Evaluation of γ-Irradiation Effects on EPDM/SBS Blends for Durability and Recycling Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Processing of Samples
2.3. Chemiluminescence Study
2.4. Analysis of the Gel Fraction (Equilibrium Solvent Swelling Measurements)
2.5. Physical and Mechanical Testing
2.6. FTIR Analysis
2.7. DSC Analysis
3. Results and Discussion
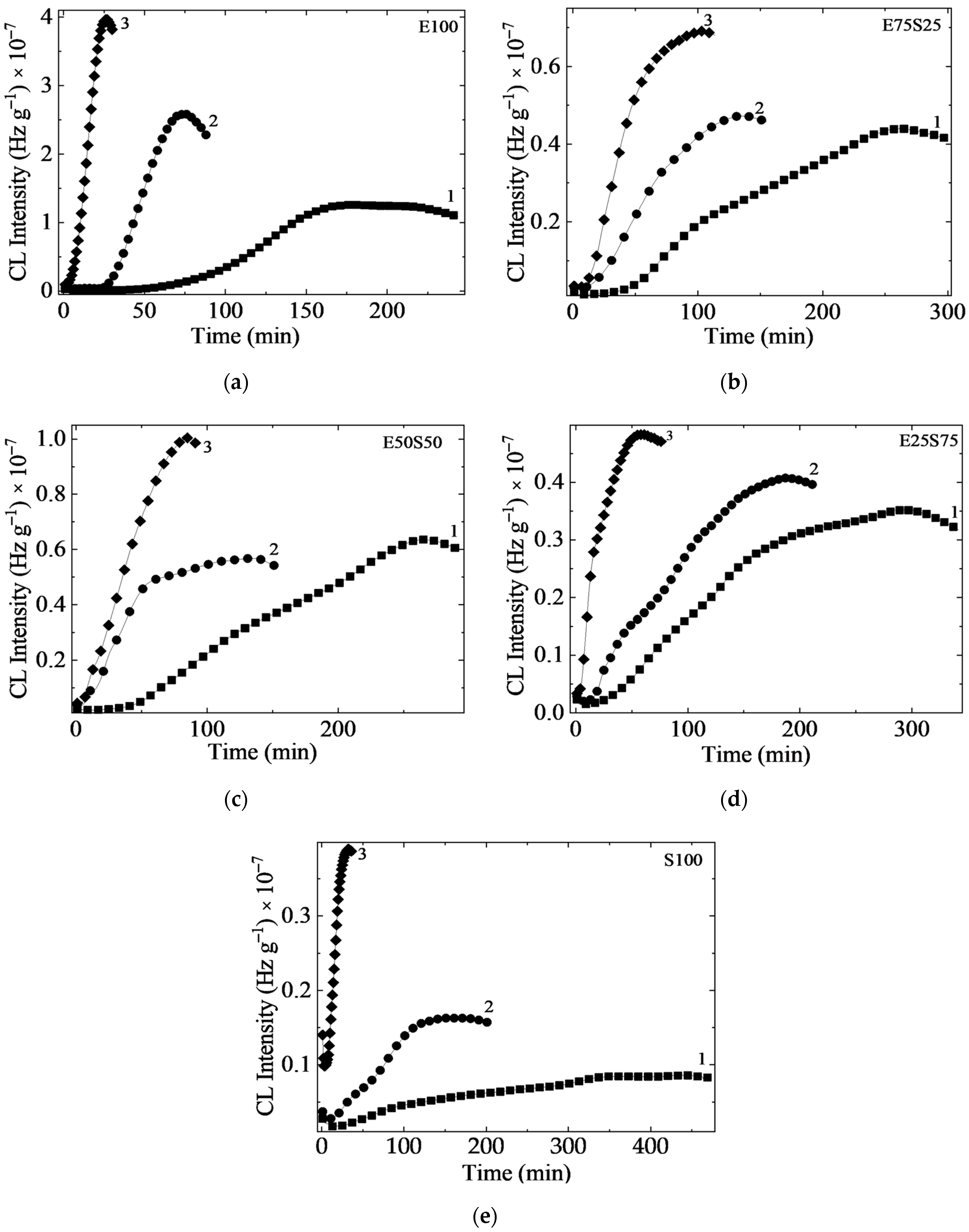
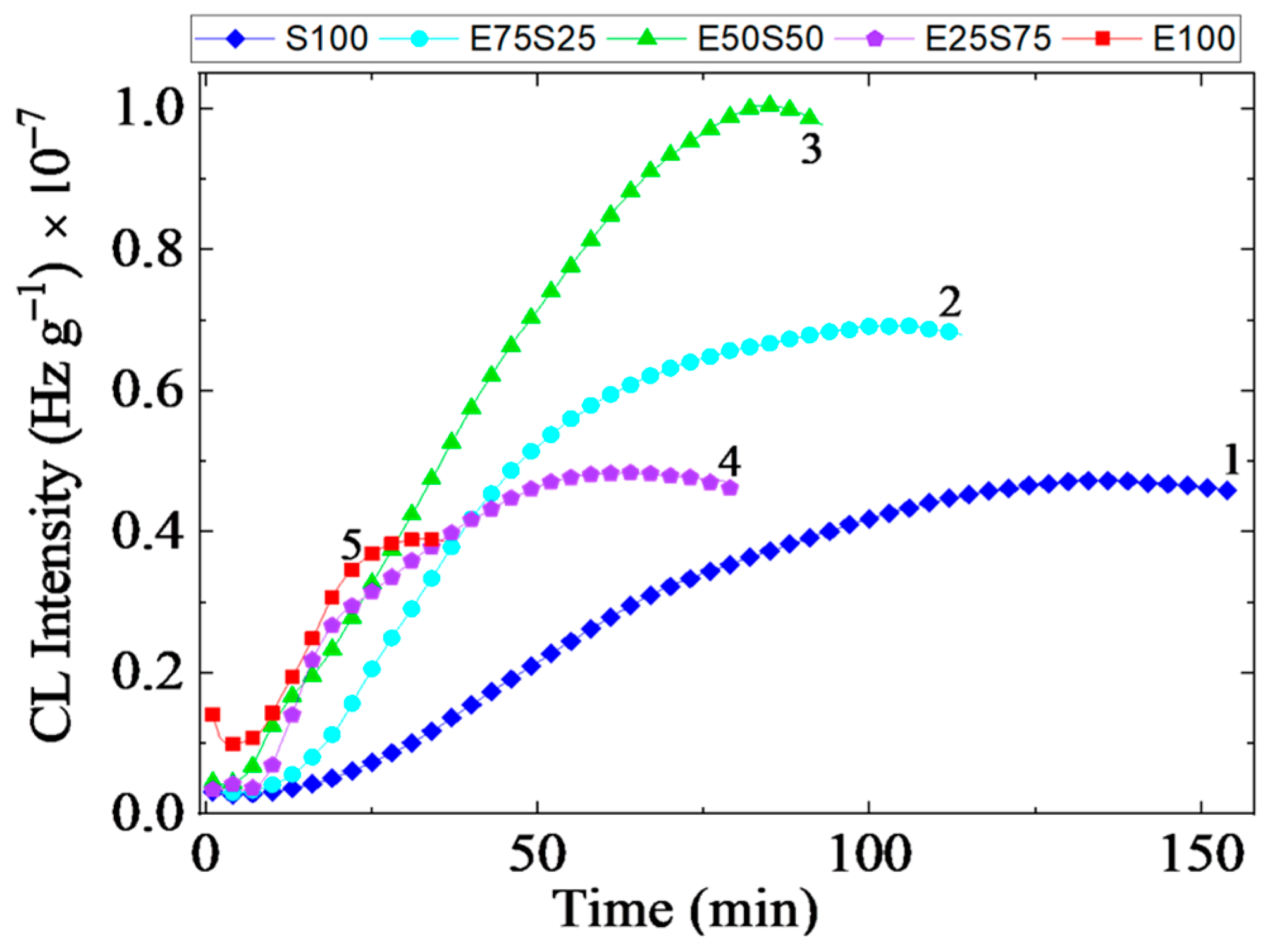
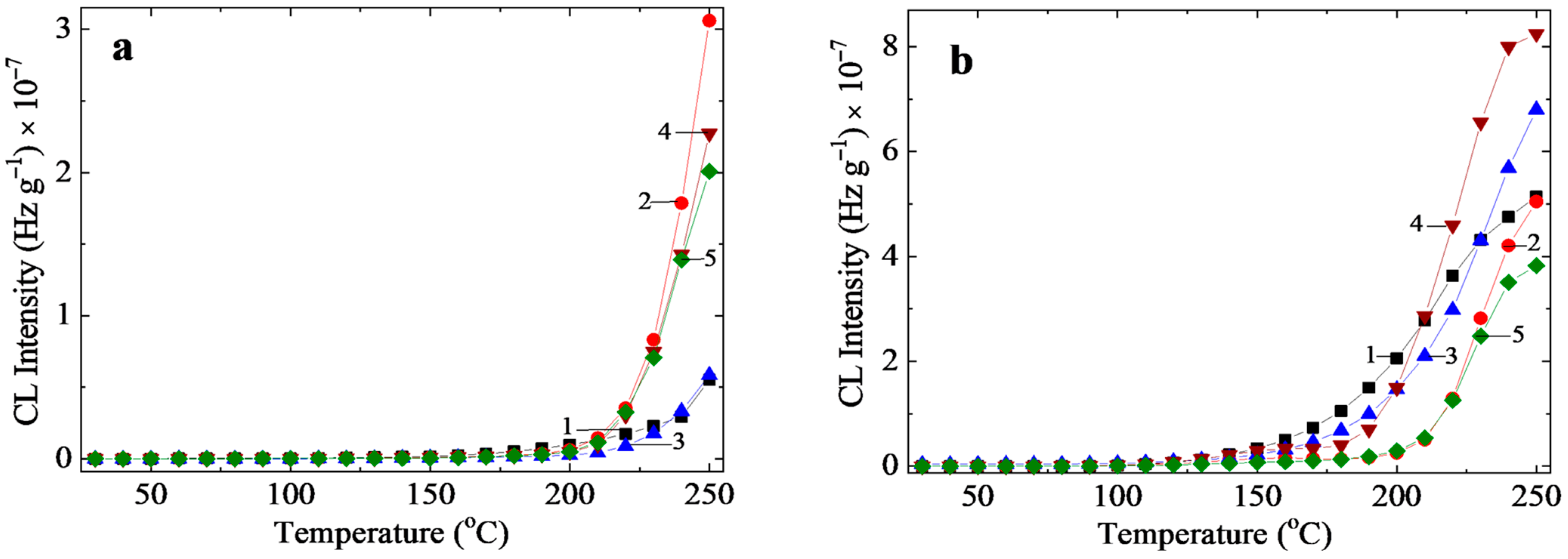
3.1. Chemiluminescence
3.1.1. Isothermal Chemiluminescence
- The oxidation induction time (Table 1) indicates the effective start of the process when the measurable quanta number in the start of the propagation step sustained by the reactions of free radicals with molecular oxygen or peroxyl entities is recorded. It means that a sufficient amount of oxygen is available and the concentration of radicals may feed the oxidation chain;
- The oxidation rate suggested by the curved slope characterizes the decay of oxidized intermediates when the ability of the included phases for the molecular scission allows the accumulation of reactive fragments to feed the aging process. The stability difference between the two polymers depicts the unlikely progress of oxidation when the blends are subjected to an energetic transfer.
3.1.2. Nonisothermal Chemiluminescence
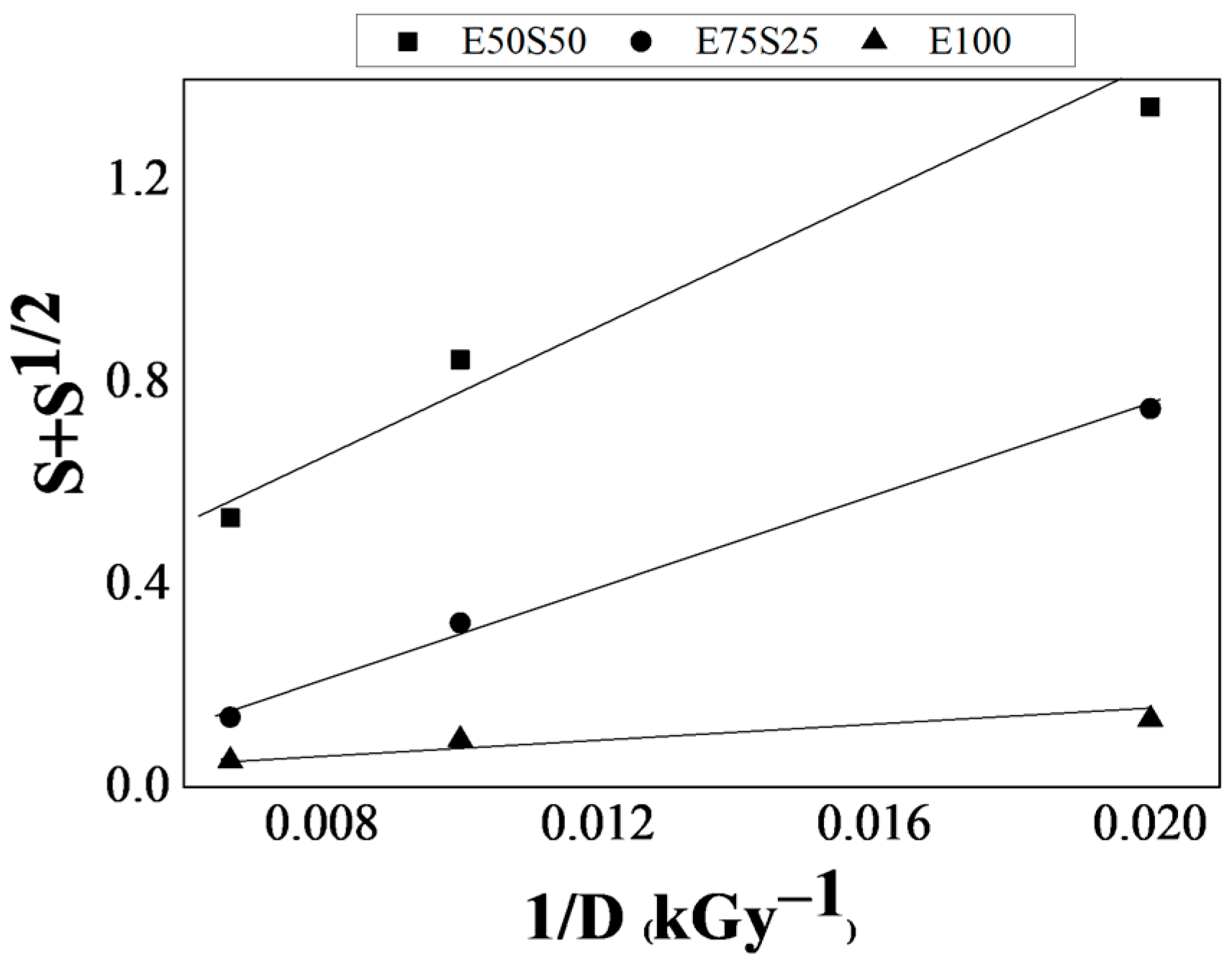
3.2. Analysis of the Gel Fraction
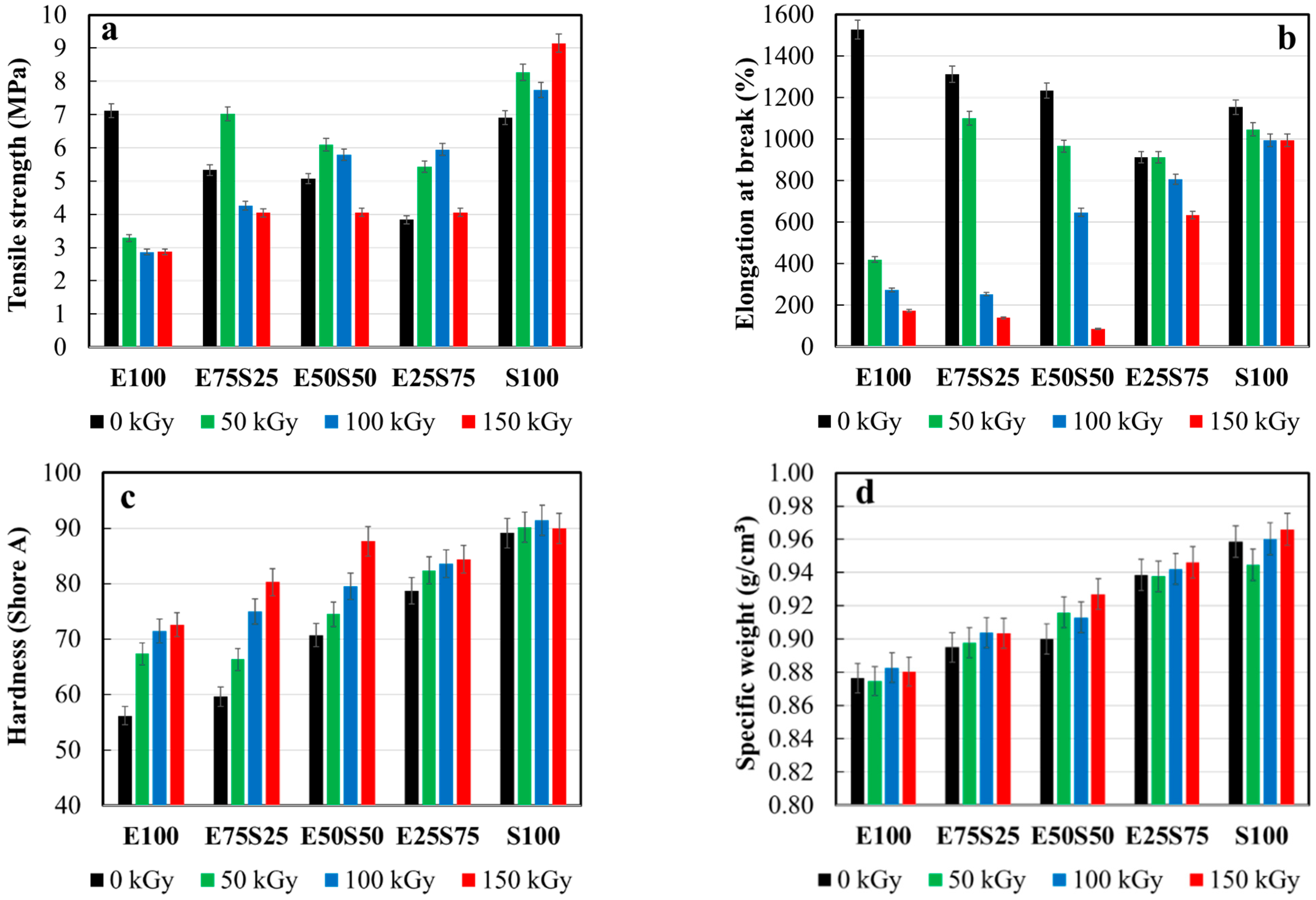
3.3. Mechanical Testing
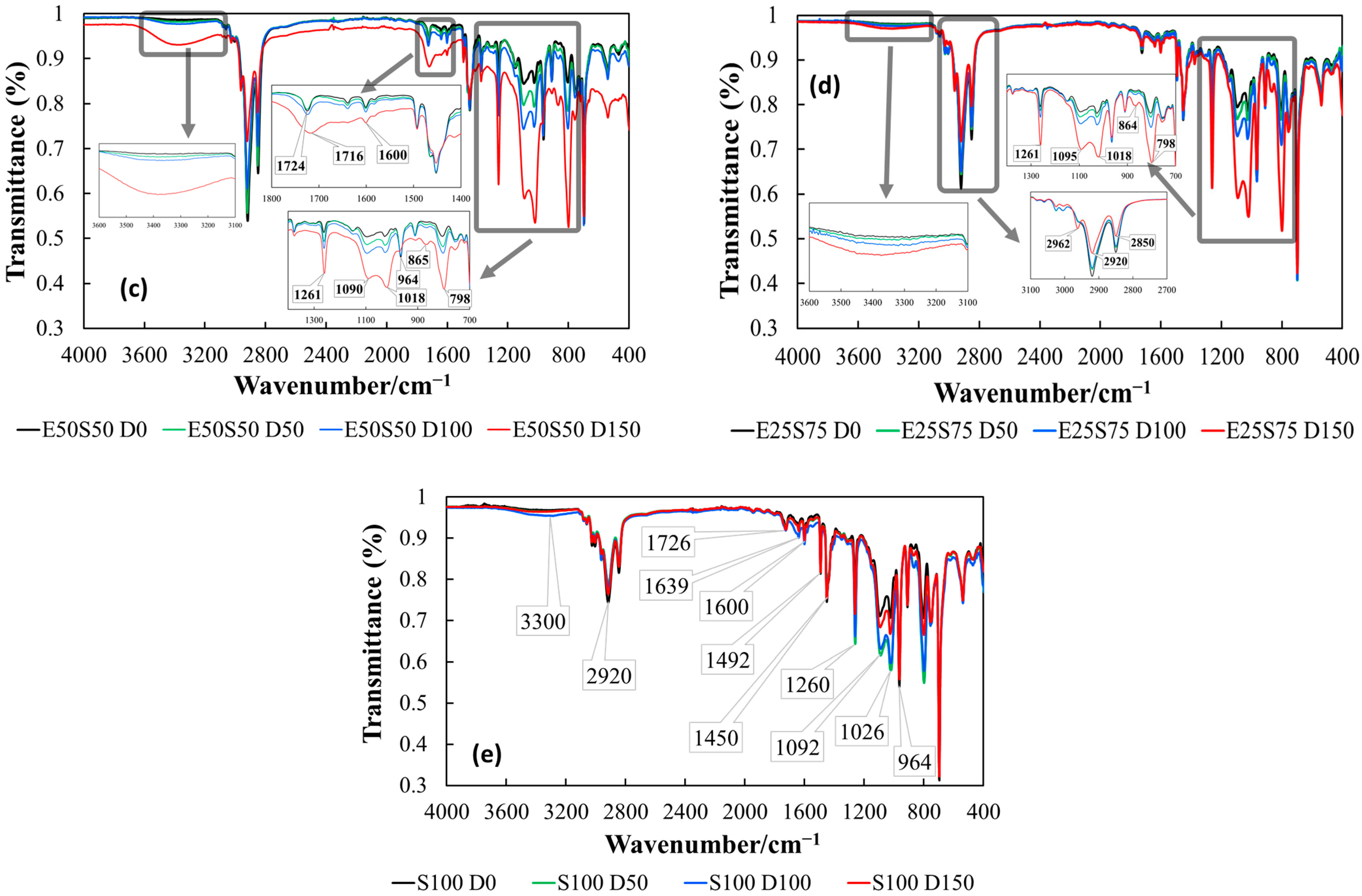
3.4. IR Analysis
- A weak band is observed in all samples due to the presence of TMPTMA as a crosslinking agent. The band persists even at low TMPTMA concentrations (3%), likely due to trace residual hydroxyl groups or slight hydrolysis.
- Its consistent presence across all spectra suggests it originates from TMPTMA, not blending byproducts in non-irradiated samples.
- Bands at 3080, 3060, 3025, and 3004 cm−1 correspond to C-H stretching in styrene (aromatic) and butadiene (olefin) groups. Intensity increases with SBS content, making these bands markers for SBS proportion.
- Bands at 2916 and 2845 cm−1 represent symmetric and asymmetric stretching of aliphatic C-H bonds. Intensity decreases with increasing SBS content, reflecting reduced ethylene proportion.
- Slight band shifts (2916 to 2918 cm−1 and 2845 to 2850 cm−1) indicate changes in intermolecular interactions due to SBS replacement by EPDM.
- Bands at 1465 and 1450 cm−1: C-H bending (scissoring) in saturated aliphatic chains; intensity remains consistent as both SBS and EPDM contain aliphatic C-H groups.
- Band at 1377 cm−1: C-H bending (rocking) in methyl/methylene groups; absent in S100 due to the lack of EPDM-specific aliphatic environments or band suppression at 100% SBS.
- Bands at 1094, 1024, and 964 cm−1 correspond to C = C stretching and bending in butadiene and styrene units, reflecting substitution patterns and C=C bond environments.
- Bands at 798, 752, and 698 cm−1 represent C-H out-of-plane bending in substituted benzene rings, confirming the aromatic styrene component in SBS.
- Radiation dose influence.
- Low doses (up to 50 kGy)—minimal changes in O−H intensity.
- High-SBS-content samples, E25S75 and S100 (Figure 8d,e), register minimal changes, indicating higher resistance to radiation-induced oxidation.
- Irradiation causes a peak shift toward 1712–1716 cm−1.
- Broadening of the absorption band due to the formation of carbonyl groups (e.g., ketones, carboxylic acids).
- Changes are more pronounced in samples with less than 50% SBS, while E25S75 and S100 blends show minimal changes, suggesting greater resistance to forming C=O structures. A higher reactivity of saturated carbon chains in EPDM contributes to C=O generation, while SBS resists oxidation due to the stabilizing effect of its styrene components [60,61].
- High-EPDM-content samples (E100, E75S25, E50S50) show significant increases in absorbance with rising radiation doses. These bands indicate radiation-induced crosslinking and/or oxidative degradation.
- The intensity of this band increases with radiation dose, suggesting the formation of epoxy structures [62].
- Band at ~1150 cm−1 (prominent in high-EPDM-content samples) decreases in intensity with increasing γ-radiation dose and disappears entirely at doses of 100–150 kGy.
- Band at 938–966 cm−1 (saturated polyolefin structures) disappears in samples containing more than 50% EPDM.

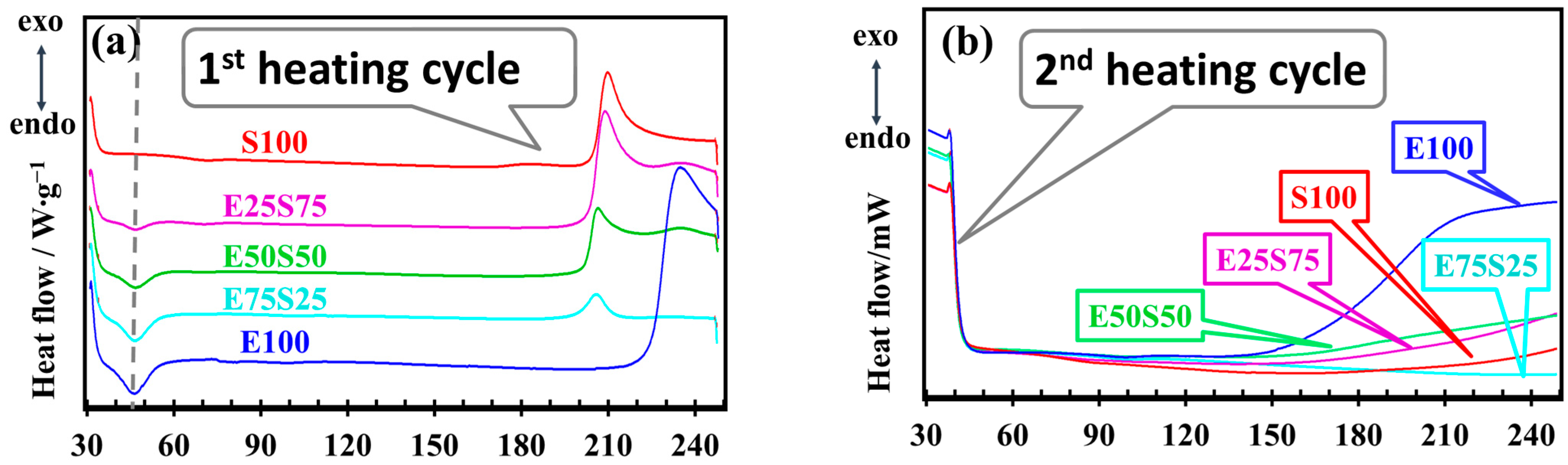
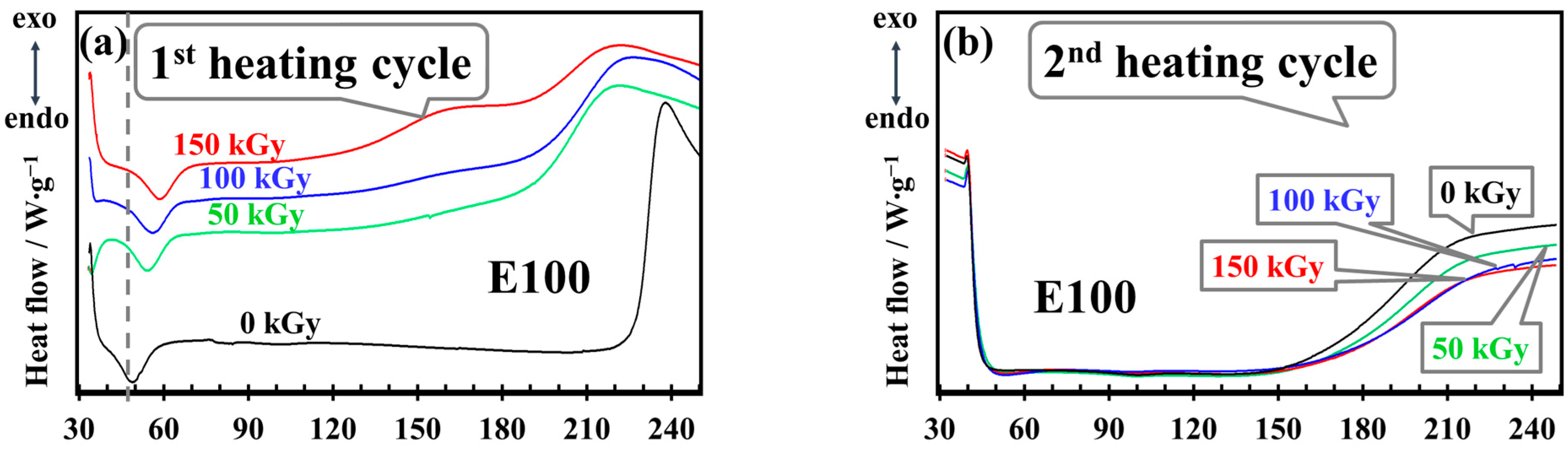
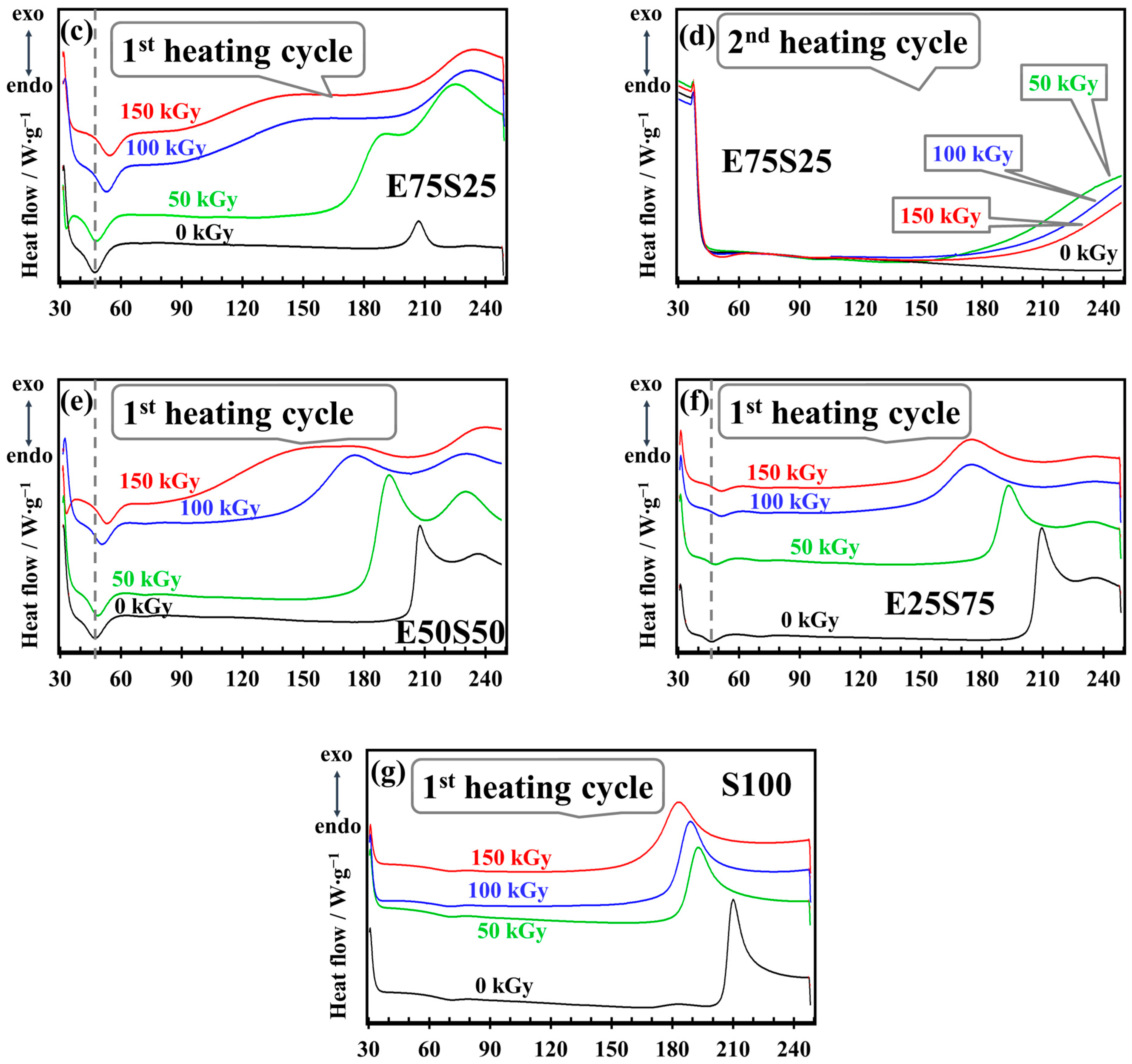
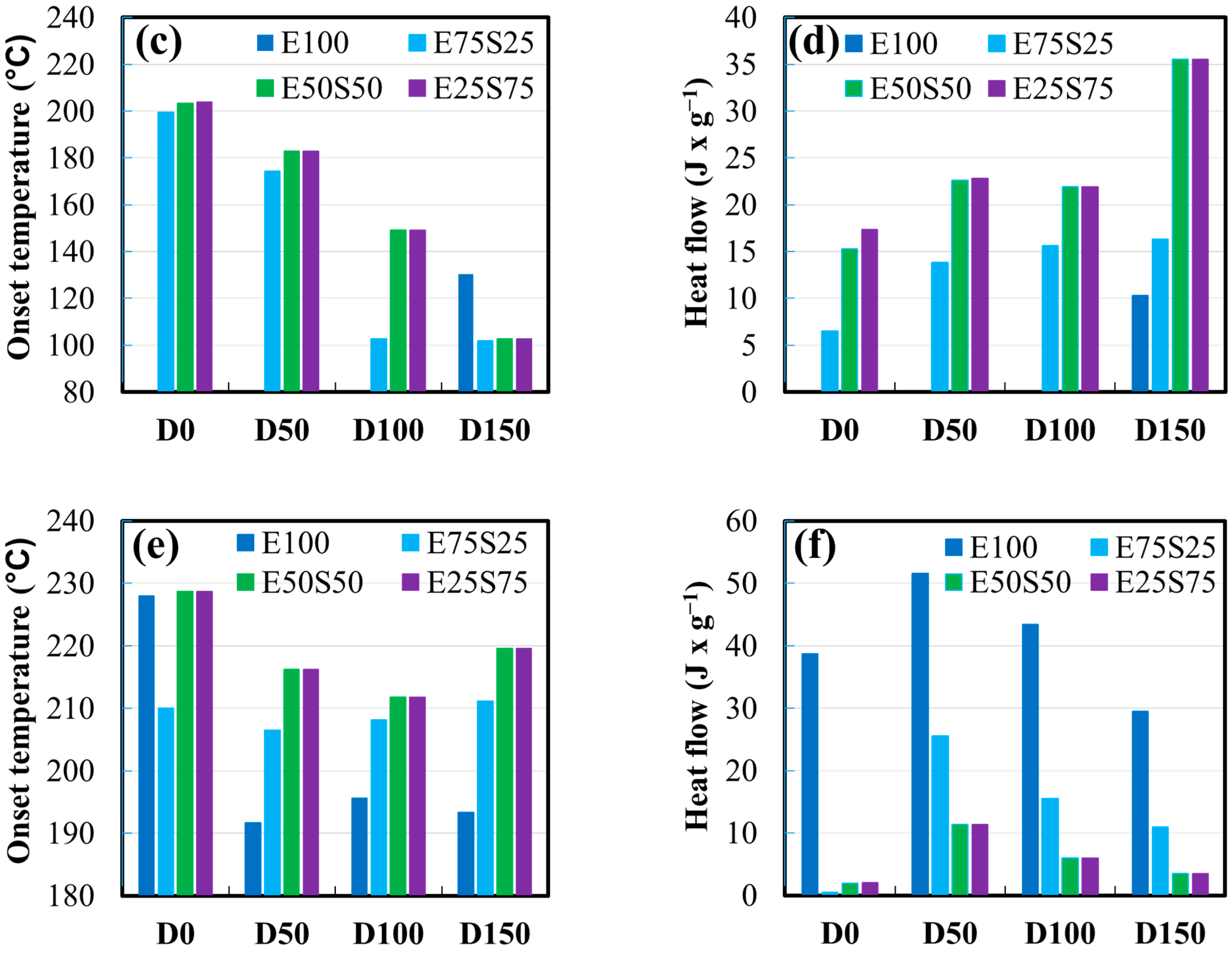
3.5. DSC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CL | Chemiluminescence |
| DSC | Differential scanning calorimetry |
| EPDM | ethylene-propylene-diene monomer |
| FTIR | Fourier transformed infrared |
| PP | polypropylene |
| SBS | styrene-butadiene-styrene |
References
- Reinaldo, J.S.; Pereira, L.M.; Silva, E.S.; de Macedo, T.C.P.; Damasceno, I.Z.; Ito, E.N. Thermal, mechanical, and morphological properties of multicomponent blends based on acrylic and styrene polymers. Polym. Test. 2020, 82, 106265. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Majka, T.M. Thermal Degradation of Polymeric Materials, 2nd ed.; Pielichowski, K., Njuguna, J., Majka, T.M., Eds.; Elsevier: London, UK, 2023; pp. 49–147. [Google Scholar]
- Barala, S.S.; Manda, V.; Jodha, A.S.; Ajay, C.; Gopalani, D. Thermal stability of gamma-irradiated ethylene propylene diene monomer composites for shielding applications. J. Appl. Polym. Sci. 2021, 139, e52975. [Google Scholar] [CrossRef]
- Kornacka, E.M. Applications of Ionizing Radiation in Materials Processing. In Applications of Ionizing Radiation in Materials Processing, Volume I; Sun, Y., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; Chapter 8. [Google Scholar]
- Majumder, P.A.; Bhowmick, A.K. Structure-property relationship of electron-beam-modified EPDM rubber. J. Appl. Polym. Sci. 1999, 77, 323–337. [Google Scholar] [CrossRef]
- De Almeida, A.; Chazeau, L.; Vigier, G.; Marque, G.; Goutille, Y. Influence of PE/PP ratio and ENB content on the degradation kinetics of γ-irradiated EPDM. Polym. Degrad. Stab. 2014, 110, 175–183. [Google Scholar] [CrossRef]
- Colom, X.; Carrillo-Navarrete, F.; Saeb, M.R.; Marin, M.; Formela, K.; Cañavate, J. Evaluation and rationale of the performance of several elastomeric composites incorporating devulcanized EPDM. Polym. Test. 2023, 121, 107976. [Google Scholar] [CrossRef]
- Morris, B.A. The Science and Technology of Flexible Packaging; Morris, B.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 167–201. [Google Scholar]
- Bernstein, S.M.; Thornberg, R.A.; Assink, R.A.; Irwin, A.N.; Hochrein, J.M.; Brown, J.R.; Derzon, D.K.; Klamo, S.B.; Clough, R.L. The origins of volatile oxidation products in the thermal degradation of polypropylene, identified by selective isotopic labeling. Polym. Degrad. Stab. 2007, 92, 2074–2094. [Google Scholar] [CrossRef]
- Zaharescu, T.; Jipa, S. Landolt-Börnstein Numerical Data and Functional Relationships in Science and Technology. In Group VIII–Polymers; Arndt, K.F., Lechner, M.D., Eds.; Springer: Amsterdam, The Netherlands, 2013; p. 103. [Google Scholar]
- Le Lay, F. Study on the lifetime of EPDM seals in nuclear-powered vessels. Radiat. Phys. Chem. 2013, 84, 210–217. [Google Scholar] [CrossRef]
- Bandzierz, K.S.; Reuvekamp, L.A.E.M.; Przybytniak, G.; Dierkes, W.K.; Blume, A.; Bielinski, D.M. Effect of electron beam irradiation on structure and properties of styrene-butadiene rubber. Radiat. Phys. Chem. 2018, 149, 14–25. [Google Scholar] [CrossRef]
- González, J.; Albano, C.; Candal, M.V.; Ichazo, M.N.; Hernández, M. Characterization of blends of PP and SBS vulcanized with gamma irradiation. Nucl. Instrum. Meth. Phys. Res. 2005, 236, 354–358. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Morreale, M.; Botta, L.; Mistretta, M.C.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Turan, S.; Park, S.; Ryu, C.Y.; Ryu, Y.; Bae, C. Functionalization of rubbery domains in SBS triblock copolymers for anion exchange membrane applications with enhanced robustness. J. Membr. Sci. 2024, 700, 122662. [Google Scholar] [CrossRef]
- Przybytniak, G.K.; Zagórski, Z.P.; Zuchowska, D. Free radicals in electron beam irradiated blends of polyethylene and butadiene-styrene block copolymer. Radiat. Phys. Chem. 1999, 55, 655–658. [Google Scholar] [CrossRef]
- Sharpaty, V.A.; Pravednikov, A.N. Formation of radicals in polymers. Polym. Sci. Part C Polym. Symp. 1967, 16, 1599–1607. [Google Scholar] [CrossRef]
- Bansal, N.; Arora, S. Exploring the impact of gamma rays and electron beam irradiation on physico-mechanical properties of polymers & polymer composites: A comprehensive review. Nucl. Instrum. Meth. Phys. Res. 2024, 549, 165297. [Google Scholar]
- Zaharescu, T.; Jipa, S.; Henderson, D. Improvement in radiation stability of EPDM by efficient antioxidants. Macromol. Symp. 2012, 315, 212–217. [Google Scholar] [CrossRef]
- Stelescu, M.D.; Airinei, A.; Bargan, A.; Fifere, N.; Georgescu, M.; Sonmez, M.; Nituica, M.; Alexandrescu, L.; Stefan, A. Mechanical properties and equilibrium swelling characteristics of some polymer composites based on ethylene propylene diene terpolymer (EPDM) reinforced with hemp fibers. Materials 2022, 15, 6838. [Google Scholar] [CrossRef]
- Pirityi, D.Z.; Pölöskei, K. Thermomechanical devulcanisation of ethylene propylene diene monomer (EPDM) rubber and its subsequent reintegration into virgin rubber. Polymers 2021, 13, 1116. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Hait, S.; De, D.; Ghosh, P.; Chanda, J.; Mukhopadhyay, R.; Dasgupta, S.; Sallat, A.; Al Aiti, M.; Stöckelhuber, K.W.; Wießner, S.; et al. Understanding the coupling effect between lignin and polybutadiene elastomer. J. Compos. Sci. 2021, 5, 154. [Google Scholar] [CrossRef]
- Díez, E.; Ovejero, G.; Romero, M.D.; Díaz, I. Polymer-solvent interaction parameters of SBS rubbers by inverse gas chromatography measurements. Fluid Phase Equilibria 2011, 308, 107–113. [Google Scholar] [CrossRef]
- ISO 23529:2016; Rubber—General Procedures for Preparing and Conditioning Test Pieces for Physical Test Methods, 3rd ed. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties, 6th ed. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 48-4:2018; Rubber, Vulcanized or Thermoplastic—Determination of Hardness—Part 4: Indentation Hardness by Durometer Method (Shore Hardness), 1st ed. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- Jacobson, K.; Eriksson, P.; Reitberger, T.; Stenberg, B. Chemiluminescence as a Tool for Polyolefin Oxidation Studies. In Long Term Properties of Polyolefins; Springer: Amsterdam, The Netherlands, 2004; pp. 151–176. [Google Scholar]
- Rosales, C.; López-Quintana, S.; Gobernado-Mitre, I.; Merino, J.C.; Pastor, J.M. Effects of electron beam irradiation on binary polyamide-6 blends with metallocene copolymers. Nucl. Instrum. Meth. Phys. Res. 2007, 265, 156–161. [Google Scholar] [CrossRef]
- Sidi, A.; Colombani, J.; Larché, J.F.; Rivaton, A. Multiscale analysis of the radio oxidative degradation of EVA/EPDM composites. ATH filler and dose rate effect. Radiat. Phys. Chem. 2018, 142, 14–22. [Google Scholar] [CrossRef]
- Achimsky, L.; Audouin, L.; Verdu, J.; Rychlá, L.; Rychlý, J. The effect of oxygen pressure on the rate of polypropylene oxidation determined by chemiluminescence. Eur. Polym. J. 1978, 35, 557–563. [Google Scholar] [CrossRef]
- Rivaton, A.; Cambon, S.; Gardette, J.L. Radiochemical ageing of EPDM elastomers. 3. Mechanism of radiooxidation. Nucl. Instrum. Meth. Phys. Res. 2005, 227, 357–368. [Google Scholar] [CrossRef]
- Zaharescu, T.; Borbath, T.; Borbath, I.; Simion, E.; Mirea, R. Thermal stability of styrene block copolymers for nuclear applications. Radiat. Phys. Chem. 2024, 223, 111828. [Google Scholar] [CrossRef]
- Jiang, X.; Yuan, X.; Guo, X.; Zeng, F.; Wang, H.; Liu, G. Study on the application of Flory-Huggins interaction parameters in swelling behavior and crosslink density of HNBR/EPDM blend. Fluid Phase Equilibria 2023, 563, 113589. [Google Scholar] [CrossRef]
- Rychlý, J.; Rychlá, L.; Novák, I.; Vanko, V.; Prečo, J.; Janigová, I.; Chodák, I. Thermooxidative stability of hot melt adhesives based on metallocene polyolefins grafted with polar acrylic acid moieties. Polym. Test. 2020, 85, 106422. [Google Scholar]
- Vernáez, O.; Dagreou, S.; Grassl, B.; Müller, A.J. Degradation of styrene butadiene rubber (SBR) in anaerobic conditions. Polym. Degrad. Stab. 2015, 111, 159–168. [Google Scholar] [CrossRef]
- Muraleedharan Nair, T.; Kumaran, M.G.; Unnikrishnan, G.; Kunchandy, S. Ageing studies of ethylene propylene diene monomer rubber/styrene-butadiene rubber blends: Effects of heat, ozone, gamma radiation, and water. J. Appl. Polym. Sci. 2008, 107, 2923–2929. [Google Scholar] [CrossRef]
- Zaharescu, T. Synergistic effect of silica nanoparticles assisted by rosemary powder in the stabilization of styrene-isoprene-styrene triblock copolymer. Radiat. Phys. Chem. 2023, 206, 110765. [Google Scholar] [CrossRef]
- Zaghdoudi, M.; Kömmling, A.; Jaunich, M.; Wolff, D. Understanding the recovery behavior and the degradative processes of EPDM during aging. Polym. Test. 2023, 121, 107987. [Google Scholar] [CrossRef]
- Bumbac, M.; Zaharescu, T.; Nicolescu, C.M.; Borbath, T.; Borbath, I. Chemiluminescence-based evaluation of styrene block copolymers’ recyclability. Sci. Rep. 2024, 14, 28221. [Google Scholar] [CrossRef]
- Ashokrao Fuke, C.; Anna Mahanwar, P.; Chowdhury, S.R. Modified ethylene-propylene-diene elastomer (EPDM)-contained silicone rubber/ethylene-propylene-diene elastomer (EPDM) blends: Effect of composition and electron beam crosslinking on mechanical, heat shrinkability, electrical, and morphological properties. J. Appl. Polym. Sci. 2019, 136, 47787. [Google Scholar] [CrossRef]
- Wang, X.; Huang, C.; Wang, X.; Luo, Y.; Wang, F. Multiscale simulation study on radiation aging of EPDM and preparation of radiation-resistant materials. Compos. Sci. Technol. 2024, 252, 110595. [Google Scholar] [CrossRef]
- Verdu, J.; Colin, X.; Audouin, L.; Rychly, J.; Matisov-Rychlá, L. Chemiluminescence from the thermal oxidation of polyisoprene and polybutadiene I. Influence of oxygen pressure on the chemiluminescence of polyisoprene during its oxidation. Polym. Degrad. Stab. 2006, 91, 1387–1394. [Google Scholar] [CrossRef]
- Dubey, K.A.; Bhardwaj, Y.K.; Chaudhari, C.V.; Bhattacharya, S.; Gupta, S.K.; Sabharwal, S. Radiation effects on SBR-EPDM blends: A correlation with blend morphology. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1676–1689. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Cheng, K. Effect of crosslink density on some properties of electron beam-irradiated styrene-butadiene rubber. Radiat. Phys. Chem. 2009, 78, 1001–1005. [Google Scholar] [CrossRef]
- Balaji, A.B.; Ratnam, C.T.; Khalid, M.; Walvekar, R. Effect of electron beam irradiation on thermal and crystallization behavior of PP/EPDM blend. Radiat. Phys. Chem. 2017, 141, 179–189. [Google Scholar] [CrossRef]
- Dubey, K.A.; Bhardwaj, Y.K.; Chaudhari, C.V.; Sabharwal, S. Radiation-processed styrene-butadiene-co-ethylene-propylene diene rubber blends: Compatibility and swelling studies. J. Appl. Polym. Sci. 2006, 99, 3638–3649. [Google Scholar] [CrossRef]
- Charlesby, A.; Pinner, S.H. Analysis of the solubility behaviour of irradiated polyethylene and other polymers. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1959, 249, 367–386. [Google Scholar]
- Zhan, K.; Elder, T.; Peng, Y. Enhancing Polypropylene/Polyethylene Blend Performance Through Compatibilization for a Sustainable Future: A Mini Review Focusing on Establishing Bio-Derived Filler Based Hybrid Compatibilizer System. Macromol. Rapid Commun. 2024, 2400724. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Kaptan, H.; Güven, O. Effect of γ-irradiation dose for the oxygen diffusion into polymers. J. Appl. Polym. Sci. 1997, 64, 1291–1294. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, A.; Zhou, T.; Cao, X.; Xie, Z. A study on thermal oxidation mechanism of styrene-butadiene-styrene block copolymer (SBS). Polym. Degrad. Stab. 2007, 92, 1682–1691. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Li, Y.; Xie, L.; Sheng, K. Radiation effects on styrene-butadiene-styrene copolymer. Nucl. Instrum. Meth. Phys. Res. 2008, 266, 3134–3136. [Google Scholar] [CrossRef]
- De Almeida, A.; Chazeau, L.; Vigier, G.; Marque, G.; Goutille, Y. Ultimate and toughness properties of γ-irradiated EPDM. Eur. Polym. J. 2017, 97, 178–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Zhao, S.; Xie, L.; Sheng, K. Compatibility effect of radiation-grafting-functionalized styrene-butadiene-styrene on polyamide 6/styrene-butadiene-styrene blends. J. Appl. Polym. Sci. 2008, 108, 1029. [Google Scholar] [CrossRef]
- Khatiwada, S.P.; Gohs, U.; Janke, A.; Jehnichen, D.; Heinrich, G.; Adhikari, R. Influence of electron beam irradiation on the morphology and mechanical properties of styrene/butadiene triblock copolymers. Radiat. Phys. Chem. 2018, 152, 56–62. [Google Scholar] [CrossRef]
- Abdel-Hakim, A.; El-Gamal, A.A.; El-Zayat, M.M.; Sade, A.M. Effect of novel sucrose-based polyfunctional monomer on physico-mechanical and electrical properties of irradiated EPDM. Radiat. Phys. Chem. 2021, 189, 109729. [Google Scholar] [CrossRef]
- Planes, E.; Chazeau, L.; Vigier, G.; Fournier, J. Evolution of EPDM networks aged by gamma irradiation—Consequences on the mechanical properties. Polymer 2009, 50, 4028–4038. [Google Scholar] [CrossRef]
- Bumbac, M.; Nicolescu, C.M.; Zaharescu, T.; Gurgu, I.V.; Bumbac, C.; Manea, E.E.; Ionescu, I.A.; Serban, B.C.; Buiu, O.; Dumitrescu, C. Influence of biogenic material content on the biodegradability of styrene-butadiene composites with incorporated Chlorella vulgaris biomass. Polymers 2024, 16, 1241. [Google Scholar] [CrossRef]
- Smith, B. The infrared spectra of polymers III: Hydrocarbon polymers. Spectroscopy 2021, 36, 22–25. [Google Scholar] [CrossRef]
- Bumbac, M.; Nicolescu, C.M.; Zaharescu, T.; Gurgu, I.V.; Bumbac, C.; Manea, E.E.; Ionescu, I.A.; Serban, B.C.; Buiu, O.; Dumitrescu, C. Biodegradation study of styrene-butadiene composites with incorporated Arthrospira platensis biomass. Polymers 2024, 16, 1218. [Google Scholar] [CrossRef] [PubMed]
- Canto, L.B.; Mantovani, G.L.; Deazevedo, E.R.; Bonagamba, T.J.; Hage, E.; Pessan, L.A. Molecular Characterization of Styrene-Butadiene-Styrene Block Copolymers (SBS) by GPC, NMR, and FTIR. Polym. Bull. 2006, 57, 513–524. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 94–107. ISBN 978-0-470-09307-8. [Google Scholar]
- Buhari, S.; Vinayak, M.V.; Mohan, A.K.; Rhee, K.Y.; Asif, A. Enhancing electrochemical degradation resistance in EPDM/CB composites: A comprehensive approach to achieve optimal physico-mechanical properties for automotive applications. Polym. Eng. Sci. 2024, 64, 3937–3951. [Google Scholar] [CrossRef]
- de Souza, E.L.; Zanzi, M.D.S.; Paiva, K.V.; Oliveira, J.L.; Monteiro, A.S.; Barra, G.M.O.; Dutra, G.B. Evaluation of the aging of elastomeric acrylonitrile-butadiene rubber and ethylene-propylene-diene monomer gaskets used to seal plates heat exchanger. Polym. Eng. Sci. 2021, 61, 3001–3016. [Google Scholar] [CrossRef]
- Antoon, M.K.; Koenig, J.L. Crosslinking mechanism of an anhydride-cured epoxy resin as studied by Fourier transform infrared spectroscopy. J. Polym. Sci. Polym. Chem. 1981, 19, 549–570. [Google Scholar] [CrossRef]
- Blanco, I.; Zaharescu, T. The effect of polyhedral oligomeric silsesquioxanes (POSSs) incorporation in ethylene-propylene-diene-terpolymer (EPDM): A thermal study. J. Therm. Anal. Calorim. 2022, 147, 5313–5321. [Google Scholar] [CrossRef]





| Material | OIT (min) | Correlation Factor | Activation Energy (kJ mol−1) | ||
|---|---|---|---|---|---|
| 160 °C | 170 °C | 180 °C | |||
| E 100 | 98 | 30 | 11 | 0.99940 | 90 |
| E75S25 | 70 | 35 | 10 | 0.98428 | 80 |
| E50S50 | 44 | 18 | 7 | 0.99958 | 76 |
| E25S75 | 48 | 25 | 9 | 0.99025 | 69 |
| S 100 | 38 | 16 | 9 | 0.94750 | 60 |
| Ageing Procedure (Radiation Dose) | OOT (°C) | ||||
|---|---|---|---|---|---|
| E100 | E75S25 | E50S50 | E25S75 | S100 | |
| 0 kGy | 171 | 206 | 211 | 222 | 220 |
| 50 kGy | 162 | 182 | 192 | 212 | 215 |
| 100 kGy | 144 | 165 | 163 | 205 | 201 |
| 150 kGy | 109 | 112 | 135 | 184 | 183 |
| Sample | Dose (kGy) | Gel Fraction (%) | Vr | ρr (g/cm3) | νcross × 10−4 (mol cm−3) | p/q |
|---|---|---|---|---|---|---|
| E50S50 | 50 | 41.81 ± 0.003 | 0.1166 ± 0.003 | 0.9219 ± 0.002 | 0.0291 ± 0.005 | 0.1927 |
| 100 | 70.17 ± 0.067 | 0.1713 ± 0.006 | 0.9427 ± 0.004 | 0.1940 ± 0.027 | ||
| 150 | 85.24 ± 0.013 | 0.2336 ± 0.013 | 0.9450 ± 0.003 | 0.6543 ± 0.133 | ||
| E75S25 | 50 | 75.12 ± 0.016 | 0.1364 ± 0.004 | 0.8699 ± 0.002 | 0.1412 ± 0.015 | 0.1468 |
| 100 | 93.37 ± 0.007 | 0.2966 ± 0.002 | 0.8932 ± 0.011 | 1.8319 ± 0.238 | ||
| 150 | 98.50 ± 0.001 | 0.3366 ± 0.002 | 0.9116 ± 0.002 | 2.7576 ± 0.064 | ||
| E100 | 50 | 98.53 ± 0.001 | 0.2728 ± 0.006 | 0.8878 ± 0.002 | 1.6316 ± 0.105 | 0.084 |
| 100 | 99.27 ± 0.001 | 0.3647 ± 0.002 | 0.8898 ± 0.004 | 4.0706 ± 0.073 | ||
| 150 | 98.98 ± 0.001 | 0.3879 ± 0.001 | 0.8950 ± 0.001 | 4.9850 ± 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharescu, T.; Bumbac, M.; Nicolescu, C.M.; Stelescu, M.D.; Borbath, T.; Borbath, I. Evaluation of γ-Irradiation Effects on EPDM/SBS Blends for Durability and Recycling Potential. Polymers 2025, 17, 1314. https://doi.org/10.3390/polym17101314
Zaharescu T, Bumbac M, Nicolescu CM, Stelescu MD, Borbath T, Borbath I. Evaluation of γ-Irradiation Effects on EPDM/SBS Blends for Durability and Recycling Potential. Polymers. 2025; 17(10):1314. https://doi.org/10.3390/polym17101314
Chicago/Turabian StyleZaharescu, Traian, Marius Bumbac, Cristina Mihaela Nicolescu, Maria Daniela Stelescu, Tunde Borbath, and Istvan Borbath. 2025. "Evaluation of γ-Irradiation Effects on EPDM/SBS Blends for Durability and Recycling Potential" Polymers 17, no. 10: 1314. https://doi.org/10.3390/polym17101314
APA StyleZaharescu, T., Bumbac, M., Nicolescu, C. M., Stelescu, M. D., Borbath, T., & Borbath, I. (2025). Evaluation of γ-Irradiation Effects on EPDM/SBS Blends for Durability and Recycling Potential. Polymers, 17(10), 1314. https://doi.org/10.3390/polym17101314








