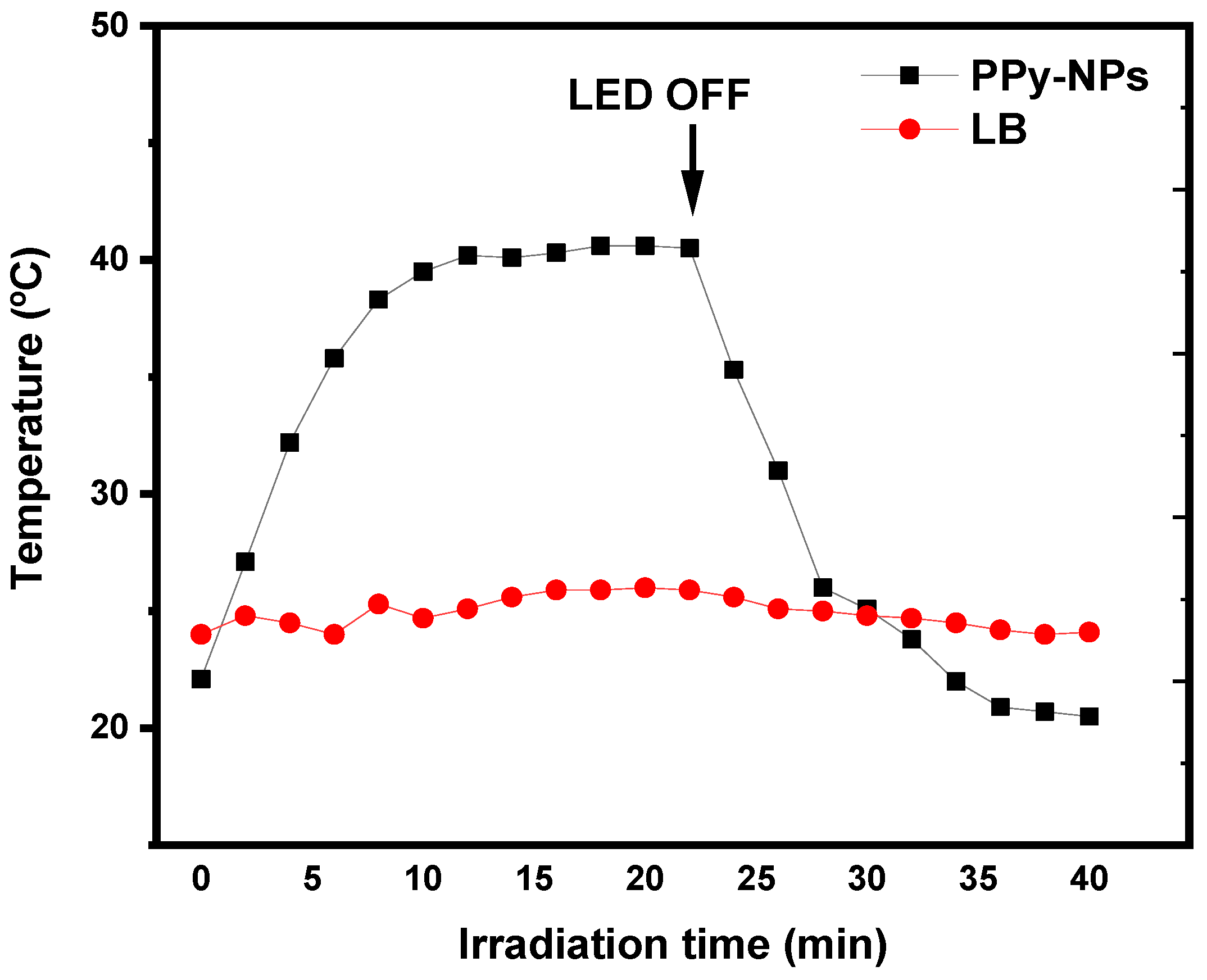
2.3.8. Photothermal Irradiation Set-Up
2.3.9. Antibacterial Photothermal Inactivation (a-PTI)
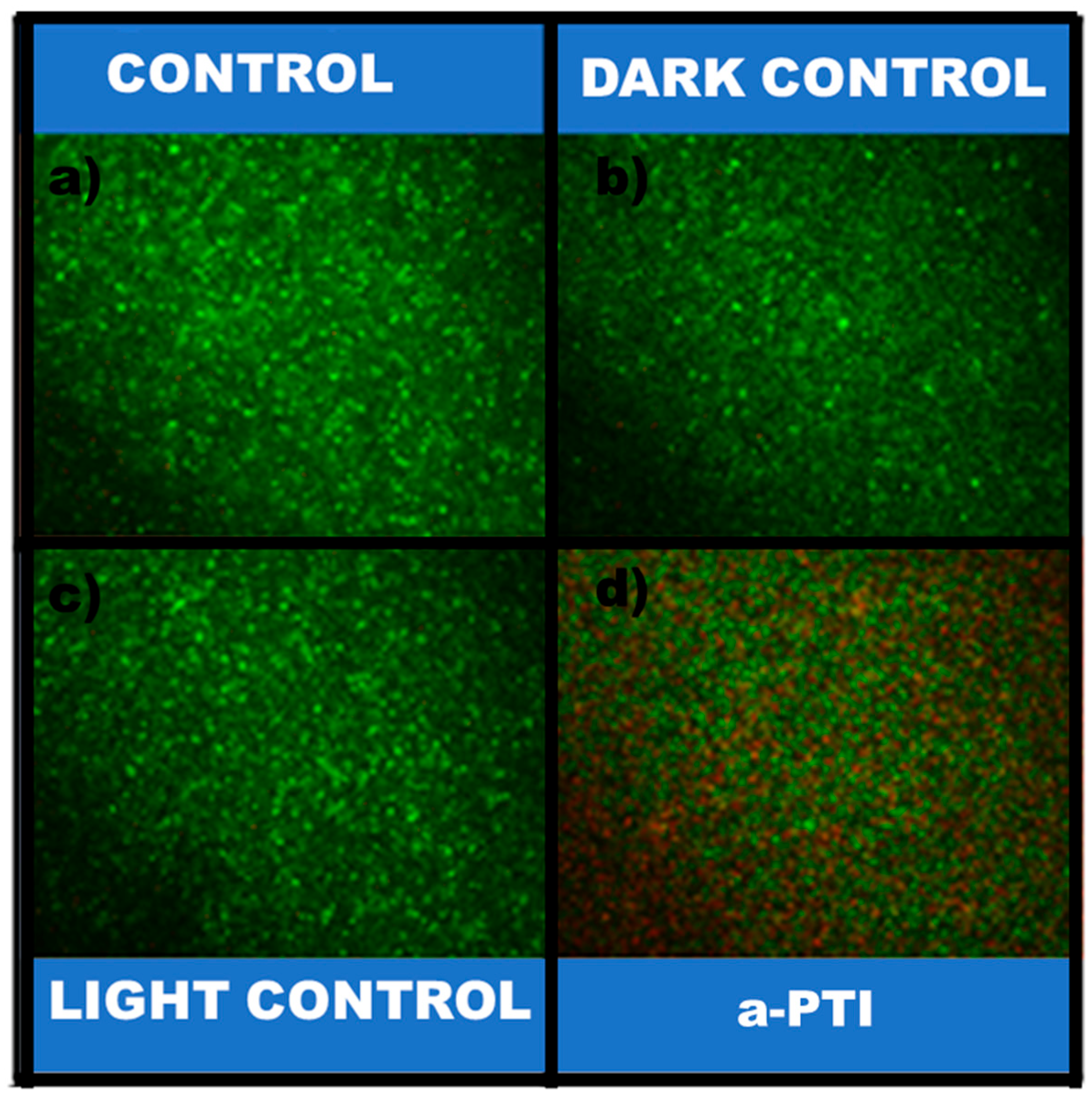
2.3.10. Determination of Reactive Oxygen Species (ROS)
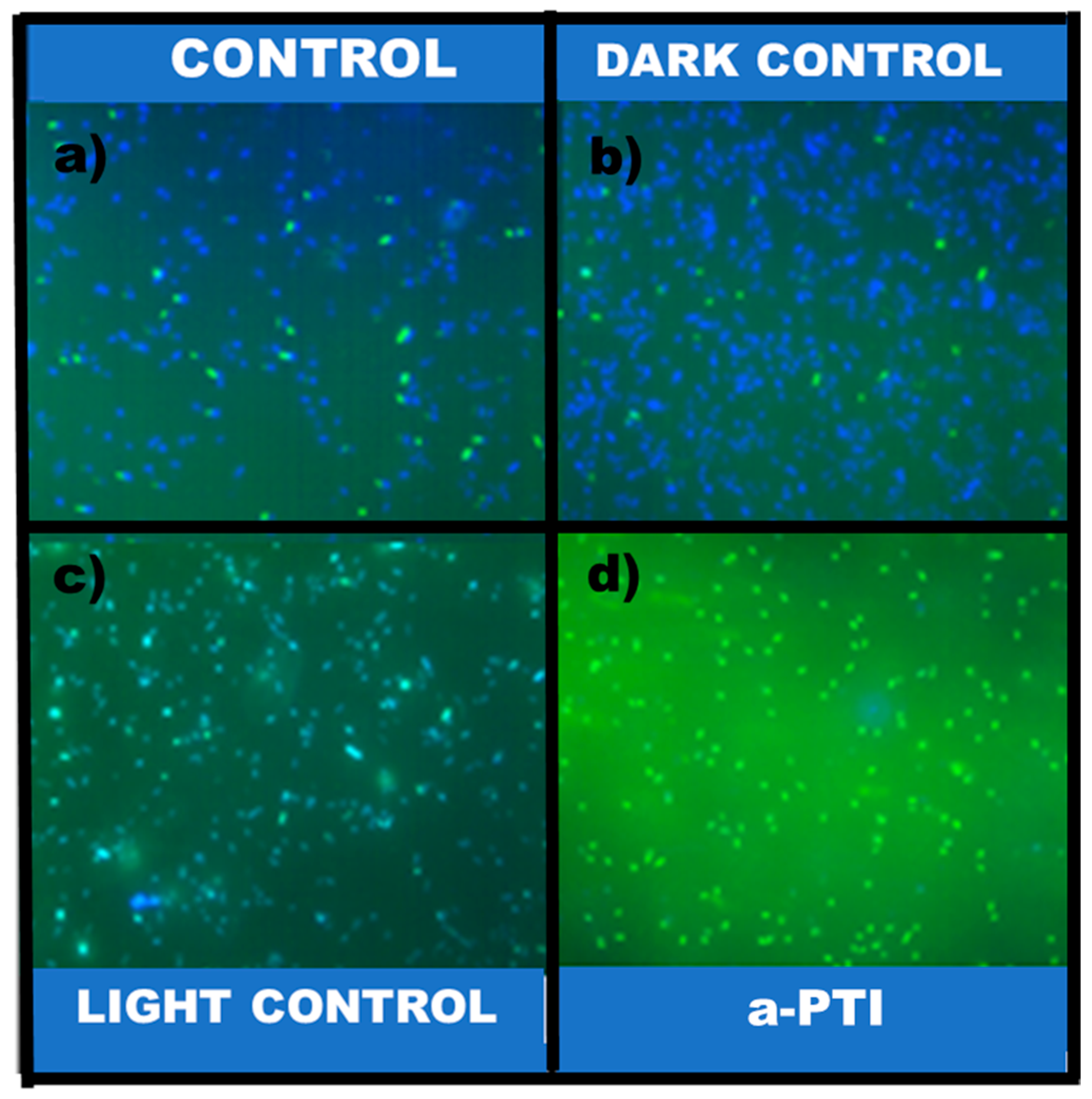
2.3.11. Metabolic Activity Analysis by MTT Assay
2.3.12. Determination of Fatty Acid by Gas Chromatography (GC)
2.3.13. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of PPy-NPs
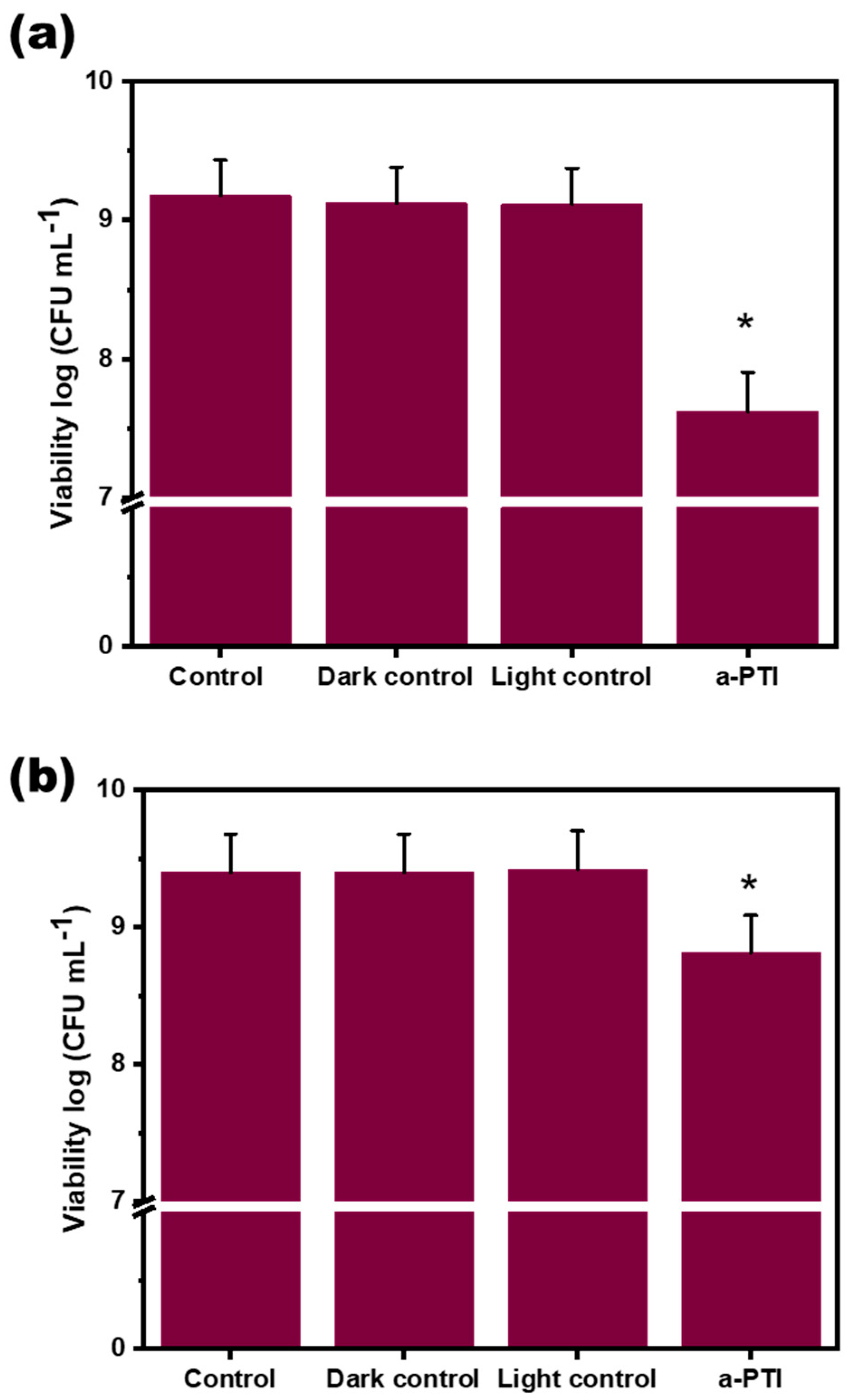
3.2. Antimicrobial Studies of PPy-NPs Against P. aeruginosa
3.2.1. Effect of PPy-NPs Per Se on P. aeruginosa Viability
3.2.2. Bacterial Inactivation Assisted by NIR Irradiation on PPy-NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial resistance and its drivers—A review. Antibiotics 2022, 11, 1362. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Gao, M.; Yun, Y.; Malmsten, M.; Rotello, V.M.; Zboril, R.; Akhavan, O.; Kraskouski, A.; Amalraj, J.; Cai, X. Antibacterial nanomaterials: Mechanisms, impacts on antimicrobial resistance and design principles. Angew. Chem. Int. Ed. 2023, 62, e202217345. [Google Scholar] [CrossRef] [PubMed]
- Stephens, L.J.; Werrett, M.V.; Sedgwick, A.C.; Bull, S.D.; Andrews, P.C. Antimicrobial innovation: A current update and perspective on the antibiotic drug development pipeline. Future Med. Chem. 2020, 12, 2035–2065. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nat. Commun. 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Caracciolo, P.C.; Abraham, G.A.; Battaglia, E.S.; Bongiovanni Abel, S. Recent progress and trends in the development of electrospun and 3D printed polymeric-based materials to overcome antimicrobial resistance (AMR). Pharmaceutics 2023, 15, 1964. [Google Scholar] [CrossRef]
- Smith, W.P.; Wucher, B.R.; Nadell, C.D.; Foster, K.R. Bacterial defences: Mechanisms, evolution and antimicrobial resistance. Nat. Rev. Microbiol. 2023, 21, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.; Kollef, M. The epidemiology and pathogenesis and treatment of Pseudomonas aeruginosa infections: An update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef]
- Tolker-Nielsen, T. Pseudomonas aeruginosa biofilm infections: From molecular biofilm biology to new treatment possibilities. Apmis 2014, 122, 1–51. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Sindeldecker, D.; Stoodley, P. The many antibiotic resistance and tolerance strategies of Pseudomonas aeruginosa. Biofilm 2021, 3, 100056. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni Abel, S.; Gallarato, L.A.; Dardanelli, M.S.; Barbero, C.A.; Rivarola, C.R.; Yslas, E.I. Photothermal lysis of Pseudomonas aeruginosa by polyaniline nanoparticles under near infrared irradiation. Biomed. Phys. Eng. Express 2018, 4, 045037. [Google Scholar] [CrossRef]
- Zhao, W.-H.; Hu, Z.-Q. β-Lactamases identified in clinical isolates of Pseudomonas aeruginosa. Crit. Rev. Microbiol. 2010, 36, 245–258. [Google Scholar] [CrossRef]
- Liu, H.; Xing, F.; Zhou, Y.; Yu, P.; Xu, J.; Luo, R.; Xiang, Z.; Rommens, P.M.; Liu, M.; Ritz, U. Nanomaterials-based photothermal therapies for antibacterial applications. Mater. Des. 2023, 233, 112231. [Google Scholar] [CrossRef]
- Doveri, L.; Diaz Fernandez, Y.A.; Dacarro, G. Nanomaterials for Photothermal Antimicrobial Surfaces. ACS Omega 2024, 9, 25575–25590. [Google Scholar] [CrossRef]
- Pezzi, L.; Pane, A.; Annesi, F.; Losso, M.A.; Guglielmelli, A.; Umeton, C.; De Sio, L. Antimicrobial effects of chemically functionalized and/or photo-heated nanoparticles. Materials 2019, 12, 1078. [Google Scholar] [CrossRef]
- Dediu, V.; Ghitman, J.; Gradisteanu Pircalabioru, G.; Chan, K.H.; Iliescu, F.S.; Iliescu, C. Trends in photothermal nanostructures for antimicrobial applications. Int. J. Mol. Sci. 2023, 24, 9375. [Google Scholar] [CrossRef] [PubMed]
- Omidiyan, M.; Srinoi, P.; Tajalli, P.; Lee, T.R. Review of Light-Activated Antimicrobial Nanoparticle–Polymer Composites for Biomedical Devices. ACS Appl. Nano Mater. 2024, 7, 8377–8391. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Huang, K.; Wei, W.; Huang, Y.; Dai, H. Photothermal antibacterial MoS2 composited chitosan hydrogel for infectious wound healing. Biomater. Adv. 2024, 156, 213701. [Google Scholar] [CrossRef]
- Liu, W.; Gu, H.; Liu, W.; Lv, C.; Du, J.; Fan, J.; Peng, X. NIR-emitting carbon dots for discriminative imaging and photo-inactivation of pathogenic bacteria. Chem. Eng. J. 2022, 450, 137384. [Google Scholar] [CrossRef]
- Sharma, C.P.; Arnusch, C. Laser-induced graphene composite adhesive tape with electro-photo-thermal heating and antimicrobial capabilities. Carbon 2022, 196, 102–109. [Google Scholar] [CrossRef]
- Asaftei, M.; Lucidi, M.; Cirtoaje, C.; Holban, A.-M.; Charitidis, C.A.; Yang, F.; Wu, A.; Stanciu, G.A.; Sağlam, Ö.; Lazar, V. Fighting bacterial pathogens with carbon nanotubes: Focused review of recent progress. RSC Adv. 2023, 13, 19682–19694. [Google Scholar] [CrossRef] [PubMed]
- Federico, S.; Catania, V.; Palumbo, F.S.; Fiorica, C.; Schillaci, D.; Pitarresi, G.; Giammona, G. Photothermal nanofibrillar membrane based on hyaluronic acid and graphene oxide to treat Staphylococcus aureus and Pseudomonas aeruginosa infected wounds. Int. J. Biol. Macromol. 2022, 214, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Omran, A.A.B.; Singh, J.; Ilyas, R. Recent trends and developments in conducting polymer nanocomposites for multifunctional applications. Polymers 2021, 13, 2898. [Google Scholar] [CrossRef]
- Bongiovanni Abel, S.; Rivarola, C.R.; Barbero, C.A.; Molina, M.J.R.a. Electromagnetic radiation driving of volume changes in nanocomposites made of a thermosensitive hydrogel polymerized around conducting polymer nanoparticles. RSC Adv. 2020, 10, 9155–9164. [Google Scholar] [CrossRef] [PubMed]
- Paramshetti, S.; Angolkar, M.; Al Fatease, A.; Alshahrani, S.M.; Hani, U.; Garg, A.; Ravi, G.; Osmani, R.A.M. Revolutionizing drug delivery and therapeutics: The biomedical applications of conductive polymers and composites-based systems. Pharmaceutics 2023, 15, 1204. [Google Scholar] [CrossRef]
- Bongiovanni Abel, S.; Molina, M.A.; Rivarola, C.R.; Kogan, M.J.; Barbero, C.A. Smart polyaniline nanoparticles with thermal and photothermal sensitivity. Nanotechnology 2014, 25, 495602. [Google Scholar] [CrossRef]
- Bongiovanni Abel, S.; Yslas, E.I.; Rivarola, C.R.; Barbero, C.A. Synthesis of polyaniline (PANI) and functionalized polyaniline (F-PANI) nanoparticles with controlled size by solvent displacement method. Application in fluorescence detection and bacteria killing by photothermal effect. Nanotechnology 2018, 29, 125604. [Google Scholar] [CrossRef]
- Pang, Q.; Wu, K.; Jiang, Z.; Shi, Z.; Si, Z.; Wang, Q.; Cao, Y.; Hou, R.; Zhu, Y. A polyaniline nanoparticles crosslinked hydrogel with excellent photothermal antibacterial and mechanical properties for wound dressing. Macromol. Biosci. 2022, 22, 2100386. [Google Scholar] [CrossRef]
- You, C.; Wu, H.; Wang, M.; Wang, S.; Shi, T.; Luo, Y.; Sun, B.; Zhang, X.; Zhu, J. A strategy for photothermal conversion of polymeric nanoparticles by polyaniline for smart control of targeted drug delivery. Nanotechnology 2017, 28, 165102. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni Abel, S.; Frontera, E.; Acevedo, D.; Barbero, C.A.J.P. Functionalization of conductive polymers through covalent postmodification. Polymers 2022, 15, 205. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: A review. J. Med. Chem. 2019, 63, 205. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Yu, D. Progress of conductive polypyrrole nanocomposites. Synth. Met. 2022, 290, 117138. [Google Scholar] [CrossRef]
- Lisyte, V.; Popov, A. Comparative analysis of structure, synthesis, and properties of polyaniline and polypyrrole: Insights into conductive polymer variability. Chem. Proc. 2023, 14, 9. [Google Scholar]
- Bendrea, A.-D.; Cianga, L.; Cianga, I. Progress in the field of conducting polymers for tissue engineering applications. J. Biomater. Appl. 2011, 26, 3–84. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Tarres, L.; Bongiovanni, S.; Barbero, C.A.; Kogan, M.J.; Rivarola, V.A.; Bertuzzi, M.L.; Yslas, E.I. Assessment of polyaniline nanoparticles toxicity and teratogenicity in aquatic environment using Rhinella arenarum model. Ecotoxicol. Environ. Saf. 2015, 114, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. Part A 2004, 68, 411–422. [Google Scholar] [CrossRef]
- da Silva, F.A., Jr.; Queiroz, J.C.; Macedo, E.R.; Fernandes, A.W.; Freire, N.B.; da Costa, M.M.; de Oliveira, H. Antibacterial behavior of polypyrrole: The influence of morphology and additives incorporation. Mater. Sci. Eng. C 2016, 62, 317–322. [Google Scholar] [CrossRef]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.-U.; Egli, T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.A.; Anbazhagan, V. Synthesis of copper sulfide nanoparticles and evaluation of in vitro antibacterial activity and in vivo therapeutic effect in bacteria-infected zebrafish. RSC Adv. 2017, 7, 36644–36652. [Google Scholar] [CrossRef]
- Mondal, S.; Kumar, V.; Singh, S.P. Oxidative stress measurement in different morphological forms of wild-type and mutant cyanobacterial strains: Overcoming the limitation of fluorescence microscope-based method. Ecotoxicol. Environ. Saf. 2020, 200, 110730. [Google Scholar] [CrossRef]
- Sadeghi, R.; Owlia, P.; Yaraee, R.; Sharif, F.; Taleghani, F. An in vitro assessment of antimicrobial and cytotoxic effects of nanosilver. J. Med. Bacteriol. 2012, 1, 44–52. [Google Scholar]
- Cesari, A.B.; Fernandez, M.; Paulucci, N.S.; Dardanelli, M.S. Long-Life Inoculant: Bradyrhizobium Stored in Biodegradable Beads for Four Years Shows Optimal Cell Vitality, Interacts with Peanut Roots, and Promotes Early Growth. Plants 2024, 13, 2983. [Google Scholar] [CrossRef]
- Sakthivel, S.; Boopathi, A. Synthesis and characterization of polypyrrole (PPy) thin film by spin coating Technique. J. Chem. Chem. Sci 2014, 4, 150–155. [Google Scholar]
- Zhao, Y.; Li, Y.; Kang, W.; He, Y.; Liu, W.; Liu, H.; Cheng, B. A novel flexible sensor for respiratory monitoring based on in situ polymerization of polypyrrole and polyurethane coating. RSC Adv. 2017, 7, 49576–49585. [Google Scholar] [CrossRef]
- Dai, H.; Ye, X.; Han, Z.; Xue, Q. Polypyrrole/zinc oxide/zeolitic imidazolate framework (PPy/ZnO/ZIF67) nanocomposites modified electrode for electrochemical analysis of Cd2+ and Pb2+ in waters. Synth. Met. 2023, 299, 117470. [Google Scholar] [CrossRef]
- Rahmani, F.; Ziyadi, H.; Baghali, M.; Luo, H.; Ramakrishna, S. Electrospun PVP/PVA nanofiber mat as a novel potential transdermal drug-delivery system for buprenorphine: A solution needed for pain management. Appl. Sci. 2021, 11, 2779. [Google Scholar] [CrossRef]
- Almeida, Y.A.d.; Bispo, D.F.; Montalvão, M.M.; Mota, K.O.; Corrêa, C.B.; Gimenez, I.F. Effect of preparation additives on the antimicrobial activity and cytotoxicity of Polypyrrole. J. Braz. Chem. Soc. 2021, 32, 1203–1212. [Google Scholar] [CrossRef]
- Kucekova, Z.; Humpolicek, P.; Kasparkova, V.; Perecko, T.; Lehocký, M.; Hauerlandova, I.; Saha, P.; Stejskal, J. Colloidal polyaniline dispersions: Antibacterial activity, cytotoxicity and neutrophil oxidative burst. Colloids Surf. B Biointerfaces 2014, 116, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Fang, X.; Tang, S.; Zheng, N. Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy. Chem. Commun. 2012, 48, 8934–8936. [Google Scholar] [CrossRef] [PubMed]
- Behzadpour, N.; Sattarahmady, N.; Akbari, N. Antimicrobial photothermal treatment of pseudomonas aeruginosa by a carbon nanoparticles-polypyrrole nanocomposite. J. Biomed. Phys. Eng. 2019, 9, 661. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Denich, T.; Beaudette, L.; Lee, H.; Trevors, J. Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J. Microbiol. Methods 2003, 52, 149–182. [Google Scholar] [CrossRef]
- Parsons, J.B.; Rock, C.O. Bacterial lipids: Metabolism and membrane homeostasis. Prog. Lipid Res. 2013, 52, 249–276. [Google Scholar] [CrossRef] [PubMed]
- Mező, E.; Hartmann-Balogh, F.; Madarászné Horváth, I.; Bufa, A.; Marosvölgyi, T.; Kocsis, B.; Makszin, L. Effect of culture conditions on fatty acid profiles of bacteria and lipopolysaccharides of the genus pseudomonas—GC-MS analysis on ionic liquid-based column. Molecules 2022, 27, 6930. [Google Scholar] [CrossRef]
- Wu, J.; Chu, Z.; Ruan, Z.; Wang, X.; Dai, T.; Hu, X. Changes of intracellular porphyrin, reactive oxygen species, and fatty acids profiles during inactivation of methicillin-resistant Staphylococcus aureus by antimicrobial blue light. Front. Physiol. 2018, 9, 1658. [Google Scholar] [CrossRef]




| Fatty Acid | P. aeruginosa (Control) | P. aeruginosa + PPy-NPs (Dark Control) | P. aeruginosa + PPy-NPs + NIR (a-PTI) |
|---|---|---|---|
| 16.0 | 37.95 ± 1.08 | 38.27 ± 0.33 | 31.57 ± 1.80 * |
| 16.1 Δ9 | 31.59 ± 1.47 | 26.55 ± 0.79 * | 15.70 ± 1.15 * |
| 18.0 | 5.06 ± 2.49 | 10.49 ± 1.59 * | 27.53 ± 3.10 * |
| 18.1 Δ11 | 25.38 ± 0.07 | 24.69 ± 1.13 | 18.18 ± 0.95 * |
| U/S | 1.33 | 1.05 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, M.D.; Bongiovanni Abel, S.; Barbero, C.A.; Paulucci, N.S.; Yslas, E.I. Low-Power NIR-Triggered Photothermal Inactivation of Pseudomonas aeruginosa with Polypyrrole Nanoparticles. Polymers 2025, 17, 1442. https://doi.org/10.3390/polym17111442
Gil MD, Bongiovanni Abel S, Barbero CA, Paulucci NS, Yslas EI. Low-Power NIR-Triggered Photothermal Inactivation of Pseudomonas aeruginosa with Polypyrrole Nanoparticles. Polymers. 2025; 17(11):1442. https://doi.org/10.3390/polym17111442
Chicago/Turabian StyleGil, Melina D., Silvestre Bongiovanni Abel, César A. Barbero, Natalia S. Paulucci, and Edith I. Yslas. 2025. "Low-Power NIR-Triggered Photothermal Inactivation of Pseudomonas aeruginosa with Polypyrrole Nanoparticles" Polymers 17, no. 11: 1442. https://doi.org/10.3390/polym17111442
APA StyleGil, M. D., Bongiovanni Abel, S., Barbero, C. A., Paulucci, N. S., & Yslas, E. I. (2025). Low-Power NIR-Triggered Photothermal Inactivation of Pseudomonas aeruginosa with Polypyrrole Nanoparticles. Polymers, 17(11), 1442. https://doi.org/10.3390/polym17111442









