Abstract
Hydrogels are pivotal in advanced materials, driving innovations in medical fields, such as targeted drug delivery, regenerative medicine, and skin repair. This systematic review explores the transformative impact of in-silico design on hydrogel development, leveraging computational tools such as molecular dynamics, finite element modeling, and artificial intelligence to optimize synthesis, characterization, and performance. We analyze cutting-edge strategies for tailoring the physicochemical properties of hydrogels, including their mechanical strength, biocompatibility, and stimulus responsiveness, to meet the needs of next-generation biomedical applications. By integrating machine learning and computational modeling with experimental validation, this review highlights how in silico approaches accelerate material innovation, addressing challenges and outlining future directions for scalable, personalized hydrogel solutions in regenerative medicine and beyond.
1. Introduction
Hydrogels, characterized by their high-water content, biocompatibility, tunable mechanical properties, and ability to emulate the extracellular matrix, are pivotal in advancing biomedical applications, such as controlled drug delivery, tissue engineering, wound healing, and biosensing [1,2,3,4]. These versatile materials have garnered significant attention because of their ability to address complex clinical challenges, including targeted therapeutic delivery and regenerative medicine. However, traditional hydrogel development, which relies on empirical and iterative experimental approaches, is often resource intensive and time consuming, limiting the pace of innovation.
The advent of in silico methodologies has revolutionized hydrogel research by introducing computational tools that increase the precision, efficiency, and predictability of material design [5,6,7]. Techniques such as molecular dynamics simulations, finite element analysis, and machine learning enable researchers to model hydrogel behavior at the molecular and macroscopic scales, elucidating the structure-property-activity relationships critical for tailoring functionalities [8,9,10,11,12]. Molecular simulations provide insights into crosslinking mechanisms, network stability, mechanical properties, and degradation kinetics, whereas machine learning-driven models analyze extensive datasets to optimize polymer compositions for specific biomedical applications [13,14,15,16,17,18,19]. These computational strategies bridge theoretical predictions with experimental outcomes, offering a deeper understanding of hydrogel interactions with biological systems, including cells, tissues, and therapeutic agents, under physiological conditions [15,20,21]. This review systematically explores how in silico approaches are transforming hydrogel design, characterization, and application, paving the way for next-generation biomedical solutions.
2. Literature Parsing and Analysis
2.1. Focused Questions
This systematic review investigates whether in silico strategies, including molecular modeling and machine learning, significantly contribute to the rational drug design, simulation, and biomedical application of hydrogels. The focus is on their role in enhancing drug delivery efficiency, mechanical properties, and the biocompatibility of hydrogel systems used in various therapeutic contexts.
2.2. Eligibility Criteria
An extensive review of the literature was performed utilizing the PubMed, Google Scholar, Web of Science, and Scopus databases to retrieve relevant research. The search methodology incorporated controlled vocabulary terms and free-text keywords to maximize the identification of relevant publications.
The review included peer-reviewed articles and research articles written in English and published up to 2025. Emphasis has been placed on the literature from the past 20 years to reflect recent advancements in computational and data-driven techniques. Eligible studies had to meet specific inclusion criteria: (1) focus on hydrogel or hydrogel-like materials with explicit biomedical applications; (2) use computational modeling methods; (3) integrate machine learning techniques; and (4) present a clear methodological framework explaining the computational tools used and the results obtained or validated.
Studies were excluded if they lacked a computational or machine learning component, focused solely on nonhydrogel biomaterials, were purely experimental without in silico analysis, or were classified as letters to the editor, opinion pieces, or other non-peer-reviewed forms of publication.
3. Classification of Hydrogels
Hydrogels can be broadly divided into natural and synthetic categories (Table 1). Natural hydrogels derived from polysaccharides such as alginate and chitosan and proteins such as collagen and elastin are valued for their biocompatibility and biodegradability but often suffer from limitations in mechanical strength and batch-to-batch consistency [22,23,24]. Synthetic hydrogels, typically fabricated from polymers such as polyethylene glycol (PEG), polyvinyl alcohol (PVA), and polyacrylamide (PAM), offer enhanced mechanical and chemical stability but may have lower biocompatibility [25,26,27].

Table 1.
Classification of hydrogels on the basis of various criteria [27].
Hydrogels are also classified by their polymer architecture into homopolymeric, copolymeric, and interpenetrating polymer network (IPN) hydrogels, each with distinct advantages and limitations [28,29]. Their swelling behavior, which is crucial for drug delivery, is quantified by the equilibrium swelling ratio, often described by the Flory–Rehner model [30]. Hydrogels can be fabricated via physical or chemical crosslinking methods, and their degradation profiles can be tuned for biomedical applications [31,32,33,34].
Analytical techniques such as Fourier transform infrared (FTIR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, X-ray diffraction (XRD), and ultraviolet–visible (UV-vis) spectroscopy are utilized to investigate the structural features and chemical composition of hydrogels [11,19,35,36]. The classification of hydrogels on the basis of physical and chemical parameters is presented in Table 2.

Table 2.
Classification of hydrogels on the basis of physical and chemical parameters [37].
Hydrogels exhibit diverse physicochemical properties, including viscoelastic and mechanical characteristics, which directly influence their performance, stability, and bioactivity [19,35,36,38]. Techniques such as rheometry and dynamic mechanical analysis (DMA) are commonly used to assess the mechanical characteristics of hydrogels [39,40,41]. Morphological characterization methods, such as atomic force microscopy (AFM), transmission electron microscopy (TEM), light microscopy (LM), scanning electron microscopy (SEM), and micro-CT (microcomputed tomography), along with wide-angle X-ray scattering (WAXS) and small-angle X-ray scattering (SAXS), support the assessment of nanoscale morphology [11,42,43,44].
Polymer networks called hydrogels are essential for many biomedical applications (Table 3). They are primarily categorized into natural and synthetic types, each with distinct advantages and limitations. Natural hydrogels, including collagen, gelatin, hyaluronic acid (HA), and chitosan, are favored for their superior biocompatibility and biodegradability but often exhibit suboptimal mechanical properties [11,45]. Synthetic hydrogels, such as polyethylene glycol (PEG) derivatives, polycaprolactone (PCL), polyvinyl alcohol (PVA), and poly-N-isopropylacrylamide (PNIPAAm), offer increased mechanical durability but typically have poor biocompatibility [46,47].

Table 3.
Summary of typical hydrogel materials and their physicochemical properties [47].
3.1. Natural Hydrogels
Among natural hydrogels, collagen stands out because of its abundance in the extracellular matrix (ECM). Collagen-based hydrogels can be created by self-assembly triggered by temperature and pH, featuring cell-adhesion peptides, such as RGD (arginine-glycine-aspartic acid), which allow for integration with cellular receptors [48,49]. However, the limited mechanical qualities and batch-to-batch variability of collagen have prevented its wider use. Chemical cross-linking techniques, such as glycation and enzyme-mediated cross-linking, have been used to increase the stability and mechanical strength of collagen hydrogels [50].
Gelatin, derived from collagen through partial hydrolysis, shares many structural similarities with its parent protein but lacks the native triple helix. It is a preferred alternative to collagen because of its biodegradability and similarity in composition. Gelatin hydrogels can be chemically cross-linked through methods such as transglutaminase-induced cross-linking or photoinduced covalent bonding, particularly with methacrylate modifications [43,44,51].
Hyaluronic acid (HA), a highly hydrophilic ECM component, plays a significant role in cellular functions, including migration and differentiation, through its interaction with CD44 receptors. The molecular weight of HA is a key determinant of its biological activity. However, the natural inability of HA to undergo self-crosslinking limits its direct application in hydrogel formulations. To overcome this, HA polymers are often chemically modified, incorporating groups such as hydrazide, thiol, or methacrylate functionalities [52].
Alginate, a naturally occurring polymer obtained from brown seaweed, forms hydrogels when cross-linked with divalent cations, such as calcium. These alginate hydrogels exhibit a nanoporous structure that mimics the basement membrane, influencing cellular behaviors such as movement, differentiation, and dissemination [53].
3.2. Synthetic Hydrogels
Synthetic hydrogels are increasingly used in biological applications because of their promising qualities. PEG substitutes are frequently utilized for hydrogel production because of their affordability, ease of synthesis, and chemical modification potential. PEG hydrogels are typically formed through the photopolymerization of PEG diacrylate (PEGDA) but are inherently bioinert, necessitating chemical modifications to incorporate cell-binding motifs and biodegradable elements [54,55].
PVA is another widely used synthetic polymer for hydrogel formation because of its high biocompatibility and versatility in medical applications. However, PVA hydrogels suffer from low mechanical strength, limiting their effectiveness in certain applications. Methods such as double cross-linking and the addition of carbon nanotubes have been developed to improve the mechanical characteristics of PVA hydrogels [56].
PNIPAAm and other thermosensitive hydrogels undergo phase transitions in response to temperature changes, making them suitable for tissue engineering and drug delivery applications. While these hydrogels exhibit excellent tunability in response to temperature, their poor biodegradability and potential cytotoxicity hinder their widespread use in clinical settings [57].
Other synthetic hydrogels, such as PCL and polyurethane (PU), offer distinct advantages. PCL is known for its long degradation time and excellent biocompatibility, making it a preferred material for tissue scaffolds. PU hydrogels can be tailored by altering the chemical structure of polyols and isocyanates to achieve the desired mechanical properties [58,59].
Composite hydrogels, such as the chitosan-PEG-PNIPAAm blend, combine the advantages of multiple hydrogel materials, offering tunable properties, including increased mechanical strength and sensitivity to pH and temperature [60].
4. Hydrogel Physicochemical Properties and Their Impact on the Cell System
4.1. Stiffness
Stiffness is one of the most crucial properties of hydrogels in regulating cell behavior. The stiffness of the ECM varies significantly between tissues. For example, the brain typically has a stiffness of approximately 1 kPa, muscle tissue has a stiffness of approximately 10 kPa, and bone tissue can be as stiff as 100 kPa. In contrast, diseased tissues, such as tumor tissues, often show altered stiffness, which has been associated with disease progression and metastasis. Hydrogels can be engineered to mimic the stiffness of specific tissues, thus providing a supportive environment for cells. The stiffness of a hydrogel scaffold can influence various aspects of cell behavior, including morphology, proliferation, migration, and differentiation [61].
The ability of cells to sense substrate stiffness is mediated primarily by integrins, focal adhesion complexes, and the cytoskeleton. Cells modulate their adhesion and internal contractility in response to changes in substrate stiffness, ensuring mechanical homeostasis. Mechanotransduction signaling pathways that are influenced by stress include integrin-mediated FAK signaling, the RhoA/ROCK pathway, the YAP/TAZ pathway, and the Wnt/beta-catenin signaling pathway, all of which play vital roles in regulating cell fate [62].
Additionally, stem cell microencapsulation for cell therapy involves the process of stem cell microencapsulation within a hydrogel matrix for therapeutic applications. The process begins with stem cell isolation, potentially involving cell differentiation or genetic modification, from a patient or donor (Figure 1). These cells are then encapsulated within a biocompatible hydrogel, allowing for nutrient and oxygen diffusion while protecting the cells from the host’s immune response (e.g., antibodies and immune cells). Growth factors can be incorporated into hydrogels to promote cell survival and function. Waste products are able to diffuse out of the hydrogel. Finally, this hydrogel-based system can be delivered via injection or transplantation for cell therapy, offering a controlled and protective environment for the cells to exert their therapeutic effects. The molecular structure exemplifies a hydrogel component. This microencapsulation strategy enables controlled release and localized delivery, enhancing the efficacy and safety of stem cell-based therapies [16,39].
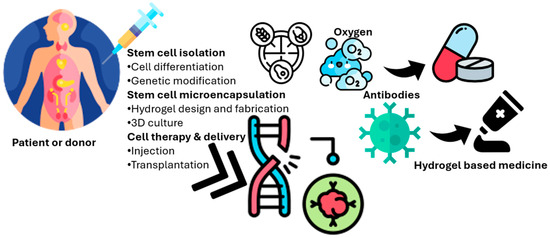
Figure 1.
Schematic representation of stem cell microencapsulation within a biocompatible hydrogel matrix for cell therapy. The process involves stem cell isolation, encapsulation, nutrient and oxygen diffusion, immune protection, and controlled delivery of therapeutic cells via injection or transplantation.
4.2. Stimulus-Responsive Hydrogels
Stimulus-responsive hydrogels, often called “smart” hydrogels, have notable benefits for regulating cell life in response to modifications in the environment. These hydrogels can undergo physical or chemical changes upon exposure to stimuli such as temperature, pH, light, or ionic strength. This responsiveness enables them to modify pore size, alter scaffold stiffness, or release bioactive chemicals under control, all of which can have an immediate impact on cellular behavior. Smart hydrogels have shown promise in several applications, such as tissue regeneration, wound healing, and controlled drug delivery [63].
While significant progress has been made in understanding the role of the physicochemical properties of hydrogels in regulating cell behavior, several challenges remain. It is still difficult to produce hydrogels that accurately replicate the intricacy of natural ECMs. Furthermore, the influence of hydrogel properties on cell behavior can vary depending on the cell type, environmental factors, and dynamic nature of in vivo conditions. Future research should focus on the development of more sophisticated hydrogel systems that integrate multiple cues (mechanical, chemical, and biological) to more effectively control cellular responses and tissue development [64].
5. Trends in Hydrogel Customization for Regenerative Medicine
The strategic research of bioactive substances, such as growth factors, bioligands, and peptides, to modify specific cellular behaviors has been highlighted by recent studies [65]. This approach enables hydrogels to actively promote biological processes, such as osteogenesis, angiogenesis, and neural differentiation [66]. The effect of bioactive molecule concentration on cell differentiation rates can be captured via logistic growth models, which consider factors such as the maximal differentiation rate, sensitivity coefficient, and threshold concentration.
Innovative fabrication techniques such as 3D bioprinting, advanced crosslinking methods, and rapid prototyping have facilitated the production of hydrogel scaffolds with finely tuned geometries and functionalities [67]. The mechanical and geometric properties can be computationally predicted via finite element modeling (FEM), ensuring compatibility with diverse tissue engineering applications, including vascular, cardiac, spinal, and urethral regeneration.
Hybrid hydrogel systems that integrate both organic and inorganic components along with nanomaterials offer mechanical resilience and controlled biofunctionality. These complex formulations are often described mathematically through multivariable optimization functions that balance mechanical strength, electrical conductivity, and cell compatibility [68]. The structure of hydrogels, their drug encapsulation and release mechanisms, and their biomedical applications underscore their importance in the biomedical field. The swollen hydrogel network is particularly notable for its capacity for water absorption and potential for drug incorporation (Figure 2). Additionally, drug encapsulation within the hydrogel matrix facilitates controlled drug release and supports tissue engineering in various formats, including cartilage, heart, and liver applications [69].
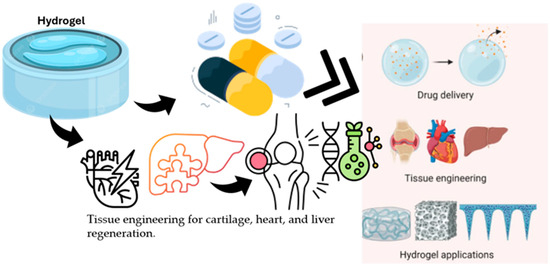
Figure 2.
Illustration of a swollen hydrogel network showing its water absorption capacity and drug encapsulation mechanism. The diagram highlights the structural features enabling controlled drug release and applications in tissue engineering for cartilage, heart, and liver regeneration.
In addition, biological conditions are gaining significant prominence. The gelation dynamics of these materials can be modeled via time-dependent functions that incorporate the equilibrium gel modulus and the gelation rate constant. Porosity and oxygenation in scaffold design are critical parameters that significantly influence cell viability and integration with host tissues. These relationships are governed by diffusion principles, in which the partial pressure of oxygen and the availability of nutrients depend on pore size and scaffold thickness [70].
In hepatic tissue engineering, controlling the hydrogel microarchitecture and mechanical properties optimizes hepatic function. Biocomposite gelatin films with growth factors support epithelial regeneration [71]. Electrospun nanofiber scaffolds mimic ECM structures, aiding tissue regeneration suitable for computational modeling [39,72].
Carrageenan-based hydrogels assist in wound repair, whereas decellularized heart ECM preserves angiogenic proteins for cardiac regeneration [73]. Patterned gelatin-methacrylate and polyethylene glycol diacrylate hydrogels form epidermal models, and 3D microchanneled gelatin hydrogels promote cardiomyocyte functionality [50,74].
Hydrogel carriers from human hair-derived keratins deliver growth factors for muscle loss treatment. Chitosan hydrogels with stem cells enhance cardiac regeneration, and antioxidant DNA hydrogels deliver interleukin-33 for diabetic wound healing [75,76].
Hydrogels absorb and retain large amounts of water, making them useful in medical and industrial applications. Their behavior depends on their composition and structural response. Chitosan-based hydrogels are thermosensitive, alginate hydrogels are photosensitive, and PEG-based hydrogels respond to reactive oxygen species. DNA-based hydrogels are biosensitive, and peptide-based hydrogels respond to pH, temperature, and ion concentration [77,78].
Hydrogels can be categorized by structure and composition: homopolymers, copolymers, or multipolymers, and amorphous, semicrystalline, or crystalline (Table 4). Crosslinking can be chemical or physiological, affecting mechanical behavior and substance movement. Their forms vary, such as those of soft matrices or thin films, and their electrical properties can be nonionic, ionic, or mixed [50].

Table 4.
The classification of hydrogels is based on their source, structure, chemical nature, and stimulus-responsiveness [44,50,68].
Polyvinyl alcohol (PVA) hydrogels are notable for their stability, strength, and safety. They are used in tissue engineering, drug delivery, wound dressings, wearable sensors, personal care, biodegradable packaging, energy storage, and water treatment [79].
Heparin hydrogel microwells optimize hepatocyte culture, and injectable hyaluronic acid-based hydrogels support spinal cord repair. Interpenetrating networks of hyaluronic acid and fibrin improve scaffold properties and cellular proliferation [80], demonstrating the potential of ECM-based scaffolds for tissue regeneration.
6. Dynamic Mechanical Properties in Tissue Development
Morphological behavior, including differentiation, is strongly influenced by the mechanical characteristics of tissues and polymers. Native tissues exhibit dynamic mechanical properties that change over time, impacting cellular responses. During chick heart development, mesodermal tissue stiffens as it matures, with its elastic modulus increasing from 0.9 kPa to 8.2 kPa between thirty-six and 408 h postfertilization [81].
To more closely resemble natural tissue conditions and direct cellular behavior appropriately, hydrogels can recreate changes in tissue stiffness via a variety of techniques.
6.1. Incorporating Dynamic Properties into Hydrogels
One approach to incorporating dynamic mechanical properties into hydrogels is through the use of thiol-modified hyaluronic acid (HA) hydrogels, which can be engineered to mimic the stiffening behavior of tissues such as the chick heart. Research by Young and Engler demonstrated that hydrogels with dynamic properties upregulated the expression of mature cardiac markers in cells, especially compared with static polyacrylamide hydrogels [49]. Other methods to introduce dynamic properties into hydrogels involve the use of supramolecular chemistry and self-assembly techniques, where specific peptides are incorporated into the hydrogel to enable responses to various physical, chemical, and biological stimuli.
6.2. Supramolecular Chemistry in Hydrogel Design
Supramolecular chemistry offers a promising strategy for designing hydrogels that can respond dynamically to various stimuli, such as light, temperature, pH, or enzyme cleavage. For example, research by Stupp and colleagues demonstrated that supramolecular nanofibers could effectively deliver bone-regenerating growth factors, such as BMP-2, while maintaining dynamic responsiveness [82]. Additionally, peptide sequences inside these materials can imitate complicated biological molecules, such as vascular endothelial growth factor (VEGF), which is critical for blood vessel creation [83]. Another notable study revealed that high-density presentation of a laminin-derived epitope on nanofibers selectively differentiated neural progenitors into neurons [84]. These findings highlight the potential of incorporating supramolecular materials into hydrogels to create dynamic systems that adapt to their surroundings, offering controlled delivery of biological molecules and promoting tissue regeneration.
Researchers are using 3D biomimetic printing to investigate dynamic biomaterials in addition to supramolecular substances, a cutting-edge technique that adds time to traditional 3D printing. For example, cellulose fibrils have been used to print hydrogels with anisotropic swelling behavior, allowing them to change shape over time upon immersion in water. Using this technique, 3D shapes inspired by nature, such as the New Guinean native Dendrobium helix orchid, have been produced [85]. Although 3D biomimetic printing systems have not yet been widely applied in regenerative medicine, their potential is vast. The ability to print dynamic structures that react to environmental conditions could revolutionize the field.
6.3. Future Applications of Regenerative Medicine in 3D Bioprinting
The future potential of 3D biomimetic systems in regenerative medicine is promising, especially for clinical applications such as heart valve repair and maxillofacial surgery. For example, significant efforts have been made to develop tissue-engineered heart valves to replace damaged or stenotic valves due to congenital conditions or disease, although these therapies often require invasive open-heart surgeries for implantation. However, a 3D bioprinted heart valve could be inserted via a minimally invasive procedure, forming its functional shape in situ and potentially revolutionizing heart valve repair by reducing the need for complex surgical interventions. Additionally, orbital floor fractures, which can lead to complications such as enophthalmos if not properly treated, can be repaired during maxillofacial procedures via 3D bioprinted structures that match a patient’s unique anatomy, allowing for minimally invasive placement that later expands to assume the full shape of the tissue [86]. The development of therapeutic drugs for human use remains challenging, with only 10.4% of all drugs examined in phase I clinical trials subsequently approved for use [87]. Over the past two decades, tissue engineering (TE)-based therapies have emerged as promising strategies for functional tissue replacement, with 371 clinical trials involving hydrogels registered globally by mid-2017, 69 of which focused on TE applications [88]. While these efforts have shown limited success in simpler organ structures such as the skin, cornea, urethra, bladder, and blood vessels, developing advanced TE strategies for more complex tissues faces challenges such as ensuring adequate oxygen and nutrient supply through vascularization, combining different cell types in precise spatial arrangements, obtaining unique mechanical characteristics, and integrating engineered tissues with host tissue. Hydrogel-based scaffolds show great promise in addressing these challenges [89,90]. Furthermore, designing hydrogels for clinical applications involves selecting appropriate materials, whether synthetic or natural, determining suitable crosslinking methods (chemical or physical) to achieve the desired mechanical strength and stability, and utilizing fabrication techniques such as 3D bioprinting, electrospinning, and in situ tissue engineering to create hydrogels with specific architectures (Figure 3). The ultimate goal is to transform these engineered hydrogels into effective clinical systems, underscoring the interdisciplinary nature of hydrogel research [91].
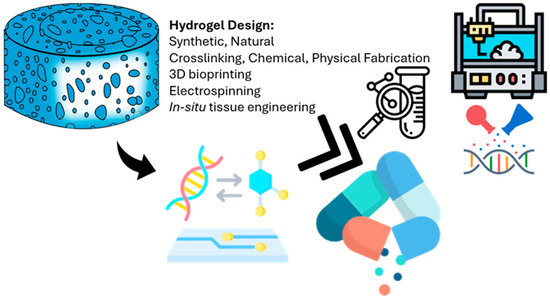
Figure 3.
Overview of how hydrogel scaffolds are designed and fabricated for use in clinical applications. The hydrogel shown at the top left represents the basic structure, which can be made from either synthetic or natural materials. Key factors in designing these scaffolds include the type of crosslinking (chemical or physical) and the fabrication methods used, such as 3D bioprinting (top right), electrospinning, and other microengineering techniques. The arrow pointing to the left shows how bioactive molecules and genetic material can be added to the hydrogel to improve its biological function. The double arrow highlights how hydrogel systems are increasingly being integrated with lab-on-a-chip platforms and other precise engineering tools to create tissue-specific designs. Finally, the icon at the bottom right represents the ultimate goal: applying these engineered hydrogels directly in patients for uses like tissue regeneration and targeted drug delivery.
6.4. Cartilage Repair
Repairing specific cartilage is one of the most promising uses of hydrogels in clinical TE. There is a significant need for efficient cartilage regeneration techniques because osteoarthritis affects 13.8% of people over 60 years of age worldwide [92]. Articular cartilage is an ideal candidate for TE approaches because it is relatively simple in structure, avascular, aneural, and composed of a single cell type. Numerous studies have explored the use of hydrogels to regenerate cartilage. These materials include thermoresponsive composites such as chitosan-PVA and hydrogels made from natural ECM constituents such as type I collagen. Other synthetic hydrogel systems, such as a PLGA–PEG–PLGA-based hydrogel, have also shown promise [93]. In one notable clinical trial, Sharma et al. developed a photoreactive, PEGDA-based hydrogel [77], which was injected into focal cartilage defects in the medial femoral condyle of 15 patients. This approach was combined with microfracture surgery, which enabled autologous cells to infiltrate the hydrogel. Compared with microfracture alone, the results demonstrated improved tissue regeneration, with greater tissue filling and better organization. Patients also reported reduced pain, a critical factor for clinical success. However, long-term follow-up is needed to confirm whether this treatment can prevent the progression of osteoarthritis [94]. Preclinical trials using various hydrogel systems have also yielded encouraging results. In rabbit models, for example, cartilage healing was facilitated by a synthetic PLGA–PEG–PLGA hydrogel loaded with kartogenin and mesenchymal stem cells (MSCs). Similarly, an oligo(poly(ethylene-glycol) fumarate)-based hydrogel containing MSCs [95] facilitated more hyaline-like cartilage regeneration than scaffolds alone in porcine models [96]. Additionally, hydrogel-based therapies for bone and cartilage regeneration involve the use of hydrogels to treat bone- and cartilage-related diseases (Figure 4). Two primary types of hydrogels, micro/nanoparticle-based hydrogels and smart drug depot hydrogels, can specifically target the bone/cartilage extracellular matrix and cells. These hydrogels are effective in treating common bone conditions such as osteoarthritis, osteoporosis, and bone fractures, highlighting the potential of local hydrogel injection as a therapeutic approach. Furthermore, the delivery of drugs via hydrogels to the bone marrow has been demonstrated, indicating a systemic treatment strategy [96].
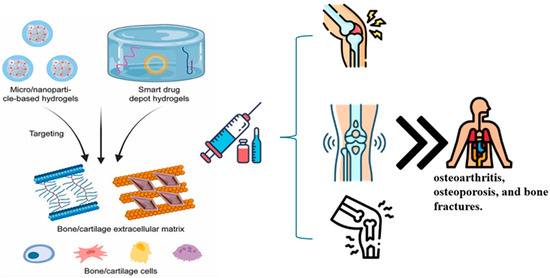
Figure 4.
Schematic representation of hydrogel-based therapies for bone and cartilage regeneration. The illustration highlights two therapeutic strategies: (1) micro/nanoparticle-based hydrogels and (2) smart drug depot hydrogels. These systems are designed for targeted delivery to the bone and cartilage extracellular matrix, enhancing local interaction with resident cells (e.g., chondrocytes, osteoblasts). Additionally, systemically delivered formulations support broader therapeutic effects, including targeting bone marrow. These approaches aim to treat degenerative and traumatic musculoskeletal conditions such as osteoarthritis, osteoporosis, and bone fractures.
7. Integration of Classical Molecular Modeling Methods in Hydrogel Research
Classical molecular modeling has transformed next-generation hydrogel design by enabling precise digital prototyping, multiscale characterization, and optimization of synthesis processes for biomedical applications. Techniques such as molecular dynamics (MD), density functional theory (DFT), finite element analysis (FEA), and computational fluid dynamics (CFD) provide insights into hydrogel behavior at the atomic, molecular, and macroscopic levels, accelerating innovations in drug delivery, tissue engineering, and biosensing [40,69,97,98,99,100].
MD simulations elucidate the nanoscale interactions, modeling of polymer crosslinking, pore structure, and water absorption dynamics critical for hydrogel swelling and mechanical strength. For example, MD distinguishes bound, intermediate, and free water states, informing stimulus-responsive hydrogel design, although challenges remain in simulating micron-sized pores owing to computational limitations [101,102,103]. Accurate MD relies on molecular force fields (e.g., AMBER, CHARMM, CVFF, PCFF), with integration algorithms such as Verlet, velocity Verlet, or Leap-Frog balancing accuracy and efficiency [80,104,105,106]. DFT, a quantum mechanical approach, probes electronic structures and water diffusion and enhances the design of hydrogels with tailored electronic or hygroscopic properties [107,108]. FEA discretizes hydrogel systems to predict mechanical responses, swelling behavior, and stress distribution, enabling the design of scaffolds with tissue-specific stiffness [109]. CFD optimizes manufacturing processes by simulating fluid and heat transport during hydrogel synthesis [110].
Multiphysics modeling integrates mechanical, thermal, and fluidic interactions, simulating complex hydrogel behaviors under physiological conditions. This approach supports the development of next-generation hydrogels with tunable degradation kinetics and stimuli responsiveness [111]. Response surface methodology (RSM) further refines synthesis conditions, optimizing parameters such as the polymer concentration and crosslinking density for enhanced performance [112,113]. By replacing labor-intensive trial-and-error methods, these computational tools enable rapid prototyping and characterization, paving the way for scalable, high-performance hydrogels in regenerative medicine and precision therapeutics.
8. Integration of Artificial Intelligence in Hydrogel Research
Artificial intelligence (AI) in computational science enables systems to perform complex tasks, such as data analysis, decision-making, and predictive modeling, which traditionally require human expertise. By leveraging sophisticated algorithms and expansive datasets, AI facilitates data-driven insights critical for next-generation hydrogel design [90,97,114,115]. Since the mid-20th century, AI has progressed through distinct phases: the symbolic era prioritized logic-based systems, the knowledge-based era focused on rule-driven expertise, and the statistical learning era introduced machine learning (ML) techniques, including random forests, support vector machines (SVMs), and deep learning. Reinforcement learning has recently gained prominence, significantly impacting materials science [116].
ML, a core AI discipline, encompasses supervised, unsupervised, and reinforcement learning, employing models such as neural networks, SVMs, and transformers [26,117,118]. In hydrogel research, AI-driven predictive models correlate composition, structure, and performance, optimizing synthesis and characterization for biomedical applications [91,119]. Techniques such as gradient descent and evolutionary algorithms identify optimal formulations that enhance hydrogel stability and functionality [92].
AI-powered hydrogels advance drug delivery, biosensing, bioprinting, wound healing, and tissue engineering by improving safety and accelerating material discovery [93]. Unlike traditional labor-intensive methods, AI enables high-throughput modeling, leveraging the ECM-mimicking properties of hydrogels, such as high water content, biocompatibility, and tunable mechanics [19,35,94,95,96]. Diverse polymer integrations further refine the mechanical and biological characteristics of the material [4,101].
Mathematical models, which incorporate variables such as the polymer concentration, molecular weight, and temperature, quantify hydrogel property relationships. Nanoparticle incorporation enhances mechanical and biological performance, whereas advanced fabrication supports dynamic scaffold design [51]. AI optimizes scaffold engineering through polymer chemistry, nanotechnology, and computational bioengineering [66,67].
For example, PEG-based hydrogels promote tissue regeneration, whereas natural polymers excel in terms of biocompatibility and ECM emulation [110,120,121]. Moreover, gelatin methacryloyl (GelMA) hydrogels offer tunable mechanics, influencing matrix metalloproteinase expression, and alginate-based systems support cryopreservation and regeneration [101,102,122,123,124]. Composite and hybrid hydrogels incorporating nanoparticles enhance mechanical and electrical properties, increasing bioactivity [13,14,125,126].
Supervised ML methods, including artificial neural networks (ANNs), deep neural networks (DNNs), convolutional neural networks (CNNs), and extreme gradient boosting (XGBoost), model input-output relationships and identify key variables [5,10,26,53,122,123,127,128,129,130,131]. Unsupervised methods such as principal component analysis (PCA) reduce data dimensionality, revealing patterns [132,133]. Reinforcement learning optimizes design parameters, whereas support vector regression (SVR) and least squares SVM (LS-SVM) refine property predictions [5,10,125,134,135,136,137]. Ensemble models, such as random forests (RFs) and SVMs, ensure robust analysis, minimizing overfitting [5,50,54,111,136,138,139,140]. Hybrid approaches integrating ANNs, fuzzy logic, and rule-based systems enhance optimization, predicting self-assembly and biofunctionality [5,121,125,134,137,141,142,143,144,145,146,147]. Collectively, these AI and ML advancements enable precise, scalable, and innovative hydrogel designs, revolutionizing biomedical applications by accelerating material discovery and optimizing performance for personalized therapeutic solutions.
9. Conclusions and Future Outlook
This systematic review explores the transformative role of in silico hydrogel design, emphasizing the integration of computational and AI techniques in advancing hydrogel properties for biomedical applications. AI, ML, and high-throughput computational screening have accelerated the development of materials for tissue regeneration, wound healing, drug delivery, and biosensing. These advancements enable accurate predictions of hydrogel characteristics, reducing the reliance on traditional experimental methods. AI-driven models and optimization strategies enhance the design of hydrogels with improved properties. Computational tools such as molecular dynamics simulations and finite element analysis contribute to a comprehensive understanding of hydrogels, supporting the development of next-generation systems. Customizing hydrogel properties for specific therapeutic purposes improves clinical outcomes and expedites material innovation.
The future of in-silico hydrogel design holds promise with evolving AI models enhancing predictive capabilities and material discovery. Integrating patient-specific data into hydrogel design methods promotes personalized medicine. Hybrid hydrogels combining nanomaterials and bioactive chemicals will unlock new possibilities in drug delivery and tissue engineering. AI-assisted design techniques enable precise control at the microscale, bolstering applications in organ regeneration and biosensing. Computational modeling and AI tools promise to revolutionize biomedical and healthcare applications, stimulating innovation across industrial sectors. Hydrogels have great potential in clinical applications, but overcoming scientific, regulatory, and practical challenges is essential.
Advanced numerical simulations have improved our understanding of hydrogel properties, complementing traditional methods and fostering innovation. AI and ML have emerged as powerful tools in hydrogel development, enabling data-driven design and optimization. Cutting-edge AI techniques such as deep learning open new possibilities for tailored hydrogels. However, challenges remain in ensuring data quality and reliability, bridging the gap between simulations and experimental validation, and addressing ethical aspects of AI and ML in hydrogel design. Collaboration across disciplines is crucial to developing standardized datasets and shared benchmarks, driving innovation, and improving patient outcomes.
Author Contributions
Individual contributions were as follows: Concept and supervision, S.S.; writing—original draft preparation, M.M.F. and S.S.; writing—review and editing, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We extend our special thanks to Samson Afolabi, a native English speaker from Sergey Shityakov’s team, for his valuable assistance in editing and proofreading the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Sofi, H.S.; Ashraf, R.; Khan, A.H.; Beigh, M.A.; Majeed, S.; Sheikh, F.A. Reconstructing nanofibers from natural polymers using surface functionalization approaches for applications in tissue engineering, drug delivery and biosensing devices. Mater. Sci. Eng. C 2019, 94, 1102–1124. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Advances in polymeric systems for tissue engineering and biomedical applications. Macromol. Biosci. 2012, 12, 286–311. [Google Scholar] [CrossRef] [PubMed]
- Abune, L.; Davis, B.; Wang, Y. Aptamer-functionalized hydrogels: An emerging class of biomaterials for protein delivery, cell capture, regenerative medicine, and molecular biosensing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1731. [Google Scholar] [CrossRef] [PubMed]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-responsive hydrogels: Intelligent materials for biosensing and drug delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef]
- Negut, I.; Bita, B. Exploring the potential of artificial intelligence for hydrogel development—A short review. Gels 2023, 9, 845. [Google Scholar] [CrossRef]
- Akhtar, Z.B.; Gupta, A.D. Advancements within molecular engineering for regenerative medicine and biomedical applications: An investigation analysis towards a computing retrospective. J. Electron. Electromed. Eng. Med. Inform. 2024, 6, 54–72. [Google Scholar] [CrossRef]
- Patel, R.A.; Webb, M.A. Data-driven design of polymer-based biomaterials: High-throughput simulation, experimentation, and machine learning. ACS Appl. Bio Mater. 2023, 7, 510–527. [Google Scholar] [CrossRef]
- Döring, A.; Birnbaum, W.; Kuckling, D. Responsive hydrogels–structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013, 42, 7391–7420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Finster, R.; Sankaran, P.; Bihar, E. Computational and AI-driven design of hydrogels for bioelectronic applications. Adv. Electron. Mater. 2025, 2025, 2400763. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Ahadian, S.; Sadeghian, R.B.; Salehi, S.; Ostrovidov, S.; Bae, H.; Ramalingam, M.; Khademhosseini, A. Bioconjugated hydrogels for tissue engineering and regenerative medicine. Bioconjug. Chem. 2015, 26, 1984–2001. [Google Scholar] [CrossRef]
- Farazin, A.; Gheisizadeh, A. Revolutionizing bone repair and regeneration: The role of machine learning in designing advanced nanocomposite hydrogels. Polym. Adv. Technol. 2025, 36, e70161. [Google Scholar] [CrossRef]
- El-Tanani, M.; Satyam, S.M.; Rabbani, S.A.; El-Tanani, Y.; Aljabali, A.A.A.; Al Faouri, I.; Rehman, A. Revolutionizing drug delivery: The impact of advanced materials science and technology on precision medicine. Pharmaceutics 2025, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Li, Z.; Xu, S.; Liu, Z. Recent advances of hydrogel network models for studies on mechanical behaviors. Acta Mech. Sin. 2021, 37, 367–386. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Qin, X.; Liu, J.; Huang, W.; Lv, Q. Current understanding of hydrogel for drug release and tissue engineering. Gels 2022, 8, 301. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Wang, C.; Varshney, R.R.; Wang, D.-A. Therapeutic cell delivery and fate control in hydrogels and hydrogel hybrids. Adv. Drug Deliv. Rev. 2010, 62, 699–710. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Hydrogels: Experimental characterization and mathematical modelling of their mechanical and diffusive behaviour. Chem. Soc. Rev. 2018, 47, 2357–2373. [Google Scholar] [CrossRef]
- Vernerey, F.J.; Sridhar, S.L.; Muralidharan, A.; Bryant, S.J. Mechanics of 3D cell–hydrogel interactions: Experiments, models, and mechanisms. Chem. Rev. 2021, 121, 11085–11148. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Francesco, D.D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Natural polymers and the hydrogels prepared from them. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. [Google Scholar]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of hydrogels: From synthetic strategies, classification, and properties to biomedical applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Dau, H.; Jones, G.R.; Tsogtgerel, E.; Nguyen, D.; Keyes, A.; Liu, Y.-S.; Rauf, H.; Ordonez, E.; Puchelle, V.; Alhan, H.B.; et al. Linear block copolymer synthesis. Chem. Rev. 2022, 122, 14471–14553. [Google Scholar] [CrossRef]
- Soman, A.; Mathew, F.; Chacko, A.J.; Alias, M.; Poosan, G.V. Interpenetrating polymer network (IPN)-hydrogels. Pharma Innov. 2014, 3 Pt A, 59. [Google Scholar]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.T.P.; Papavasiliou, G.; Teymour, F. Characterizing the molecular architecture of hydrogels and crosslinked polymer networks beyond Flory–Rehner theory. Biomacromolecules 2020, 21, 5104–5118. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental concepts of hydrogels: Synthesis, properties, and their applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Mukherjee, C.; Varghese, D.; Krishna, J.S.; Boominathan, T.; Rakeshkumar, R.; Dineshkumar, S.; Brahmananda Rao, C.V.S.; Sivaramakrishna, A. Recent advances in biodegradable polymers–properties, applications and future prospects. Eur. Polym. J. 2023, 192, 112068. [Google Scholar] [CrossRef]
- Garcia-Garcia, A.; Muñana-González, S.; Lanceros-Mendez, S.; Ruiz-Rubio, L.; Alvarez, L.P.; Vilas-Vilela, J.L. Biodegradable natural hydrogels for tissue engineering, controlled release, and soil remediation. Polymers 2024, 16, 2599. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nami, S.A.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Hubbell, J.A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 2010, 31, 7836–7845. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Wu, S.; Fan, Y.; Li, X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: Current progress and challenges. Biomater. Sci. 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Lee, S.C.; Gillispie, G.; Prim, P.; Lee, S.J. Physical and chemical factors influencing the printability of hydrogel-based extrusion bioinks. Chem. Rev. 2020, 120, 10834–10886. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Reguieg, F.; Ricci, L.; Bouyacoub, N.; Belbachir, M.; Bertoldo, M. Thermal characterization by DSC and TGA analyses of PVA hydrogels with organic and sodium MMT. Polym. Bull. 2020, 77, 929–948. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.; Tomás, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Chartoff, R.P.; Menczel, J.D.; Dillman, S.H. Dynamic mechanical analysis (DMA). In Thermal Analysis of Polymers: Fundamentals and Applications; John and Wiley Sons: Hoboken, NJ, USA, 2009; pp. 387–495. [Google Scholar]
- Oyen, M.L. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Kravanja, K.A.; Finšgar, M. Analytical techniques for the characterization of bioactive coatings for orthopaedic implants. Biomedicines 2021, 9, 1936. [Google Scholar] [CrossRef]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; El-Moshy, S.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; El-Sayed, K.M.F. Hydrogels and dentin–pulp complex regeneration: From the benchtop to clinical translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-based hydrogels for tissue engineering applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Radulescu, D.-M.; Neacsu, I.A.; Grumezescu, A.-M.; Andronescu, E. New insights of scaffolds based on hydrogels in tissue engineering. Polymers 2022, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Samiraninezhad, N.; Asadi, K.; Rezazadeh, H.; Gholami, A. Using chitosan, hyaluronic acid, alginate, and gelatin-based smart biological hydrogels for drug delivery in oral mucosal lesions: A review. Int. J. Biol. Macromol. 2023, 252, 126573. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Evolution of hybrid hydrogels: Next-generation biomaterials for drug delivery and tissue engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Liu, K.; Gao, F. Hydrogel scaffolds in bone regeneration: Their promising roles in angiogenesis. Front. Pharmacol. 2023, 14, 1050954. [Google Scholar] [CrossRef]
- Brandl, F.; Kastner, F.; Gschwind, R.M.; Blunk, T.; Teßmar, J.; Göpferich, A. Hydrogel-based drug delivery systems: Comparison of drug diffusivity and release kinetics. J. Control. Release 2010, 142, 221–228. [Google Scholar] [CrossRef]
- Monfared, V. Application of artificial intelligence (machine learning) in additive manufacturing, bio-systems, bio-medicine, and composites. In Additive Manufacturing for Biocomposites and Synthetic Composites; CRC Press: New York, NY, USA, 2023; pp. 152–203. [Google Scholar]
- Wang, S.; Sun, Z. Hydrogel and machine learning for soft robots’ sensing and signal processing: A Review. J. Bionic Eng. 2023, 20, 845–857. [Google Scholar] [CrossRef]
- Zhao, J.; Santino, F.; Giacomini, D.; Gentilucci, L. Integrin-targeting peptides for the design of functional cell-responsive biomaterials. Biomedicines 2020, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Ma, Y.; Han, T.; Yang, Q.; Wang, J.; Feng, B.; Jia, Y.; Wei, Z.; Xu, F. Viscoelastic cell microenvironment: Hydrogel-based strategy for recapitulating dynamic ECM mechanics. Adv. Funct. Mater. 2021, 31, 2100848. [Google Scholar] [CrossRef]
- Khansari, M.M.; Sorokina, L.V.; Mukherjee, P.; Mukhtar, F.; Shirdar, M.R.; Shahidi, M.; Shokuhfar, T. Classification of hydrogels based on their source: A review and application in stem cell regulation. JOM 2017, 69, 1340–1347. [Google Scholar] [CrossRef]
- Morales, X.; Cortes-Dominguez, I.; Solorzano, C.O. Modeling the mechanobiology of cancer cell migration using 3D biomimetic hydrogels. Gels 2021, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Niethammer, P. Components and mechanisms of nuclear mechanotransduction. Annu. Rev. Cell Dev. Biol. 2021, 37, 233–256. [Google Scholar] [CrossRef]
- Claudino, M.; Zhang, X.; Alim, M.D.; Podgórski, M.; Bowman, C.N. Mechanistic kinetic modeling of thiol–Michael addition photopolymerizations via photocaged “superbase” generators: An analytical approach. Macromolecules 2016, 49, 8061–8074. [Google Scholar] [CrossRef]
- Aseervatham, J. Cytoskeletal remodeling in cancer. Biology 2020, 9, 385. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef]
- Wang, X.; Schmidt, C.K.; Sanchez, S.; Gracias, D.H.; Carazo-Salas, R.E.; Jackson, S.P.; Schmidt, O.G. Rolled-up functionalized nanomembranes as three-dimensional cavities for single cell studies. Nano Lett. 2014, 14, 4197–4204. [Google Scholar]
- Park, S. Development of the Engineered Biological Model Systems and Chemoselective Redox Responsive Ligation (CRRL). Ph.D. Thesis, The University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, 2010. [Google Scholar]
- Soleymani, S.; Naghib, S.M. 3D and 4D printing hydroxyapatite-based scaffolds for bone tissue engineering and regeneration. Heliyon 2023, 9, e19363. [Google Scholar] [CrossRef]
- Wellman, S.M.; Eles, J.R.; Ludwig, K.A.; Seymour, J.P.; Michelson, N.J.; McFadden, W.E.; Vazquez, A.L.; Kozai, T.D.Y. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 2018, 28, 1701269. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y. Rational design of smart hydrogels for biomedical applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef]
- Han, X.; Chang, S.; Zhang, M.; Bian, X.; Li, C.; Li, D. Advances of hydrogel-based bioprinting for cartilage tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 746564. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Chouhan, D.; Mandal, B.B. Tissue engineered skin and wound healing: Current strategies and future directions. Curr. Pharm. Des. 2017, 23, 3455–3482. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wang, Y.; Cui, W. Advanced electrospun hydrogel fibers for wound healing. Compos. Part B Eng. 2021, 223, 109101. [Google Scholar] [CrossRef]
- Rode, M.P.; Angulski, A.B.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.S.; de Carvalho, R.G.; IV, D.G.; Oliveira, L.F.C.; des Maia, L.F.; Trentin, A.G.; et al. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Chowdhury, S.D.; Wilson, R.L. Advancements and challenges in hydrogel engineering for regenerative medicine. Gels 2024, 10, 238. [Google Scholar] [CrossRef]
- Wang, H.; Shi, J.; Wang, Y.; Yin, Y.; Wang, L.; Liu, J.; Liu, Z.; Duan, C.; Zhu, P.; Wang, C. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials 2014, 35, 3986–3998. [Google Scholar] [CrossRef]
- Hamsayegan, S.; Raissi, H.; Ghahari, A. Selective detection of food contaminants using engineered gallium-organic frameworks with MD and metadynamics simulations. Sci. Rep. 2024, 14, 18144. [Google Scholar] [CrossRef]
- Hinderer, S.; Layland, S.L.; Schenke-Layland, K. ECM and ECM-like materials—Biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 2016, 97, 260–269. [Google Scholar] [CrossRef]
- Sharma, S.; Bhende, M.; Goel, A. A review: Polysaccharide-based hydrogels and their biomedical applications. Polym. Bull. 2024, 81, 8573–8594. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Rangel-Argote, M.; Claudio-Rizo, J.A.; Mata-Mata, J.L.; Mendoza-Novelo, B. Characteristics of collagen-rich extracellular matrix hydrogels and their functionalization with poly(ethylene glycol) derivatives for enhanced biomedical applications: A review. ACS Appl. Bio Mater. 2018, 1, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, J.; Qi, C.; Fang, Y.; Zhang, L.; Zhuo, R.; Jiang, X. Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater. 2016, 35, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, H.; Ahlfeld, T.; Kilian, D.; Liu, Y.; Gelinsky, M.; Hu, Q. Evaluation of different crosslinking methods in altering the properties of extrusion-printed chitosan-based multi-material hydrogel composites. Bio-Des. Manuf. 2023, 6, 150–173. [Google Scholar] [CrossRef]
- Lin, F.-Y.; Chang, C.-Y.; Nguyen, H.; Li, H.; Fishel, M.L.; Lin, C.-C. Viscoelastic hydrogels for interrogating pancreatic cancer-stromal cell interactions. Mater. Today Bio 2023, 19, 100576. [Google Scholar] [CrossRef]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-healing injectable hydrogels for tissue regeneration. Chem. Rev. 2022, 123, 834–873. [Google Scholar] [CrossRef]
- Simińska-Stanny, J.; Hachemi, F.; Dodi, G.; Cojocaru, F.D.; Gardikiotis, I.; Podstawczyk, D.; Delporte, C.; Jiang, G.; Nie, L.; Shavandi, A. Optimizing phenol-modified hyaluronic acid for designing shape-maintaining biofabricated hydrogel scaffolds in soft tissue engineering. Int. J. Biol. Macromol. 2023, 244, 125201. [Google Scholar] [CrossRef]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R. Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 87. [Google Scholar] [CrossRef]
- Thanaskody, K.; Jusop, A.S.; Tye, G.J.; Zaman, W.S.W.K.; Dass, S.A.; Nordin, F. MSCs vs. iPSCs: Potential in therapeutic applications. Front. Cell Dev. Biol. 2022, 10, 1005926. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Wang, X.; Xiao, J. Insight into heart-tailored architectures of hydrogel to restore cardiac functions after myocardial infarction. Mol. Pharm. 2022, 20, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Bonde, S.; Chandarana, C.; Prajapati, P.; Vashi, V. A comprehensive review on recent progress in chitosan composite gels for biomedical uses. Int. J. Biol. Macromol. 2024, 272, 132723. [Google Scholar] [CrossRef]
- Abdelaziz, A.G.; Nageh, H.; Abdo, S.M.; Abdalla, M.S.; Amer, A.A.; Abdal-Hay, A.; Barhoum, A. A review of 3D polymeric scaffolds for bone tissue engineering: Principles, fabrication techniques, immunomodulatory roles, and challenges. Bioengineering 2023, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, L.; Gharibshahian, M.; Salehi, M.; Zamani, S.; Abpeikar, Z.; Ghaderzadeh, O.; Alizadeh, M.; Masoudi, A.; Rezaei, N.; Cheraghali, D. Recent advances in hydrogels applications for tissue engineering and clinical trials. Regen. Ther. 2024, 26, 635–645. [Google Scholar] [CrossRef]
- Li, C.-S.; Xu, Y.; Li, J.; Qin, S.-H.; Huang, S.-W.; Chen, X.-M.; Luo, Y.; Gao, C.-T.; Xiao, J.-H. Ultramodern natural and synthetic polymer hydrogel scaffolds for articular cartilage repair and regeneration. Biomed. Eng. Online 2025, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, D.A.; Wu, D.; He, F.; Wang, H.; Huang, L.; Shi, D.; Liu, Q.; Ni, N.; Pakvasa, M.; et al. Applications of biocompatible scaffold materials in stem cell-based cartilage tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 603444. [Google Scholar] [CrossRef]
- Sofat, N.; Kuttapitiya, A. Future directions for the management of pain in osteoarthritis. Int. J. Clin. Rheumatol. 2014, 9, 197. [Google Scholar] [CrossRef]
- Hashemi-Afzal, F.; Fallahi, H.; Bagheri, F.; Collins, M.N.; Eslaminejad, M.B.; Seitz, H. Advancements in hydrogel design for articular cartilage regeneration: A comprehensive review. Bioact. Mater. 2025, 43, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Ren, X.; Afizah, M.H.; Tarigan-Panjaitan, S.; Yang, Z.; Wu, Y.; Chian, K.S.; Mikos, A.G.; Hui, J.H.P. Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model. Tissue Eng. Part A 2013, 19, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, H.B.; Dogru, S.; Lashkarinia, S.S.; Pekkan, K. Soft-tissue material properties and mechanogenetics during cardiovascular development. J. Cardiovasc. Dev. Dis. 2022, 9, 64. [Google Scholar] [CrossRef]
- Kim, B.S.; Das, S.; Jang, J.; Cho, D.-W. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem. Rev. 2020, 120, 10608–10661. [Google Scholar] [CrossRef] [PubMed]
- Sreekumaran, S.; Radhakrishnan, A.; Rauf, A.A.; Kurup, M. Nanohydroxyapatite incorporated photocrosslinked gelatin methacryloyl/poly(ethylene glycol) diacrylate hydrogel for bone tissue engineering. Prog. Biomater. 2021, 10, 43–51. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin methacrylate (GelMA)-based hydrogels for cell transplantation: An effective strategy for tissue engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef]
- Krishna, K.S.; Li, Y.; Li, S.; Kumar, C.S.S.R. Lab-on-a-chip synthesis of inorganic nanomaterials and quantum dots for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 1470–1495. [Google Scholar] [CrossRef]
- Aguirre, M.; Ballard, N.; Gonzalez, E.; Hamzehlou, S.; Sardon, H.; Calderon, M.; Paulis, M.; Tomovska, R.; Dupin, D.; Bean, R.H.; et al. Polymer colloids: Current challenges, emerging applications, and new developments. Macromolecules 2023, 56, 2579–2607. [Google Scholar] [CrossRef]
- Thiele, J.; Ma, Y.; Bruekers, S.M.C.; Ma, S.; Huck, W.T.S. 25th anniversary article: Designer hydrogels for cell cultures: A materials selection guide. Adv. Mater. 2014, 26, 125–148. [Google Scholar] [CrossRef]
- Boateng, D.; Li, X.; Zhu, Y.; Zhang, H.; Wu, M.; Liu, J.; Kang, Y.; Zeng, H.; Han, L. Recent advances in flexible hydrogel sensors: Enhancing data processing and machine learning for intelligent perception. Biosens. Bioelectron. 2024, 261, 116499. [Google Scholar] [CrossRef]
- Spreiter, Q.; Walter, M. Classical molecular dynamics simulation with the velocity Verlet algorithm at strong external magnetic fields. J. Comput. Phys. 1999, 152, 102–119. [Google Scholar] [CrossRef]
- Marchetto, A.; Chaib, Z.S.; Rossi, C.A.; Ribeiro, R.P.; Pantano, S.; Rossetti, G.; Giorgetti, A. CGMD platform: Integrated web servers for the preparation, running, and analysis of coarse-grained molecular dynamics simulations. Molecules 2020, 25, 5934. [Google Scholar] [CrossRef]
- Lamas, C.P.; Sanz, E.; Vega, C.; Noya, E.G. Estimation of bubble cavitation rates in a symmetrical Lennard-Jones mixture by NVT seeding simulations. J. Chem. Phys. 2023, 158, 124109. [Google Scholar] [CrossRef]
- Chakraborty, P.; Mitra, S.; Kim, A.R.; Zhao, B.; Mitra, S.K. Density Functional Theory approach to interpret elastowetting of hydrogels. Langmuir 2024, 40, 7168–7177. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, Y.; Dong, X.; Yin, Q.; Zhu, H.; Li, S.; Zhou, J.; Wang, C. Cell-derived extracellular matrix-coated silk fibroin scaffold for cardiogenesis of brown adipose stem cells through modulation of TGF-β pathway. Regen. Biomater. 2020, 7, 403–412. [Google Scholar] [CrossRef]
- Musharaf, H.M.; Roshan, U.; Mudugamuwa, A.; Trinh, Q.T.; Zhang, J.; Nguyen, N.-T. Computational fluid–structure interaction in microfluidics. Micromachines 2024, 15, 897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Yan, Z.; Ji, S.; Xiao, S.; Gao, J. Metal nanoparticle hybrid hydrogels: The state-of-the-art of combining hard and soft materials to promote wound healing. Theranostics 2024, 14, 1534. [Google Scholar] [CrossRef]
- Filipecka-Szymczyk, K.; Makowska-Janusik, M.; Marczak, W. Molecular dynamics simulations of HEMA-based hydrogels for ophthalmological applications. Molecules 2024, 29, 5784. [Google Scholar] [CrossRef]
- Jiang, S.; Li, Y.; Yong, Z. Compatibility analysis between natural rubber/polybutadiene rubber and protective waxes based on experiments and Materials Studio simulations. J. Appl. Polym. Sci. 2024, 141, e55468. [Google Scholar] [CrossRef]
- Salave, S.; Rana, D.; Sharma, A.; Bharathi, K.; Gupta, R.; Khode, S.; Benival, D.; Kommineni, N. Polysaccharide-based implantable drug delivery: Development strategies, regulatory requirements, and future perspectives. Polysaccharides 2022, 3, 625–654. [Google Scholar] [CrossRef]
- Arif, Z.U. The role of polysaccharide-based biodegradable soft polymers in the healthcare sector. Adv. Ind. Eng. Polym. Res. 2024, 8, 132–156. [Google Scholar] [CrossRef]
- D’Andrea, L.D.; Iaccarino, G.; Fattorusso, R.; Sorriento, D.; Carannante, C.; Capasso, D.; Trimarco, B.; Pedone, C. Targeting angiogenesis: Structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 14215–14220. [Google Scholar] [CrossRef]
- de Sousa, M.C.A.; Rodrigues, C.A.V.; Ferreira, I.A.F.; Diogo, M.M.; Linhardt, R.J.; Cabral, J.M.S.; Ferreira, F.C. Functionalization of electrospun nanofibers and fiber alignment enhance neural stem cell proliferation and neuronal differentiation. Front. Bioeng. Biotechnol. 2020, 8, 580135. [Google Scholar] [CrossRef] [PubMed]
- Foyt, D.A.; Norman, M.D.A.; Yu, T.T.L.; Gentleman, E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 2018, 7, 1700939. [Google Scholar] [CrossRef]
- Deore, A.B.; Dhumane, J.R.; Wagh, R.; Sonawane, R. The stages of drug discovery and development process. Asian J. Pharm. Res. Dev. 2019, 7, 62–67. [Google Scholar] [CrossRef]
- Ogata, S.; Lidorikis, E.; Shimojo, F.; Nakano, A.; Vashishta, P.; Kalia, R.K. Hybrid finite-element/molecular-dynamics/electronic-density-functional approach to materials simulations on parallel computers. Comput. Phys. Commun. 2001, 138, 143–154. [Google Scholar] [CrossRef]
- Li, Z.; Song, P.; Li, G.; Han, Y.; Ren, X.; Bai, L.; Su, J. AI energized hydrogel design, optimization and application in biomedicine. Mater. Today Bio 2024, 25, 101014. [Google Scholar] [CrossRef]
- Mahesh, B. Machine learning algorithms—A review. Int. J. Sci. Res. (IJSR) 2020, 9, 381–386. [Google Scholar] [CrossRef]
- Singh, A.; Thakur, N.; Sharma, A. A review of supervised machine learning algorithms. In Proceedings of the2016 3rd International Conference on Computing for Sustainable Global Development (INDIACom), New Delhi, India, 16–18 March 2016; pp. 1310–1315. [Google Scholar]
- Batra, R.; Song, L.; Ramprasad, R. Emerging materials intelligence ecosystems propelled by machine learning. Nat. Rev. Mater. 2021, 6, 655–678. [Google Scholar] [CrossRef]
- Wang, C.; He, T.; Zhou, H.; Zhang, Z.; Lee, C. Artificial intelligence enhanced sensors-enabling technologies to next-generation healthcare and biomedical platform. Bioelectron. Med. 2023, 9, 17. [Google Scholar] [CrossRef]
- Nosrati, H.; Nosrati, M. Artificial intelligence in regenerative medicine: Applications and implications. Biomimetics 2023, 8, 442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Soft-Body Simulation with CUDA based on Mass-Spring Model and Verlet Integration Scheme; American Society of Mechanical Engineers: New York, NY, USA, 2020; Volume 7A, ISBN 978-0-7918-8454-6. [Google Scholar]
- Orio, M.; Pantazis, D.A.; Neese, F. Density functional theory. Photosynth. Res. 2009, 102, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Bagayoko, D. Understanding density functional theory (DFT) and completing it in practice. AIP Adv. 2014, 4, 127104. [Google Scholar] [CrossRef]
- Panagiotopoulou, O. Finite element analysis (FEA): Applying an engineering method to functional morphology in anthropology and human biology. Ann. Hum. Biol. 2009, 36, 609–623. [Google Scholar] [CrossRef]
- Chen, G.; Xian, W.; Wang, Q.; Li, Y. Molecular simulation guided and physics-informed mechanistic modeling of multifunctional polymers. Acta Mech. Sin. 2021, 37, 725–745. [Google Scholar] [CrossRef]
- Pissis, P.; Kyritsis, A. Electrical conductivity studies in hydrogels. Solid State Ion. 1997, 97, 105–113. [Google Scholar] [CrossRef]
- Díaz-Marín, C.D.; Zhang, L.; Lu, Z.; Alshrah, M.; Grossman, J.C.; Wang, E.N. Kinetics of sorption in hygroscopic hydrogels. Nano Lett. 2022, 22, 1100–1107. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Beresford, R. Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. J. Pharm. Biomed. Anal. 2000, 22, 717–727. [Google Scholar] [CrossRef]
- Bemani, A.; Madani, M.; Kazemi, A. Machine learning-based estimation of nano-lubricants viscosity in different operating conditions. Fuel 2023, 352, 129102. [Google Scholar] [CrossRef]
- Xu, J.; Tsai, Y.-L.; Hsu, S.-h. Design strategies of conductive hydrogel for biomedical applications. Molecules 2020, 25, 5296. [Google Scholar] [CrossRef]
- Xu, S.; Chen, X.; Wang, S.; Chen, Z.; Pan, P.; Huang, Q. Integrating machine learning for the optimization of polyacrylamide/alginate hydrogel. Regen. Biomater. 2024, 11, rbae109. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Brooks, B.R. A double exponential potential for van der Waals interaction. AIP Adv. 2019, 9, 065304. [Google Scholar] [CrossRef] [PubMed]
- Younes, K.; Kharboutly, Y.; Antar, M.; Chaouk, H.; Obeid, E.; Mouhtady, O.; Abu-Samha, M.; Halwani, J.; Murshid, N. Application of unsupervised machine learning for the evaluation of aerogels’ efficiency towards ion removal—A principal component analysis (PCA) approach. Gels 2023, 9, 304. [Google Scholar] [CrossRef]
- Sumiea, E.H.; Abdulkadir, S.J.; Alhussian, H.S.; Al-Selwi, S.M.; Alqushaibi, A.; Ragab, M.G.; Fati, S.M. Deep deterministic policy gradient algorithm: A systematic review. Heliyon 2024, 10, e30697. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of scaffolds from bio-based natural materials for tissue regeneration applications: A review. Gels 2023, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Champa-Bujaico, E.; García-Díaz, P.; Díez-Pascual, A.M. Machine learning for property prediction and optimization of polymeric nanocomposites: A state-of-the-art. Int. J. Mol. Sci. 2022, 23, 10712. [Google Scholar] [CrossRef]
- Tiong, T.; Saad, I.; Teo, K.T.K.; Lago, H. Deep reinforcement learning with robust deep deterministic policy gradient. In Proceedings of the 2020 2nd International Conference on Electrical, Control and Instrumentation Engineering (ICECIE), Kuala Lumpur, Malaysia, 28 November 2020; pp. 1–5. [Google Scholar]
- Zhu, J.-A.; Jia, Y.; Lei, J.; Liu, Z. Deep learning approach to mechanical property prediction of single-network hydrogel. Mathematics 2021, 9, 2804. [Google Scholar] [CrossRef]
- Wang, Y.; Wallmersperger, T.; Ehrenhofer, A. Application of back propagation neural networks and random forest algorithms in material research of hydrogels. PAMM 2023, 23, e202200278. [Google Scholar] [CrossRef]
- Seifermann, M.; Reiser, P.; Friederich, P.; Levkin, P.A. High-throughput synthesis and machine learning assisted design of photodegradable hydrogels. Small Methods 2023, 7, 2300553. [Google Scholar] [CrossRef]
- Liu, D.; Ma, S.; Yuan, H.; Markert, B. Modelling and simulation of coupled fluid transport and time-dependent fracture in fibre-reinforced hydrogel composites. Comput. Methods Appl. Mech. Eng. 2022, 390, 114470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).