Production of Graphite Nanoplatelets via Functionalized Polyketone-Assisted Diels–Alder Chemistry: Evidence of Reduced Layer Thickness and Enhanced Exfoliation Efficiency
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
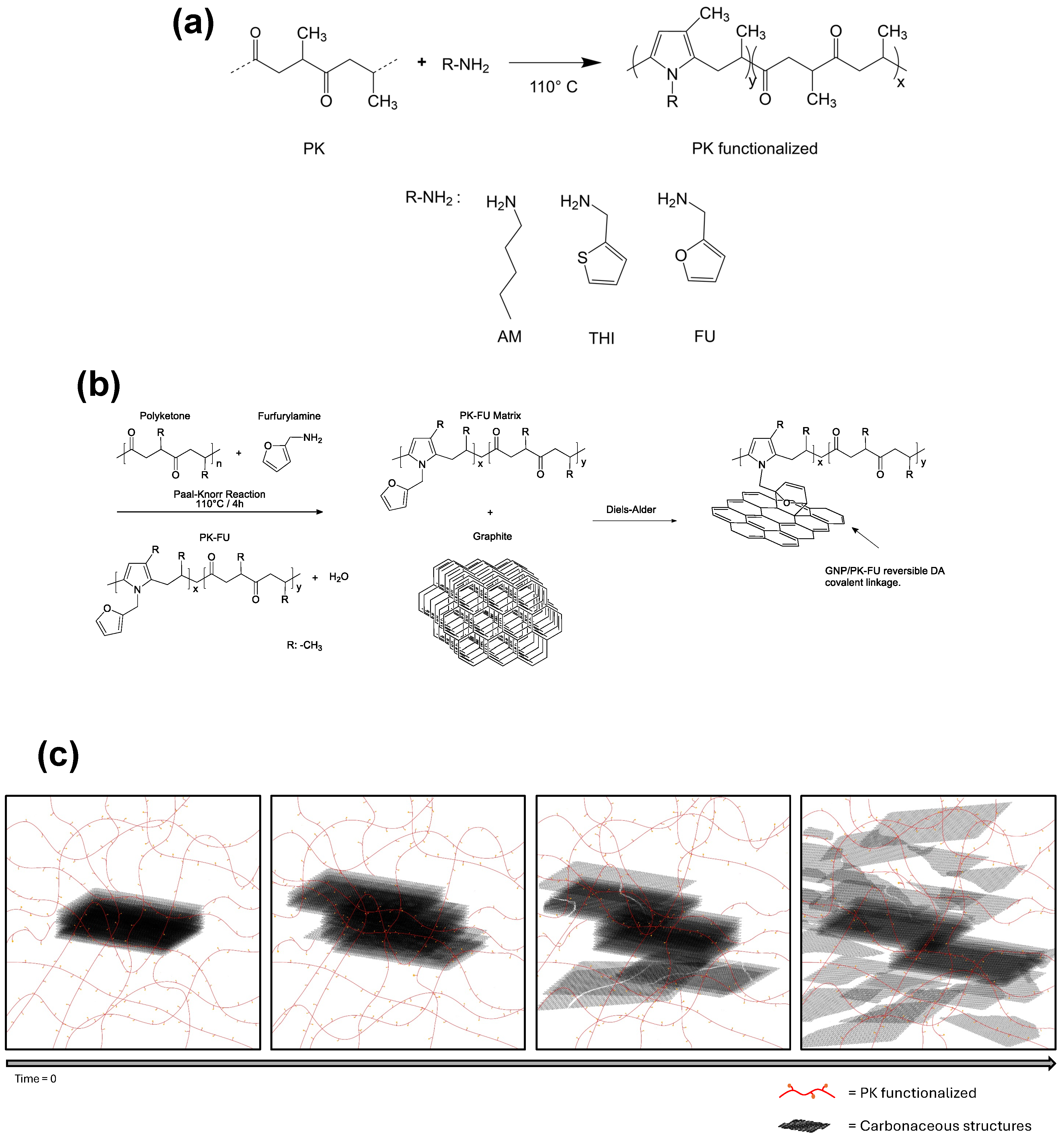
2.2.1. Functionalization of Polyketone with Aliphatic, Furan, and Thiophene Groups
2.2.2. Graphite Exfoliation Assisted by Polyketones
2.2.3. Mass Yield of Exfoliated Graphite
2.3. Characterization
3. Results and Discussion
3.1. Functionalization of Polyketone with Aliphatic, Furan, and Thiophene Groups
3.2. Polymer/Graphite Composites
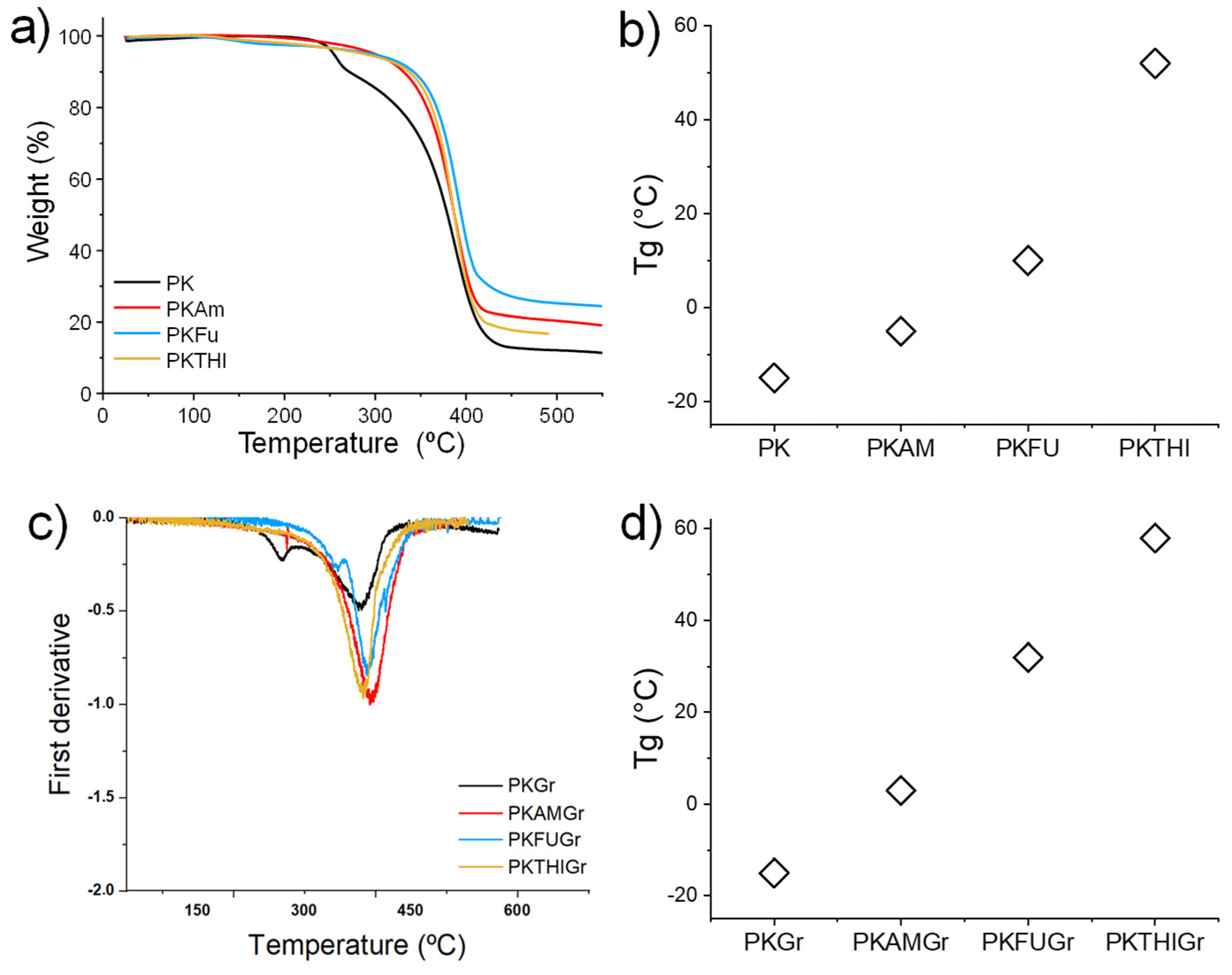
3.3. Thermal Characterization
3.4. Rheology Characterization
3.5. Microscopy Characterization
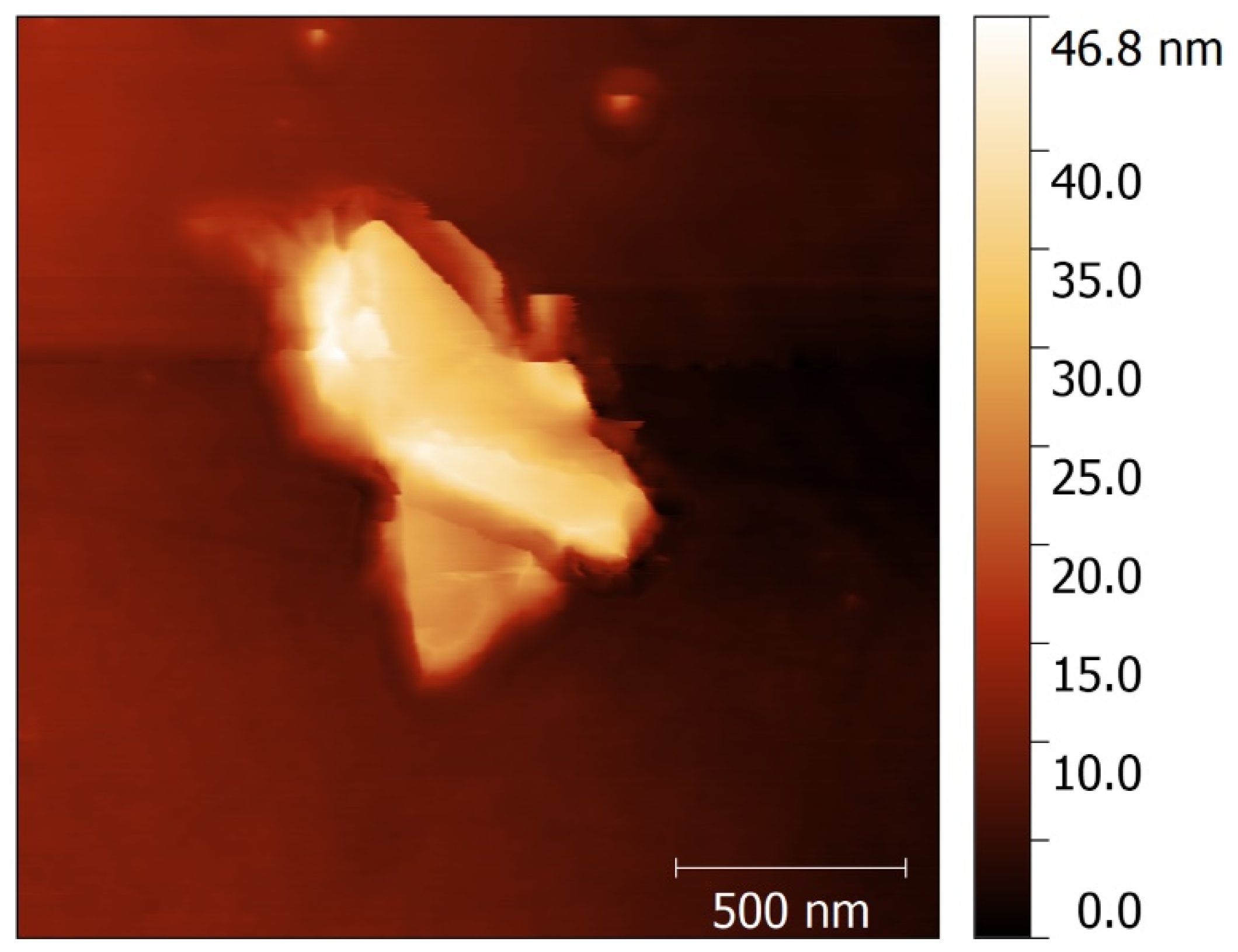
3.6. AFM Characterization
3.7. Raman Spectroscopy
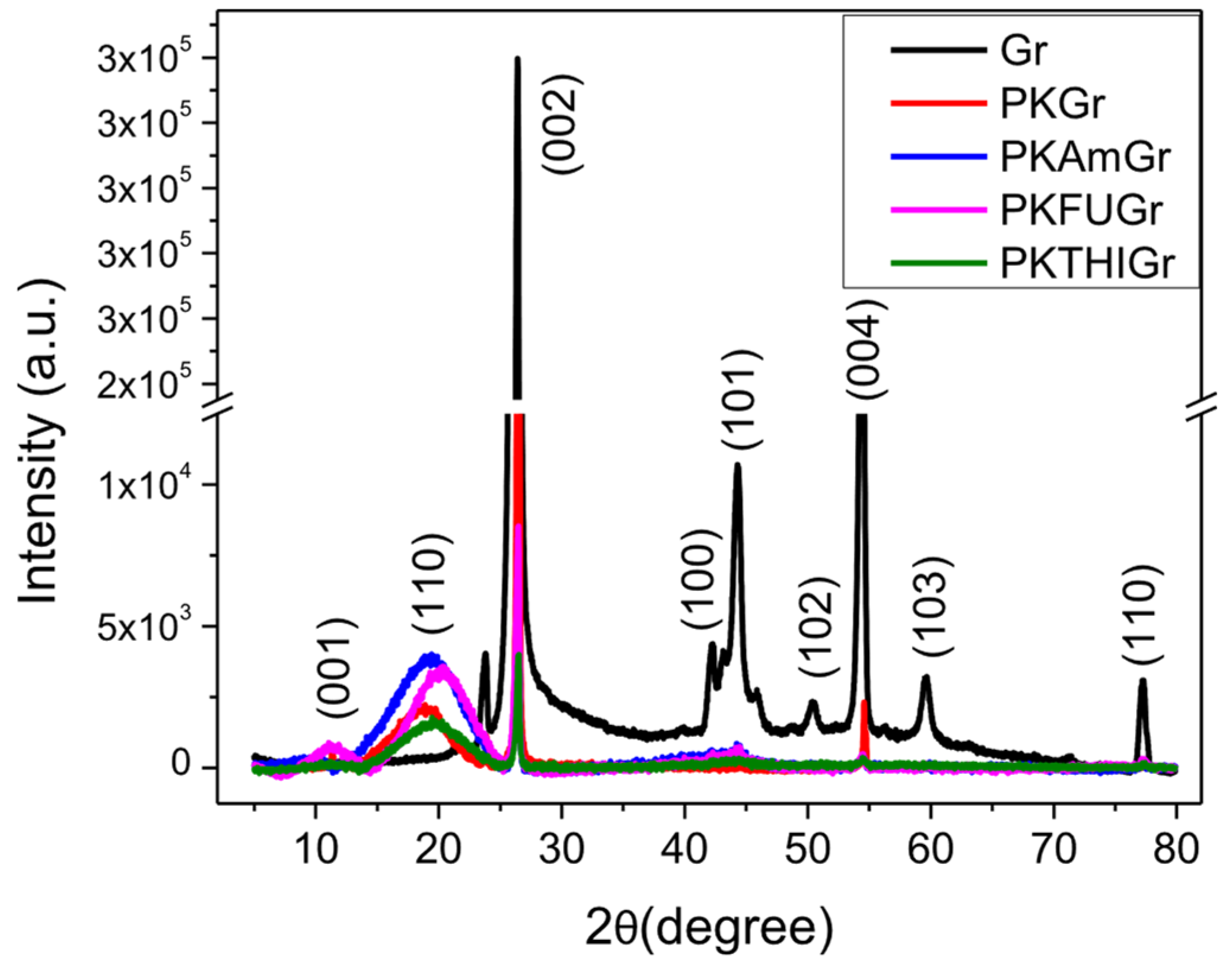
3.8. X-Ray Diffraction (XRD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miculescu, M.; Thakur, V.K.; Miculescu, F.; Voicu, S.I. Graphene-Based Polymer Nanocomposite Membranes: A Review. Polym. Adv. Technol. 2016, 27, 844–859. [Google Scholar] [CrossRef]
- Shahryari, Z.; Yeganeh, M.; Gheisari, K.; Ramezanzadeh, B. A Brief Review of the Graphene Oxide-Based Polymer Nanocomposite Coatings: Preparation, Characterization, and Properties. J. Coat. Technol. Res. 2021, 18, 945–969. [Google Scholar] [CrossRef]
- Shah, R.; Kausar, A.; Muhammad, B.; Shah, S. Progression from Graphene and Graphene Oxide to High Performance Polymer-Based Nanocomposite: A Review. Polym. Plast. Technol. Eng. 2015, 54, 173–183. [Google Scholar] [CrossRef]
- Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. The Structure of Suspended Graphene Sheets. Nature 2007, 446, 60–63. [Google Scholar] [CrossRef]
- Pal, N.; Dubey, P.; Gopinath, P.; Pal, K. Combined Effect of Cellulose Nanocrystal and Reduced Graphene Oxide into Poly-Lactic Acid Matrix Nanocomposite as a Scaffold and Its Anti-Bacterial Activity. Int. J. Biol. Macromol. 2017, 95, 94–105. [Google Scholar] [CrossRef]
- Kymakis, E.; Savva, K.; Stylianakis, M.M.; Fotakis, C.; Stratakis, E. Flexible Organic Photovoltaic Cells with In Situ Nonthermal Photoreduction of Spin-Coated Graphene Oxide Electrodes. Adv. Funct. Mater. 2013, 23, 2742–2749. [Google Scholar] [CrossRef]
- Lu, C.H.; Yang, H.H.; Zhu, C.L.; Chen, X.; Chen, G.N. A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Ed. Engl. 2009, 48, 4785–4787. [Google Scholar] [CrossRef]
- Gutiérrez-Cruz, A.; Ruiz-Hernández, A.R.; Vega-Clemente, J.F.; Luna-Gazcón, D.G.; Campos-Delgado, J. A Review of Top-down and Bottom-up Synthesis Methods for the Production of Graphene, Graphene Oxide and Reduced Graphene Oxide. J. Mater. Sci. 2022, 57, 14543–14578. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Lombardo, A.; Hasan, T.; Sun, Z.; Colombo, L.; Ferrari, A.C. Production and Processing of Graphene and 2d Crystals. Mater. Today 2012, 15, 564–589. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical Vapour Deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Yan, Y.; Nashath, F.Z.; Chen, S.; Manickam, S.; Lim, S.S.; Zhao, H.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of Graphene: Potential Carbon Precursors and Approaches. Nanotechnol. Rev. 2020, 9, 1284–1314. [Google Scholar] [CrossRef]
- Rudrapati, R.; Rudrapati, R. Graphene: Fabrication Methods, Properties, and Applications in Modern Industries. In Graphene Production and Application; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- Sumdani, M.G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I. Recent Advances of the Graphite Exfoliation Processes and Structural Modification of Graphene: A Review. J. Nanopart. Res. 2021, 23, 253. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Gupta, R.K. Graphite, Graphene, and Their Polymer Nanocomposites; Routledge: London, UK, 2013; p. 609. [Google Scholar]
- Zhao, L.; Tang, J.; Zhou, M.; Shen, K. A Review of the Coefficient of Thermal Expansion and Thermal Conductivity of Graphite. New Carbon Mater. 2022, 37, 544–555. [Google Scholar] [CrossRef]
- Backes, C.; Abdelkader, A.M.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R.; et al. Production and Processing of Graphene and Related Materials. 2D Mater. 2020, 7, 022001. [Google Scholar] [CrossRef]
- Kairi, M.I.; Dayou, S.; Kairi, N.I.; Bakar, S.A.; Vigolo, B.; Mohamed, A.R. Toward High Production of Graphene Flakes—A Review on Recent Developments in Their Synthesis Methods and Scalability. J. Mater. Chem. A Mater. 2018, 6, 15010–15026. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, F.; Wang, H.; Huang, F.; Meng, F.; Hui, D.; Zhou, Z. Intercalation Polymerization Approach for Preparing Graphene/Polymer Composites. Polymers 2018, 10, 61. [Google Scholar] [CrossRef]
- Shi, Z.; He, P.; Wang, N.; Liu, Y.; Chen, X.; Li, Y.; Ding, G.; Yu, Q.; Xie, X. Bubble-Mediated Mass Production of Graphene: A Review. Adv. Funct. Mater. 2022, 32, 2203124. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of Graphene in Energy Storage Device—A Review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Eslami-Farsani, R.; Torabian, M.; Amousa, N. Recent Advances in One-Pot Functionalization of Graphene Using Electrochemical Exfoliation of Graphite: A Review Study. Synth. Met. 2020, 269, 116549. [Google Scholar] [CrossRef]
- Toyoda, M.; Hou, S.; Huang, Z.H.; Inagaki, M. Exfoliated Graphite: Room Temperature Exfoliation and Their Applications. Carbon Lett. 2023, 33, 335–362. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of Graphene Materials by Electrochemical Exfoliation: Recent Progress and Future Potential. Carbon Energy 2019, 1, 173–199. [Google Scholar] [CrossRef]
- Rana, N.; Narang, J.; Chauhan, A. Advancing Frontiers: Graphene-Based Nano-Biosensor Platforms for Cutting-Edge Research and Future Innovations. Indian J. Microbiol. 2024, 1, 1–24. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the Graphene Family—A Recommended Nomenclature for Two-Dimensional Carbon Materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Dhar, S.; Barman, A.R.; Ni, G.X.; Wang, X.; Xu, X.F.; Zheng, Y.; Tripathy, S.; Ariando; Rusydi, A.; Loh, K.P.; et al. A New Route to Graphene Layers by Selective Laser Ablation. AIP Adv. 2011, 1, 22109. [Google Scholar] [CrossRef]
- Pirzado, A.A.; Le Normand, F.; Romero, T.; Paszkiewicz, S.; Papaefthimiou, V.; Ihiawakrim, D.; Janowska, I. Few-Layer Graphene from Mechanical Exfoliation of Graphite-Based Materials: Structure-Dependent Characteristics. ChemEngineering 2019, 3, 37. [Google Scholar] [CrossRef]
- Komeily-Nia, Z.; Qu, L.T.; Li, J.L. Progress in the Understanding and Applications of the Intrinsic Reactivity of Graphene-Based Materials. Small Sci. 2021, 1, 2000026. [Google Scholar] [CrossRef]
- Seo, J.M.; Jeon, I.Y.; Baek, J.B. Mechanochemically Driven Solid-State Diels–Alder Reaction of Graphite into Graphene Nanoplatelets. Chem. Sci. 2013, 4, 4273–4277. [Google Scholar] [CrossRef]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of Graphite Exfoliation. J. Mater. Chem. 2012, 22, 24992–25002. [Google Scholar] [CrossRef]
- Schmidt, C.; Rosen, M.E.; Caplan, D.F.; Pines, A.; Quinton, M.F. Orientation and Motion of Tetrahydrofuran in Graphite Intercalation Compounds. Proton NMR Studies of Cs(THF)1.3C24 and K(THF)2.5C24. J. Phys. Chem. 1995, 99, 10565–10572. [Google Scholar] [CrossRef]
- Torkaman, N.F.; Kley, M.; Bremser, W.; Wilhelm, R. Reversible Functionalization and Exfoliation of Graphite by a Diels–Alder Reaction with Furfuryl Amine. RSC Adv. 2022, 12, 17249–17256. [Google Scholar] [CrossRef]
- Wang, Z.; Tong, J.; Li, W.; Zhang, H.; Hu, M.; Chen, H.; He, H. Highly Enhancing Electrical, Thermal, and Mechanical Properties of Polypropylene/Graphite Intercalation Compound Composites by In Situ Expansion during Melt Mixing. Polymers 2021, 13, 3095. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Weng, S.; Niu, R.; Zhen, W.; Xu, F.; Zhu, W.; Zhang, C. Poly(Vinyl Alcohol)-Assisted Exfoliation of van Der Waals Materials. ACS Omega 2022, 7, 38774–38781. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, C.; Weng, W.; Wu, D.; Yan, W. Preparation of Polystyrene/Graphite Nanosheet Composite. Polymer 2003, 44, 1781–1784. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Georgakilas, V.; Zboril, R.; Steriotis, T.A.; Stubos, A.K.; Trapalis, C. Aqueous-Phase Exfoliation of Graphite in the Presence of Polyvinylpyrrolidone for the Production of Water-Soluble Graphenes. Solid. State Commun. 2009, 149, 2172–2176. [Google Scholar] [CrossRef]
- Ho, Q.B.; Osazuwa, O.; Modler, R.; Daymond, M.; Gallerneault, M.T.; Kontopoulou, M. Exfoliation of Graphite and Expanded Graphite by Melt Compounding to Prepare Reinforced, Thermally and Electrically Conducting Polyamide Composites. Compos. Sci. Technol. 2019, 176, 111–120. [Google Scholar] [CrossRef]
- Nasir, A.; Kausar, A.; Younus, A. Polymer/Graphite Nanocomposites: Physical Features, Fabrication and Current Relevance. Polym. Plast. Technol. Eng. 2015, 54, 750–770. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Borriello, A.; Zarrelli, M. Polymer Nanocomposites Based on Graphite Nanoplatelets and Amphiphilic Graphene Platelets. Compos. B Eng. 2022, 246, 110223. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Morphology and Properties of Polyester/Exfoliated Graphite Nanocomposites. Macromolecules 2008, 41, 3317–3327. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Development of Graphene-Based Polymeric Nanocomposites: A Brief Overview. Polymers 2021, 13, 2978. [Google Scholar] [CrossRef]
- Chen, W.; Wu, K.; Liu, Q.; Lu, M. Functionalization of Graphite via Diels-Alder Reaction to Fabricate Poly (Vinyl Alcohol) Composite with Enhanced Thermal Conductivity. Polymer 2020, 186, 122075. [Google Scholar] [CrossRef]
- Seo, J.M.; Baek, J.B. A Solvent-Free Diels–Alder Reaction of Graphite into Functionalized Graphene Nanosheets. Chem. Commun. 2014, 50, 14651–14653. [Google Scholar] [CrossRef] [PubMed]
- Zabihi, O.; Ahmadi, M.; Abdollahi, T.; Nikafshar, S.; Naebe, M. Collision-Induced Activation: Towards Industrially Scalable Approach to Graphite Nanoplatelets Functionalization for Superior Polymer Nanocomposites. Sci. Rep. 2017, 7, 3560. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Bekyarova, E.; Niyogi, S.; Haddon, R.C. Diels-Alder Chemistry of Graphite and Graphene: Graphene as Diene and Dienophile. J. Am. Chem. Soc. 2011, 133, 3324–3327. [Google Scholar] [CrossRef]
- Feng, Z.; Zuo, H.; Hu, J.; Yu, B.; Ning, N.; Tian, M.; Zhang, L. In Situ Exfoliation of Graphite into Graphene Nanosheets in Elastomer Composites Based on Diels-Alder Reaction during Melt Blending. Ind. Eng. Chem. Res. 2019, 58, 13182–13189. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Pucci, A.; Raffa, P.; Santosa, D.; Pescarmona, P.P.; Gengler, R.Y.N.; Rudolf, P.; Moreno-Villoslada, I.; Picchioni, F. Electrically-Responsive Reversible Polyketone/MWCNT Network through Diels-Alder Chemistry. Polymers 2018, 10, 1076. [Google Scholar] [CrossRef]
- Migliore, N.; Polgar, L.M.; Araya-Hermosilla, R.; Picchioni, F.; Raffa, P.; Pucci, A. Effect of the Polyketone Aromatic Pendent Groups on the Electrical Conductivity of the Derived MWCNTs-Based Nanocomposites. Polymers 2018, 10, 618. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Pucci, A.; Araya-Hermosilla, E.; Pescarmona, P.P.; Raffa, P.; Polgar, L.M.; Moreno-Villoslada, I.; Flores, M.; Fortunato, G.; Broekhuis, A.A.; et al. An Easy Synthetic Way to Exfoliate and Stabilize MWCNTs in a Thermoplastic Pyrrole-Containing Matrix Assisted by Hydrogen Bonds. RSC Adv. 2016, 6, 85829–85837. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.A.; Carlotti, M.; Picchioni, F.; Mattoli, V.; Pucci, A. Electrically-Conductive Polyketone Nanocomposites Based on Reduced Graphene Oxide. Polymers 2020, 12, 923. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Giannetti, A.; Lima, G.M.R.; Orozco, F.; Picchioni, F.; Mattoli, V.; Bose, R.K.; Pucci, A. Thermally Switchable Electrically Conductive Thermoset RGO/PK Self-Healing Composites. Polymers 2021, 13, 339. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Stuart, M.C.A.; Picchioni, F. Polymeric Amines by Chemical Modifications of Alternating Aliphatic Polyketones. J. Appl. Polym. Sci. 2008, 107, 262–271. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Roscam Abbing, M.; Catalán-Toledo, J.; Oyarzun-Ampuero, F.; Pucci, A.; Raffa, P.; Picchioni, F.; Moreno-Villoslada, I. Synthesis of Tuneable Amphiphilic-Modified Polyketone Polymers, Their Complexes with 5,10,15,20-Tetrakis-(4-Sulfonatophenyl)Porphyrin, and Their Role in the Photooxidation of 1,3,5-Triphenylformazan Confined in Polymeric Nanoparticles. Polymer 2019, 167, 215–223. [Google Scholar] [CrossRef]
- Lima, G.M.R.; Orozco, F.; Picchioni, F.; Moreno-Villoslada, I.; Pucci, A.; Bose, R.K.; Araya-Hermosilla, R. Electrically Self-Healing Thermoset MWCNTs Composites Based on Diels-Alder and Hydrogen Bonds. Polymers 2019, 11, 1885. [Google Scholar] [CrossRef]
- Zhang, Y.; Broekhuis, A.A.; Picchioni, F. Aqueous Polymer Emulsions by Chemical Modifications of Thermosetting Alternating Polyketones. J. Appl. Polym. Sci. 2007, 106, 3237–3247. [Google Scholar] [CrossRef]
- Toncelli, C.; Schoonhoven, M.J.; Broekhuis, A.A.; Picchioni, F. Paal-Knorr Kinetics in Waterborne Polyketone-Based Formulations as Modulating Cross-Linking Tool in Electrodeposition Coatings. Mater. Des. 2016, 108, 718–724. [Google Scholar] [CrossRef]
- Mul, W.P.; Dirkzwager, H.; Broekhuis, A.A.; Heeres, H.J.; Van der Linden, A.J.; Guy Orpen, A. Highly Active, Recyclable Catalyst for the Manufacture of Viscous, Low Molecular Weight, CO–Ethene–Propene-Based Polyketone, Base Component for a New Class of Resins. Inorg. Chim. Acta 2002, 327, 147–159. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’ko, Y.K.; et al. High-Yield Production of Graphene by Liquid-Phase Exfoliation of Graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef]
- Popova, A.N. Crystallographic Analysis of Graphite by X-Ray Diffraction. Coke Chem. 2017, 60, 361–365. [Google Scholar] [CrossRef]
- Barnakov, C.N.; Khokhlova, G.P.; Malysheva, V.Y.; Popova, A.N.; Ismagilov, Z.R. X-Ray Diffraction Analysis of the Crystal Structures of Different Graphites. Solid. Fuel Chem. 2015, 49, 25–29. [Google Scholar] [CrossRef]
- Bendrea, A.D.; Cianga, L.; Göen Colak, D.; Constantinescu, D.; Cianga, I. Thiophene End-Functionalized Oligo-(D,L-Lactide) as a New Electroactive Macromonomer for the “Hairy-Rod” Type Conjugated Polymers Synthesis. Polymers 2023, 15, 1094. [Google Scholar] [CrossRef]
- Pachariyangkun, A.; Suda, M.; Hadsadee, S.; Jungsuttiwong, S.; Nalaoh, P.; Pattanasattayavong, P.; Sudyoadsuk, T.; Yamamoto, H.M.; Promarak, V. Effect of Thiophene/Furan Substitution on Organic Field Effect Transistor Properties of Arylthiadiazole Based Organic Semiconductors. J. Mater. Chem. C Mater. 2020, 8, 17297–17306. [Google Scholar] [CrossRef]
- Calvo-Martín, G.; Plano, D.; Sanmartín, C. New Experimental Conditions for Diels–Alder and Friedel-Crafts Alquilation Reactions with Thiophene: A New Selenocyanate with Potent Activity against Cancer. Molecules 2022, 27, 982. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, Y.; Liu, T.; Su, X.; Qian, L.; Song, Y.; Xu, H. Synthesis and Nonlinear Optical Properties of Polyacetylenes Containing Oxadiazole and Thiophene Pendant Groups with High Thermal Stability. J. Polym. Sci. A Polym. Chem. 2010, 48, 5498–5504. [Google Scholar] [CrossRef]
- Galimberti, M.; Barbera, V.; Guerra, S.; Conzatti, L.; Castiglioni, C.; Brambilla, L.; Serafini, A. Biobased Janus Molecule for the Facile Preparation of Water Solutions of Few Layer Graphene Sheets. RSC Adv. 2015, 5, 81142–81152. [Google Scholar] [CrossRef]
- Barbera, V.; Brambilla, L.; Milani, A.; Palazzolo, A.; Castiglioni, C.; Vitale, A.; Bongiovanni, R.; Galimberti, M. Domino Reaction for the Sustainable Functionalization of Few-Layer Graphene. Nanomaterials 2018, 9, 44. [Google Scholar] [CrossRef]
- Barra, G.; Guadagno, L.; Raimondo, M.; Santonicola, M.G.; Toto, E.; Vecchio Ciprioti, S. A Comprehensive Review on the Thermal Stability Assessment of Polymers and Composites for Aeronautics and Space Applications. Polymers 2023, 15, 3786. [Google Scholar] [CrossRef]
- Tayouri, M.I.; Estaji, S.; Mousavi, S.R.; Salkhi Khasraghi, S.; Jahanmardi, R.; Nouranian, S.; Arjmand, M.; Khonakdar, H.A. Degradation of Polymer Nanocomposites Filled with Graphene Oxide and Reduced Graphene Oxide Nanoparticles: A Review of Current Status. Polym. Degrad. Stab. 2022, 206, 110179. [Google Scholar] [CrossRef]
- Guerra, V.; Wan, C.; Degirmenci, V.; Sloan, J.; Presvytis, D.; Watson, M.; McNally, T. Characterisation of Graphite Nanoplatelets (GNP) Prepared at Scale by High-Pressure Homogenisation. J. Mater. Chem. C Mater. 2019, 7, 6383–6390. [Google Scholar] [CrossRef]
- Shtein, M.; Pri-Bar, I.; Varenik, M.; Regev, O. Characterization of Graphene-Nanoplatelets Structure via Thermogravimetry. Anal. Chem. 2015, 87, 4076–4080. [Google Scholar] [CrossRef]
- Hope, J.T.; Sun, W.; Kewalramani, S.; Saha, S.; Lakhe, P.; Shah, S.A.; Mason, M.J.; Green, M.J.; Hule, R.A. Scalable Production of Graphene Nanoplatelets for Energy Storage. ACS Appl. Nano Mater. 2020, 3, 10303–10309. [Google Scholar] [CrossRef]
- Wu, J.B.; Lin, M.L.; Cong, X.; Liu, H.N.; Tan, P.H. Raman Spectroscopy of Graphene-Based Materials and Its Applications in Related Devices. Chem. Soc. Rev. 2018, 47, 1822–1873. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Zydziak, N.; Yameen, B.; Barner-Kowollik, C. Diels–Alder Reactions for Carbon Material Synthesis and Surface Functionalization. Polym. Chem. 2013, 4, 4072–4086. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman Spectroscopy in Graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, Q.; Cheng, H.; Hu, M.; Zhang, S. Classification and Carbon Structural Transformation from Anthracite to Natural Coaly Graphite by XRD, Raman Spectroscopy, and HRTEM. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 249, 119286. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-Ray Diffraction Patterns of Graphite and Turbostratic Carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Hadi, A.; Zahirifar, J.; Karimi-Sabet, J.; Dastbaz, A. Graphene Nanosheets Preparation Using Magnetic Nanoparticle Assisted Liquid Phase Exfoliation of Graphite: The Coupled Effect of Ultrasound and Wedging Nanoparticles. Ultrason. Sonochem. 2018, 44, 204–214. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Gao, Y.; Zhao, Y. Control of Number of Graphene Layers Using Ultrasound in Supercritical CO2 and Their Application in Lithium-Ion Batteries. J. Supercrit. Fluids 2014, 85, 95–101. [Google Scholar] [CrossRef]
- Orellana, J.; Araya-Hermosilla, E.; Pucci, A.; Araya-Hermosilla, R. Polymer-Assisted Graphite Exfoliation: Advancing Nanostructure Preparation and Multifunctional Composites. Polymers 2024, 16, 2273. [Google Scholar] [CrossRef]
- Sajid, H.M.; Afzal, H.; Irfan, M.; Saleem, M.; Jan, R.; Javed, S.; Akram, M.A. Design of Multilayered 2D Nanomaterial Composite Structures for EMI Shielding Analysis. ACS Omega 2022, 7, 35586–35594. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jang, H.; Park, S.; An, J.H.; Jang, J. Comparative Studies on Crystallinity, Thermal and Mechanical Properties of Polyketone Grown on Plasma Treated CVD Graphene. Polymers 2021, 13, 919. [Google Scholar] [CrossRef] [PubMed]
- Sariyev, B.; Abdikadyr, A.; Baitikenov, T.; Anuarbekov, Y.; Golman, B.; Spitas, C. Thermal Properties and Mechanical Behavior of Hot Pressed PEEK/Graphite Thin Film Laminate Composites. Sci. Rep. 2023, 13, 12785. [Google Scholar] [CrossRef]
- Araya-Hermosilla, E.; Parlanti, P.; Gemmi, M.; Mattoli, V.; Di Pietro, S.; Iacopini, D.; Granchi, C.; Turchi, B.; Fratini, F.; Di Bussolo, V.; et al. Functionalized Aliphatic Polyketones with Germicide Activity. RSC Adv. 2022, 12, 35358–35366. [Google Scholar] [CrossRef]
- Orozco, F.; Kaveh, M.; Santosa, D.S.; Lima, G.M.R.; Gomes, D.R.; Pei, Y.; Araya-Hermosilla, R.; Moreno-Villoslada, I.; Picchioni, F.; Bose, R.K. Electroactive Self-Healing Shape Memory Polymer Composites Based on Diels–Alder Chemistry. ACS Appl. Polym. Mater. 2021, 3, 6147–6156. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer: Berlin/Heidelberg, Germany, 2009; Volume 433. [Google Scholar]
- Araya-Hermosilla, R.; Fortunato, G.; Pucci, A.; Raffa, P.; Polgar, L.; Broekhuis, A.A.; Pourhossein, P.; Lima, G.M.R.; Beljaars, M.; Picchioni, F. Thermally Reversible Rubber-Toughened Thermoset Networks via Diels-Alder Chemistry. Eur. Polym. J. 2016, 74, 229–240. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable Production of Large Quantities of Defect-Free Few-Layer Graphene by Shear Exfoliation in Liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef]
- Achee, T.C.; Sun, W.; Hope, J.T.; Quitzau, S.G.; Sweeney, C.B.; Shah, S.A.; Habib, T.; Green, M.J. High-Yield Scalable Graphene Nanosheet Production from Compressed Graphite Using Electrochemical Exfoliation. Sci. Rep. 2018, 8, 14525. [Google Scholar] [CrossRef]
- León, V.; Rodriguez, A.M.; Prieto, P.; Prato, M.; Vázquez, E. Exfoliation of Graphite with Trriazine Derivatives under Ball-Milling Conditions: Preparation of Few-Layer Graphene via Selective Noncovalent Interactions. ACS Nano 2014, 8, 563–571. [Google Scholar] [CrossRef]


| Samples | Storage Modulus (MPa) | Loss Modulus (MPa) |
|---|---|---|
| PK | 8.04 × 10−8 | 2.33 × 10−5 |
| PKAM | 5.30 × 10−6 | 3.02 × 10−4 |
| PKFU | 1.64 × 10−5 | 5.21 × 10−4 |
| PKTHI | 4.81 × 10−5 | 2.16 × 10−3 |
| Samples | Storage Modulus (MPa) | Loss Modulus (MPa) |
|---|---|---|
| PKGr | 1.45 × 10−6 | 5.44 × 10−5 |
| PKAMGr | 7.31 × 10−4 | 6.71 × 10−4 |
| PKFUGr | 1.06 × 10−3 | 1.48 × 10−2 |
| PKTHIGr | 2.39 × 10−1 | 4.49 × 10−1 |
| Samples | Filter 0.45 μm | Filter 0.22 μm | ||
|---|---|---|---|---|
| ID/IG | I2D/IG | ID/IG | I2D/IG | |
| Gr | 0.12 | 0.6 | - | - |
| PKGr | 0.38 | 0.7 | 0.32 | 0.6 |
| PKAMGr | 0.21 | 0.6 | 0.25 | 0.6 |
| PKFUGr | 0.21 | 0.6 | 0.43 | 0.9 |
| PKTHIGr | 0.21 | 0.5 | 0.25 | 0.5 |
| Sample | Graphite | PK | ||
|---|---|---|---|---|
| 2θ002 (°) | Intensity | d002 (nm) | 2θ110 (°) | |
| Gr | 26.4 | 290,957 | 0.337 | - |
| PKGr | 26.5 | 14,336 | 0.336 | 18.9 |
| PKAMGr | 26.5 | 5998 | 0.336 | 19.7 |
| PKFUGr | 26.5 | 8527 | 0.336 | 20.2 |
| PKTHIGr | 26.5 | 3999 | 0.336 | 19.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisternas, R.; Orellana, J.; Silva, N.; Correa-Puerta, J.; Pucci, A.; Bose, R.K.; Picchioni, F.; Araya-Hermosilla, E.; Araya-Hermosilla, R. Production of Graphite Nanoplatelets via Functionalized Polyketone-Assisted Diels–Alder Chemistry: Evidence of Reduced Layer Thickness and Enhanced Exfoliation Efficiency. Polymers 2025, 17, 1333. https://doi.org/10.3390/polym17101333
Cisternas R, Orellana J, Silva N, Correa-Puerta J, Pucci A, Bose RK, Picchioni F, Araya-Hermosilla E, Araya-Hermosilla R. Production of Graphite Nanoplatelets via Functionalized Polyketone-Assisted Diels–Alder Chemistry: Evidence of Reduced Layer Thickness and Enhanced Exfoliation Efficiency. Polymers. 2025; 17(10):1333. https://doi.org/10.3390/polym17101333
Chicago/Turabian StyleCisternas, Ricardo, Jaime Orellana, Nataly Silva, Jonathan Correa-Puerta, Andrea Pucci, Ranjita K. Bose, Francesco Picchioni, Esteban Araya-Hermosilla, and Rodrigo Araya-Hermosilla. 2025. "Production of Graphite Nanoplatelets via Functionalized Polyketone-Assisted Diels–Alder Chemistry: Evidence of Reduced Layer Thickness and Enhanced Exfoliation Efficiency" Polymers 17, no. 10: 1333. https://doi.org/10.3390/polym17101333
APA StyleCisternas, R., Orellana, J., Silva, N., Correa-Puerta, J., Pucci, A., Bose, R. K., Picchioni, F., Araya-Hermosilla, E., & Araya-Hermosilla, R. (2025). Production of Graphite Nanoplatelets via Functionalized Polyketone-Assisted Diels–Alder Chemistry: Evidence of Reduced Layer Thickness and Enhanced Exfoliation Efficiency. Polymers, 17(10), 1333. https://doi.org/10.3390/polym17101333









