Enhanced Lithium Recovery from Salt-Lake Brines via Advanced Nanofiltration Membranes: Polymeric Structure–Sieving Performance Relationships
Abstract
1. Introduction
2. Ionic Screening Mechanisms for NF Membranes
2.1. Nanopore Sieving Effect
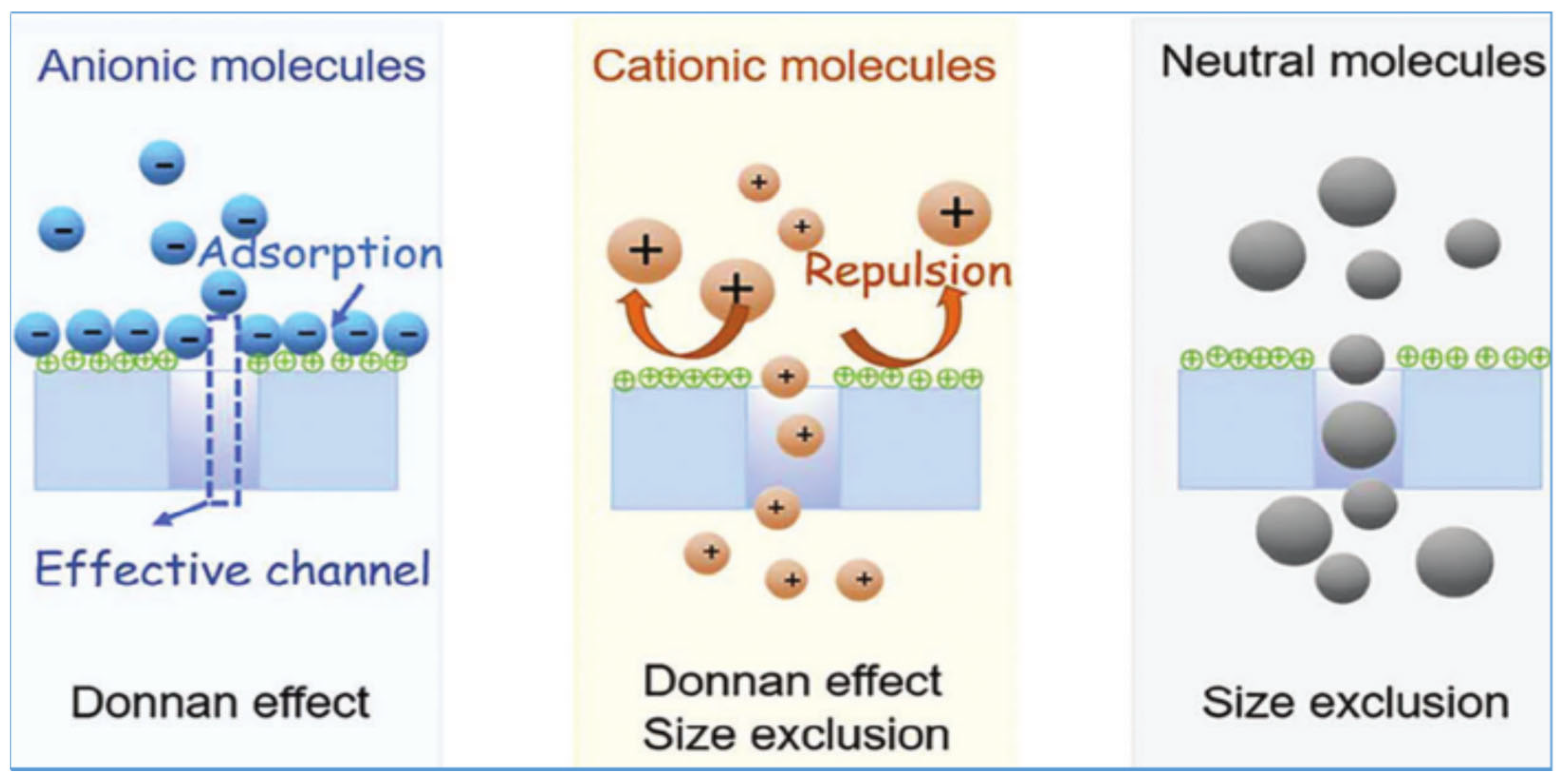
2.2. Donnan Electrostatic Effect
2.3. Dielectric Repulsion in Membrane Pores
3. Research Progress on Li Extraction Using Commercial Nanofiltration Membranes
4. Application of Positively Charged NF Membranes in Li Extraction from Salt-Lake Brines
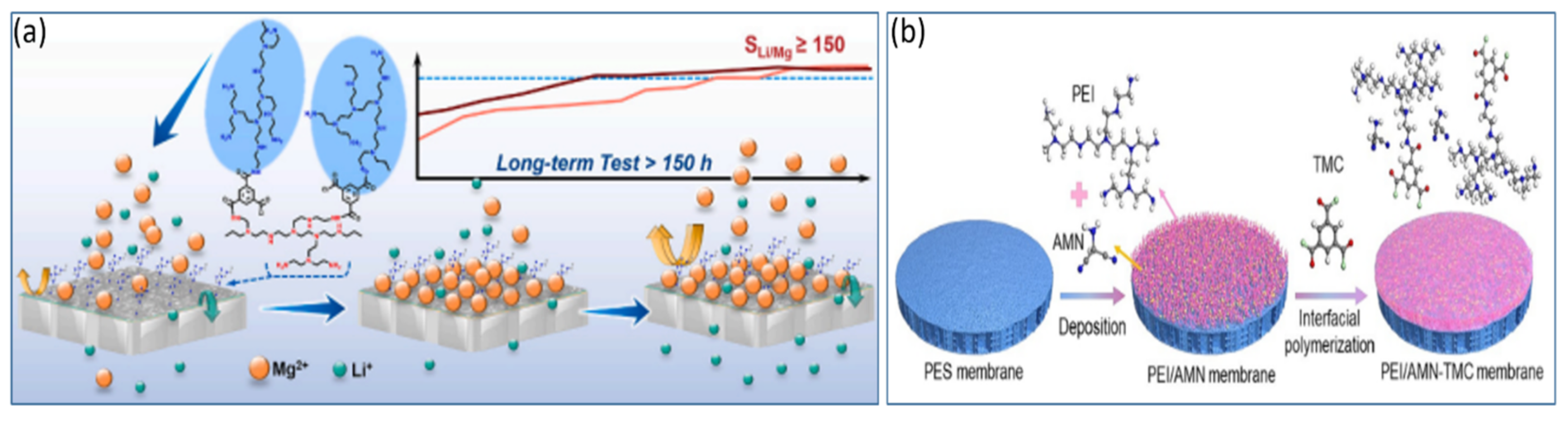
4.1. Positively Charged Modification of the Amine Group-Based Monolayer NF Membrane
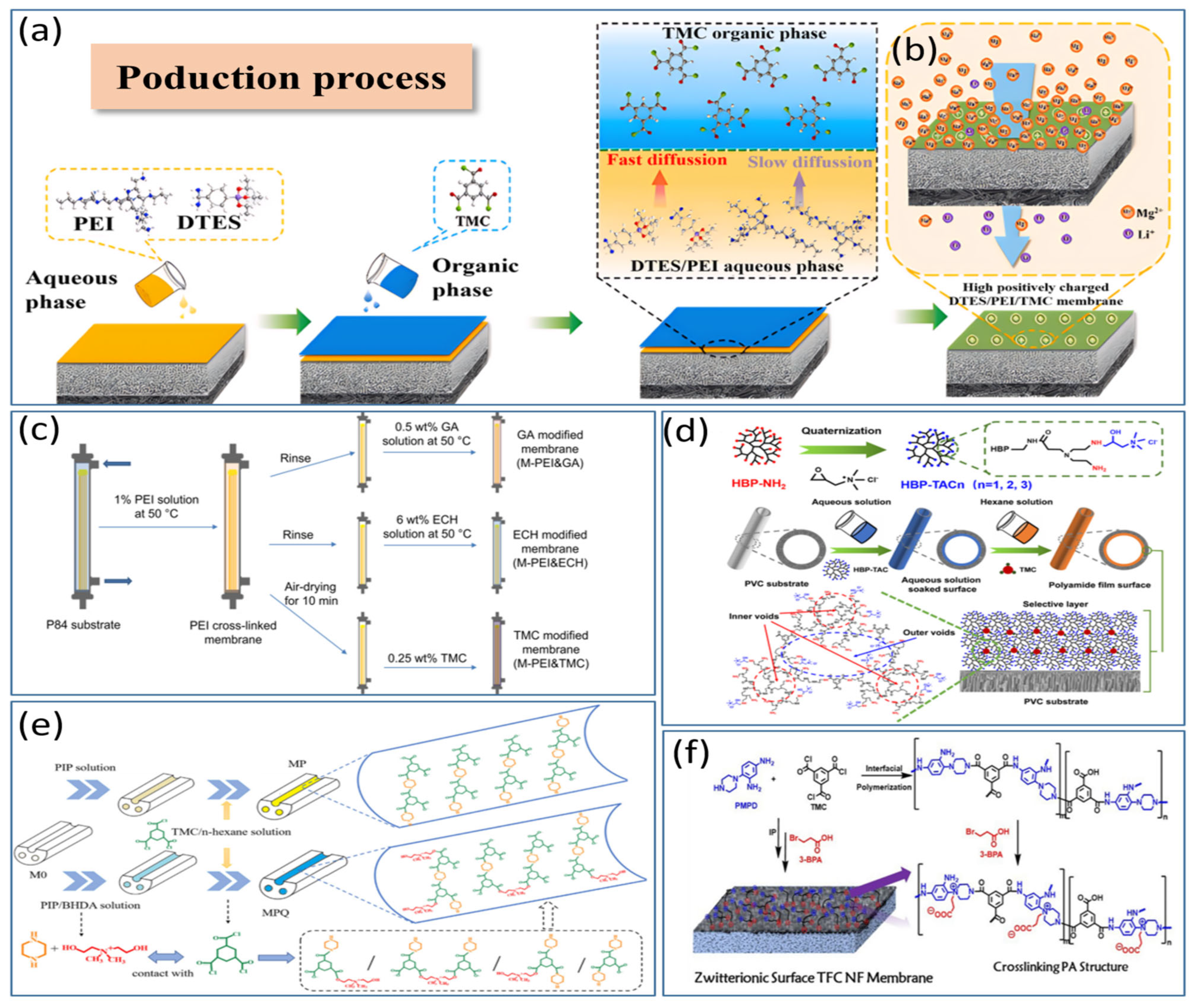
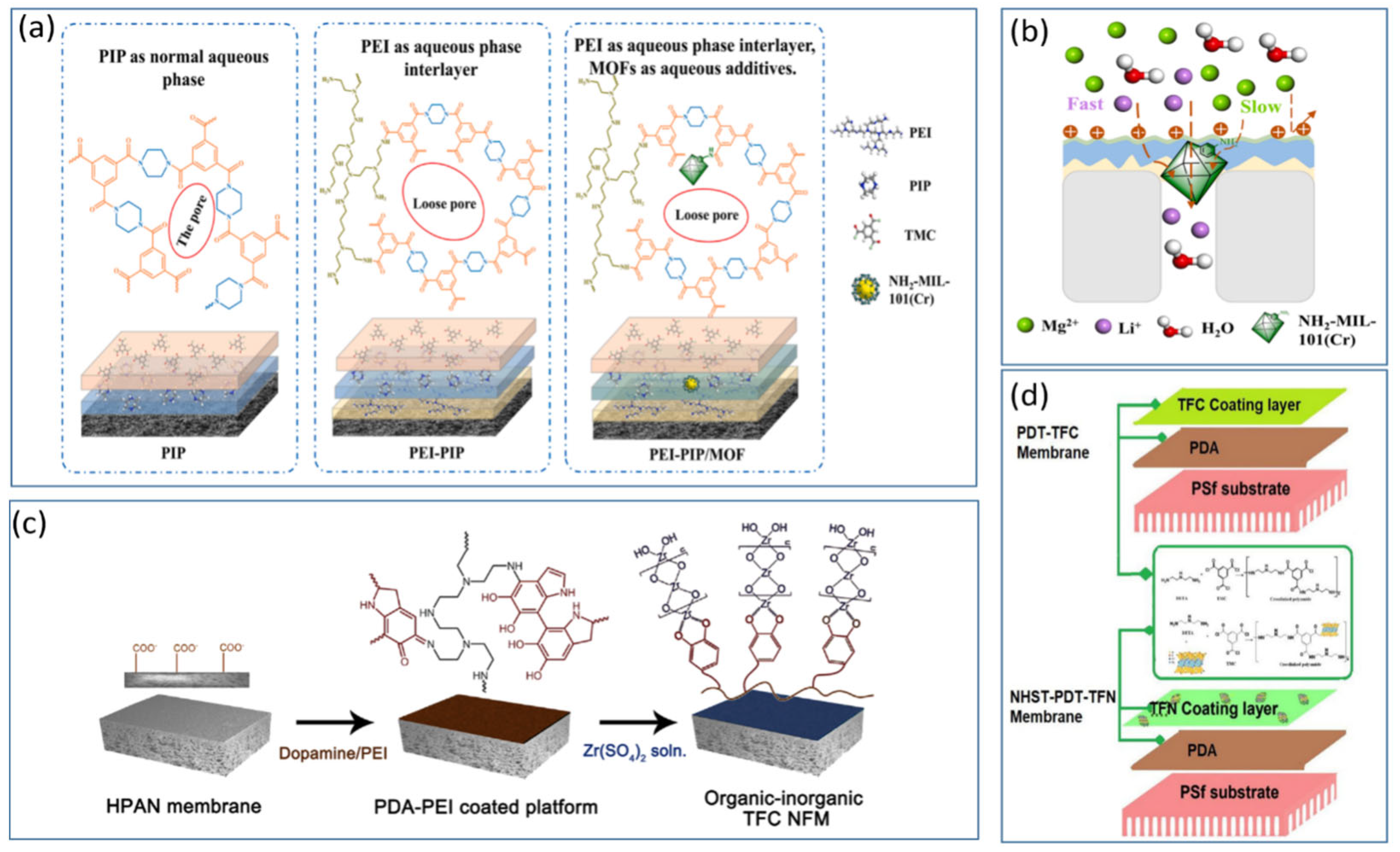
4.2. Modification of NF Membranes with Positively Charged Intermediate Composite Layers
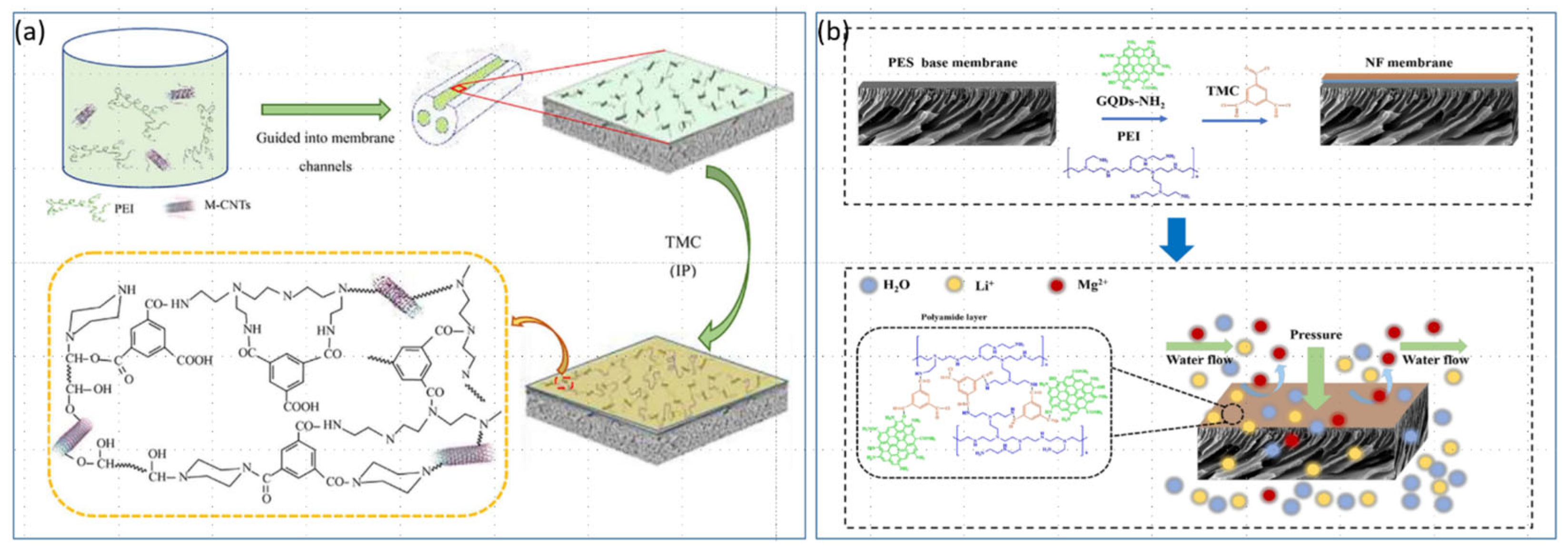
4.3. Modification of NF Membranes with Composite Inorganic Nanomaterials
4.4. Surface Grafting Modification of NF Membranes
5. Anti-Fouling Properties of NF Membranes with High Stability
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Su, H.; Zhu, Z.; Wang, L.; Qi, T. Progress and prospects of lithium extraction and recovery technologies from salt-lake brine. Mater. Rep. 2019, 33, 2119–2126. [Google Scholar] [CrossRef]
- Greim, P.; Solomon, A.A.; Breyer, C. Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat. Commun. 2020, 11, 4570. [Google Scholar] [CrossRef] [PubMed]
- Whelan, J.A. LITHIUM RESOURCES OF UTAH¹. 1976. Available online: https://books.google.com.hk/books?id=dzVSAQAAIAAJ&ots=tKCQuWFGMF&dq=Lithium%20Resources%20of%20Utah&lr&pg=PA75#v=onepage&q=Lithium%20Resources%20of%20Utah&f=false (accessed on 2 April 2025).
- Huston, D.L.; Doublier, M.; Downes, P. Geological Setting, Age and Endowment of Major Australian Mineral Deposits: A Compilation; Geoscience Australia: Canberra, Australia, 2021. [Google Scholar]
- Steinmetz, R.L.L.; Salvi, S. Brine grades in Andean salars: When basin size matters A review of the Lithium Triangle. Earth-Sci. Rev. 2021, 217, 103615. [Google Scholar] [CrossRef]
- Sabugueiro, A.J.S.S.E. Albemarle: Riding the EV Revolution—Is Lithium the Future? Master’s Thesis, Universidade NOVA de Lisboa (Portugal), Lisbon, Portugal, 2021. [Google Scholar]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Minmetals Securities. Yankuang Lithium (000792) Is Deeply Engaged in the Exploration and Development in Qarhan, Qinghai. It Is a Strategic Leader in China’s Salt Lake Potassium and Lithium Resources. Available online: https://pdf.dfcfw.com/pdf/H3_AP202108181510788872_1.pdf?1647441087000.pdf (accessed on 17 August 2021).
- ASX Announcement. Manono Project Mineral Resource Increases 47% to 842 Mt as Roche Dure Tonnages Expanded. Available online: https://static1.squarespace.com/static/5934d2ae6b8f5beeb5ba23f3/t/65b9b7436b31114064e7c0f4/170669896690/20240131 (accessed on 31 January 2024).
- ABH Engineering Inc. Updated Resource Estimate Zeus Lithium Project. Available online: https://noramlithiumcorp.com/site/assets/files/3997/2023-03-20-updated-resource-estimate-zeus.pdf (accessed on 17 March 2023).
- ASE Release. Greenbushes CY23 Resources and Reserves. Available online: https://www.igo.com.au/site/pdf/483721a4-afe7-4723-a907-6efce0c66748/Greenbushes-CY23-Resources-and-Reserves (accessed on 19 February 2024).
- Kidman Resources. Substantial Increase in Earl Grey Lithium Mineral Resource Estimate. Available online: https://wcsecure.weblink.com.au/pdf/KDR/01963105.pdf (accessed on 19 March 2018).
- Liontown. Mineral Resources, Reserves and CP Statements. Available online: https://www.ltresources.com.au/wp-content/uploads/2023/05/62d128633552295d6f75292b_Resources-Reserves-and-CP-Statements-2207.pdf (accessed on 21 June 2023).
- Kodal Minerals Plc. Mining Licence Application Lodged Feasibility Study Demonstrates Robust Economics for Development of the Bougouni Lithium Project. Available online: https://polaris.brighterir.com/public/kodal_minerals/news/rns/story/xl3g13x (accessed on 27 January 2020).
- Allkem Limited. Mt Cattlin Resource Update with Higher Grade. Available online: https://www.globenewswire.com/news-release/2023/04/17/2647623/0/en/Mt-Cattlin-Resource-Update-with-Higher-Grade.html (accessed on 17 April 2023).
- Global Lithium Resources. Manna Lithium Project Resource Grows. Available online: https://wcsecure.weblink.com.au/pdf/GL1/02690209.pdf (accessed on 26 July 2023).
- Ioneer Ltd. Technical Report Summary of the Rhyolite Ridge Lithium-Boron Project. Available online: https://www.sec.gov/Archives/edgar/data/1896084/000114036121040692/filename5.htm (accessed on 30 September 2021).
- Rio Tinto. Rio Tinto Updates Ore Reserves and Mineral Resources at Jadar. Available online: https://www.riotinto.com/-/media/Content/Documents/Invest/Reserves-and-resources/2021/RT-Jadar-reserves-resources-2021.pdf?rev=e1220b9671424ad9b275dd6bdd2ed480 (accessed on 23 February 2022).
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. Water Res. Technol. 2017, 3, 37–53. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, H.; Xu, Z. Current status and progress of lithium extraction from salt-lake brine and seawater by membrane method. Technol. Water Treat. 2017, 43, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Q.-B.; Wang, J.; Li, P.-F.; Zhao, J. Fractionation of monovalent ions from seawater brine via softening nanofiltration and selective electrodialysis: Which is better? Desalination 2022, 533, 115717. [Google Scholar] [CrossRef]
- Yang, Z.; Fang, W.; Wang, Z.; Zhang, R.; Zhu, Y.; Jin, J. Dual-skin layer nanofiltration membranes for highly selective Li+/Mg2+ separation. J. Membr. Sci. 2021, 620, 118862. [Google Scholar] [CrossRef]
- Wang, S.H. Study on Preparation of Crown Ether Functionalized Nanofiltration Membrane and Its Lithium-Magnesium Separation Performance. Master’s Thesis, Tianjin Polytechnic University, Tianjin, China, 2021. [Google Scholar] [CrossRef]
- Xu, Y. Research on Quaternary Ammonium Salt Modified Polyethyleneimine Composite Nanofiltration Membrane and Magnesium-Lithium Separation Performance. Master’s Thesis, Wuhan Institute of Technology, Wuhan, China, 2022. [Google Scholar] [CrossRef]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent advances in nanofibrous membranes: Production and applications in water treatment and desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Xu, P.; Hong, J.; Xu, Z.; Xia, H.; Ni, Q.-Q. MWCNTs-COOK-assisted high positively charged composite membrane: Accelerating Li+ enrichment and Mg2+ removal. Compos. Part B Eng. 2021, 212, 118796. [Google Scholar] [CrossRef]
- Shen, K.; Cheng, C.; Zhang, T.; Wang, X. High performance polyamide composite nanofiltration membranes via reverse interfacial polymerization with the synergistic interaction of gelatin interlayer and trimesoyl chloride. J. Membr. Sci. 2019, 588, 117192. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Trilles, C.A.; De Guzman, M.R.; Pereira, J.M.; Aquino, R.R.; Huang, S.-H.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Improved performance of thin-film nanocomposite nanofiltration membranes as induced by embedded polydopamine-coated silica nanoparticles. Sep. Purif. Technol. 2019, 224, 113–120. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, W.; Wan, Z.; Qin, S.; Huang, Q.; Cai, W.; Wang, Q.; Yao, M.; Zhang, Y. Electrochemically Mediated Lithium Extraction for Energy and Environmental Sustainability. Adv. Funct. Mater. 2024, 34, 2400416. [Google Scholar] [CrossRef]
- Zhao, H. Research on the Preparation of Positively Charged PEI/PSf Nanocomposite Nanofiltration Membrane and the Separa-tion of Lithium and Magnesium. Master’s Thesis, Beijing Jiaotong University, Beijing, China, 2022. [Google Scholar] [CrossRef]
- Han, M.J.; Baroña, G.N.B.; Jung, B. Effect of surface charge on hydrophilically modified poly(vinylidene fluoride) membrane for microfiltration. Desalination 2011, 270, 76–83. [Google Scholar] [CrossRef]
- Guo, C. Construction of Charged-Regulated and Ultrathin Nanofiltration Membranes and Their Magnesium-Lithium Separation Performance. Master’s Thesis, Tianjin Polytechnic University, Tianjin, China, 2021. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Chen, H.; Rose, M.; Fleming, M.; Souizi, S.; Shashvatt, U.; Blaney, L. Recent advances in Donnan dialysis processes for water/wastewater treatment and resource recovery: A critical review. Chem. Eng. J. 2023, 455, 140522. [Google Scholar] [CrossRef]
- Labban, O.; Liu, C.; Chong, T.H.; Lienhard, J.H. Relating transport modeling to nanofiltration membrane fabrication: Navigating the permeability-selectivity trade-off in desalination pretreatment. J. Membr. Sci. 2018, 554, 26–38. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Wang, H.; Wang, M. The application of nanofiltration membrane for recovering lithium from salt lake brine. Desalination 2019, 468, 114081. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lau, S.K.; Yong, W.F. Recent advances of thin film composite nanofiltration membranes for Mg2+/Li+ separation. Adv. Membr. 2024, 4, 100093. [Google Scholar] [CrossRef]
- Liu, W.; Wang, K.; Wang, X.; Huang, X. High-Selectivity Nanofiltration Separation Technology Facilitates Resource Extraction and Recovery in High-Salt Environments. Environ. Eng. 2024, 42, 29–41. [Google Scholar] [CrossRef]
- Lu, D.; Xiang, X.; Geng, Y.; Wang, M.; Yao, Z.; Gao, Z.; Fang, C.; Zhu, L.; Zhang, L. Design strategy of polyamide nanofiltration membrane for salt-lake lithium extraction. Salt-Lake Res. 2025, 33, 100–112. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.M.; Li, D.; Gao, Y.; Wang, K.; Huang, X. Dominant Mechanism of Nanofiltration for Chloride/Sulfate Ion Separation in High Salinity Solutions: The Quantification of Pore Size-Influenced Dielectric Exclusion. Environ. Sci. Technol. 2025, 59, 5848–5855. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lin, S.; Li, X.; Wang, Z. The Hidden Role of the Dielectric Effect in Nanofiltration: A Novel Perspective to Unravel New Ion Separation Mechanisms. Environ. Sci. Technol. 2024, 58, 15874–15884. [Google Scholar] [CrossRef]
- Yang, G.; Shi, H.; Liu, W.; Xing, W.; Xu, N. Investigation of Mg2+/Li+ Separation by Nanofiltration. Chin. J. Chem. Eng. 2011, 19, 586–591. [Google Scholar] [CrossRef]
- Bi, Q.; Zhang, Z.; Zhao, C.; Tao, Z. Study on the recovery of lithium from high Mg2+/Li+ ratio brine by nanofiltration. Water Sci. Technol. 2014, 70, 1690–1694. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-Y.; Cai, L.-J.; Nie, X.-Y.; Song, X.; Yu, J.-G. Separation of magnesium and lithium from brine using a Desal nanofiltration membrane. J. Water Process Eng. 2015, 7, 210–217. [Google Scholar] [CrossRef]
- Somrani, A.; Hamzaoui, A.H.; Pontie, M. Study on lithium separation from salt lake brines by nanofiltration (NF) and low pressure reverse osmosis (LPRO). Desalination 2013, 317, 184–192. [Google Scholar] [CrossRef]
- Nan, Q.; Li, P.; Cao, B. Fabrication of positively charged nanofiltration membrane via the layer-by-layer assembly of graphene oxide and polyethylenimine for desalination. Appl. Surf. Sci. 2016, 387, 521–528. [Google Scholar] [CrossRef]
- Dey, T.K.; Bindal, R.C.; Prabhakar, S.; Tewari, P.K. Development, Characterization and Performance Evaluation of Positively-Charged Thin Film-Composite Nanofiltration Membrane Containing Fixed Quaternary Ammonium Moieties. Sep. Sci. Technol. 2011, 46, 933–943. [Google Scholar] [CrossRef]
- Leniz-Pizarro, F.; Liu, C.; Colburn, A.; Escobar, I.C.; Bhattacharyya, D. Positively charged nanofiltration membrane synthesis, transport models, and lanthanides separation. J. Memb. Sci. 2021, 620, 118973. [Google Scholar] [CrossRef]
- Zhao, Z.; Di, N.; Zha, Z.; Wang, J.; Wang, Z.; Zhao, S. Positively Charged Polyamine Nanofiltration Membrane for Precise Ion-Ion Separation. ACS Appl. Mater. Interfaces 2023, 15, 48695–48704. [Google Scholar] [CrossRef]
- Xu, P.; Wang, W.; Qian, X.; Wang, H.; Guo, C.; Li, N.; Xu, Z.; Teng, K.; Wang, Z. Positive charged PEI-TMC composite nanofiltration membrane for separation of Li+ and Mg2+ from brine with high Mg2+/Li+ ratio. Desalination 2019, 449, 57–68. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, H.; Lin, Y.; Liu, X.; Wang, L.; Yao, H.; Tang, Y.; Yu, L.; Wang, H.; Wang, X. Positively-charged PEI/TMC nanofiltration membrane prepared by adding a diamino-silane coupling agent for Li+/Mg2+ separation. J. Membr. Sci. 2023, 672, 121468. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, X.; Wang, J.; Tang, C.Y. Novel polyethyleneimine/TMC-based nanofiltration membrane prepared on a polydopamine coated substrate. Front. Chem. Sci. Eng. 2017, 12, 273–282. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Wang, Y.; Ding, Y.; Zhang, W.; Liu, F. Interfacial polymerized polyamide nanofiltration membrane by demulsification of hexane-in-water droplets through hydrophobic PTFE membrane: Membrane performance and formation mechanism. Sep. Purif. Technol. 2021, 275, 119227. [Google Scholar] [CrossRef]
- Bi, Q.; Zhang, C.; Liu, J.; Liu, X.; Xu, S. Positively charged zwitterion-carbon nitride functionalized nanofiltration membranes with excellent separation performance of Mg2+/Li+ and good antifouling properties. Sep. Purif. Technol. 2021, 257, 117959. [Google Scholar] [CrossRef]
- Gao, J.; Sun, S.-P.; Zhu, W.-P.; Chung, T.-S. Green modification of outer selective P84 nanofiltration (NF) hollow fiber membranes for cadmium removal. J. Membr. Sci. 2016, 499, 361–369. [Google Scholar] [CrossRef]
- Gu, K.; Wang, K.; Zhou, Y.; Gao, C. Ion-promoting-penetration phenomenon in the polyethyleneimine/trimesic acid nanofiltration membrane. Sep. Purif. Technol. 2021, 257, 117958. [Google Scholar] [CrossRef]
- Mu, T.; Zhang, H.-Z.; Sun, J.-Y.; Xu, Z.-L. Three-channel capillary nanofiltration membrane with quaternary ammonium incorporated for efficient heavy metals removal. Sep. Purif. Technol. 2020, 248, 117133. [Google Scholar] [CrossRef]
- Qiu, Z.-L.; Yu, W.-H.; Shen, Y.-J.; Zhu, B.-K.; Fang, L.-F. Cationic hyperbranched poly(amido-amine) engineered nanofiltration membrane for molecular separation. J. Membr. Sci. 2021, 629, 119275. [Google Scholar] [CrossRef]
- Li, S.-L.; Wu, P.; Wang, J.; Hu, Y. High-performance zwitterionic TFC polyamide nanofiltration membrane based on a novel triamine precursor. Sep. Purif. Technol. 2020, 251, 117380. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, G.; Cheng, Q.; Yu, S.; Liu, M.; Gao, C. Positively charged thin-film composite hollow fiber nanofiltration membrane for the removal of cationic dyes through submerged filtration. Desalination 2013, 328, 42–50. [Google Scholar] [CrossRef]
- Wang, M.; Dong, W.; Guo, Y.; Zhai, Z.; Feng, Z.; Hou, Y.; Li, P.; Niu, Q.J. Positively charged nanofiltration membranes mediated by a facile polyethyleneimine-Noria interlayer deposition strategy. Desalination 2021, 513, 114836. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Fang, L.F.; Shen, Y.J.; Yu, W.H.; Zhu, B.K.; Helix-Nielsen, C.; Zhang, W. Ionic Dendrimer Based Polyamide Membranes for Ion Separation. ACS Nano 2021, 15, 7522–7535. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Zheng, J.; Hou, S.; Mao, H.; Zhang, S. Green fabrication of a positively charged nanofiltration membrane by grafting poly(ethylene imine) onto a poly (arylene ether sulfone) membrane containing tertiary amine groups. J. Membr. Sci. 2016, 517, 39–46. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; He, M.; Zhang, Z.; Chen, J.; Yang, G. Fabrication of high-performance nanofiltration membranes by using sulfated cellulose nanofibril as the intermediate support layer. Desalination 2022, 532, 115741. [Google Scholar] [CrossRef]
- Li, B.; Yang, Z.; Dou, Y.; Zhang, J.; Lu, J.; Han, J. Two-Dimensional LDH Film Templating for Controlled Preparation and Performance Enhancement of Polyamide Nanofiltration Membranes. Angew. Chem Int. Ed. Engl. 2023, 62, e202304442. [Google Scholar] [CrossRef]
- Wu, M.-B.; Lv, Y.; Yang, H.-C.; Liu, L.-F.; Zhang, X.; Xu, Z.-K. Thin film composite membranes combining carbon nanotube intermediate layer and microfiltration support for high nanofiltration performances. J. Membr. Sci. 2016, 515, 238–244. [Google Scholar] [CrossRef]
- Ma, L.; Bi, Q.; Zhou, W.; Liu, X.; Qi, F.; Zhang, H.; Gao, Y.; Xu, S. Nanofiltration membrane with a zwitterion-g-C3N4 composite interlayer for Mg2+/Li+ separation. J. Water Process Eng. 2023, 53, 103751. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Xu, Z.-L.; Ding, H.; Tang, Y.-J. Positively charged capillary nanofiltration membrane with high rejection for Mg2+ and Ca2+ and good separation for Mg2+ and Li+. Desalination 2017, 420, 158–166. [Google Scholar] [CrossRef]
- Ma, X.-H.; Yang, Z.; Yao, Z.-K.; Xu, Z.-L.; Tang, C.Y. A facile preparation of novel positively charged MOF/chitosan nanofiltration membranes. J. Membr. Sci. 2017, 525, 269–276. [Google Scholar] [CrossRef]
- Xu, P.; Hong, J.; Xu, Z.; Xia, H.; Ni, Q.-Q. Novel aminated graphene quantum dots (GQDs-NH2)-engineered nanofiltration membrane with high Mg2+/Li+ separation efficiency. Sep. Purif. Technol. 2021, 258, 118042. [Google Scholar] [CrossRef]
- Jia, R.; Wu, L.-K.; Xu, Z.-L.; Hedar, M.; Luo, L.-H.; Wu, Y.-Z.; Li, H.-X.; Tong, Y.-H.; Xu, S.-J. Efficient separation of Li+/Mg2+ via positively charged TFN membrane based on the PEI interlayer. Chem. Eng. Sci. 2024, 284, 119523. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, H.-C.; Liang, H.-Q.; Wan, L.-S.; Xu, Z.-K. Novel nanofiltration membrane with ultrathin zirconia film as selective layer. J. Membr. Sci. 2016, 500, 265–271. [Google Scholar] [CrossRef]
- Karki, S.; Gohain, M.B.; Yadav, D.; Thakare, N.R.; Pawar, R.R.; Hazarika, S.; Ingole, P.G. Building rapid water transport channels within thin-film nanocomposite membranes based on 2D mesoporous nanosheets. Desalination 2023, 547, 116222. [Google Scholar] [CrossRef]
- Fang, L.-F.; Zhou, M.-Y.; Cheng, L.; Zhu, B.-K.; Matsuyama, H.; Zhao, S. Positively charged nanofiltration membrane based on cross-linked polyvinyl chloride copolymer. J. Membr. Sci. 2019, 572, 28–37. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Zhou, A.; He, X.; Sun, Y.; Zhang, J. A positively charged composite nanofiltration membrane modified by EDTA for LiCl/MgCl2 separation. Sep. Purif. Technol. 2017, 186, 233–242. [Google Scholar] [CrossRef]
- Gu, T.; Zhang, R.; Zhang, S.; Shi, B.; Zhao, J.; Wang, Z.; Long, M.; Wang, G.; Qiu, T.; Jiang, Z. Quaternary ammonium engineered polyamide membrane with high positive charge density for efficient Li+/Mg2+ separation. J. Membr. Sci. 2022, 659, 120802. [Google Scholar] [CrossRef]
- Wu, H.; Lin, Y.; Feng, W.; Liu, T.; Wang, L.; Yao, H.; Wang, X. A novel nanofiltration membrane with [MimAP][Tf2N] ionic liquid for utilization of lithium from brines with high Mg2+/Li+ ratio. J. Membr. Sci. 2020, 603, 117997. [Google Scholar] [CrossRef]
- Feng, Y.; Peng, H.; Zhao, Q. Fabrication of high performance Mg2+/Li+ nanofiltration membranes by surface grafting of quaternized bipyridine. Sep. Purif. Technol. 2022, 280, 119848. [Google Scholar] [CrossRef]
- Lu, D.; Ma, T.; Lin, S.; Zhou, Z.; Li, G.; An, Q.; Yao, Z.; Sun, Q.; Sun, Z.; Zhang, L. Constructing a selective blocked-nanolayer on nanofiltration membrane via surface-charge inversion for promoting Li+ permselectivity over Mg2+. J. Membr. Sci. 2021, 635, 119504. [Google Scholar] [CrossRef]
- Vatanpour, V.; Esmaeili, M.; Safarpour, M.; Ghadimi, A.; Adabi, J. Synergistic effect of carboxylated-MWCNTs on the performance of acrylic acid UV-grafted polyamide nanofiltration membranes. React. Funct. Polym. 2019, 134, 74–84. [Google Scholar] [CrossRef]
- Peng, H.; Zhao, Q. A Nano-Heterogeneous Membrane for Efficient Separation of Lithium from High Magnesium/Lithium Ratio Brine. Adv. Funct. Mater. 2021, 31, 2009430. [Google Scholar] [CrossRef]
- Soyekwo, F.; Wen, H.; Liao, D.; Liu, C. Fouling-resistant ionic graft-polyamide nanofiltration membrane with improved permeance for lithium separation from MgCl2/LiCl mixtures. J. Membr. Sci. 2022, 659, 120773. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, Y.; Xu, X.; Peng, X.; Niu, Y.; Xu, P.; Li, T. Research on the factors influencing nanofiltration membrane fouling and the prediction of membrane fouling. J. Water Process Eng. 2024, 59, 104876. [Google Scholar] [CrossRef]
- Schafer, A.I.; Akanyeti, I.; Semiao, A.J. Micropollutant sorption to membrane polymers: A review of mechanisms for estrogens. Adv. Colloid Interface Sci. 2011, 164, 100–117. [Google Scholar] [CrossRef]
- Khan, I.A.; Kim, J.O. Role of inorganic foulants in the aging and deterioration of low-pressure membranes during the chemical cleaning process in surface water treatment: A review. Chemosphere 2023, 341, 140073. [Google Scholar] [CrossRef]
- Zhu, L. Rejection of Organic Micropollutants by Clean and Fouled Nanofiltration Membranes. J. Chem. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Fang, W.; Shi, L.; Wang, R. Interfacially polymerized composite nanofiltration hollow fiber membranes for low-pressure water softening. J. Membr. Sci. 2013, 430, 129–139. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, F.; Wang, L.; Zhen, H.; Zhang, N.; Yin, S.; Zhou, G.; Ruan, X.; He, G.; Jiang, X. Nanofiltration membrane with modified nano-gradient structure and positive charge for Li separation from high Mg/Li ratio brine. Desalination 2024, 577, 117394. [Google Scholar] [CrossRef]
- Li, J.; Fang, L.; Xu, D.; Zhang, X.; Jiang, L.; Zhu, Q.; Chen, Q.; Jin, P.; Volodine, A.; Dewil, R.; et al. Intercalation of small molecules in the selective layer of polyamide nanofiltration membranes facilitates the separation of Mg2+/Li+. Chem. Eng. J. 2024, 487, 150659. [Google Scholar] [CrossRef]



| Lake Name | Region/ Country | Lake Type | Lithium Content | References |
|---|---|---|---|---|
| 1. Great Salt Lake | Utah, USA | Chloride type | Mean 48 mg/L, Resource 0.44 Mt Li | [3] |
| 2. Clayton Valley | Nevada, USA | Sulfate type | Mean 146 mg/L, Resource 0.05 Mt Li | [4] |
| 3. Salar de Uyuni | Potosi, Bolivia | Chloride type | Mean 715 mg/L, Resource 9.00 Mt Li | [5] |
| 4. Salar de Atacama | Antofagasta, Chile | Sulfate type | Mean 1880 mg/L, Resource 9.60 Mt Li | [6] |
| 5. Salar de Uyuni | Salar de Uyuni, Bolivia | Sulfate type | Mean 715 mg/L, Resource 9.00 Mt Li | [5] |
| 6. Zhabuye Salt Lake | Shigatse, Tibet, China | Carbonate type | Mean 1467 mg/L, Resource 1.53 Mt Li | [7] |
| 7. West/East Taijinar Lake | Qinghai, China | Magnesium sulfate subtype | Mean 171 mg/L, Resource 0.90 Mt Li | [7] |
| 8. Qarhan | Qinghai, China | Chloride type | Mean 126 mg/L, Resource 4.82 Mt Li | [8] |
| 9. Salar del Hombre Muerto | Catamarca, Argentina | Sulfate type | Mean 628 mg/L, Resource 3.61 Mt Li | [5] |
| 10. Salar de Coipasa | Potosi, Bolivia | Sulfate type | Mean 258 mg/L, Resource 0.20 Mt Li | [5] |
| Salt Mine Name | Region/Country | Deposit Type | Lithium Content | References |
|---|---|---|---|---|
| 1. Manono | Tanganyika, DR Congo | Pegmatite | Average 7563 ppm, Resource 3.78 Mt Li | [9] |
| 2. Cyprees—Zeus | Nevada, USA | Volcano-sedimentary | Average 1189 ppm, Resource 0.48 Mt Li | [10] |
| 3. Greenbushes | Western Australia | Pegmatite | Average 6960 ppm, Resource 2.84 Mt Li | [11] |
| 4. Earl Grey—Mt Holland | Western Australia | Pegmatite | Average 7162 ppm, Resource 1.23 Mt Li | [12] |
| 5. Mina de Cachoeira | Minas Gerais, Brazil | Pegmatite | Average 6496 ppm, Resource 0.72 Mt Li | [13] |
| 6. Bougouni | Sikaso, Mali | Pegmatite | Average 5243 ppm, Resource 0.06 Mt Li | [14] |
| 7. Mt Cattlin | Great Southern Australia | Pegmatite | Average 6044 ppm, Resource 0.07 Mt Li | [15] |
| 8. Manna | Western Australia | Pegmatite | Average 5197 ppm, Resource 0.10 Mt Li | [16] |
| 9. Rhyolite Ridge | Nevada, USA | Volcano-sedimentary | Average 1600 ppm, Resource 0.12 Mt Li | [17] |
| 10. Jadar | Western Serbia | Volcano-sedimentary | Average 7795 ppm, Resource 0.67 Mt Li | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Zheng, Y.; Zhang, X.; Tan, M.; Wang, J.; Tian, G. Enhanced Lithium Recovery from Salt-Lake Brines via Advanced Nanofiltration Membranes: Polymeric Structure–Sieving Performance Relationships. Polymers 2025, 17, 1440. https://doi.org/10.3390/polym17111440
Li R, Zheng Y, Zhang X, Tan M, Wang J, Tian G. Enhanced Lithium Recovery from Salt-Lake Brines via Advanced Nanofiltration Membranes: Polymeric Structure–Sieving Performance Relationships. Polymers. 2025; 17(11):1440. https://doi.org/10.3390/polym17111440
Chicago/Turabian StyleLi, Ruilin, Yong Zheng, Xu Zhang, Mengfei Tan, Jinhui Wang, and Guiying Tian. 2025. "Enhanced Lithium Recovery from Salt-Lake Brines via Advanced Nanofiltration Membranes: Polymeric Structure–Sieving Performance Relationships" Polymers 17, no. 11: 1440. https://doi.org/10.3390/polym17111440
APA StyleLi, R., Zheng, Y., Zhang, X., Tan, M., Wang, J., & Tian, G. (2025). Enhanced Lithium Recovery from Salt-Lake Brines via Advanced Nanofiltration Membranes: Polymeric Structure–Sieving Performance Relationships. Polymers, 17(11), 1440. https://doi.org/10.3390/polym17111440








