pH-Sensitive Chitosan-Based Hydrogels Trap Poloxamer Micelles as a Dual-Encapsulating Responsive System for the Loading and Delivery of Curcumin
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Formulation of Cross-Linked Chitosan Hydrogels Charged with Cur-Loaded P407 Micelles
2.3. Curcumin Encapsulation Efficiency (%Cur EE) and Loading Capacity (%Cur LC)
2.4. Characterization Techniques
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR-ATR)
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Rheological Characterization
2.4.5. Cur Release from the Hydrogels
2.4.6. Hemolysis Test
2.4.7. Cytotoxicity Test with VERO CCL-81 Cells (MTT Assay)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Curcumin Loading Capacity and Encapsulation Efficiency
3.2. Chemical Characterization by FTIR-ATR
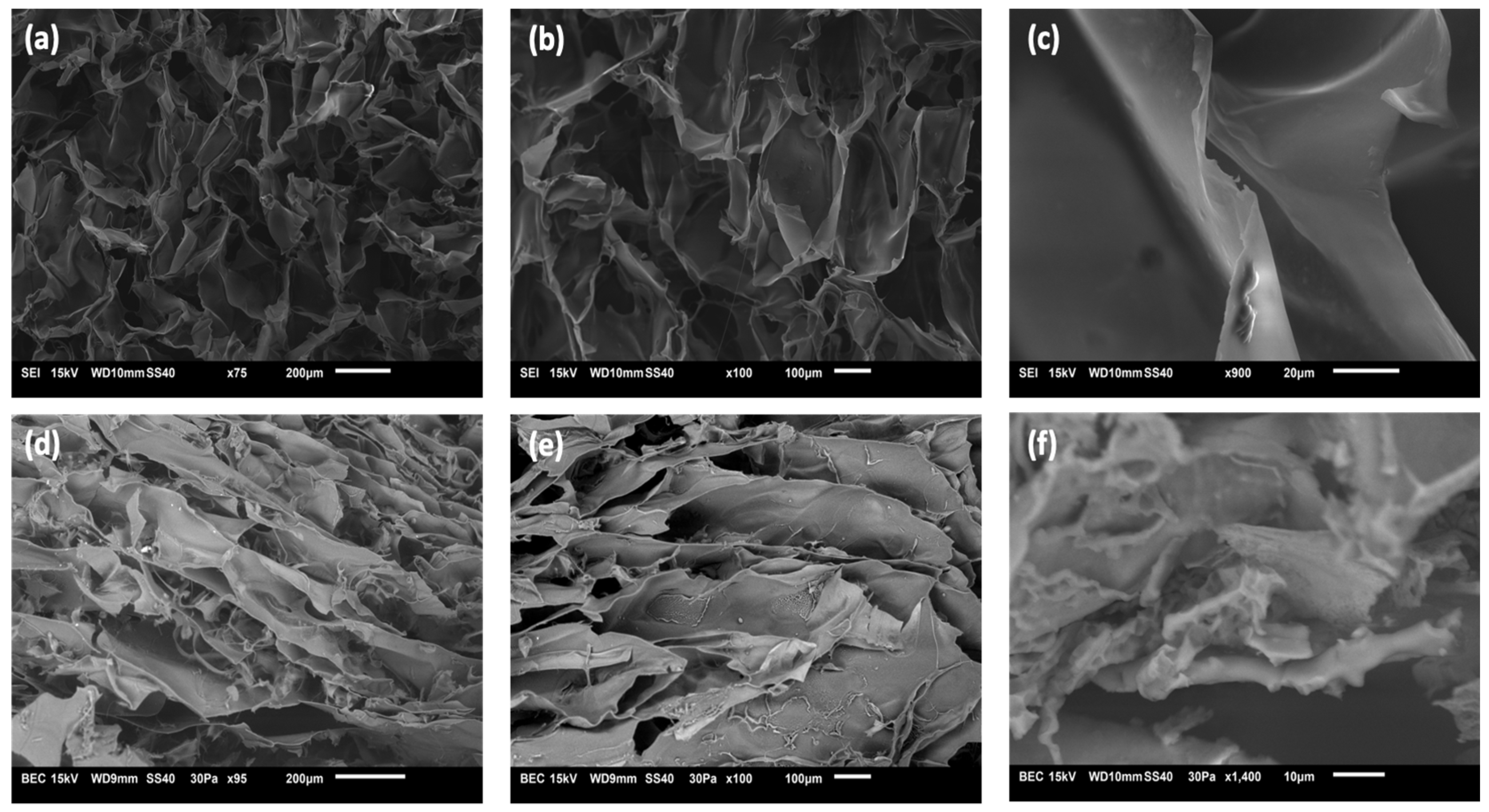
3.3. Morphology of Hydrogels
3.4. Differential Scanning Calorimetry (DSC)
3.5. Rheological Properties
3.6. Curcumin Release
3.7. In Vitro Biocompatibility Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
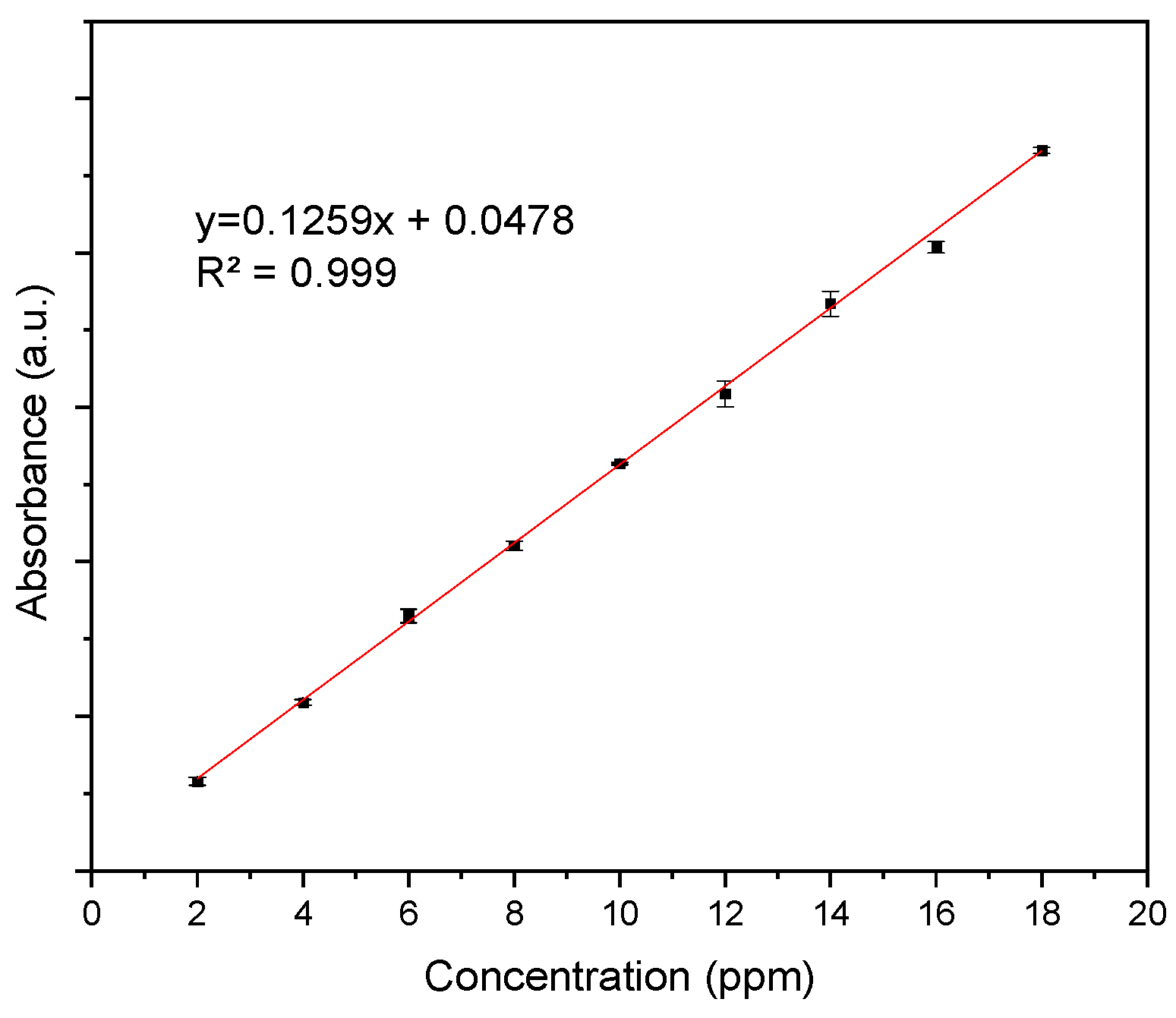
Appendix A.1. Calibration Curve of Curcumin
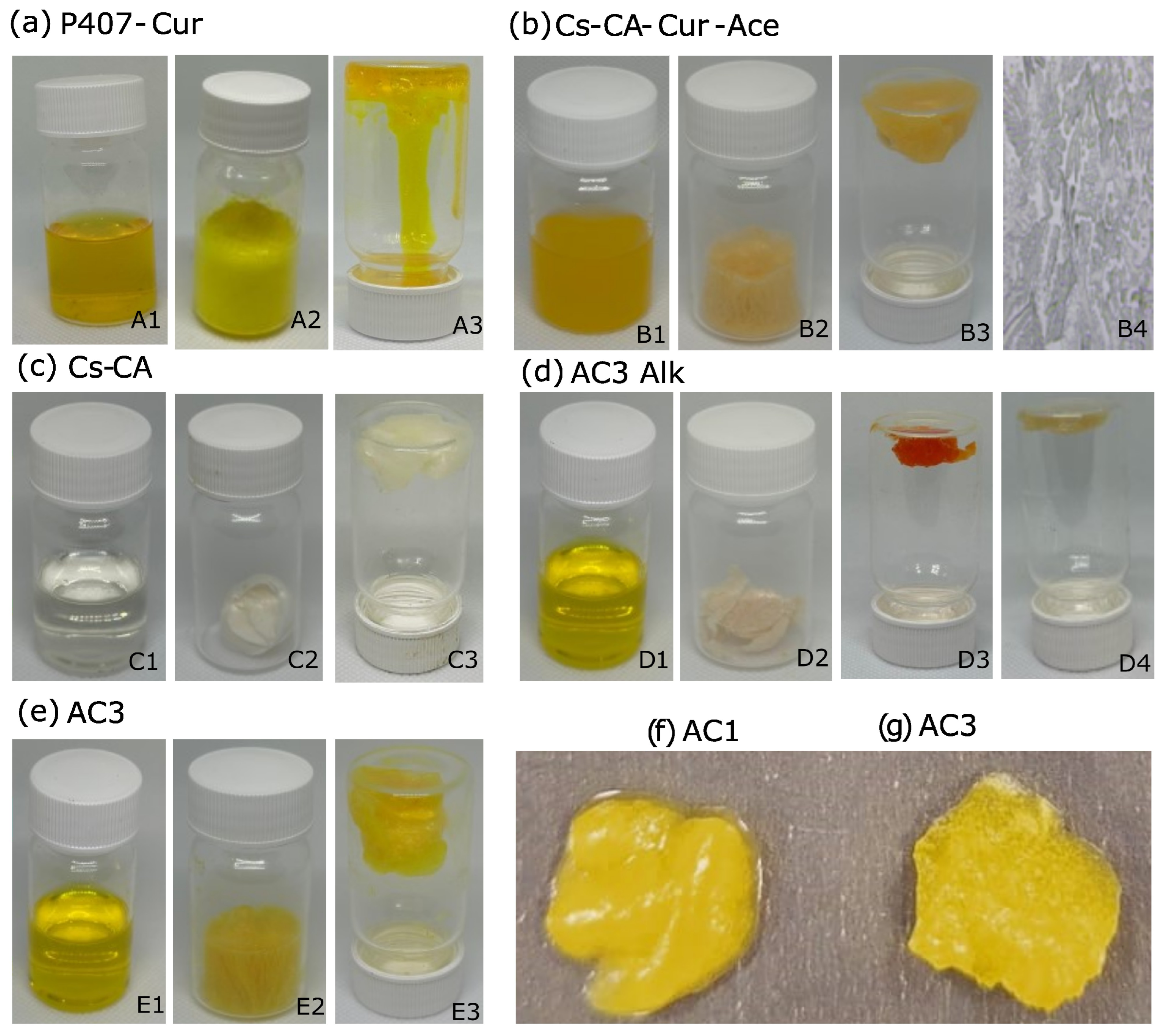
Appendix A.2. Macroscopic Characteristics of Hydrogels and Control Samples
References
- Zhao, X.; Tang, J.; Liu, Y.; Hu, B.; Chen, Q.; Liu, Y. Reaction Kinetics of Chitosan Nanogels Crosslinked by Genipin. J. Chromatogr. A 2023, 1710, 464427. [Google Scholar] [CrossRef] [PubMed]
- Joshy, K.S.; Thomas, S.; Kumar, V.; Editors, T. Gels Horizons: From Science to Smart Materials Nanoparticles for Drug Delivery; Springer: Singapore, 2021; ISBN 9789811622700. [Google Scholar]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan Hydrogels Incorporating Colloids for Sustained Drug Delivery. Carbohydr. Polym. 2022, 275, 118689. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Goswami, S.; Das, S.; Bhattacharjee, S. PH-Responsive, Stable, and Biocompatible Functional Nanogels Based on Chitosan (CS)/Poly Methacrylic Acid (PMAA) Polymers: Synthesis and Characterization. Mater. Today Commun. 2023, 36, 106541. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.D.; Selvanathan, V.; Sonsudin, F.; Abouloula, C.N. PH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A Versatile Semi-Synthetic Polymer in Biomedical Applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Li, L.; Xia, Y.; Hu, X.; Fang, Y. The Incorporated Hydrogel of Chitosan-Oligoconjugated Linoleic Acid Vesicles and the Protective Sustained Release for Curcumin in the Gel. Int. J. Biol. Macromol. 2023, 227, 17–26. [Google Scholar] [CrossRef]
- Fangueiro, J.S.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Cross-Linked Chitosan Microspheres for Oral Delivery of Insulin: Taguchi Design and in Vivo Testing. Colloids Surf. B Biointerfaces 2012, 92, 175–179. [Google Scholar] [CrossRef]
- Peidayesh, H.; Ahmadi, Z.; Khonakdar, H.A.; Abdouss, M.; Chodák, I. Baked Hydrogel from Corn Starch and Chitosan Blends Cross-Linked by Citric Acid: Preparation and Properties. Polym. Adv. Technol. 2020, 31, 1256–1269. [Google Scholar] [CrossRef]
- Yang, J.; Duan, A.; Shen, L.; Liu, Q.; Wang, F.; Liu, Y. Preparation and Application of Curcumin Loaded with Citric Acid Crosslinked Chitosan-Gelatin Hydrogels. Int. J. Biol. Macromol. 2024, 264, 130801. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Anghel, N.; Dinu, M.V. Dextran-Chitosan Composites: Antioxidant and Anti-Inflammatory Properties. Polymers 2023, 15, 1980. [Google Scholar] [CrossRef] [PubMed]
- Almajidi, Y.Q.; Gupta, J.; Sheri, F.S.; Zabibah, R.S.; Faisal, A.; Ruzibayev, A.; Adil, M.; Saadh, M.J.; Jawad, M.J.; Alsaikhan, F.; et al. Advances in Chitosan-Based Hydrogels for Pharmaceutical and Biomedical Applications: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 253, 127278. [Google Scholar] [CrossRef] [PubMed]
- Cas, M.D.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Sanz, D.; Claramunt, R.M.; Alkorta, I. Curcumin and Curcuminoids: Chemistry, Structural Studies and Biological Properties New Inhibitors of Plasmodium Falciparum View Project. An. Real Acad. Nac. Farm. 2015, 81, 278–310. [Google Scholar]
- Kang, N.; Wang, M.M.; Wang, Y.H.; Zhang, Z.N.; Cao, H.R.; Lv, Y.H.; Yang, Y.; Fan, P.H.; Qiu, F.; Gao, X.M. Tetrahydrocurcumin Induces G2/M Cell Cycle Arrest and Apoptosis Involving P38 MAPK Activation in Human Breast Cancer Cells. Food Chem. Toxicol. 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and Carboxymethyl Cellulose-Based 3D Multifunctional Bioactive Hydrogels Loaded with Nano-Curcumin for Synergistic Diabetic Wound Repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef]
- Górnicka, J.; Mika, M.; Wróblewska, O.; Siudem, P.; Paradowska, K. Methods to Improve the Solubility of Curcumin from Turmeric. Life 2023, 13, 207. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Shoji, M.; Nakagawa, K.; Watanabe, A.; Tsuduki, T.; Yamada, T.; Kuwahara, S.; Kimura, F.; Miyazawa, T. Comparison of the Effects of Curcumin and Curcumin Glucuronide in Human Hepatocellular Carcinoma HepG2 Cells. Food Chem. 2014, 151, 126–132. [Google Scholar] [CrossRef]
- Alavi, F.; Ciftci, O.N. Increasing the Bioavailability of Curcumin Using a Green Supercritical Fluid Technology-Assisted Approach Based on Simultaneous Starch Aerogel Formation-Curcumin Impregnation. Food Chem. 2024, 455, 139468. [Google Scholar] [CrossRef]
- Wu, C.; Ning, X.; Liu, Q.; Zhou, X.; Guo, H. Sustained Release of Curcumin from Cur-LPs Loaded Adaptive Injectable Self-Healing Hydrogels. Polymers 2024, 16, 3451. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.U.; Zia, K.M.; Nazir, A.; Iqbal, J.; Ejaz, S.A.; Akash, M.S.H. Pluronic-Based Mixed Polymeric Micelles Enhance the Therapeutic Potential of Curcumin. AAPS PharmSciTech 2018, 19, 2719–2739. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.J.; Patel, H.S.; Ray, D.; Aswal, V.K.; Singh, S.; Vijayvargia, R.; Sheth, U.; Sharma, R.K. Enhanced Solubility and Oral Bioavailability of Hydrophobic Drugs Using Pluronic Nanomicelles: An In-Vitro Evaluation. ChemistrySelect 2021, 6, 7040–7048. [Google Scholar] [CrossRef]
- Kang, Y.; Ha, W.; Zhang, S.; Li, B.J. Studies on PH-Sensitive High Drug Loading Prodrug Micelles for Anticancer Drug Delivery. J. Control. Release 2013, 172, E81–E82. [Google Scholar] [CrossRef]
- López, G.G. Nanomedicina Contra El Cáncer. Investig. Cienc. 2009, 391, 24–31. [Google Scholar]
- Dellali, M.; Iurciuc, C.E.; Savin, C.L.; Spahis, N.; Djennad, M.; Popa, M. Hydrogel Films Based on Chitosan and Oxidized Carboxymethylcellulose Optimized for the Controlled Release of Curcumin with Applications in Treating Dermatological Conditions. Molecules 2021, 26, 2185. [Google Scholar] [CrossRef]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Abnous, K.; Ramezani, M. Synthesis and Preparation of Biodegradable Hybrid Dextran Hydrogel Incorporated with Biodegradable Curcumin Nanomicelles for Full Thickness Wound Healing. Int. J. Pharm. 2017, 532, 466–477. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Peng, X.; Zheng, Y.; Cheng, Z.; Sun, S.; Ding, Q.; Liu, W.; Ding, C. A Poloxamer/Hyaluronic Acid/Chitosan-Based Thermosensitive Hydrogel That Releases Dihydromyricetin to Promote Wound Healing. Int. J. Biol. Macromol. 2022, 216, 475–486. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Varghese, S.; Manjusha, V. Hyaluronic Acid Coated Pluronic F127/Pluronic P123 Mixed Micelle for Targeted Delivery of Paclitaxel and Curcumin. Int. J. Biol. Macromol. 2021, 192, 950–957. [Google Scholar] [CrossRef]
- Park, K.M.; Joung, Y.K.; Park, K.D.; Lee, S.Y.; Lee, M.C. RGD-Conjugated Chitosan-Pluronic Hydrogels as a Cell Supported Scaffold for Articular Cartilage Regeneration. Macromol. Res. 2008, 16, 517–523. [Google Scholar] [CrossRef]
- Proksch, E. PH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of Curcumin in Pluronic Block Copolymer Micelles for Drug Delivery Applications. J. Biomater. Appl. 2011, 25, 619–639. [Google Scholar] [CrossRef] [PubMed]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors Influencing the Drug Release from Calcium Phosphate Cements. Bioact. Mater. 2022, 7, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Talevi, A.; Ruiz, M.E. Korsmeyer-Peppas, Peppas-Sahlin, and Brazel-Peppas: Models of Drug Release. In The ADME Encyclopedia; Springer International Publishing: Cham, Switzerland, 2022; pp. 613–621. [Google Scholar]
- Longoria-García, S.; Sánchez-Domínguez, C.N.; Sánchez-Domínguez, M.; Delgado-Balderas, J.R.; Islas-Cisneros, J.F.; Vidal-Gutiérrez, O.; Gallardo-Blanco, H.L. Design and Characterization of PMyc/PMax Peptide-Coupled Gold Nanosystems for Targeting Myc in Prostate Cancer Cell Lines. Nanomaterials 2023, 13, 2802. [Google Scholar] [CrossRef]
- Menezes, C.; Valério, E.D.E. The Kidney Vero-E6 Cell Line: A Suitable Model to Study the Toxicity of Microcystins. In New Insights into Toxicity and Drug Testing; IntechOpen: London, UK, 2013; p. 254. ISBN 978-953-51-0946-4. [Google Scholar]
- Worthen, A.J.; Irving, K.S.; Lapitsky, Y. Supramolecular Strategy Effects on Chitosan Bead Stability in Acidic Media: A Comparative Study. Gels 2019, 5, 11. [Google Scholar] [CrossRef]
- Li, C.P.; Weng, M.C.; Huang, S.L. Preparation and Characterization of PH Sensitive Chitosan/3- Glycidyloxypropyl Trimethoxysilane (GPTMS) Hydrogels by Sol-Gel Method. Polymers 2020, 12, 1326. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Sukumaran, H.G.; Dara, P.K.; Ganesan, B.; Ashraf, M.; Anandan, R.; Mathew, S.; Nagarajarao, R.C. Nano-Encapsulation of Curcumin in Fish Collagen Grafted Succinyl Chitosan Hydrogel Accelerates Wound Healing Process in Experimental Rats. Food Hydrocoll. Health 2022, 2, 100061. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of PH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Song, W.; Xu, J.; Gao, L.; Zhang, Q.; Tong, J.; Ren, L. Preparation of Freeze-Dried Porous Chitosan Microspheres for the Removal of Hexavalent Chromium. Appl. Sci. 2021, 11, 4217. [Google Scholar] [CrossRef]
- Ren, L.; Xu, J.; Zhang, Y.; Zhou, J.; Chen, D.; Chang, Z. Preparation and Characterization of Porous Chitosan Microspheres and Adsorption Performance for Hexavalent Chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N.; et al. Injectable and in Situ-Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of in Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 17936–17948. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.; Hang, L.; Dung, M.; Hiep, T.; Le, L.; Le, V.T. A Dual Synergistic of Curcumin and Gelatin on Thermal-Responsive Hydrogel Based on Chitosan-P123 in Wound Healing Application. Biomed. Pharmacother. 2019, 117, 109183. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of Curcumin in Alginate-Chitosan-Pluronic Composite Nanoparticles for Delivery to Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Tsao, C.T.; Chang, C.H.; Lin, Y.Y.; Wu, M.F.; Han, J.L.; Hsieh, K.H. Kinetic Study of Acid Depolymerization of Chitosan and Effects of Low Molecular Weight Chitosan on Erythrocyte Rouleaux Formation. Carbohydr. Res. 2011, 346, 94–102. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Zarrintaj, P.; Jafari, S.H.; Hosseini, S.M.; Aliakbari, S.; Pourbadie, H.G.; Naderi, N.; Zibaii, M.I.; Gholizadeh, S.S.; Ramsey, J.D.; et al. Fabricating an Electroactive Injectable Hydrogel Based on Pluronic-Chitosan/Aniline-Pentamer Containing Angiogenic Factor for Functional Repair of the Hippocampus Ischemia Rat Model. Mater. Sci. Eng. C 2020, 117, 111328. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, C.; Liu, R.; Liu, Q.; Xu, J.; Yao, J. Structure and Properties of Citric Acid Cross-Linked Chitosan/Poly(Vinyl Alcohol) Composite Films for Food Packaging Applications. Carbohydr. Polym. 2023, 312, 120842. [Google Scholar] [CrossRef]
- Jadwiga Ostrowska-Czubenko, M.P. State of Water in Citrate Crosslinked Chitosan Membrane. Prog. Chem. Appl. Chitin Its Deriv. 2010, XV15, 33–40. [Google Scholar]
- Guerrero, P.; Muxika, A.; Zarandona, I.; de la Caba, K. Crosslinking of Chitosan Films Processed by Compression Molding. Carbohydr. Polym. 2019, 206, 820–826. [Google Scholar] [CrossRef]
- Yadav, A.; Lomash, V.; Samim, M.; Flora, S.J.S. Curcumin Encapsulated in Chitosan Nanoparticles: A Novel Strategy for the Treatment of Arsenic Toxicity. Chem. Biol. Interact. 2012, 199, 49–61. [Google Scholar] [CrossRef]
- Khouri, J.; Penlidis, A.; Moresoli, C. Viscoelastic Properties of Crosslinked Chitosan Films. Processes 2019, 7, 157. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Adhikari, B.; Zhang, L. Preparation and Properties of Citric Acid-Crosslinked Chitosan Salt Microspheres through Radio Frequency Assisted Method. Food Hydrocoll. 2023, 139, 108538. [Google Scholar] [CrossRef]
- Bertz, A.; Wöhl-Bruhn, S.; Miethe, S.; Tiersch, B.; Koetz, J.; Hust, M.; Menzel, H. Encapsulation of Proteins in Hydrogel Carrier Systems for Controlled Drug Delivery: Influence of Network Structure and Drug Size on Release. J. Biotechnol. 2013, 2, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Gander, B.; Gurny, R.; Doelker, E.; Peppas, N.A. Effect of Polymeric Network Structure on Drug Release from Cross-Linked Poly (Vinyl Alcohol) Micromatrices. Pharm. Res. 1989, 6, 578–684. [Google Scholar] [CrossRef]
- Nahla, E.; Rabab, K.; Azza, A.M.; Dufresne, A.; Abouzeid, R.E.; Mahmoud, T.A.; Amr, M. Risedronate-Loaded Aerogel Scaffolds for Bone Regeneration. Drug Deliv. 2022, 30, 51–63. [Google Scholar] [CrossRef]
- Salazar, M.C.; Negrón, A.V. Preparación y Caracterización de Películas de Quitosano Depolimerizado y Reticulado Con Tripolifosfato de Sodio. Rev. Soc. Quím. Perú 2013, 79, 195–208. [Google Scholar]
- Kalaimani, N.; Ramya, K.; Vinitha, G.; Aarthi, R.; Raja, R.C. Structural, Spectral, Thermal and Nonlinear Optical Analysis of Anhydrous Citric Acid Crystal. Optik 2019, 192, 162960. [Google Scholar] [CrossRef]
- Sopade, P.A.; Halley, P.J.; Junming, L.L. Gelatinisation of Starch in Mixtures of Sugars. II. Application of Differential Scanning Calorimetry. Carbohydr. Polym. 2004, 58, 311–321. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.; Ding, J.; Huang, B.; Wang, P.; Zhao, Y.; Mu, Q.; Zhang, S.; Ren, C.; Xu, W. Hydrogels for Underwater Adhesion: Adhesion Mechanism, Design Strategies and Applications. J. Mater. Chem. A 2022, 10, 11823–11853. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Gónzalez-Ulloa, G.; González, M.A.; Jiménez-Rosado, M.; Rafii-El-Idrissi, B.M.; Romero, A.; Ostros, F.J.; Perez-Puyana, V.M. Influence of Natural Crosslinkers on Chitosan Hydrogels ForPotential Biomedical Applications. Macromol. Mater. Eng. 2023, 308, 1–13. [Google Scholar] [CrossRef]
- Ling, Z.; Jinfeng, Z.; Xiong, L.; Qiang, W.; Qin, X. Physicochemical Properties and Intermolecular Interactions of a Novel Diacylglycerol Oil Oleogel Made with Ethyl Cellulose as Affected by γ-Oryzanol. Food Hydrocoll. 2023, 138, 108484. [Google Scholar]
- Guoshan, S.L.; Zhang, C.; He De-Cai, F.; Philip, G.W.; Huiliang, W. Facile Fabrication of Tough Hydrogels Physically Cross-Linked by Strong Cooperative Hydrogen Bonding. Macromolecules 2013, 46, 2013. [Google Scholar]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Rubio-Valle, J.F.; Romero, A.; Ostos, F.J.; Benhnia, R.E.I.; Perez-Puyana, V. Biocompatible and Thermoresistant Hydrogels Based on Collagen and Chitosan. Polymers 2022, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Paarvanova, B.; Tacheva, B.; Savova, G.; Karabaliev, M.; Georgieva, R. Hemolysis by Saponin Is Accelerated at Hypertonic Conditions. Molecules 2023, 28, 7096. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Dev, A.; Das, S.S.; Kim, H.J.; Kumar, A.; Thakur, V.K.; Han, S.S. Curcumin-Loaded Alginate Hydrogels for Cancer Therapy and Wound Healing Applications: A Review. Int. J. Biol. Macromol. 2023, 232, 123283. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.; Hu, W.; Han, X.; Fan, L.; Tao, S. Preparation and Characterization of Carboxymethyl Chitosan/Collagen Peptide/Oxidized Konjac Composite Hydrogel. Int. J. Biol. Macromol. 2020, 149, 31–40. [Google Scholar] [CrossRef]
- ASTM F756; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM International: West Conshohocken, PA, USA, 2015.
- Ravikumar, S.; Hsieh, C.; Rajashekharaiah, V. Prospects of Curcumin as an Additive in Storage Solutions: A Study on Erythrocytes. Turk. J. Med. Sci. 2016, 46, 825–833. [Google Scholar] [CrossRef]
- Jafari, H.; Namazi, H. PH-Sensitive Biosystem Based on Laponite RD/Chitosan/Polyvinyl Alcohol Hydrogels for Controlled Delivery of Curcumin to Breast Cancer Cells. Colloids Surf. B Biointerfaces 2023, 231, 113585. [Google Scholar] [CrossRef]
- Manaargadoo-Catin, M.; Ali-Cherif, A.; Pougnas, J.L.; Perrin, C. Hemolysis by Surfactants—A Review. Adv. Colloid. Interface Sci. 2016, 228, 1–16. [Google Scholar] [CrossRef]
- Zare-Gachi, M.; Daemi, H.; Mohammadi, J.; Baei, P.; Bazgir, F.; Hosseini-Salekdeh, S.; Baharvand, H. Improving Anti-Hemolytic, Antibacterial and Wound Healing Properties of Alginate Fibrous Wound Dressings by Exchanging Counter-Cation for Infected Full-Thickness Skin Wounds. Mater. Sci. Eng. C 2020, 107, 110321. [Google Scholar] [CrossRef]
- Ong, T.H.D.; Yu, N.; Meenashisundaram, G.K.; Schaller, B.; Gupta, M. Insight into Cytotoxicity of Mg Nanocomposites Using MTT Assay Technique. Mater. Sci. Eng. C 2017, 78, 647–652. [Google Scholar] [CrossRef]
- Zeng, N.; Mignet, N.; Dumortier, G.; Olivier, E.; Seguin, J.; Maury, M.; Scherman, D.; Rat, P.; Boudy, V. Poloxamer Bioadhesive Hydrogel for Buccal Drug Delivery: Cytotoxicity and Trans-Epithelial Permeability Evaluations Using TR146 Human Buccal Epithelial Cell Line. Int. J. Pharm. 2015, 495, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Facchi, S.P.; Scariot, D.B.; Bueno, P.V.A.; Souza, P.R.; Figueiredo, L.C.; Follmann, H.D.M.; Nunes, C.S.; Monteiro, J.P.; Bonafé, E.G.; Nakamura, C.V.; et al. Preparation and Cytotoxicity of N-Modified Chitosan Nanoparticles Applied in Curcumin Delivery. Int. J. Biol. Macromol. 2016, 87, 237–245. [Google Scholar] [CrossRef] [PubMed]





| Sample | CA (wt%) | Reaction Time (h) | Cs (wt%) |
|---|---|---|---|
| AC1 | 0.05 | 6 | 0.5 |
| AC2 | 0.05 | 6 | 1 |
| AC3 | 0.05 | 24 | 1 |
| AC4 | 0.05 | 24 | 0.5 |
| AC5 | 0.1 | 6 | 0.5 |
| AC6 | 0.1 | 6 | 1 |
| AC7 | 0.1 | 24 | 1 |
| AC8 | 0.1 | 24 | 0.5 |
| AC9 | 0.075 | 15 | 0.75 |
| AC10 | 0.075 | 15 | 0.75 |
| AC11 | 0.05 | 15 | 0.75 |
| AC12 | 0.1 | 15 | 0.75 |
| AC13 | 0.075 | 6 | 0.75 |
| AC14 | 0.075 | 24 | 0.75 |
| AC15 | 0.075 | 15 | 0.5 |
| AC16 | 0.075 | 15 | 1 |
| Cs-CA | 0.05 | 24 | 1 |
| Sample | % Cs (wt%) | % CA (wt%) | % P407 (wt%) | % Water (wt%) | %Cur LC | %Cur EE |
|---|---|---|---|---|---|---|
| AC1 | 14.66 | 0.73 | 34.21 | 50.38 | 0.38 | 67.55 ± 0.38 |
| AC2 | 22.42 | 1.12 | 26.16 | 50.29 | 0.29 | 75.69 ± 0.43 |
| AC3 | 22.42 | 1.12 | 26.16 | 50.29 | 0.29 | 80.07 ± 0.45 |
| AC4 | 14.66 | 0.73 | 34.21 | 50.38 | 0.38 | 69.01 ± 0.39 |
| AC5 | 14.45 | 1.44 | 33.72 | 50.38 | 0.38 | 66.86 ± 0.38 |
| AC6 | 21.92 | 2.19 | 25.58 | 50.28 | 0.28 | 70.38 ± 0.40 |
| AC7 | 21.92 | 2.19 | 25.58 | 50.28 | 0.28 | 74.91 ± 0.42 |
| AC8 | 14.45 | 1.44 | 33.72 | 50.38 | 0.38 | 70.44 ± 0.40 |
| AC9 | 18.87 | 1.41 | 29.37 | 50.33 | 0.33 | 69.93 ± 0.40 |
| AC10 | 18.87 | 1.41 | 29.37 | 50.33 | 0.33 | 66.76 ± 0.38 |
| AC11 | 19.05 | 0.95 | 29.65 | 50.33 | 0.33 | 70.01 ± 0.40 |
| AC12 | 18.70 | 1.87 | 29.09 | 50.32 | 0.32 | 71.71 ± 0.41 |
| AC13 | 18.87 | 1.41 | 29.37 | 50.33 | 0.33 | 72.48 ± 0.41 |
| AC14 | 18.87 | 1.41 | 29.37 | 50.33 | 0.33 | 71.39 ± 0.41 |
| AC15 | 14.55 | 1.09 | 33.96 | 50.38 | 0.38 | 68.03 ± 0.39 |
| AC16 | 22.17 | 1.66 | 25.87 | 50.29 | 0.29 | 78.33 ± 0.44 |
| Cs-CA | 47.61 | 2.38 | 0 | 50.00 | - | - |
| Cs-CA-Cur Ace | 47.61 | 2.38 | 0 | 50.00 | - | - |
| P407-Cur | 0.00 | 0.00 | 26.16 | 73.84 | - | - |
| AC3 Alk | 22.93 | 0.00 | 26.76 | 50.30 | - | - |
| System | %Cur LC | %Cur EE | Reference |
|---|---|---|---|
| Cur loaded into PEG-PLA micelles incorporated into dextran hydrogel | 0.72 | 81.56 | [28] |
| Cur encapsulated into liposomes coated with thiolated chitosan | 0.21 | 88.75 | [44] |
| Cur nano-encapsulated into fish collagen–succinyl chitosan composite hydrogel | 0.23 | 96.2 | [40] |
| Hybrid hydrogel made of chitosan and P123, containing gelatin and Cur | 0.027 | - | [45] |
| Composite of alginate, chitosan and P407 micelles loaded with Cur | 0.10 | 13.0 | [46] |
| Chitosan hydrogel cross-linked with citric acid charged with Cur-loaded P407 micelles | 0.38 | 69.0 | This work |
| Systems | Temperature (°C) | Critical Strain (-) | G′1 (Pa) | Tan(δ)1 (-) |
|---|---|---|---|---|
| Cs-CA | 22 | 0.411 ± 0.072 | 1211 ± 117 | 0.083 ± 0.011 |
| 37 | 0.179 ± 0.019 | 67 ± 11 | 0.015 ± 0.003 | |
| AC3 | 22 | 0.479 ± 0.083 | 1898 ± 317 | 0.081 ± 0.013 |
| 37 | 0.191 ± 0.023 | 83 ± 19 | 0.021 ± 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Alonso, A.E.; Rodríguez-Chávez, D.F.; Toxqui-Terán, A.; Rubio-Valle, J.F.; Martín-Alfonso, J.E.; Longoria-García, S.; Gallardo-Blanco, H.L.; Sánchez-Domínguez, C.N.; Sánchez-Domínguez, M. pH-Sensitive Chitosan-Based Hydrogels Trap Poloxamer Micelles as a Dual-Encapsulating Responsive System for the Loading and Delivery of Curcumin. Polymers 2025, 17, 1335. https://doi.org/10.3390/polym17101335
Herrera-Alonso AE, Rodríguez-Chávez DF, Toxqui-Terán A, Rubio-Valle JF, Martín-Alfonso JE, Longoria-García S, Gallardo-Blanco HL, Sánchez-Domínguez CN, Sánchez-Domínguez M. pH-Sensitive Chitosan-Based Hydrogels Trap Poloxamer Micelles as a Dual-Encapsulating Responsive System for the Loading and Delivery of Curcumin. Polymers. 2025; 17(10):1335. https://doi.org/10.3390/polym17101335
Chicago/Turabian StyleHerrera-Alonso, Alejandra E., Daniela F. Rodríguez-Chávez, Alberto Toxqui-Terán, José F. Rubio-Valle, José E. Martín-Alfonso, Samuel Longoria-García, Hugo L. Gallardo-Blanco, Celia N. Sánchez-Domínguez, and Margarita Sánchez-Domínguez. 2025. "pH-Sensitive Chitosan-Based Hydrogels Trap Poloxamer Micelles as a Dual-Encapsulating Responsive System for the Loading and Delivery of Curcumin" Polymers 17, no. 10: 1335. https://doi.org/10.3390/polym17101335
APA StyleHerrera-Alonso, A. E., Rodríguez-Chávez, D. F., Toxqui-Terán, A., Rubio-Valle, J. F., Martín-Alfonso, J. E., Longoria-García, S., Gallardo-Blanco, H. L., Sánchez-Domínguez, C. N., & Sánchez-Domínguez, M. (2025). pH-Sensitive Chitosan-Based Hydrogels Trap Poloxamer Micelles as a Dual-Encapsulating Responsive System for the Loading and Delivery of Curcumin. Polymers, 17(10), 1335. https://doi.org/10.3390/polym17101335










