Recent Developments in Enzyme-Free PANI-Based Electrochemical Nanosensors for Pollutant Detection in Aqueous Environments
Abstract
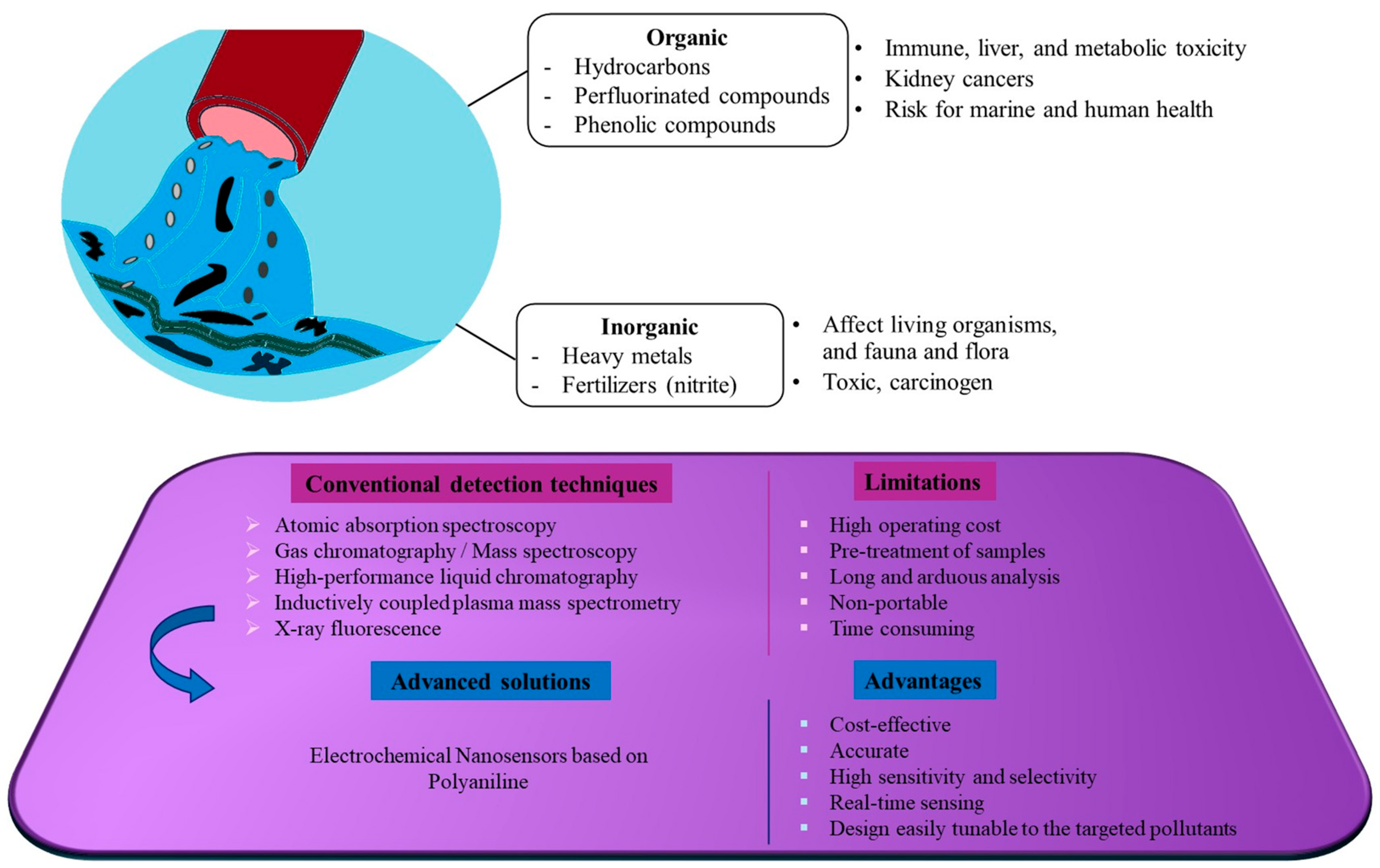
1. Introduction
Search Methodology
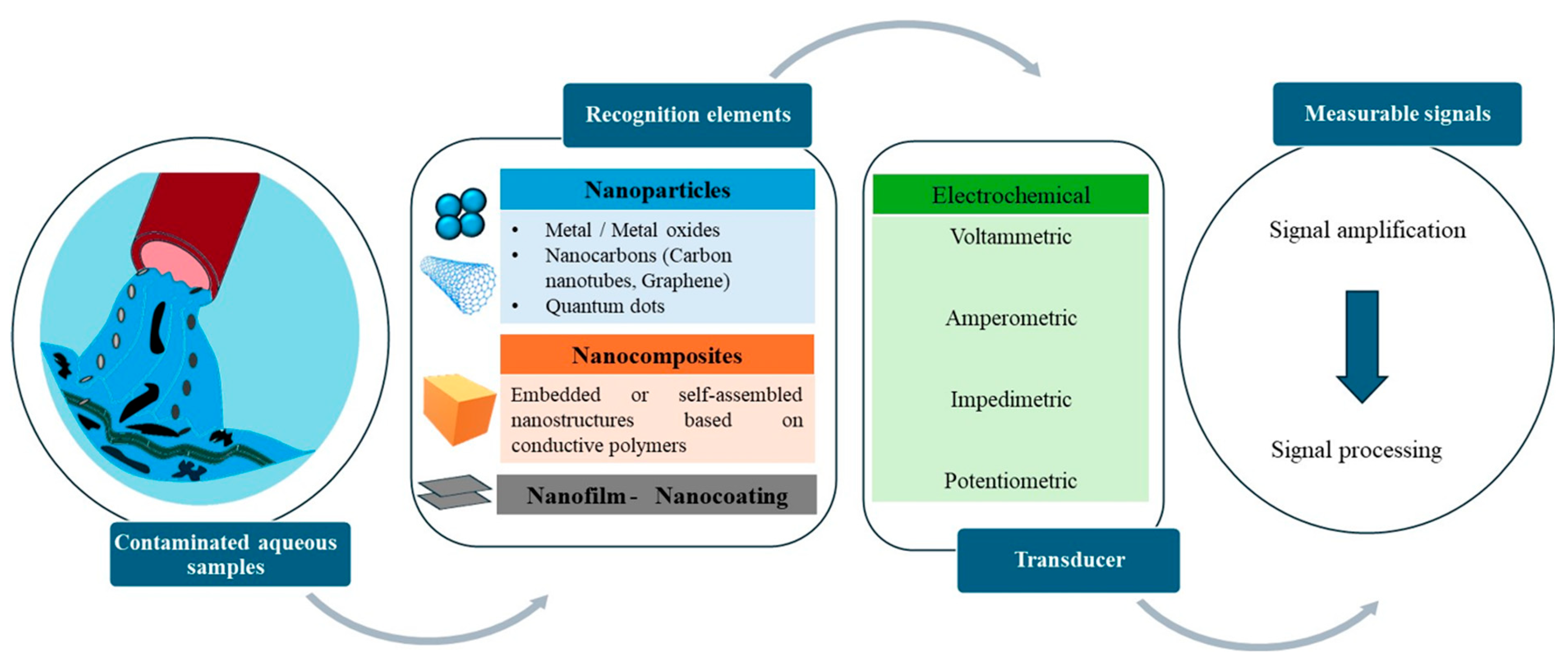
2. Principles and Preparations of Nanomaterial-Based Nanosensors Using Conducting Materials
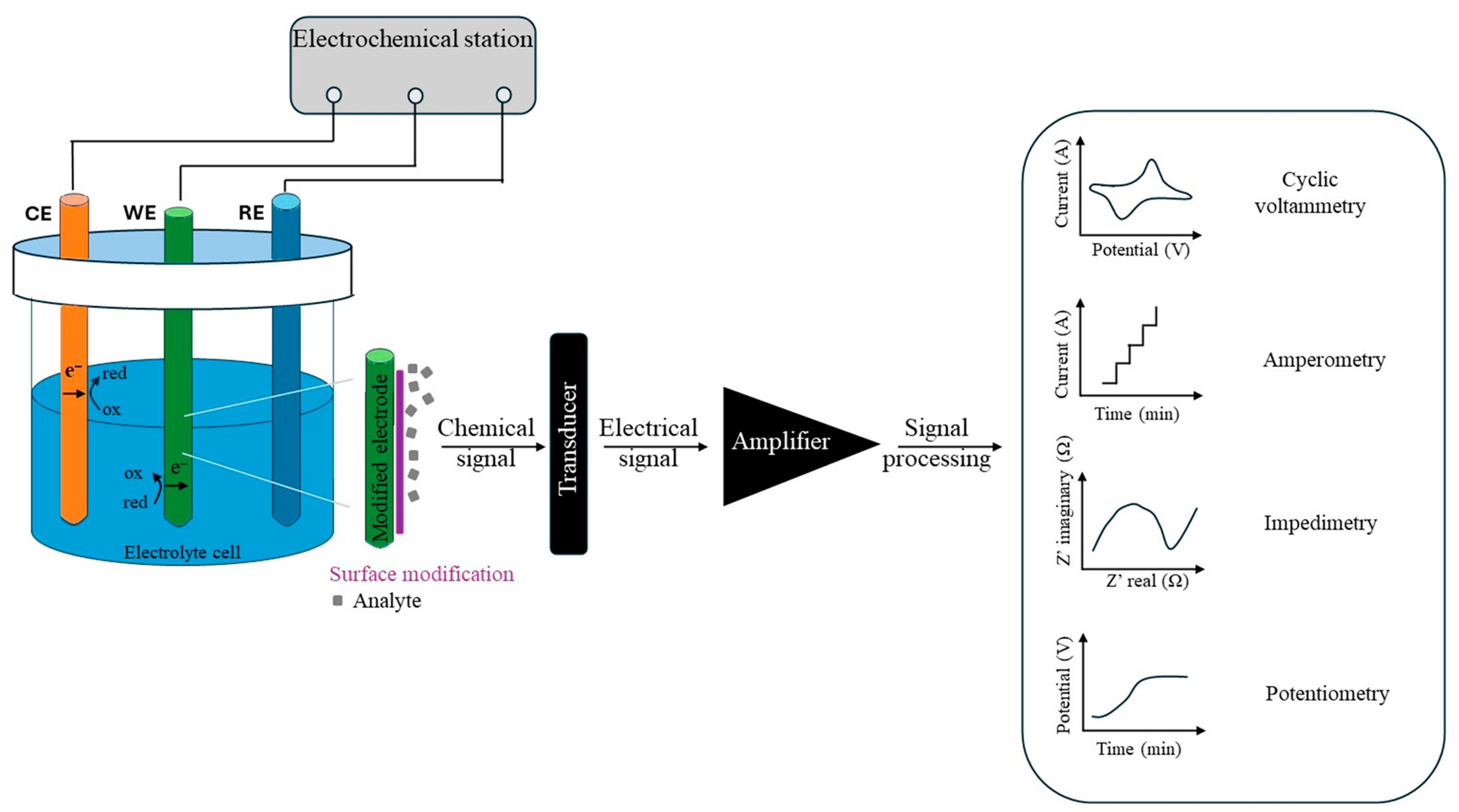
2.1. How Electrochemical Nanosensors Work
2.2. Nanomaterials
2.2.1. Fabrication
2.2.2. Conductive Polymers (CPs)
3. Application of PANI-Based Electrochemical Nanosensors for Pollutant Detection in Aqueous Environments
3.1. Heavy Metal Ions
3.2. Phenolic Compounds
3.3. Organic Compounds
3.4. Inorganic Compounds
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Hairom, N.H.H.; Soon, C.F.; Mohamed, R.M.S.R.; Morsin, M.; Zainal, N.; Nayan, N.; Zulkifli, C.Z.; Harun, N.H. A review of nanotechnological applications to detect and control surface water pollution. Environ. Technol. Innov. 2021, 24, 102032. [Google Scholar] [CrossRef]
- Negi, S.; Batoye, S.; Singh, K.; Waraich, J.S. Environmental Pollution, Its Causes and Impact on Ecosystem. In New Frontiers of Nanomaterials in Environmental Science; Springer: Singapore, 2021; ISBN 9789811592393. [Google Scholar]
- Odumbe, E.; Murunga, S.; Ndiiri, J. Heavy Metals in Wastewater Effluent: Causes, Effects, and Removal Technologies Evans. In Trace Metals in the Environment; IntechOpen: London, UK, 2023; Volume i, p. 13. [Google Scholar]
- Ahamad, A.; Madhav, S.; Singh, A.K.; Kumar, A.; Singh, P. Types of Water Pollutants: Conventional and Emerging. In Sensors in Water Pollutants Monitoring: Role of Material. Advanced Functional Materials and Sensors; Springer: Singapore, 2020; pp. 21–41. [Google Scholar] [CrossRef]
- Sankhla, M.S.; Kumari, M.; Agrawal, M.N.; Kumar, R.; Prashant, A. Heavy metals contamination in water and their hazardous effect on human health—A review. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 759–766. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality; The World Health Organization: Geneva, Switzerland, 2022.
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef] [PubMed]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M. Sensing methods for hazardous phenolic compounds based on graphene and conducting polymers-based materials. Chemosensors 2021, 9, 291. [Google Scholar] [CrossRef]
- De, A.; Kalita, D. Bio-Fabricated Gold and Silver Nanoparticle Based Plasmonic Sensors for Detection of Environmental Pollutants: An Overview. Crit. Rev. Anal. Chem. 2023, 53, 672–688. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Zhen, H.; Chen, X.; Sheng, M.; Li, K.; Xue, W.; Zhao, H.; Meng, S.; Cao, G. Determination of estrogens and estrogen mimics by solid-phase extraction with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1168, 122559. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I. Sample Preparation for X-Ray Fluorescence Analysis. Encycl. Anal. Chem. 2016, 1–29. [Google Scholar] [CrossRef]
- Wu, D.; Hu, Y.; Cheng, H.; Ye, X. Detection Techniques for Lead Ions in Water: A Review. Molecules 2023, 28, 3601. [Google Scholar] [CrossRef]
- Hu, J.; Xian, Y.; Wu, Y.; Chen, R.; Dong, H.; Hou, X.; Liang, M.; Wang, B.; Wang, L. Perchlorate occurrence in foodstuffs and water: Analytical methods and techniques for removal from water—A review. Food Chem. 2021, 360, 130146. [Google Scholar] [CrossRef]
- Potes-Lesoinne, H.A.; Ramirez-Alvarez, F.; Perez-Gonzalez, V.H.; Martinez-Chapa, S.O.; Gallo-Villanueva, R.C. Nanomaterials for electrochemical detection of pollutants in water: A review. Electrophoresis 2022, 43, 249–262. [Google Scholar] [CrossRef]
- Chauhan, S.; Dahiya, D.; Sharma, V.; Khan, N.; Chaurasia, D.; Nadda, A.K.; Varjani, S.; Pandey, A.; Bhargava, P.C. Advances from conventional to real time detection of heavy metal(loid)s for water monitoring: An overview of biosensing applications. Chemosphere 2022, 307, 136124. [Google Scholar] [CrossRef] [PubMed]
- Olabintan, A.B.; Abdullahi, A.H.S.; Yusuf, B.O.; Ganiyu, S.A.; Saleh, T.A.; Basheer, C. Prospects of polymer Nanocomposite-Based electrochemical sensors as analytical devices for environmental Monitoring: A review. Microchem. J. 2024, 204, 111053. [Google Scholar] [CrossRef]
- Munawar, A.; Ong, Y.; Schirhagl, R.; Tahir, M.A.; Khan, W.S.; Bajwa, S.Z. Nanosensors for diagnosis with optical, electric and mechanical transducers. RSC Adv. 2019, 9, 6793–6803. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.-Y.; Cheng, R.; Shi, L.; Ma, Z.; Zheng, X. Nanomaterials for water pollution monitoring and remediation. Environ. Chem. Lett. 2017, 15, 23–27. [Google Scholar] [CrossRef]
- Grozdanov, A.; Dimitrievska, I.; Paunovic, P. Recent advancements in nano sensors for air and water pollution control. Mater. Sci. Eng. Int. J. 2023, 7, 113–128. [Google Scholar] [CrossRef]
- Thenrajan, T.; Wilson, J. Conducting Polymers Based Nanocomposites for the Environmental Pollutants Detection. In Bio and Nanoremediation of Hazardous Environmental Pollutants; Taylor and Francis Group: Abingdon, UK, 2023; ISBN 9781000954432. [Google Scholar]
- Wen, J.; Wang, S.; Feng, J.; Ma, J.; Zhang, H.; Wu, P.; Li, G.; Wu, Z.; Meng, F.; Li, L.; et al. Recent progress in polyaniline-based chemiresistive flexible gas sensors: Design, nanostructures, and composite materials. J. Mater. Chem. A 2024, 12, 6190–6210. [Google Scholar] [CrossRef]
- Kale, R.A.; Dhawale, S.C.; Mulik, B.B.; Adhikari, A.; Sathe, B.R. Polyaniline based highly selective electrochemical sensor for ascorbic acid determination: Performance studies towards real sample analysis. J. Ind. Eng. Chem. 2024, 136, 167–176. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Hossein Baghdadi, A.; Kartom Kamarudin, S.; Abdul Rashid, S.; Jakmunee, J.; Shaari, N. Recent progress in polyaniline and its composites; Synthesis, properties, and applications. Eur. Polym. J. 2024, 210, 112948. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and electrochemical properties of conducting polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Brook, I.; Tchoudakov, R.; Suckeveriene, R.Y.; Narkis, M. Electro-mechanical sensors based on conductive hybrid nanocomposites. Polym. Adv. Technol. 2015, 26, 889–897. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Bodkhe, G.A.; Shirsat, S.; Ramanavicius, A.; Shirsat, M.D. Nanocomposite platform based on EDTA Modified Ppy/SWNTs for the sensing of Pb(II) ions by electrochemical method. Front. Chem. 2018, 6, 451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Huang, W.; Zhang, T.; Hu, X.; Perman, J.A.; Ma, S. A metal-organic framework and conducting polymer based electrochemical sensor for high performance cadmium ion detection. J. Mater. Chem. A 2017, 5, 8385–8393. [Google Scholar] [CrossRef]
- Okpara, E.C.; Nde, S.C.; Fayemi, O.E.; Ebenso, E.E. Electrochemical Characterization and Detection of Lead in Water Using SPCE Modified with BiONPs/PANI. Nanomaterials 2021, 11, 1294. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.R.; Alam, M.M.; Aijaz, M.O.; Asiri, A.M.; Almubaddel, F.S.; Rahman, M.M. The fabrication of a chemical sensor with PANI-TiO2 nanocomposites. RSC Adv. 2020, 10, 12224–12233. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Li, X.; Zhao, Q.; Zhao, H.; Quan, X.; Chen, J. An electrochemical sensor based on molecularly imprinted polypyrrole/graphene quantum dots composite for detection of bisphenol A in water samples. Sens. Actuators B Chem. 2016, 233, 599–606. [Google Scholar] [CrossRef]
- Maity, J.; Ghosh, S. Advancement in Biosensors Based on Emerging Polymers. In Advanced Polymers. Advances in Material Research and Technology; Ikhmayies, S.J., Ed.; Springer: Cham, Switzerland, 2024. [Google Scholar]
- Hui, Y.; Huang, Z.; Alahi, M.E.E.; Nag, A.; Feng, S.; Mukhopadhyay, S.C. Recent Advancements in Electrochemical Biosensors for Monitoring the Water Quality. Biosensors 2022, 12, 551. [Google Scholar] [CrossRef]
- Kilic, N.M.; Singh, S.; Keles, G.; Cinti, S.; Kurbanoglu, S.; Odaci, D. Novel Approaches to Enzyme-Based Electrochemical Nanobiosensors. Biosensors 2023, 13, 622. [Google Scholar] [CrossRef]
- Islam, M.S.; Sazawa, K.; Sugawara, K.; Kuramitz, H. Electrochemical Biosensor for Evaluation of Environmental Pollutants Toxicity. Environments 2023, 10, 63. [Google Scholar] [CrossRef]
- Middelhoek, S.; Noorlag, D.J.W.; Steenvoorden, G.K. Silicon and Hybrid Micro-Electronic Sensors. Electrocompon. Sci. Technol. 1983, 10, 217–229. [Google Scholar] [CrossRef]
- Privett, B.J.; Shin, J.H.; Schoenfisch, M.H. Electrochemical sensors. Anal. Chem. 2008, 80, 4499–4517. [Google Scholar] [CrossRef]
- Saputra, H.A. Electrochemical sensors: Basic principles, engineering, and state of the art. Monatsh. Chem. 2023, 154, 1083–1100. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Vikesland, P. Nanotechnology for water quality monitoring. Nat. Nanotechnol. 2018, 13, 651–660. [Google Scholar] [CrossRef]
- Alshehri, E.M.; Alarfaj, N.A.; Al-Tamimi, S.A.; El-Tohamy, M.F. Ultrasensitive Functionalized Polymeric-Nanometal Oxide Sensors for Potentiometric Determination of Ranitidine Hydrochloride. Polymers 2022, 14, 4150. [Google Scholar] [CrossRef]
- Simões, F.R.; Xavier, M.G. Nanoscience and Its Applications; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 6; ISBN 9780323497800. [Google Scholar]
- Terán-Alcocer, Á.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef]
- Sulthana, S.F.; Iqbal, U.M.; Suseela, S.B.; Anbazhagan, R.; Chinthaginjala, R.; Chitathuru, D.; Ahmad, I.; Kim, T.H. Electrochemical Sensors for Heavy Metal Ion Detection in Aqueous Medium: A Systematic Review. ACS Omega 2024, 9, 25493–25512. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Touhami, A. Biosensors and Nanobiosensors: Design and Applications. Nanomedicine 2014, 391–462. [Google Scholar] [CrossRef]
- Aref, M.; Ranjbari, E.; García-Guzmán, J.J.; Hu, K.; Lork, A.; Crespo, G.A.; Ewing, A.G.; Cuartero, M. Potentiometric pH Nanosensor for Intracellular Measurements: Real-Time and Continuous Assessment of Local Gradients. Anal. Chem. 2021, 93, 15744–15751. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Sel. 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, C.; Kwon, O.S. Conducting polymer based nanobiosensors. Polymers 2016, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Zelikman, E.; Suckeveriene, R.Y. Ultrasonically induced polymerization and polymer grafting in the presence of carbonaceous nanoparticles. Processes 2020, 8, 1680. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Makgopa, J.; Elugoke, S.E. Comparative electrochemical properties of polyaniline/carbon quantum dots nanocomposites modified screen-printed carbon and gold electrodes. Mater. Res. Express 2024, 10, 125603. [Google Scholar] [CrossRef]
- Khokhar, D.; Jadoun, S.; Arif, R.; Jabin, S. Functionalization of conducting polymers and their applications in optoelectronics. Polym. Technol. Mater. 2021, 60, 463–485. [Google Scholar] [CrossRef]
- Al-Haidary, Q.N.; Al-Mokaram, A.M.; Hussein, F.M.; Ismail, A.H. Development of polyaniline for sensor applications: A review. J. Phys. Conf. Ser. 2021, 1853, 012062. [Google Scholar] [CrossRef]
- Suckeveriene, R.Y.; Zelikman, E.; Mechrez, G.; Narkis, M. Literature review: Conducting carbon nanotube/polyaniline nanocomposites. Rev. Chem. Eng. 2011, 27, 15–21. [Google Scholar] [CrossRef]
- Horev, Y.D.; Maity, A.; Zheng, Y.; Milyutin, Y.; Khatib, M.; Yuan, M.; Suckeveriene, R.Y.; Tang, N.; Wu, W.; Haick, H. Stretchable and Highly Permeable Nanofibrous Sensors for Detecting Complex Human Body Motion. Adv. Mater. 2021, 33, 2102488. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Kyomuhimbo, H.D.; Feleni, U. Electroconductive Green Metal-polyaniline Nanocomposites: Synthesis and Application in Sensors. Electroanalysis 2023, 35, e202100636. [Google Scholar] [CrossRef]
- Desai, N.; Sudhakar, Y.N.; Patil, R.R.; Chandran, A.; Nidhin, M.; Agnihotri, A.S. Electrochemical sensor based on PVP coated cobalt ferrite/graphite/PANI nanocomposite for the detection of hydrazine. Mater. Res. Express 2023, 10, 125505. [Google Scholar] [CrossRef]
- Suckeveriene, R.Y.; Zelikman, E.; Mechrez, G.; Tzur, A.; Frisman, I.; Cohen, Y.; Narkis, M. Synthesis of Hybrid Polyaniline/Carbon Nanotube Nanocomposites by Dynamic Interfacial Inverse Emulsion Polymerization Under Sonication. J. Appl. Polym. Sci. 2011, 120, 676–682. [Google Scholar] [CrossRef]
- Radhi, M.M.; Mossa, A.A.; Al-Mulla, E.A.J.; Lafta, A.N. Electrochemical Study of Modified Glassy Carbon Electrode With Polyaniline Nanoparticles Using Cyclic Voltammetry. Bull. Chem. Soc. Ethiop. 2022, 36, 687–696. [Google Scholar] [CrossRef]
- Numan, A.; Gill, A.A.S.; Rafique, S.; Guduri, M.; Zhan, Y.; Maddiboyina, B.; Li, L.; Singh, S.; Nguyen Dang, N. Rationally engineered nanosensors: A novel strategy for the detection of heavy metal ions in the environment. J. Hazard. Mater. 2021, 409, 124493. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union EUR-Lex-32020L2184-EN-EUR-Lex. Off. J. Eur. Union 2020, L435, 1–62.
- US-EPA National Primary Drinking Water Regulations|Ground Water and Drinking Water|US EPA. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 9 June 2021).
- Roh, H.; Kim, D.H.; Cho, Y.; Jo, Y.M.; del Alamo, J.A.; Kulik, H.J.; Dincă, M.; Gumyusenge, A. Robust Chemiresistive Behavior in Conductive Polymer/MOF Composites. Adv. Mater. 2024, 36, 2312382. [Google Scholar] [CrossRef]
- Alsafrani, A.E.; Adeosun, W.A.; Marwani, H.M.; Khan, I.; Jawaid, M. Efficient Synthesis and Characterization of Polyaniline @ Aluminium—Succinate Metal-Organic Frameworks Nanocomposite and Its Application for Zn(II) Ion Sensing. Polymers 2021, 13, 3383. [Google Scholar] [CrossRef]
- Milikić, J.; Savić, M.; Janošević Ležaić, A.; Šljukić, B.; Ćirić-Marjanović, G. Electrochemical Sensing of Cadmium and Lead Ions in Water by MOF-5/PANI Composites. Polymers 2024, 16, 683. [Google Scholar] [CrossRef]
- Feng, T.; Chen, K.; Zhong, J.; Cheng, Y.; Zhao, H.; Lan, M. In-situ polymerization of dendritic polyaniline nanofibers network embedded with Ag@SiO2 core-shell nanoparticles for electrochemical determination of trace arsenic(III). Sens. Actuators B Chem. 2022, 369, 132265. [Google Scholar] [CrossRef]
- Motaghedifard, M.H.; Pourmortazavi, S.M.; Mirsadeghi, S. Selective and sensitive detection of Cr(VI) pollution in waste water via polyaniline/sulfated zirconium dioxide/multi walled carbon nanotubes nanocomposite based electrochemical sensor. Sens. Actuators B Chem. 2021, 327, 128882. [Google Scholar] [CrossRef]
- Kumara, K.S.M.; Shivakumar, P.; Ganesh, V.; Budagumpi, S.; Bose, S.K.; Hareesh, K.; Nagaraju, D.H. Hydrogels of PANI doped with Fe3O4 and GO for highly stable sensor for sensitive and selective determination of heavy metal ions. Inorg. Chem. Commun. 2023, 158, 111553. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Bahrani, S.; Mousavi, S.M.; Omidifar, N.; Arjmand, M.; Lankarani, K.B.; Ramakrishna, S. Simultaneous electrochemical detection of Cd and Pb in aquatic samples via coupled graphene with brominated white polyaniline flakes. Eur. Polym. J. 2021, 162, 110926. [Google Scholar] [CrossRef]
- Akhtar, M.; Tahir, A.; Zulfiqar, S.; Hanif, F.; Warsi, M.F.; Agboola, P.O.; Shakir, I. Ternary hybrid of polyaniline-alanine-reduced graphene oxide for electrochemical sensing of heavy metal ions. Synth. Met. 2020, 265, 116410. [Google Scholar] [CrossRef]
- Alruwais, R.S.; Adeosun, W.A.; Marwani, H.M.; Jawaid, M.; Asiri, A.M.; Khan, A. Novel Aminosilane (APTES)-Grafted Polyaniline@Graphene Oxide (PANI-GO) Nanocomposite for Electrochemical Sensor. Polymers 2021, 13, 2562. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, R.; Xue, Q.; Liu, Z.; Liu, Y.; Wang, J.; Zhu, C. Detection of cadmium (II) ion in water by a novel electrochemical sensor based on modification of graphite carbon nitride and polyaniline composite. Diam. Relat. Mater. 2023, 140, 110427. [Google Scholar] [CrossRef]
- Maheshwaran, M.; Satheesh Kumar, K.K. DFT and electrochemical determination of Hg2+ and Pb2+ in water using polyaniline–quinoxaline composite modified GCE electrode. J. Mol. Liq. 2024, 398, 124317. [Google Scholar] [CrossRef]
- Zarejousheghani, M.; Rahimi, P.; Borsdorf, H.; Zimmermann, S.; Joseph, Y. Molecularly imprinted polymer-based sensors for priority pollutants. Sensors 2021, 21, 2406. [Google Scholar] [CrossRef]
- Setiyanto, H.; Purwaningsih, D.R.; Saraswaty, V.; Mufti, N.; Zulfikar, M.A. Highly selective electrochemical sensing based on electropolymerized ion imprinted polyaniline (IIPANI) on a bismuth modified carbon paste electrode (CPE-Bi) for monitoring Nickel(ii) in river water. RSC Adv. 2022, 12, 29554–29561. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Li, Y. Electrochemical sensors based on polyaniline nanocomposites for detecting Cd(II) in wastewater. Int. J. Electrochem. Sci. 2024, 19, 100519. [Google Scholar] [CrossRef]
- Chajanovsky, I.; Cohen, S.; Muthukumar, D.; Shtenberg, G.; Suckeveriene, R.Y. Enhancement of integrated nano-sensor performance comprised of electrospun PANI/carbonaceous material fibers for phenolic detection in aqueous solutions. Water Res. 2023, 246, 120709. [Google Scholar] [CrossRef]
- Al-Ghamdi, Y.O.; Jabli, M.; Alhalafi, M.H.; Khan, A.; Alamry, K.A. Oxidized carboxymethyl cellulose/polyaniline-based hybrid nanocomposite for sensitive detection of environmentally hazardous nitrophenol in real samples. Microchem. J. 2024, 199, 109913. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Bahrani, S.; Ramakrishna, S. Integrated polyaniline with graphene oxide-iron tungsten nitride nanoflakes as ultrasensitive electrochemical sensor for precise detection of 4-nitrophenol within aquatic media. J. Electroanal. Chem. 2020, 873, 114406. [Google Scholar] [CrossRef]
- Patel, B.R.; Noroozifar, M.; Kerman, K. Prussian blue-doped nanosized polyaniline for electrochemical detection of benzenediol isomers. ECS Meet. Abstr. 2020, MA2020-01, 2835. [Google Scholar] [CrossRef]
- Saleem, Q.; Shahid, S.; Javed, M.; Iqbal, S.; Rahim, A.; Mansoor, S.; Bahadur, A.; Awwad, N.S.; Ibrahium, H.A.; Almufarij, R.S.; et al. Synchronized electrochemical detection of hydroquinone and catechol in real water samples using a Co@SnO2-polyaniline composite. RSC Adv. 2023, 13, 10017–10028. [Google Scholar] [CrossRef]
- Kumar, H.; Kumari, N.; Singh, D. Quantum dots decorated polyaniline plastic nanocomposites as a novel amperometric sensor for formaldehyde: Experimental and theoretical approach. Talanta Open 2022, 6, 100141. [Google Scholar] [CrossRef]
- Suhaimi, N.F.; Baharin, S.N.A.; Jamion, N.A.; Mohd Zain, Z.; Sambasevam, K.P. Polyaniline-chitosan modified on screen-printed carbon electrode for the electrochemical detection of perfluorooctanoic acid. Microchem. J. 2023, 188, 108502. [Google Scholar] [CrossRef]
- Chi, T.Y.; Chen, Z.; Kameoka, J. Perfluorooctanesulfonic acid detection using molecularly imprinted polyaniline on a paper substrate. Sensors 2020, 20, 7301. [Google Scholar] [CrossRef]
- Masibi, K.K.; Fayemi, O.E.; Adekunle, A.S.; Al-Mohaimeed, A.M.; Fahim, A.M.; Mamba, B.B.; Ebenso, E.E. Electrochemical detection of endosulfan using an aonp-pani-swcnt modified glassy carbon electrode. Materials 2021, 14, 723. [Google Scholar] [CrossRef]
- Goswami, B.; Mahanta, D. Fe3O4-Polyaniline Nanocomposite for Non-enzymatic Electrochemical Detection of 2,4-Dichlorophenoxyacetic Acid. ACS Omega 2021, 6, 17239–17246. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, L.Z.; Pang, Y.H.; Shen, X.F. Cerium metal-organic framework composited with polyaniline on carbon cloth for high-sensitively electrochemical sensing of carbendazim. Microchem. J. 2024, 197, 109862. [Google Scholar] [CrossRef]
- Palsaniya, S.; Pal, T.; Mukherji, S. Highly sensitive detection of amoxicillin by polyaniline-AgBr amperometry sensor: Fabrication and application in tap water and lake water. Chem. Eng. J. 2023, 466, 143025. [Google Scholar] [CrossRef]
- Pan, Z.; Wei, Y.; Guo, H.; Liu, B.; Sun, L.; Lu, Z.; Wei, X.; Zhang, H.; Chen, Y.; Yang, W. Sensitive detection of sulfamethoxazole by an electrochemical sensing platform with a covalent organic framework in situ grown on polyaniline. Microporous Mesoporous Mater. 2023, 348, 112409. [Google Scholar] [CrossRef]
- Chuiprasert, J.; Srinives, S.; Boontanon, N.; Polprasert, C.; Ramungul, N.; Lertthanaphol, N.; Karawek, A.; Boontanon, S.K. Electrochemical Sensor Based on a Composite of Reduced Graphene Oxide and Molecularly Imprinted Copolymer of Polyaniline−Poly(o-phenylenediamine) for Ciprofloxacin Determination: Fabrication, Characterization, and Performance Evaluation. ACS Omega 2023, 8, 2564–2574. [Google Scholar] [CrossRef]
- Qiu, Y.; Qu, K. Binary organic-inorganic nanocomposite of polyaniline-MnO2 for non-enzymatic electrochemical detection of environmental pollutant nitrite. Environ. Res. 2022, 214, 114066. [Google Scholar] [CrossRef]
- Patri, S.B.; Karekuladh, S.M.; Malingappa, P. ZIF-8/CNFs/PANI composite as an electrochemical platform in trace-level nitrite sensing. Carbon Lett. 2023, 34, 421–435. [Google Scholar] [CrossRef]
- Chu, G.; Huang, J.; Yin, J.; Guo, Y.; Li, M.; Zhang, Y.; Sun, X. Novel anti-oxidation electrochemical sensor based on rod-shaped polyaniline-carboxymethyl cellulose-copper nanoparticles for nitrite determination. Chin. J. Anal. Chem. 2021, 49, 1–9. [Google Scholar] [CrossRef]
- Al-Kadhi, N.S.; Hefnawy, M.A.; Alamro, F.S.; Pashameah, R.A.; Ahmed, H.A.; Medany, S.S. Polyaniline-Supported Nickel Oxide Flower for Efficient Nitrite Electrochemical Detection in Water. Polymers 2023, 15, 1804. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Dhakal, G.; Nguyen, V.Q.; Lee, J.; Lee, Y.R.; Shim, J.J. Ammonium heptamolybdate preloaded on flexible carbon-matrix film electrode for the electrochemical phosphate sensor in a river water sample. Microchem. J. 2021, 170, 106639. [Google Scholar] [CrossRef]
- Farina, R.; Scalese, S.; Corso, D.; Capuano, G.E.; Screpis, G.A.; Coniglio, M.A.; Condorelli, G.G.; Libertino, S. Chronoamperometric Ammonium Ion Detection in Water via Conductive Polymers and Gold Nanoparticles. Molecules 2024, 29, 3028. [Google Scholar] [CrossRef]
- Kaur, R.; Tripathy, S.K.; Sharma, S.K. Advantages and Limitations of Environmental Nanosensors; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128174562. [Google Scholar]
- Kamel, M.; El-Fatah, G.A.; Zaher, A.; Farghali, A.A.; Othman, S.I.; Allam, A.A.; Rudayni, H.A.; Sala, A.M.; Hassouna, M.E.M.; Mahmoud, R. Cost-effective layered double hydroxides/conductive polymer nanocomposites for electrochemical detection of wastewater pollutants. Chin. J. Anal. Chem. 2024, 52, 100368. [Google Scholar] [CrossRef]
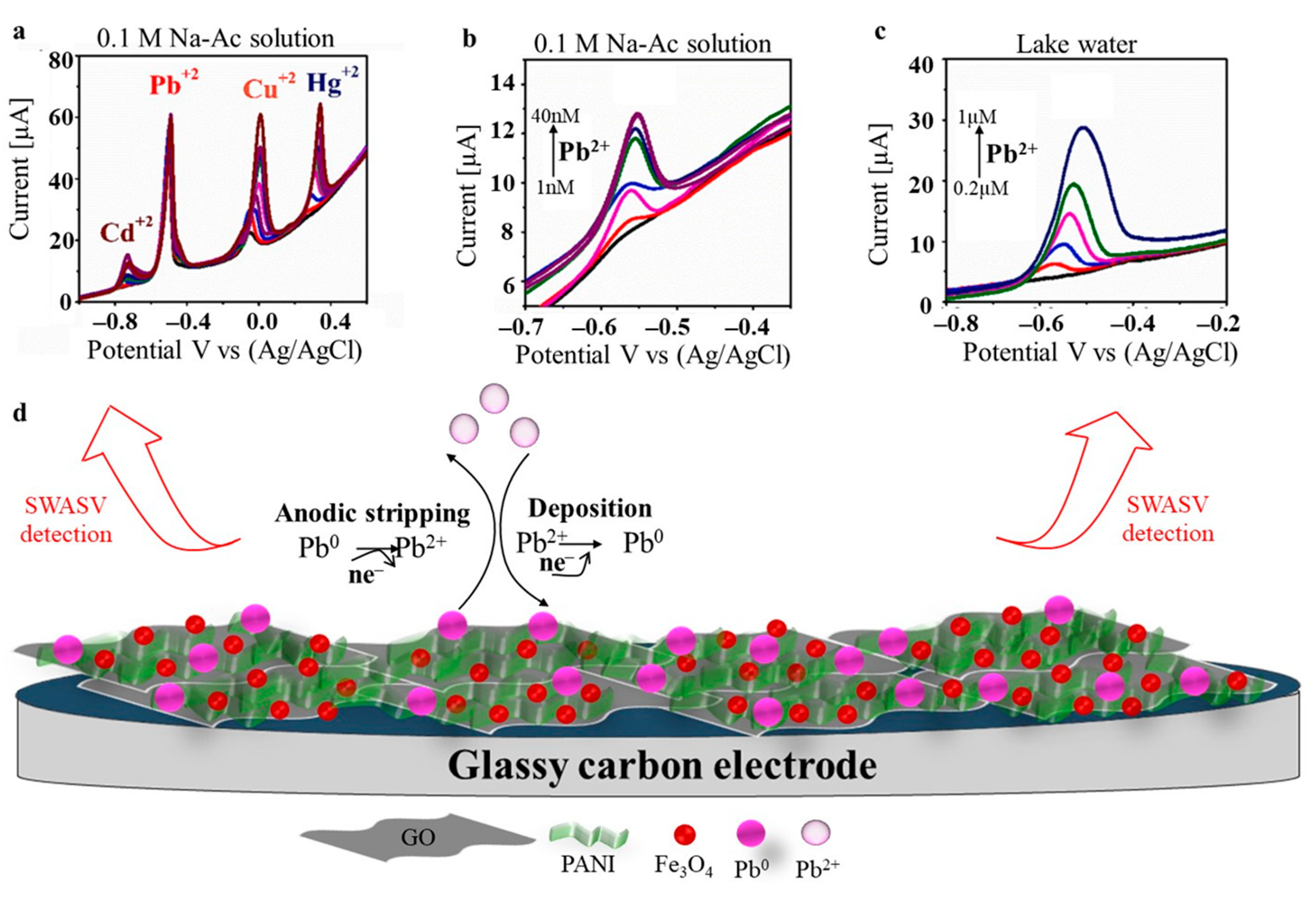
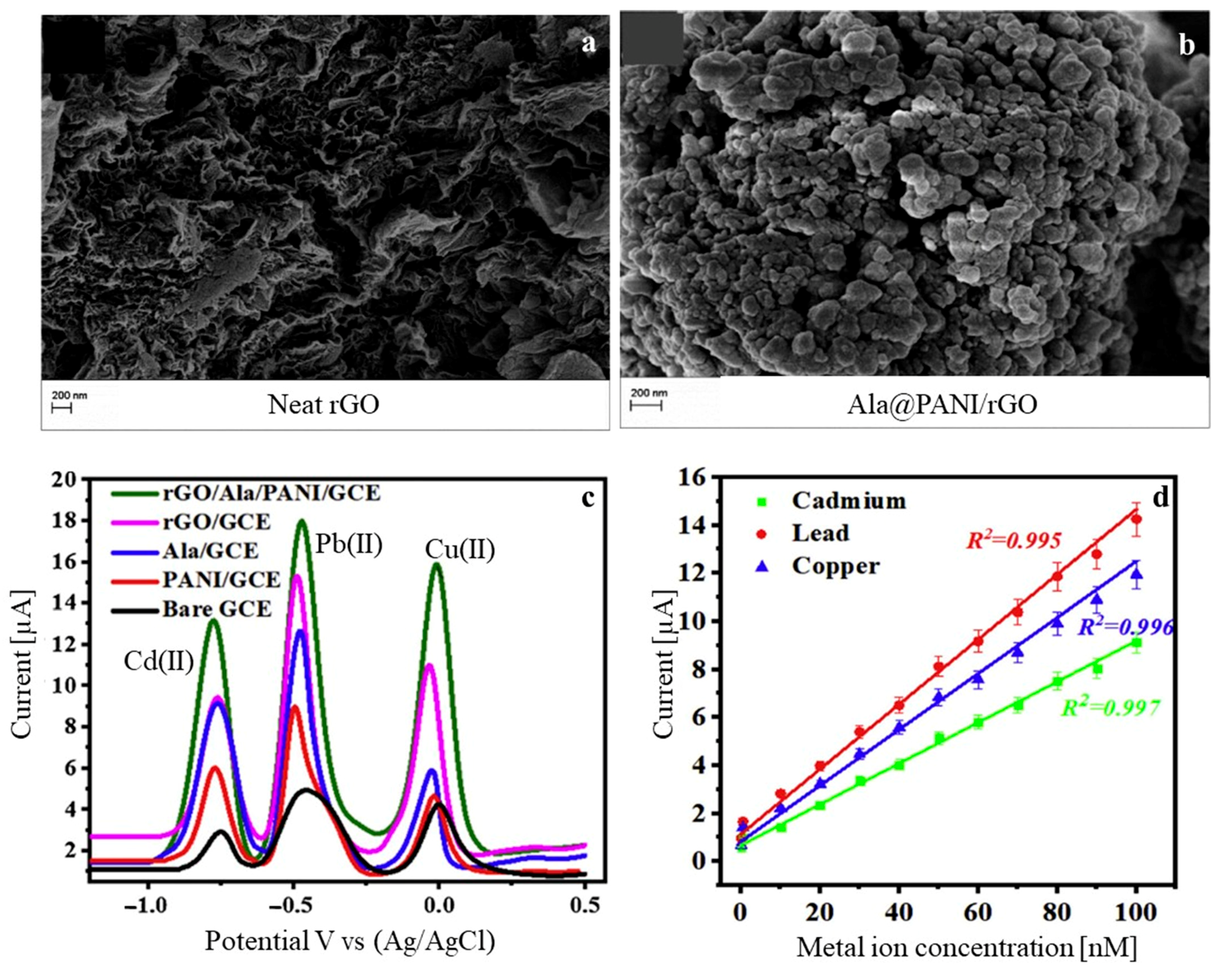

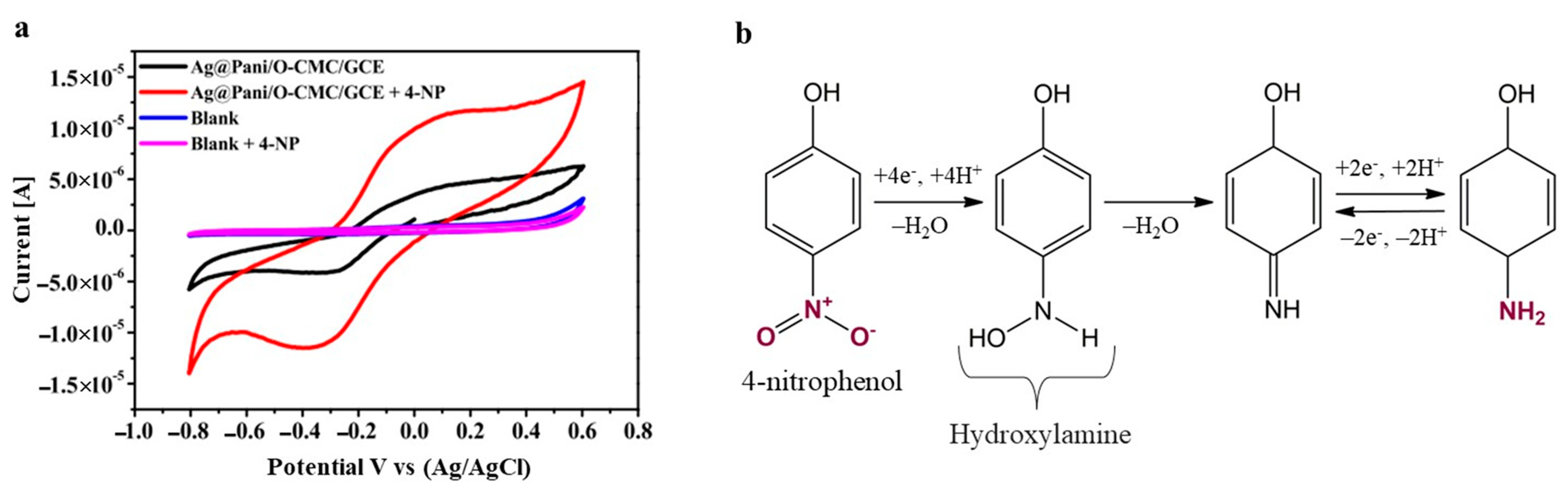
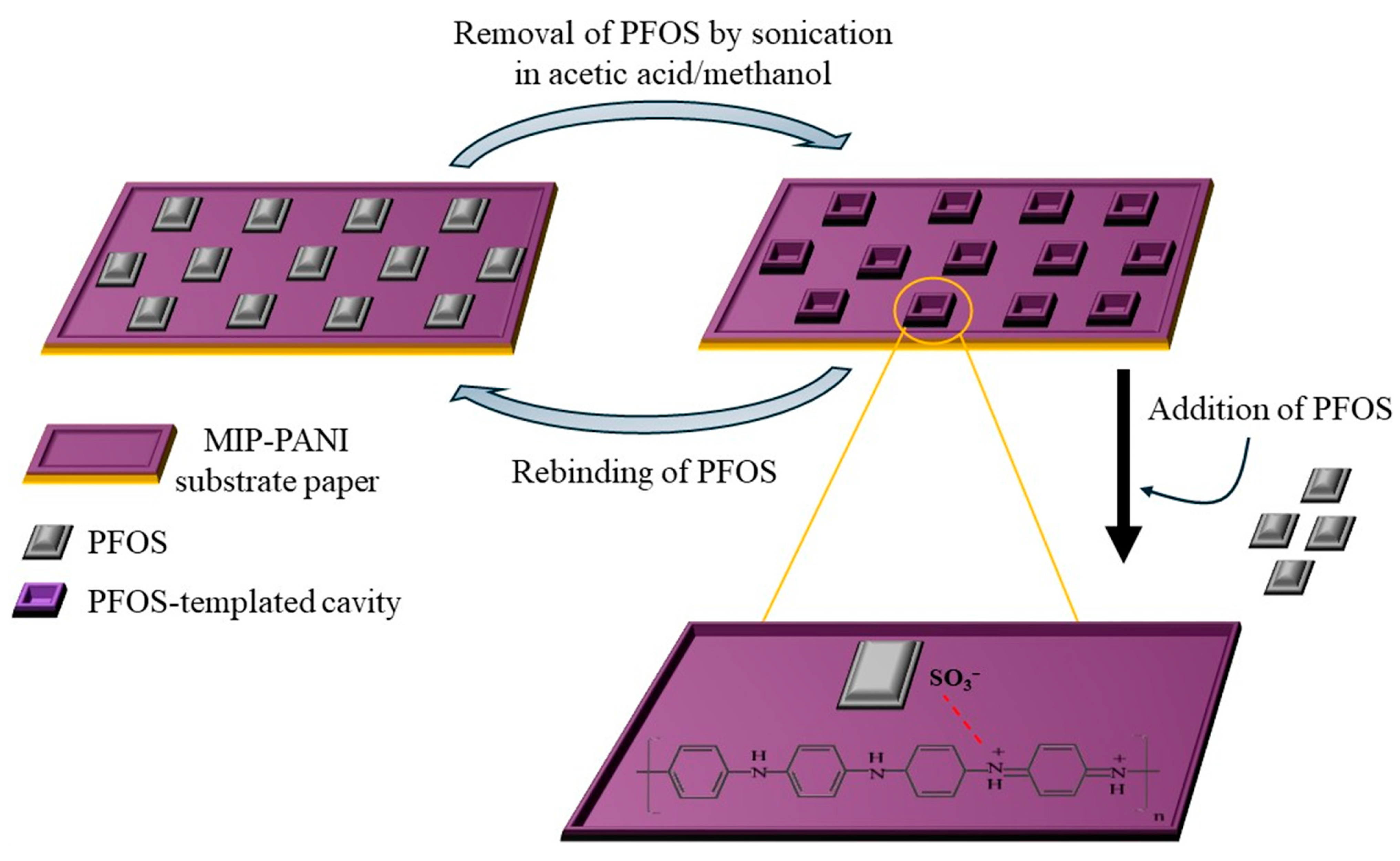

| Technique | Analyte | Electrochemical Platform | LOD [nM] | Sensitivity [µA µM−1 cm−2] | Linear Range [µM] | Ref |
|---|---|---|---|---|---|---|
| CV | – | PANI NPs/GCE | – | – | 60–180 | [60] |
| LSV | Zn(II) | Al-SA MOF@PANI/GCE | 590 | 7.14 | 2.8–228.6 | [65] |
| ASV | Cd(II) Pb(II) | MOF-5/PANI-EB | 103 100 | 156 27 [AM−1] | 0.7–1.5 0.7–1.2 | [66] |
| SWASV | As(III) | Ag@SiO2-PANI NFs/SPCE | 0.013 [μg L−1] | 0.83 [μA μg−1 L] | 0.1–100 [μg] | [67] |
| DVP | Cr(VI) | PANI@ZrO2-SO42−@MWCNT/GCE | 64.3 | 1.3693 0.9832 [μA Lμmol−1] | 0.55–13.7, 13.7–39.5 | [68] |
| SWASV | Pb(II) | H-PANI/Fe-GO/GCE | 5.15 | 0.0003304 | 1–40 (×10−3) | [69] |
| SDPV | Cd(II) Pb(II) | rGO-Brominated White PANi Flakes/GCE | 6.5 7.3 | 3914.01 4547.77 | 0.01–0.23 0.01–0.18 | [70] |
| SWASV | Cd(II) Pb(II) Cu(II) | Ala@PANI-rGO/GCE | 0.03 0.045 0.063 | 0.00043 0.00061 0.00071 | 0.08–100 (×10−3) | [71] |
| LSV | Pb(II) | PANI@APTES-GO/Nafion/GCE | 5.3 | 165.71 [µA µM−1 cm−1] | 0.01–0.4 | [72] |
| DPASV | Cd(II) | PANI@g-C3N4/GCE | 0.05 [μg L−1] | – | 0.1–140 [μg L−1] | [73] |
| DVP | Hg(II) Pb(II) | PANI@QUA/GCE | 1.28 5.91 | – | 1–20 | [74] |
| DPSAV | Ni(II) | IIPANI/CPE-Bi | 4.82 | – | 0.01–1 | [76] |
| SWASV | Cd(II) | PANI-AuNPs/GCE | 1.2 [μg L−1] | – | 5–100 | [77] |
| pH sensor | AP | F-PANI/rGO/PCL | 8.34 | 123.8 [kΩ µM−1] | 0.061–0.31 | [78] |
| DVP | 4-NP | Ag@PANI/O-CMC/GCE | 0.58 | 0.00248 | 0.01–0.1 | [79] |
| CV | 4-NP | PANI-GITN/GCE (oxidation/reduction) | 5.2 2.4 | 253.08 354.92 | 0.03–3 0.01–4 | [80] |
| DVP | HQ CH RS | Pb@NS-PANI/GPE | 180 10 20 | – | 1–350.5 2–350.5 | [81] |
| DVP | HQ CH | Co@SnO2–PANI/GCE | 4.94 1.5786 | 9.68 12.80 [µA cm−2] | 2 × 10−2–2 × 10−1 M | [82] |
| Amperometric | FA | PANI@CuO | 10−6 [mol/L] | – | – | [83] |
| DVP | PFOA | PANI@CHT/SPCE | 1.08 [μg L−1] | – | 5–150 [μg L−1] | [84] |
| pH sensor | PFOS | MIP-PANI | 1.02 [ppt] | – | 1–100 [ppt] | [85] |
| SWV | EDS | AONP@PANI-SWCNT/GCE | 6800 | 0.2086 [µA µM−1] | 32.3–77.6 | [86] |
| Amperometric | 2,4-D | Fe3O4-PANI/GCE | 210 | 4.62 × 10−7 | 1.35–2.7 | [87] |
| DVP | CBZ | Ce-MOF@PANI/CC | 12.6 | – | 0.1–80 | [88] |
| Amperometric | AX | PANI-AgBr/SPCE | 0.193 | 0.04 | 0.193–0.855 (×10−3) | [89] |
| DVP | SMX | TFAB-COF@PANI/GCE | 107 | – | 1–450 | [90] |
| DVP | CIP | PANI−o-PDA MIP@rGO/GCE | 0.05 | 5.78 × 106 [μA μmol−1 L] | 0.001−0.5 | [91] |
| CA | Nitrite | PANI@MnO2/GCE | 1080 | 0.225 | 19.98–732.17 | [92] |
| SWV | Nitrite | ZIF-8/CNF/PANI/GCE | 8100 | – | 16–835 | [93] |
| Amperometric | Nitrite | PANI-CMC@CuNPs/GCE | 170 | 0.113 0.049 | 3–15,000 15,000–29,000 | [94] |
| Amperometric | Nitrite | PANI/NiOnf/GCE | 9.7 64 | – | 0.1–1 1–500 | [95] |
| CA | Phosphate | AHM@PANI/CC/PVDF | 600 | 0.0648 [µA µM−1] | 10–114 | [96] |
| CA | NH4+ | PANIep@Au/GCE | 30 70 | 0.34 [mA/µM] 0.18 [mA/µM] | 0.35–1.5 2–7 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, S.; Chajanovsky, I.; Suckeveriene, R.Y. Recent Developments in Enzyme-Free PANI-Based Electrochemical Nanosensors for Pollutant Detection in Aqueous Environments. Polymers 2025, 17, 1320. https://doi.org/10.3390/polym17101320
Cohen S, Chajanovsky I, Suckeveriene RY. Recent Developments in Enzyme-Free PANI-Based Electrochemical Nanosensors for Pollutant Detection in Aqueous Environments. Polymers. 2025; 17(10):1320. https://doi.org/10.3390/polym17101320
Chicago/Turabian StyleCohen, Sarah, Itamar Chajanovsky, and Ran Yosef Suckeveriene. 2025. "Recent Developments in Enzyme-Free PANI-Based Electrochemical Nanosensors for Pollutant Detection in Aqueous Environments" Polymers 17, no. 10: 1320. https://doi.org/10.3390/polym17101320
APA StyleCohen, S., Chajanovsky, I., & Suckeveriene, R. Y. (2025). Recent Developments in Enzyme-Free PANI-Based Electrochemical Nanosensors for Pollutant Detection in Aqueous Environments. Polymers, 17(10), 1320. https://doi.org/10.3390/polym17101320






