Effect of Acid Hydrolysis Conditions on the Extraction of Cellulose Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
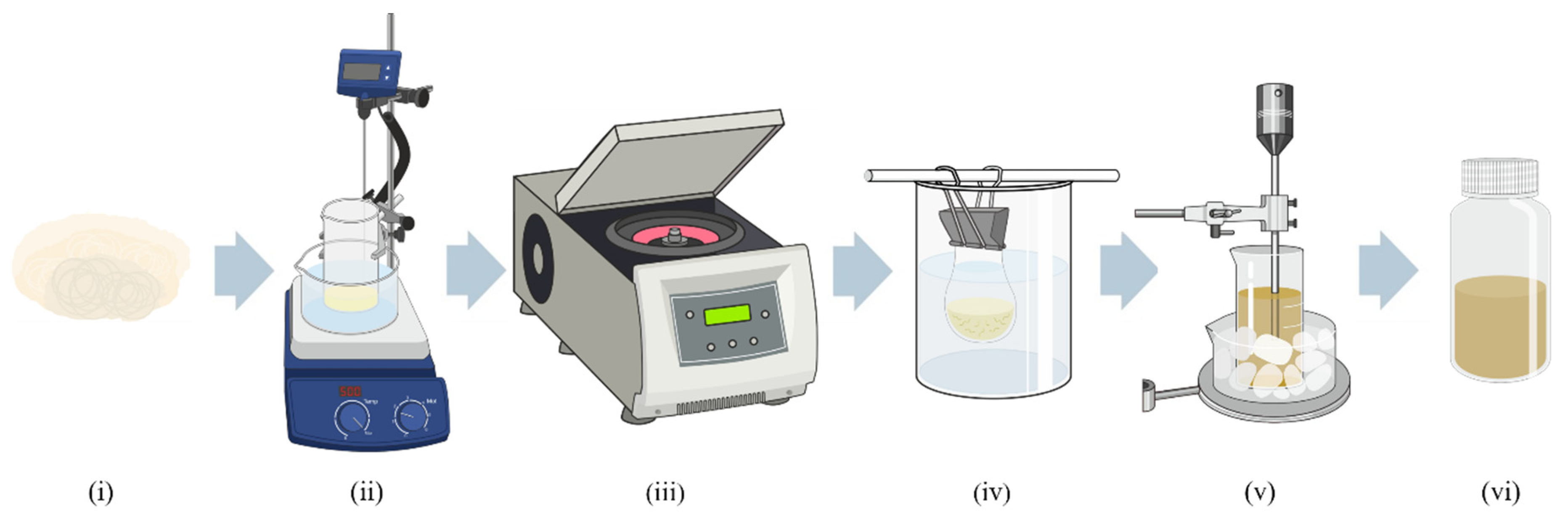
2.2. Extraction of Cellulose Nanocrystals from Rice-Straw-Extracted Cellulose
2.3. Experimental Techniques
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Transmission Electron Microscopy (TEM)
2.3.3. X-Ray Diffraction (XRD)
2.3.4. Zeta Potential
2.3.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, P.R.; Huang, X.; Yang, M.; Sharma, S.K.; Hsiao, B.S. Cellulose Nanofibers for Sustainable Separations. In Sustainable Separation Engineering; Wiley: Hoboken, NJ, USA, 2022; pp. 563–589. [Google Scholar]
- Ghaffar, A.; Salman, M.; Yameen, M.; Iqbal, S.Z.; Altaf, S.; Munir, B. Sustainable Biomedical Applications of Cellulose. In Regenerated Cellulose and Composites; Springer Nature: Cham, Switzerland, 2023; pp. 347–379. [Google Scholar] [CrossRef]
- Kramar, A.; González-Benito, F.J. Cellulose-Based Nanofibers Processing Techniques and Methods Based on Bottom-Up Approach—A Review. Polymers 2022, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Phuong, H.T.; Thoa, N.K.; Tuyet, P.T.A.; Van, Q.N.; Hai, Y.D. Cellulose Nanomaterials as a Future, Sustainable and Renewable Material. Crystals 2022, 12, 106. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kamalesh, T.; Kumar, P.S.; Hemavathy, R.V.; Rangasamy, G. A Critical Review on Sustainable Cellulose Materials and Its Multifaceted Applications. Ind. Crops Prod. 2023, 203, 117221. [Google Scholar] [CrossRef]
- Wasim, M.; Shi, F.; Liu, J.; Khan, M.R.; Farooq, A.; Sanbhal, N.; Alfred, M.; Xin, L.; Yajun, C.; Zhao, X. Extraction of Cellulose to Progress in Cellulosic Nanocomposites for Their Potential Applications in Supercapacitors and Energy Storage Devices. J. Mater. Sci. 2021, 56, 14448–14486. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Abdelaziz, A.; Bessa, W.; Hussin, M.H.; Brosse, N.; Thakur, V.K. Cellulose Nanofibrils–Graphene Hybrids: Recent Advances in Fabrication, Properties, and Applications. Nanoscale 2022, 14, 12515–12546. [Google Scholar] [CrossRef]
- Jenol, M.A.; Norrrahim, M.N.F.; Nurazzi, N.M. Nanocellulose Nanocomposites in Textiles. In Industrial Applications of Nanocellulose and Its Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2022; pp. 397–408. [Google Scholar]
- Gabriel, T.; Belete, A.; Hause, G.; Neubert, R.H.H.; Gebre-Mariam, T. Isolation and Characterization of Cellulose Nanocrystals from Different Lignocellulosic Residues: A Comparative Study. J. Polym. Environ. 2021, 29, 2964–2977. [Google Scholar] [CrossRef]
- Ma, L.; Xu, Y.; Chen, J.; Dong, C.; Pang, Z. Preparation of Cellulose Nanocrystals by Synergistic Action of Ionic Liquid and Recyclable Solid Acid under Mild Conditions. Molecules 2023, 28, 3070. [Google Scholar] [CrossRef]
- Rovera, C.; Carullo, D.; Bellesia, T.; Büyüktaş, D.; Ghaani, M.; Caneva, E.; Farris, S. Extraction of High-Quality Grade Cellulose and Cellulose Nanocrystals from Different Lignocellulosic Agri-Food Wastes. Front. Sustain. Food Syst. 2023, 6, 1087867. [Google Scholar] [CrossRef]
- Anusiya, G.; Jaiganesh, R. A Review on Fabrication Methods of Nanofibers and a Special Focus on Application of Cellulose Nanofibers. Carbohydr. Polym. Technol. Appl. 2022, 4, 100262. [Google Scholar] [CrossRef]
- Nasution, H.; Yahya, E.B.; Abdul Khalil, H.P.S.; Shaah, M.A.; Suriani, A.B.; Mohamed, A.; Alfatah, T.; Abdullah, C.K. Extraction and Isolation of Cellulose Nanofibers from Carpet Wastes Using Supercritical Carbon Dioxide Approach. Polymers 2022, 14, 326. [Google Scholar] [CrossRef] [PubMed]
- Kerwald, J.; de Moura Junior, C.F.; Freitas, E.D.; de Moraes Segundo, J.d.D.P.; Vieira, R.S.; Beppu, M.M. Cellulose-Based Electrospun Nanofibers: A Review. Cellulose 2022, 29, 25–54. [Google Scholar] [CrossRef]
- Djafari Petroudy, S.R.; Chabot, B.; Loranger, E.; Naebe, M.; Shojaeiarani, J.; Gharehkhani, S.; Ahvazi, B.; Hu, J.; Thomas, S. Recent Advances in Cellulose Nanofibers Preparation through Energy-Efficient Approaches: A Review. Energies 2021, 14, 6792. [Google Scholar] [CrossRef]
- Mohomane, S.M.; Motloung, S.V.; Koao, L.F.; Motaung, T.E. Effects of acid hydrolysis on the extraction of cellulose nanocrystals (cncs): A review. Cellul. Chem. Technol. 2022, 56, 691–703. [Google Scholar] [CrossRef]
- Samarawickrama, K.G.R.; Wijayapala, U.G.S.; Fernando, C.A.N. Extraction and Analysis of Cellulose Nanocrystals from Cotton Balls by Acid Hydrolysis. J. Sci. Univ. Kelaniya 2023, 16, 23–29. [Google Scholar] [CrossRef]
- Hernández Pérez, R.; Salgado Delgado, R.; Olarte Paredes, A.; Salgado Delgado, A.; García Hernández, E.; Medrano Valis, A.; Martínez Candia, F. Comparing Acid and Enzymatic Hydrolysis Methods for Cellulose Nanocrystals (CNCs) Obtention from Agroindustrial Rice Husk Waste. J. Nanotechnol. 2022, 2022, 5882113. [Google Scholar] [CrossRef]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Jordan, J.H.; Easson, M.W.; Condon, B.D. Cellulose Hydrolysis Using Ionic Liquids and Inorganic Acids under Dilute Conditions: Morphological Comparison of Nanocellulose. RSC Adv. 2020, 10, 39413–39424. [Google Scholar] [CrossRef]
- Pantamanatsopa, P.; Ariyawiriyanan, W.; Ekgasit, S. Production of Cellulose Nanocrystals Suspension with High Yields from Water Hyacinth. J. Nat. Fibers 2023, 20, 2134266. [Google Scholar] [CrossRef]
- Serrano-Martínez, V.M.; Pérez-Aguilar, H.; Carbonell-Blasco, M.P.; Arán-Ais, F.; Orgilés-Calpena, E. Steam Explosion-Based Method for the Extraction of Cellulose and Lignin from Rice Straw Waste. Appl. Sci. 2024, 14, 2059. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C. Montgomery Response Surface Methods and Designs. In Design and Analysis of Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 478–553. [Google Scholar]
- Purwanti, E.; Dampang, S. Pengaruh Perbedaan Kondisi Hidrolisis Terhadap Hasil Isolasi Nanokristalin Selulosa Dari Bonggol Jagung. Indo. J. Chem. Res. 2017, 5, 12–16. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Putri, E.; Gea, S. Isolasi Dan Karakterisasi Nanokistral Selulosa Dari Tandan Sawit (Elaeis Guineensis Jack). Elkawnie 2018, 4, 13–22. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and Application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Wickaramasinghe, W.A.W.I.C.; Lasitha, D.S.; Samarasekara, A.M.P.B.; Amarasinghe, D.A.S.; Karunanayake, L. Extraction and Characterization of Nano Crystalline Cellulose (NCC) From Sri Lankan Agricultural Waste. In Proceedings of the 2019 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 3–5 July 2019; IEEE: Piscataway, NJ, USA; pp. 616–620. [Google Scholar]
- Henrique, M.A.; Flauzino Neto, W.P.; Silvério, H.A.; Martins, D.F.; Gurgel, L.V.A.; Barud, H.d.S.; de Morais, L.C.; Pasquini, D. Kinetic Study of the Thermal Decomposition of Cellulose Nanocrystals with Different Polymorphs, Cellulose I and II, Extracted from Different Sources and Using Different Types of Acids. Ind. Crops Prod. 2015, 76, 128–140. [Google Scholar] [CrossRef]
- Dias, Y.J.; Silva, V.D.; Pourdeyhimi, B.; Medeiros, E.S.; Yarin, A.L. Freestanding Carbon Nanofibers Derived from Biopolymer (Kraft Lignin) as Ultra-Microporous Electrodes for Supercapacitors. Batteries 2023, 9, 566. [Google Scholar] [CrossRef]
- Ashikbayeva, Z.; Aitkulov, A.; Jelbuldina, M.; Issatayeva, A.; Beisenova, A.; Molardi, C.; Saccomandi, P.; Blanc, W.; Inglezakis, V.J.; Tosi, D. Distributed 2D Temperature Sensing during Nanoparticles Assisted Laser Ablation by Means of High-Scattering Fiber Sensors. Sci. Rep. 2020, 10, 12593. [Google Scholar] [CrossRef]
- Carbonell-Blasco, P.; Martín-Martínez, J.M.; Antoniac, I.V. Synthesis and Characterization of Polyurethane Sealants Containing Rosin Intended for Sealing Defect in Annulus for Disc Regeneration. Int. J. Adhes. Adhes. 2013, 42, 11–20. [Google Scholar] [CrossRef]
- Khan, A.; Toufiq, A.M.; Tariq, F.; Khan, Y.; Hussain, R.; Akhtar, N.; Rahman, S. ur Influence of Fe Doping on the Structural, Optical and Thermal Properties of α-MnO2 Nanowires. Mater. Res. Express 2019, 6, 065043. [Google Scholar] [CrossRef]
- Vinila, V.S.; Isac, J. Synthesis and Structural Studies of Superconducting Perovskite GdBa2Ca3Cu4O10.5+δ Nanosystems. In Design, Fabrication, and Characterization of Multifunctional Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 319–341. [Google Scholar]
- Abou-Sekkina, M.M.; Sakran, M.A.; Saafan, A.A. Development of Correlations among the Spectral, Structural, and Electrical Properties and Chemical Treatment of Egyptian Cotton Fabric Strips. Ind. Eng. Chem. Prod. Res. Dev. 1986, 25, 676–680. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Statgraphics Technologies STATGRAPHICS® Centurion XVII Manual de Usuario. Statgraphics Technologies: The Plains, VA, USA, 2015. Available online: https://www.statgraphics.net/wp-content/uploads/2015/03/Centurion-XVII-Manual-Principal.pdf (accessed on 7 May 2025).
- Douglas, C. Montgomery. In Design and Analysis of Experiments, 10th ed.; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-49244-3. [Google Scholar]
- Batanero, C.; Díaz Batanero, M.C. Análisis de Datos Con Statgraphics; Departamento de Didáctica de la Matemática, Universidad de Granada: Granada, Spain, 2008; ISBN 9788469147962. [Google Scholar]
- Leenen, I. La Prueba de La Hipótesis Nula y Sus Alternativas: Revisión de Algunas Críticas y Su Relevancia Para Las Ciencias Médicas. Investig. En Educ. Medica 2012, 1, 225–234. [Google Scholar] [CrossRef]
- Feng, Y.; Kilker, S.R.; Lee, Y. Surface Charge (Zeta-Potential) of Nanoencapsulated Food Ingredients. In Characterization of Nanoencapsulated Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2020; pp. 213–241. [Google Scholar]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Alhaji Mohammed, M.; Basirun, W.J.; Abd Rahman, N.M.M.; Shalauddin, M.; Salleh, N.M. The Effect of Acid Hydrolysis Parameters on the Properties of Nanocellulose Extracted from Almond Shells. J. Nat. Fibers 2022, 19, 14102–14114. [Google Scholar] [CrossRef]
- Ioelovich, M. Peculiarities of Cellulose Nanoparticles. Tappi J. 2014, 13, 45–51. [Google Scholar] [CrossRef]
- Kanchanalai, P.; Temani, G.; Kawajiri, Y.; Realff, M.J. Reaction Kinetics of Concentrated-Acid Hydrolysis for Cellulose and Hemicellulose and Effect of Crystallinity. Bioresources 2016, 11, 1672–1689. [Google Scholar] [CrossRef]
- Jannah, A.N.; Fuadi, A.M. Effect of Hydrolysis Time and Sulfuric Acid Concentration on Reducing Sugar Content on Corn Cob Hydrolysis. Chem. J. Tek. Kim. 2022, 9, 10. [Google Scholar] [CrossRef]
- Rana, M.S.; Rahim, M.A.; Mosharraf, M.P.; Tipu, M.F.K.; Chowdhury, J.A.; Haque, M.R.; Kabir, S.; Amran, M.S.; Chowdhury, A.A. Morphological, Spectroscopic and Thermal Analysis of Cellulose Nanocrystals Extracted from Waste Jute Fiber by Acid Hydrolysis. Polymers 2023, 15, 1530. [Google Scholar] [CrossRef]
- Zhao, G.; Du, J.; Chen, W.; Pan, M.; Chen, D. Preparation and Thermostability of Cellulose Nanocrystals and Nanofibrils from Two Sources of Biomass: Rice Straw and Poplar Wood. Cellulose 2019, 26, 8625–8643. [Google Scholar] [CrossRef]
- Sharma, N.; Allardyce, B.J.; Rajkhowa, R.; Agrawal, R. Rice Straw-Derived Cellulose: A Comparative Study of Various Pre-Treatment Technologies and Its Conversion to Nanofibres. Sci. Rep. 2023, 13, 16327. [Google Scholar] [CrossRef] [PubMed]
- Whba, F.; Mohamed, F.; Idris, M.I.; Yahya, M.S. Characterization of Cellulose Nanocrystals (CNCs) Derived from Microcrystalline Cellulose (MCC) Synthesized Using Acid Hydrolysis Method. 2022. Available online: https://www.researchsquare.com/article/rs-2078344/v2 (accessed on 7 May 2025).
- Sarı, B.; Kaynak, C. Obtaining Cellulose Nanocrystals by Acid Hydrolysis Procedure; and Their Use as Reinforcement in Polylactide Biocomposites. J. Thermoplast. Compos. Mater. 2024, 38, 1040–1062. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley: Hoboken, NJ, USA, 2017; pp. 1–49. [Google Scholar]




| Sample | Temperature (°C) | Time (min) | H2SO4 50% (mL) |
|---|---|---|---|
| PE1 | 39 | 60 | 50.0 |
| PE2 | 60 | 60 | 85.4 |
| PE3 | 60 | 60 | 50.0 |
| PE4 | 75 | 80 | 25.0 |
| PE5 | 60 | 60 | 50.0 |
| PE6 | 45 | 40 | 25.0 |
| PE7 | 75 | 80 | 75.0 |
| PE8 | 45 | 80 | 75.0 |
| PE9 | 75 | 40 | 75.0 |
| PE10 | 60 | 60 | 50.0 |
| PE11 | 45 | 80 | 25.0 |
| PE12 | 75 | 40 | 25.0 |
| PE13 | 60 | 60 | 14.7 |
| PE14 | 60 | 60 | 50.0 |
| PE15 | 60 | 88 | 50.0 |
| PE16 | 60 | 32 | 50.0 |
| PE17 | 45 | 40 | 75.0 |
| PE18 | 81 | 60 | 50.0 |
| Sample | Z Potential (mV) |
|---|---|
| PE3 | −29.0 |
| PE5 | −34.4 |
| PE7 | −33.4 |
| PE9 | −37.4 |
| PE18 | −37.3 |
| Sample | Crystallinity Index (%) | Crystallite Size (nm) |
|---|---|---|
| PE3 | 50.2 | 1.2 |
| PE5 | 51.0 | 1.7 |
| PE7 | 55.4 | 1.7 |
| PE9 | 51.3 | 1.5 |
| PE18 | 51.6 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Martínez, V.M.; Pérez-Aguilar, H.; Carbonell-Blasco, M.P.; García-García, A.; Arán-Ais, F.; Orgilés-Calpena, E. Effect of Acid Hydrolysis Conditions on the Extraction of Cellulose Nanocrystals. Polymers 2025, 17, 1313. https://doi.org/10.3390/polym17101313
Serrano-Martínez VM, Pérez-Aguilar H, Carbonell-Blasco MP, García-García A, Arán-Ais F, Orgilés-Calpena E. Effect of Acid Hydrolysis Conditions on the Extraction of Cellulose Nanocrystals. Polymers. 2025; 17(10):1313. https://doi.org/10.3390/polym17101313
Chicago/Turabian StyleSerrano-Martínez, Víctor M., Henoc Pérez-Aguilar, María Pilar Carbonell-Blasco, Avelina García-García, Francisca Arán-Ais, and Elena Orgilés-Calpena. 2025. "Effect of Acid Hydrolysis Conditions on the Extraction of Cellulose Nanocrystals" Polymers 17, no. 10: 1313. https://doi.org/10.3390/polym17101313
APA StyleSerrano-Martínez, V. M., Pérez-Aguilar, H., Carbonell-Blasco, M. P., García-García, A., Arán-Ais, F., & Orgilés-Calpena, E. (2025). Effect of Acid Hydrolysis Conditions on the Extraction of Cellulose Nanocrystals. Polymers, 17(10), 1313. https://doi.org/10.3390/polym17101313








