Changes in the Thermal and Structural Properties of Polylactide and Its Composites During a Long-Term Degradation Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasticizer Synthesis
2.3. Sample Preparation Procedure
2.4. Methods
2.4.1. Sampling
2.4.2. Gel Permeation Chromatography (GPC)
2.4.3. Attenuated Total Reflection–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.4.4. Thermogravimetric Analysis (TGA)
2.4.5. Differential Scanning Calorimetry (DSC)
2.4.6. X-Ray Diffraction (XRD)
2.4.7. Abiotic Degradation
2.4.8. Scanning Electron Microscopy
3. Results and Discussion
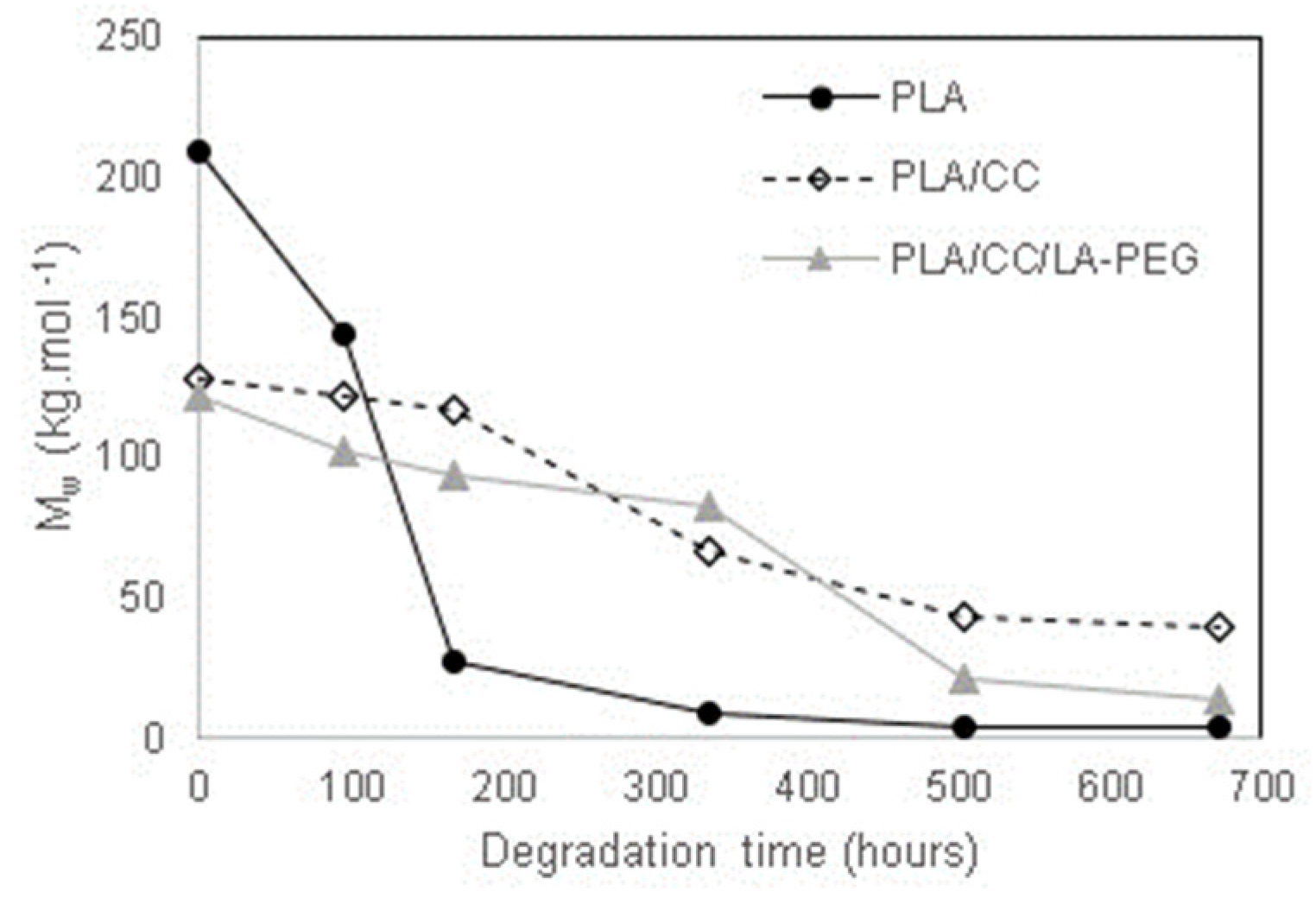
3.1. GPC
3.2. ATR-FTIR Analysis
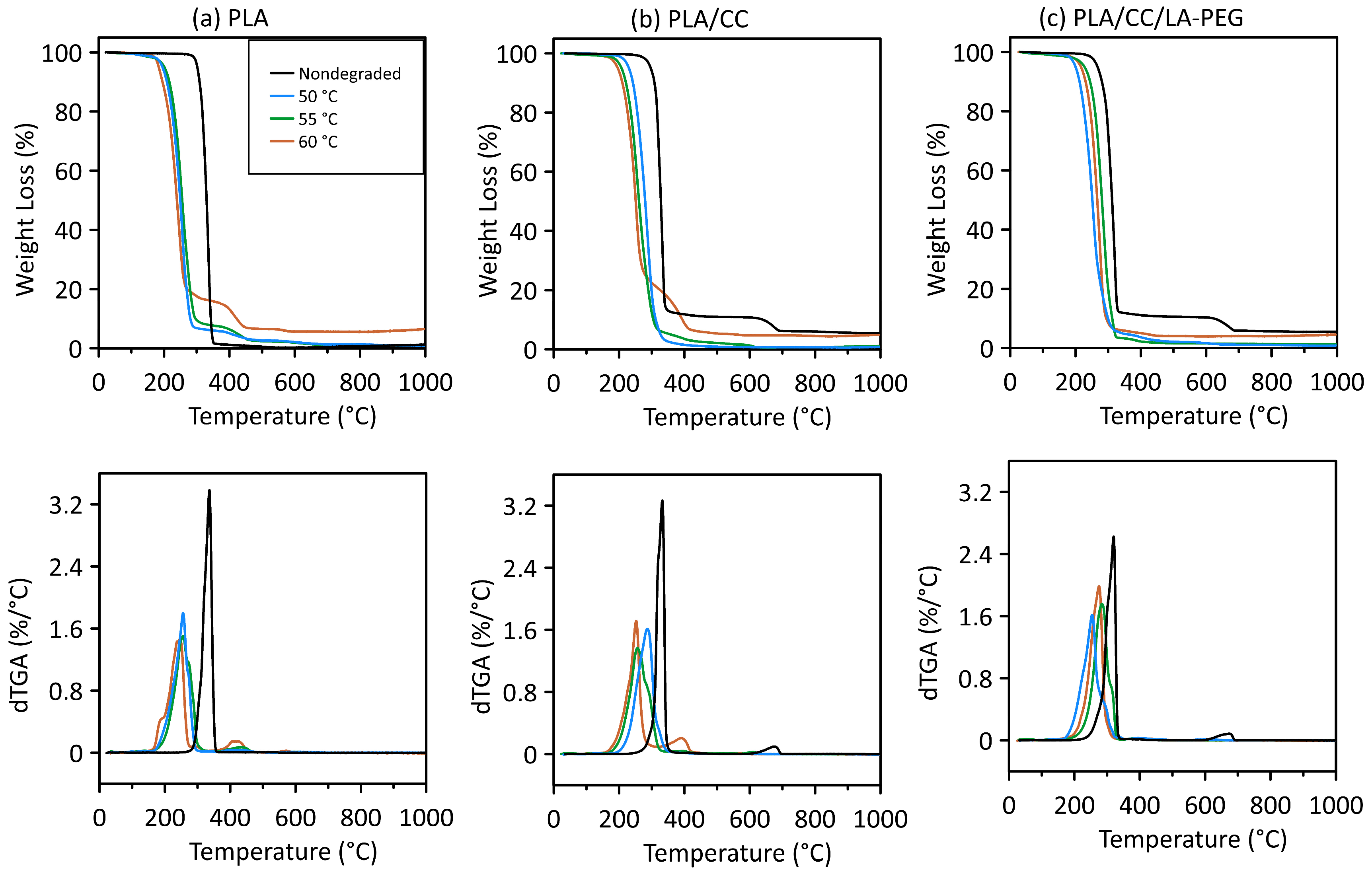
3.3. TGA
3.4. DSC
3.5. Abiotic Degradation—Degradation Rate Versus Crystalline Phase Content
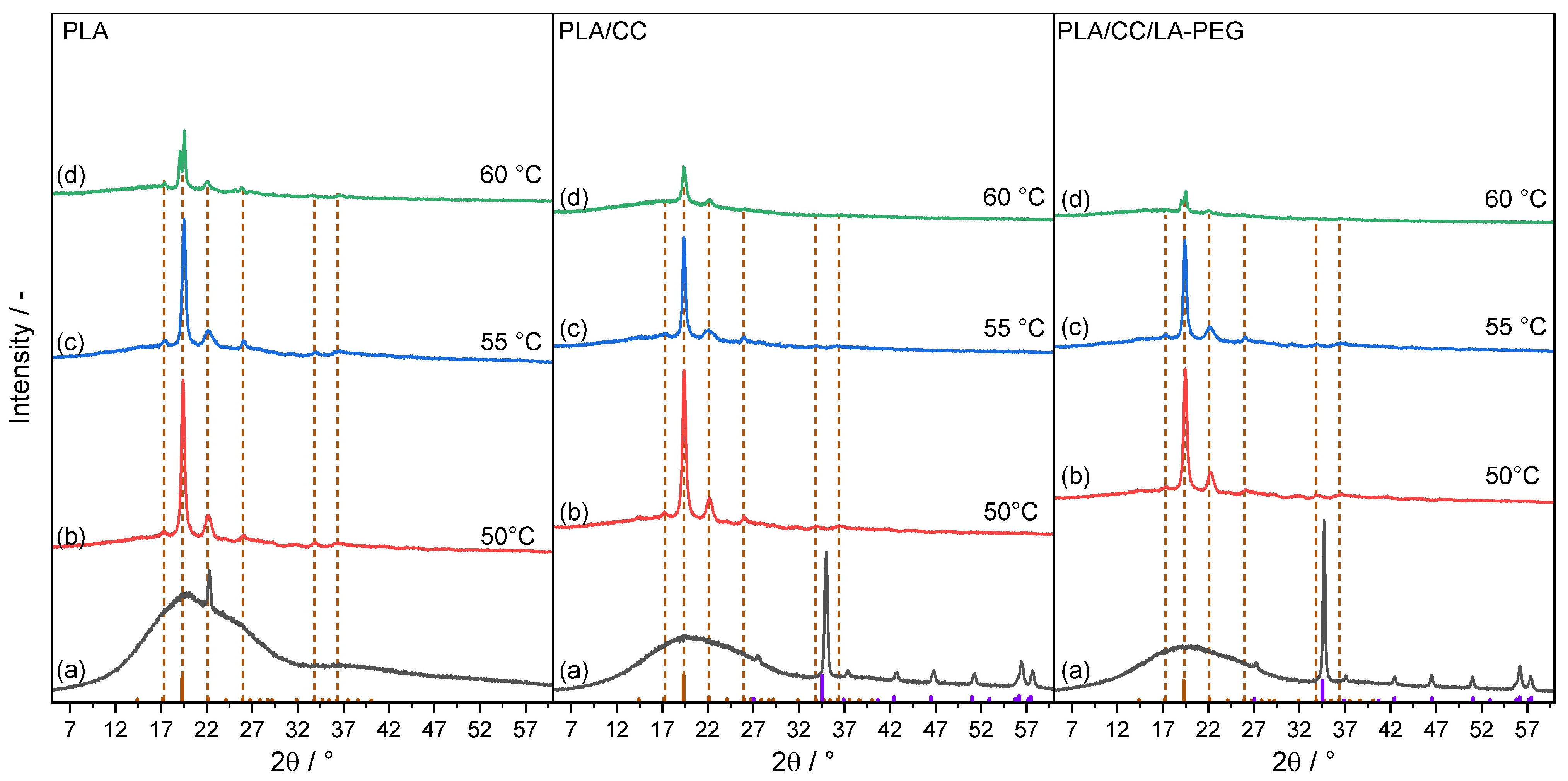
3.6. X-Ray Diffraction (XRD)
3.7. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kontou, E.; Niaounakis, M.; Panayiotis, G. Comparative study of PLA nanocomposites reinforced with clay and silica nanofillers and their mixtures. J Appl. Polym. Sci. 2011, 122, 1519–1529. [Google Scholar] [CrossRef]
- Balaguer, M.; Aliaga, C.; Fito, C.; Hortal, M. Compostability assessment of nano-reinforced poly(lactic acid) films. Waste Manag. 2016, 48, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Gigante, V.; Coltelli, M.-B.; Vannozzi, A.; Panariello, L.; Fusco, A.; Trombi, L.; Lazzeri, A. Flat die extruded biocompatible poly(lactic acid) (PLA)/poly(butylene succinate) (PBS) based films. Polymers 2019, 11, 1857. [Google Scholar] [CrossRef]
- Pantani, R.; Sorrentino, A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stab. 2013, 51, 1089–1096. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E. Influence of the type of buffer solution on thermal and structural properties of polylactide-based composites. Polym. Degrad. Stab. 2016, 129, 87–95. [Google Scholar] [CrossRef]
- Shi, N.; Dou, Q. Non-isothermal cold crystallization kinetics of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/treated calcium carbonate composites. J. Therm. Anal. Calorim. 2015, 119, 635–642. [Google Scholar] [CrossRef]
- Guo, H.; Zou, X.; Dai, W.; Zhang, P.; Xiao, B. Properties and morphology of polylactic acid composites reinforced by orientation aligned calcium carbonate whisker. J Appl. Polym. Sci. 2022, 140, e53622. [Google Scholar] [CrossRef]
- Chow, W.S.; Leu, Y.Y.; Mohd Ishak, Z.A. Water absorption of poly(lactic acid) nanocomposites: Effects of nanofillers and maleated rubbers. Polym. Plast. Technol. Mater. 2014, 53, 858–863. [Google Scholar] [CrossRef]
- Leu, Y.Y.; Chow, W.S. Kinetics of water absorption and thermal properties of poly(lactic acid)/organo montmorillonite/poly(ethylene glycol) nanocomposites. J. Vinyl Addit. Technol. 2011, 17, 40–47. [Google Scholar] [CrossRef]
- Turan, D.; Sirin, H.; Ozkoc, G. Effects of POSS particles on the mechanical, thermal, and morphological properties of PLA and plasticised PLA. J. Appl. Polym. Sci. 2010, 121, 1067–1075. [Google Scholar] [CrossRef]
- Zuo, U.; Chen, X.; Ding, Y.; Cui, L.; Fan, B.; Pan, L.; Zhang, K. Novel designed PEG-dicationic imidazolium-based ionic liquids as effective plasticizers for sustainable polylactide. Chin. J. Chem. 2021, 39, 2234–2240. [Google Scholar] [CrossRef]
- Cisar, J.; Drosler, P.; Pummerova, M.; Sedlarik, V.; Skoda, D. Composite based on PLA with improved shape stability under high-temperature conditions. Polymer 2023, 276, 125943. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Yu, Y.; Xiao, L.; Shao, Y. Crystallization behaviors of poly(lactic acid) composites fabricated using functionalized eggshell powder and poly(ethylene glycol). Thermochim. Acta 2018, 663, 67–76. [Google Scholar] [CrossRef]
- Bhiogade, A.; Kannan, M.; Devanathan, S. Degradation kinetics study of Poly lactic acid (PLA) based biodegradable green composites. Mater. Today 2020, 24, 806–814. [Google Scholar] [CrossRef]
- Rocha, D.B.; Souza de Carvalho, J.; Aparecida de Oliveira, S. A new approach for flexible PBAT/PLA/CaCO3 films into agriculture. J. Appl. Polym. Sci 2018, 135, 46660. [Google Scholar] [CrossRef]
- Vidović, E.; Faraguna, F.; Jukić, A. Influence of inorganic fillers on PLA crystallinity and thermal properties. J. Therm. Anal. Calorim. 2017, 127, 371–380. [Google Scholar] [CrossRef]
- Gayer, C.; Ritter, J.; Bullemer, M.; Grom, S.; Jauer, L.; Meiners, W.; Schleifenbaum, J.H. Development of a solvent-free polylactide/calcium carbonate composite for selective laser sintering of bone tissue engineering scaffolds. Mater. Sci. Eng. C 2019, 101, 660–673. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E.; Ortega, Z. Enzymatic degradation study of PLA-based composite scaffolds. Rev. Adv. Mater. Sci. 2020, 59, 170–175. [Google Scholar] [CrossRef]
- Polyák, P.; Nagy, K.; Vértessy, B.; Pukánszky, B. Self-regulating degradation technology for the biodegradation of poly(lactic acid). Environ. Technol. Innov. 2023, 29, 103000. [Google Scholar] [CrossRef]
- Kalita, N.K.; Damare, N.A.; Hazarika, D.; Bhagabati, P.; Kalamdhad, A.; Katiyar, V. Biodegradation and characterization study of compostable PLA bioplastic containing algae biomass as potential degradation accelerator. Environ. Chall. 2021, 3, 100067. [Google Scholar] [CrossRef]
- Ruggero, F.; Belardi, S.; Carretti, E.; Lotti, T.; Lubello, C.; Gori, R. Rigid and film bioplastics degradation under suboptimal composting conditions: A kinetic study. Waste Manag. Res. 2022, 40, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D.; Pikasi, A.; Hiskakis, M. Organic recycling of post-consumer /industrial bio-based plastics through industrial aerobic composting and anaerobic digestion—Techno-economic sustainability criteria and indicators. Polym. Degrad. Stab. 2021, 190, 109642. [Google Scholar] [CrossRef]
- Hottle, T.A.; Agüero, M.L.; Bilec, M.M.; Landis, A.E. Alkaline amendment for the enhancement of compost degradation for polylactic acid biopolymer products. Compost Sci. Util. 2016, 24, 159–173. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P.; Narayan, R. Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 2007, 26, 1049–1061. [Google Scholar] [CrossRef]
- Guzman-Sielicka, A.; Janik, H.; Sielicki, P. Proposal of new starch-blends composition quickly degradable in marine environment. J. Polym. Environ. 2013, 21, 802–806. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E.; Rodríguez-Esparragón, F. Effects of ceramic additives and bioactive coatings on the degradation of polylactic acid-based bone scaffolds under hydrolytic conditions. J. Biomed. Mater. Res. B 2023, 111, 429–441. [Google Scholar] [CrossRef]
- Kucharczyk, P.; Hnatkova, E.; Dvorak, Z.; Sedlarik, V. Novel aspects of the degradation process of PLA based bulky samples under conditions of high partial pressure of water vapour. Polym. Degrad. Stab. 2013, 98, 150–157. [Google Scholar] [CrossRef]
- Liao, R.; Yang, B.; Yu, W.; Zhou, C. Isothermal cold crystallization kinetics of polylactide/nucleating agents. J. Appl. Polym. Sci. 2007, 104, 310–317. [Google Scholar] [CrossRef]
- Hoque, E.M.; Ghorban, D.M.; Khalid, M. Next generation biomimetic bone tissue engineering matrix from poly (L- lactic acid) PLA/calcium carbonate composites doped with silver nanoparticles. Curr. Anal. Chem. 2018, 14, 268–277. [Google Scholar] [CrossRef]
- De Santis, F.; Pantani, R.; Titomanlio, G. Nucleation and crystallization kinetics of poly(lactic acid). Thermochim. Acta 2011, 522, 128–1334. [Google Scholar] [CrossRef]
- Pantani, R.; De Santis, F.; Sorrentino, A.; De Maio, F.; Titomanlio, G. Crystallization kinetics of virgin and processed poly(lactic acid). Polym. Degrad. Stab. 2010, 95, 1148–1159. [Google Scholar] [CrossRef]
- Muller, J.; Jimenez, A.; Gonzalez-Martinez, C.; Chiralt, A. Influence of plasticizers on thermal properties and crystallization behaviour of poly(lactic acid) films obtained by compression moulding. Polym. Int. 2016, 65, 970–978. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Klonos, P.A.; Kyritsis, A.; Tarani, E.; Chrissafis, K.; Masek, O.; Tsachouridis, K.; Anastasiou, A.D.; Bikiaris, D.N. Synthesis and characterization of PLA/biochar bio-composites containing different biochar types and content. Polymers 2025, 17, 263. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.H.; Pires, L.D.; Costa, L.C.; Passador, F.R.; Pessan, L.A. Analysis of the degradation during melt processing of PLA/Biosilicate® composites. J. Compos. Sci. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Drohsler, P.; Yasir, M.; Fabian, D.R.C.; Cisar, J.; Yadollahi, Z.; Sedlarik, V. Comparative degradation study of a biodegradable composite based on polylactide with halloysite nanotubes and a polyacrylic acid copolymer. Mater. Today Commun. 2022, 33, 10440. [Google Scholar] [CrossRef]
- Krishnudu, M.D.; Reddy, V.P.; Kumar, V.M.; Reddy, S.R.; Rao, U.A. Effect of CaCO3 filler reinforcement on PLA matrix composites fabricated through injection moulding. Phys. Scr. 2024, 99, 065053. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, B.; Ding, Y.; Zhou, C.; Ge, S. Impacts of poly(lactic acid) microplastics on organic compound leaching and heavy metal distribution during hydrothermal treatment of sludge. Sci. Total Environ. 2023, 901, 166012. [Google Scholar] [CrossRef]
- Gbadeyan, O.L. Thermomechanical characterization of bioplastic films produced using a combination of polylactic acid and bionano calcium carbonate. Sci. Rep. 2022, 15538, 1–9. [Google Scholar] [CrossRef]
- Nekhamanurak, B.; Patanathabutr, P.; Hongsriphan, N. The influence of micro-/nano-CaCO3 on thermal stability and melt rheology behavior of poly(lactic acid). Energy Procedia 2014, 56, 118–128. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, B.H.; Choi, J.H.; Yoon, J.-S. Mechanical properties and thermal stability of poly(L-lactide)/calcium carbonate composites. J. Appl. Polym. Sci. 2008, 109, 3087–3092. [Google Scholar] [CrossRef]
- Tsuji, H.; Echizen, Y.; Saha, S.K.; Nishimura, Y. Photodegradation of poly(L-lactic acid): Effects of photosensitizer. Macromol. Mater. Eng. 2008, 290, 1192–1203. [Google Scholar] [CrossRef]
- Kalia, S.; Avérous, L. Biodegradable and Biobased Polymers for Environmental and Biomedical Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 171–224. [Google Scholar]
- Dreier, J.; Brütting, C.; Ruckdäschel, H.; Altstädt, V.; Bonten, C. Investigation of the thermal and hydrolytic degradation of polylactide during autoclave foaming. Polymers 2021, 16, 2624. [Google Scholar] [CrossRef] [PubMed]
- Odelius, K.; Hoglund, A.; Kumar, S.; Hakkarainen, M.; Ghosh, A.K.; Bhatnagar, N.; Albertsson, A.-C. Porosity and pore size regulate the degradation product profile of polylactide. Biomacromolecules 2011, 12, 1250–1258. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, J.; Yi, Q.; Liu, L.; Zhao, Y.; Nie, H.; Liu, X.; Zou, J.; Chen, L. Biological evaluation of poly-L-lactic acid composite containing bioactive glass. Polym. Bull. 2010, 65, 411–423. [Google Scholar] [CrossRef]
- Dobircau, L.; Delpouve, N.; Herbinet, R.; Domenek, S.; Le Pluart, L.; Delbreilh, L.; Dacrue, V.; Dargent, E. Molecular mobility and physical ageing of plasticized poly(lactide). Polym. Eng. Sci. 2015, 55, 858–865. [Google Scholar] [CrossRef]
- Wolf, M.H.; Gil-Castel, O.; Cea, J.; Carrasco, J.C.; Ribes-Greus, A. Degradation of Plasticised Poly(lactide) Composites with nanofibrillated cellulose in different hydrothermal environments. J. Polym. Environ. 2023, 31, 2055–2072. [Google Scholar] [CrossRef]
- Gonzala, G.L.; Babetto, A.S.; Goncalves, L.M.; Bettini, S.H.; Souza, A.M. Biodegradation behavior of poly (lactic acid) samples obtained by three-dimensional printing: Influence of temperature and pigment presence. Polym. Eng. Sci. 2024, 64, 2812–2823. [Google Scholar] [CrossRef]
- Kara, Y.; Molnar, K. Decomposition behavior of stereocomplex PLA melt-blown fine fiber mats in water and in compost. J. Polym. Environ. 2022, 31, 1398–1414. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Barbale, M.; Zaccheo, S.; Scavone, M.; Visai, L.; Kenny, J.M. New multifunctional poly(lactide acid) composites: Mechanical, antibacterial, and degradation properties. J. Appl. Polym. Sci. 2012, 124, 1. [Google Scholar] [CrossRef]





| Name | PLA Content (wt.%) | CaCO3 Content (wt.%) | Plasticizer Content (wt.%) |
|---|---|---|---|
| PLA | 100 | - | - |
| PLA/CC | 90 | 10 | - |
| PLA/CC/LA-PEG | 86 | 10 | 4 |
| Name | Mn (g·mol−1) | Mw (g·mol−1) | Mp (g·mol−1) | PDI (-) |
|---|---|---|---|---|
| PLA | 103,000 ± 9000 | 209,000 ± 5000 | 215,000 ± 1000 | 1.89 ± 0.37 |
| PLA/CC | 63,000 ± 5000 | 128,000 ± 2000 | 147,000 ± 4000 | 2.05 ± 0.16 |
| PLA/CC/LA-PEG | 64,000 ± 4000 | 122,000 ± 3000 | 143,000 ± 3000 | 1.91 ± 0.07 |
| T5 (°C) | T50 (°C) | T95 (°C) | Δ T5–95 (°C) | Tp (°C) | |
|---|---|---|---|---|---|
| PLA | |||||
| Nondegraded | 301.8 | 330.5 | 346.8 | 16.3 | 336.3 |
| 50 °C | 221.1 | 276.9 | 357.6 | 80.7 | 266.3 |
| 55 °C | 206.3 | 263.7 | 373.9 | 110.2 | 260.8 |
| 60 °C | 183.8 | 239.7 | 598.9 | 359.2 | 246.7 |
| PLA/CC | |||||
| Nondegraded | 297.1 | 328.1 | - | - | 332.8 676.4 |
| 50 °C | 229.6 | 280.0 | 326.6 | 46.6 | 286.7 |
| 55 °C | 207.9 | 260.6 | 360.7 | 100.1 | 256.0 395.6 |
| 60 °C | 195.5 | 250.6 | 555.5 | 305.9 | 252.1 391.0 |
| PLA/CC/LA-PEG | |||||
| Nondegraded | 267.6 | 311.8 | - | - | 320.0 674.2 |
| 50 °C | 200.1 | 251.3 | 344.4 | 93.1 | 253.7 |
| 55 °C | 203.2 | 256.8 | 388.7 | 131.9 | 255.2 |
| 60 °C | 216.4 | 266.8 | 402.6 | 135.8 | 276.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisar, J.; Pummerova, M.; Drohsler, P.; Masar, M.; Sedlarik, V. Changes in the Thermal and Structural Properties of Polylactide and Its Composites During a Long-Term Degradation Process. Polymers 2025, 17, 1326. https://doi.org/10.3390/polym17101326
Cisar J, Pummerova M, Drohsler P, Masar M, Sedlarik V. Changes in the Thermal and Structural Properties of Polylactide and Its Composites During a Long-Term Degradation Process. Polymers. 2025; 17(10):1326. https://doi.org/10.3390/polym17101326
Chicago/Turabian StyleCisar, Jaroslav, Martina Pummerova, Petra Drohsler, Milan Masar, and Vladimir Sedlarik. 2025. "Changes in the Thermal and Structural Properties of Polylactide and Its Composites During a Long-Term Degradation Process" Polymers 17, no. 10: 1326. https://doi.org/10.3390/polym17101326
APA StyleCisar, J., Pummerova, M., Drohsler, P., Masar, M., & Sedlarik, V. (2025). Changes in the Thermal and Structural Properties of Polylactide and Its Composites During a Long-Term Degradation Process. Polymers, 17(10), 1326. https://doi.org/10.3390/polym17101326






