Silicon-Containing π-Conjugated Schiff Base Oligomers with Naphthalene or Binaphthalene Moieties in the Backbone: Synthesis and Study of Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Precursors and Monomers
2.3. Synthesis of Oligo-SBs
2.4. Measurements
2.5. DFT Simulations
3. Results and Discussion
3.1. Synthesis and Characterization of Monomers
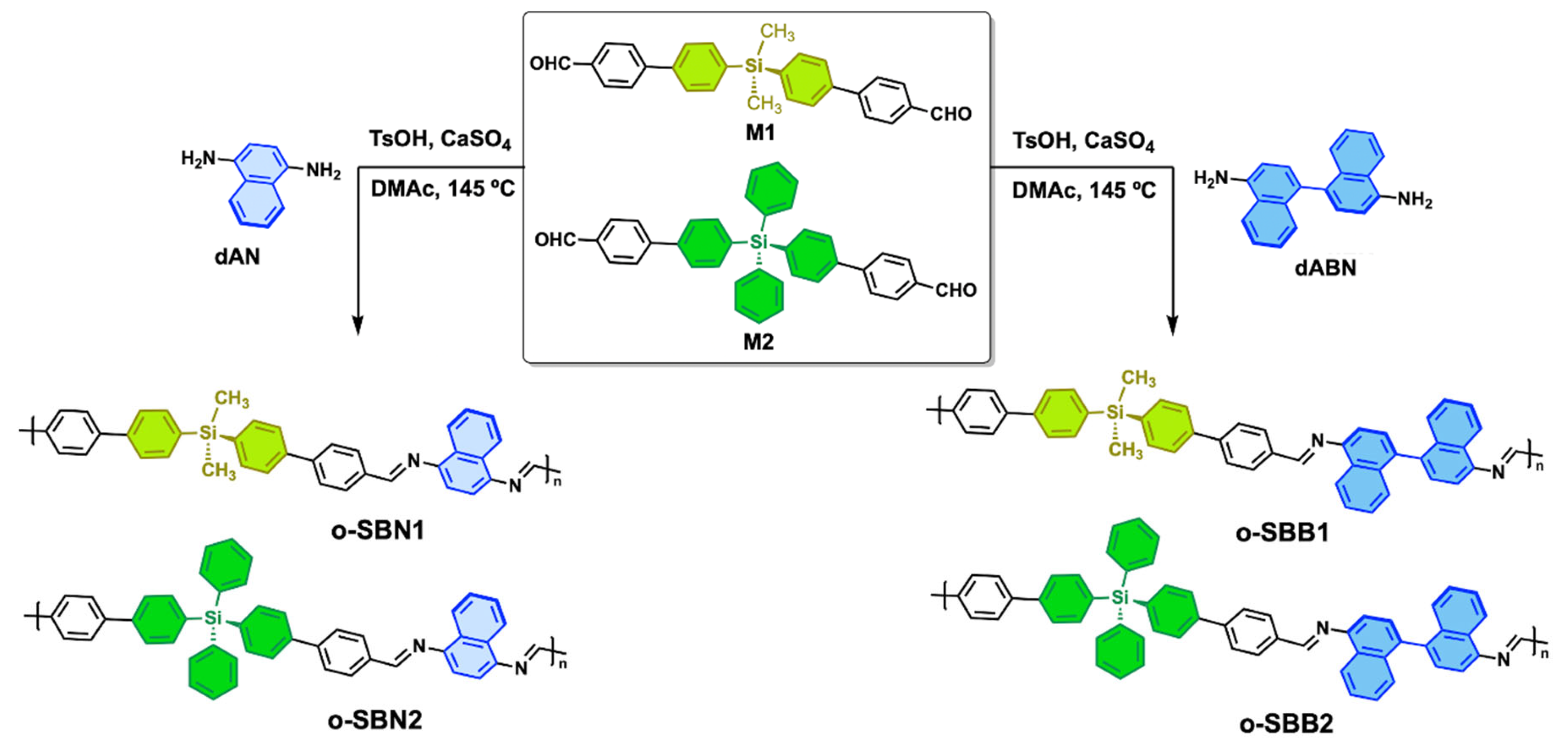
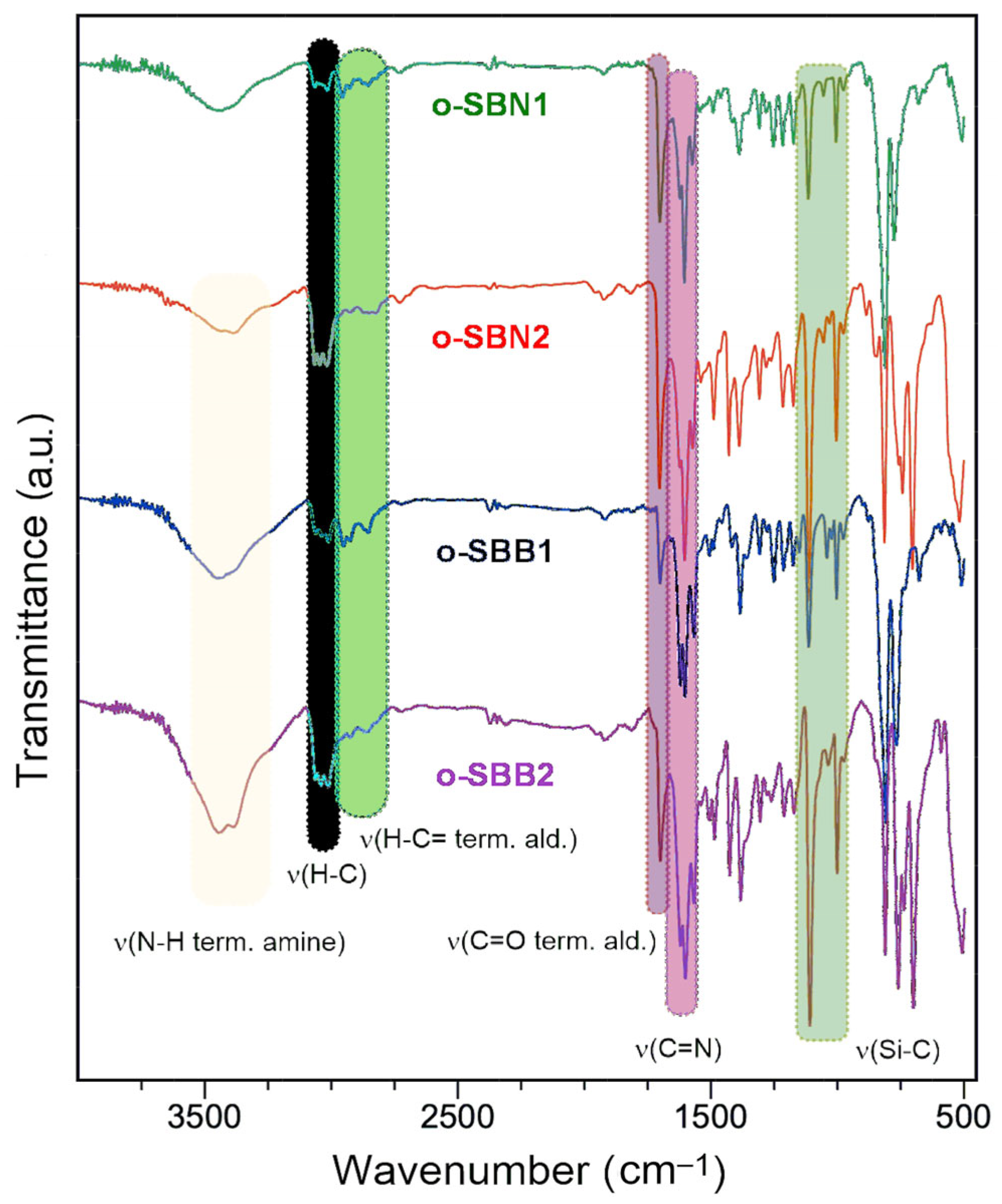
3.2. Synthesis and Characterization of Oligo-SBs
3.3. Solubility, Molecular Weights and Thermal Properties of Oligo-SBs
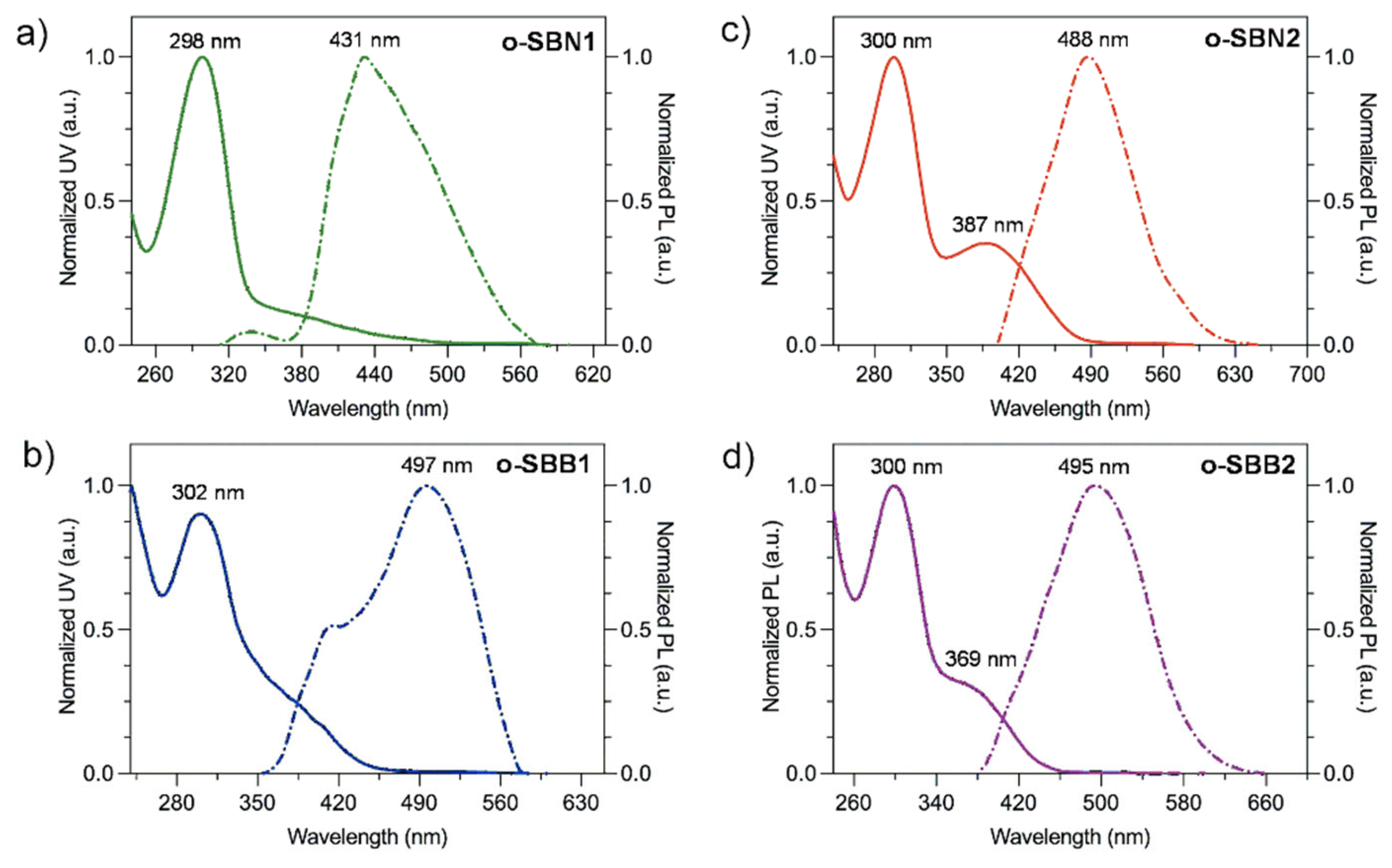
3.4. Optical and Electronic Properties of Oligo-SBs
3.5. Computational Details of Oligo-SBs
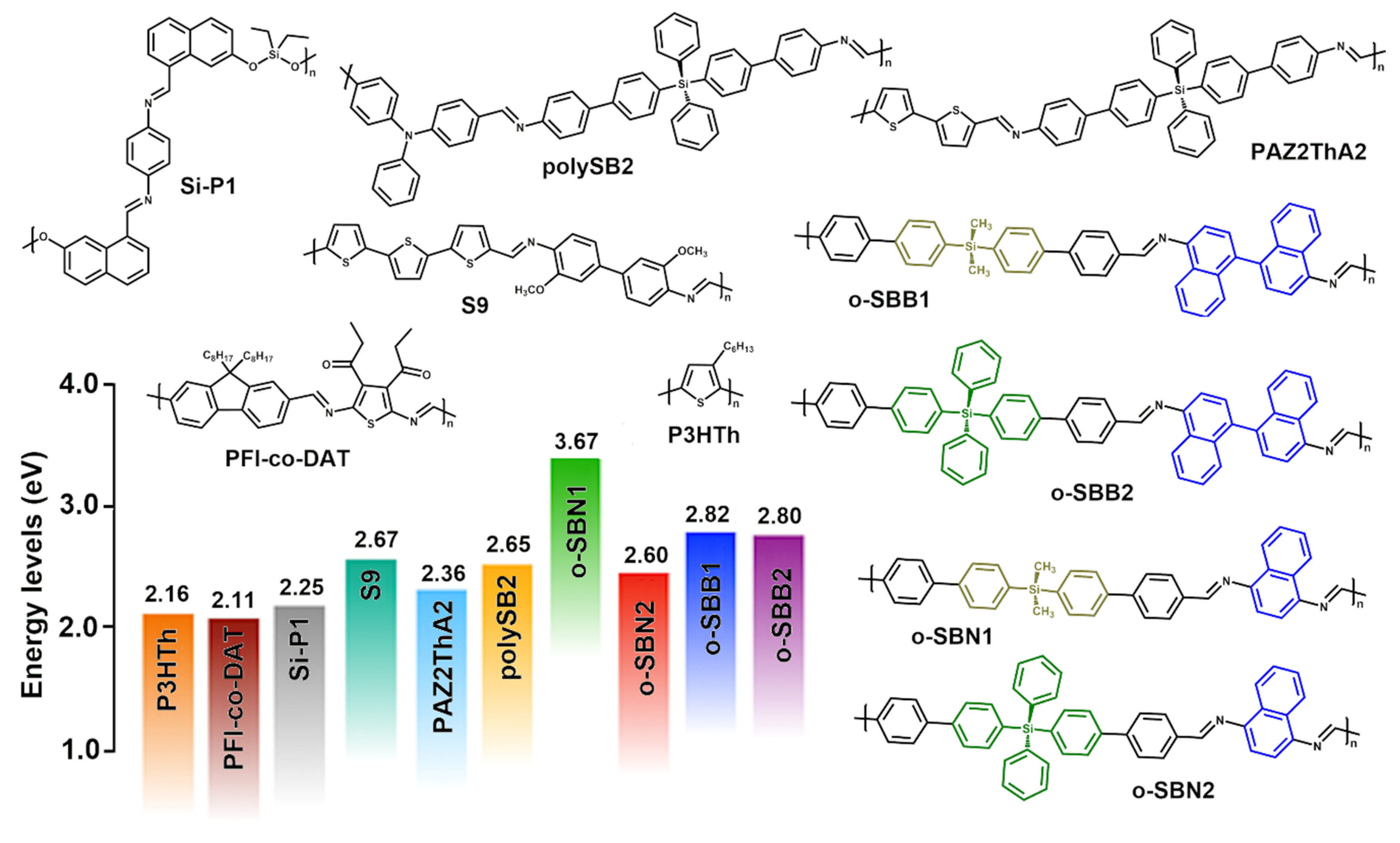
3.6. Comparative Analysis of Oligo-SBs with π-Conjugated Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Fu, W.; Chen, H. Conductive Polymers for Flexible and Stretchable Organic Optoelectronic Applications. ACS Appl. Polym. Mater. 2022, 4, 4609–4623. [Google Scholar] [CrossRef]
- Khelifi, W.; Luscombe, C.K. Recent Developments in Indacenodithiophene and Indacenodithienothiophene-Based Donor-Acceptor Conjugated Polymers: From Design to Device Performance in Organic Electronics. Prog. Polym. Sci. 2024, 151, 101804. [Google Scholar] [CrossRef]
- Yang, C.; Cheng, B.; Xu, J.; Yu, J.; Cao, S. Donor-Acceptor-Based Conjugated Polymers for Photocatalytic Energy Conversion. EnergyChem 2024, 6, 100116. [Google Scholar] [CrossRef]
- Ding, H.; Hussein, A.M.; Ahmad, I.; Latef, R.; Abbas, J.K.; Ali, A.T.A.; Saeed, S.M.; Abdulwahid, A.S.; Ramadan, M.F.; Rasool, H.A.; et al. Conducting Polymers in Industry: A Comprehensive Review on the Characterization, Synthesis and Application. Alex. Eng. J. 2024, 88, 253–267. [Google Scholar] [CrossRef]
- Liu, C.; Shao, L.; Chen, S.; Hu, Z.; Cai, H.; Huang, F. Recent Progress in π-Conjugated Polymers for Organic Photovoltaics: Solar Cells and Photodetectors. Prog. Polym. Sci. 2023, 143, 101711. [Google Scholar] [CrossRef]
- Grankowska Ciechanowicz, S.; Korona, K.P.; Wolos, A.; Drabinska, A.; Iwan, A.; Tazbir, I.; Wojtkiewicz, J.; Kaminska, M. Toward Better Efficiency of Air-Stable Polyazomethine-Based Organic Solar Cells Using Time-Resolved Photoluminescence and Light-Induced Electron Spin Resonance as Verification Methods. J. Phys. Chem. C 2016, 120, 11415–11425. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Tazbir, I.; Malinowski, M.; Filapek, M.; Kłab, T.; Luszczynska, B.; Glowacki, I.; Korona, K.P.; Kaminska, M.; et al. New Environmentally Friendly Polyazomethines with Thiophene Rings for Polymer Solar Cells. Sol. Energy 2015, 117, 246–259. [Google Scholar] [CrossRef]
- Gawlinska, K.; Iwan, A.; Starowicz, Z.; Kulesza-Matlak, G.; Stan-Glowinska, K.; Janusz, M.; Lipinski, M.; Boharewicz, B.; Tazbir, I.; Sikora, A. Searching of New, Cheap, Air- and Thermally Stable Hole Transporting Materials for Perovskite Solar Cells. Opto-Electron. Rev. 2017, 25, 274–284. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, C.; Xiao, L.; Wei, Q.; Yin, Q.; He, Y.; Song, S.; Qiang, R.; Yang, Y.; Li, Z.; et al. Synthesis and Capability Evaluation of Quinone-Enriched Polymer with Extended π-Conjugated and Contorted Structures for Efficient Energy Storage. Electrochim. Acta 2024, 476, 143693. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Gwag, J.S.; Nguyen-Dinh, L.; Truong, H.B.; Do, H.H.; Lee, Y.-C.; Tran, N.T.; Trung, L.G. Heterostructural Covalent Organic Framework/Polymer Composite Materials: Recent Advances in Multidisciplinary Applications. Nano Today 2024, 55, 102211. [Google Scholar] [CrossRef]
- Sobarzo, P.A.; Terraza, C.A.; Maya, E.M. New Efficient Tetraphenyl Silylated Poly(Azomethine)s Based on “Pincer-like” Bis(Imino)Pyridine Iron(III) Complexes as Heterogeneous Catalysts for CO2 Conversion. Eur. Polym. J. 2020, 126, 109567. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Design and Preparation of Triphenylamine-Based Polymeric Materials towards Emergent Optoelectronic Applications. Prog. Polym. Sci. 2019, 89, 250–287. [Google Scholar] [CrossRef]
- González, A.F.; Mariman, A.P.; Hauyon, R.A.; Pavez-Lizana, D.; Saldías, C.; Schott, E.; Zarate, X.; Garcia, L.; González-Henríquez, C.M.; Jessop, I.A.; et al. Thiophene- and Bithiophene-Based π-Conjugated Schiff Base Oligomers Containing Binaphthalene Moieties in the Backbone. Properties and Computational Simulations. Polym. Chem. 2024, 15, 639–651. [Google Scholar] [CrossRef]
- Abdellah, I.M.; Yildirim, E.; El-Shafei, A. Low-Cost Novel X-Shaped Hole Transport Materials for Efficient Perovskite Solar Cells: Molecular Modelling of the Core and Schiff Base Effects on Photovoltaic and Photophysical Properties. Mater. Chem. Phys. 2023, 296, 127188. [Google Scholar] [CrossRef]
- Hayat, A.; Raza, S.; Amin, M.A.; Ajmal, Z.; Alghamdi, M.M.; El-Zahhar, A.A.; Ali, H.; Ghernaout, D.; Al-Hadeethi, Y.; Sohail, M.; et al. Developing New-Generation Covalent Organic Frameworks as Sustainable Catalysts: Synthesis, Properties, Types and Solar Energy Production. Mat. Sci. Eng. R Rep. 2024, 157, 100771. [Google Scholar] [CrossRef]
- Marin, L.; Bejan, A.; Ailincai, D.; Belei, D. Poly(Azomethine-Phenothiazine)s with Efficient Emission in Solid State. Eur. Polym. J. 2017, 95, 127–137. [Google Scholar] [CrossRef]
- Gao, X.; Qin, Z. Facile Synthesis of near White Light Emitting Polymeric Nanoparticles Based on Schiff-Base Networks. Opt. Mater. 2021, 117, 111151. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, F.; Ming, S.; Zhang, Y.; Zhao, J. Construction of Two-Dimensional Porous Polymers with Crystalline or Amorphous Forms for near-Infrared Electrochromism. Eur. Polym. J. 2024, 208, 112853. [Google Scholar] [CrossRef]
- Saravanan, C.; Ashwin, B.C.M.A.; Manoj, S.; Senthilkumaran, M.; Sethuraman, V.; Balaji Bhargav, P.; Ramasamy, P.; Muthu Mareeswaran, P. Schiff Base Covalent Organic Polymers as Electrode Material for Supercapacitor Application. Mater. Lett. 2022, 320, 132348. [Google Scholar] [CrossRef]
- Hung, M.K.; Tsai, K.W.; Sharma, S.; Lei, J.; Wu, J.Y.; Chen, S.A. Optoelectronic Properties of High Triplet σ-π-Conjugated Poly[(Biphenyl Group IV-A Atom (C, Si, Ge, Sn)] Backbones. ACS Appl. Mater. Interfaces 2019, 11, 36895–36904. [Google Scholar] [CrossRef]
- Li, X.; Bai, Q.; Li, J.; Lu, F.; Sun, X.; Lu, P. Synthesis and Properties of Wide Bandgap Polymers Based on Tetraphenylsilane and Their Applications as Hosts in Electrophosphorescent Devices. New J. Chem. 2018, 42, 3344–3349. [Google Scholar] [CrossRef]
- He, M.; Hu, J.; Yan, H.; Zhong, X.; Zhang, Y.; Liu, P.; Kong, J.; Gu, J. Shape Anisotropic Chain-Like CoNi/Polydimethylsiloxane Composite Films with Excellent Low-Frequency Microwave Absorption and High Thermal Conductivity. Adv. Funct. Mater. 2025, 35, 2316691. [Google Scholar] [CrossRef]
- He, M.; Zhong, X.; Lu, X.; Hu, J.; Ruan, K.; Guo, H.; Zhang, Y.; Guo, Y.; Gu, J. Excellent Low-Frequency Microwave Absorption and High Thermal Conductivity in Polydimethylsiloxane Composites Endowed by Hydrangea-Like CoNi@BN Heterostructure Fillers. Adv. Mater. 2024, 36, 2410186. [Google Scholar] [CrossRef]
- Zhan, H.; Cheng, W.; Liu, F.; Yang, C.; Wang, Y.; Yan, K.; Liu, K.; Wang, D.; Wang, W. Resilient and Robust Mechanoresponsive Polydimethylsiloxane/SiO2 Composites Induced by Interfacial Enhancement. J. Colloid Interface Sci. 2025, 690, 137361. [Google Scholar] [CrossRef]
- Yeh, H.C.; Chien, C.H.; Shih, P.I.; Yuan, M.C.; Shu, C.F. Polymers Derived from 3,6-Fluorene and Tetraphenylsilane Derivatives: Solution-Processable Host Materials for Green Phosphorescent OLEDs. Macromolecules 2008, 41, 3801–3807. [Google Scholar] [CrossRef]
- Kim, G.W.; Yang, D.R.; Kim, Y.C.; Yang, H.I.; Fan, J.G.; Lee, C.H.; Chai, K.Y.; Kwon, J.H. Di(Biphenyl)Silane and Carbazole Based Bipolar Host Materials for Highly Efficient Blue Phosphorescent OLEDs. Dyes Pigments 2017, 136, 8–16. [Google Scholar] [CrossRef]
- Hauyon, R.A.; Fuentealba, D.; Pizarro, N.; Ortega-Alfaro, M.C.; Ugalde-Saldívar, V.M.; Sobarzo, P.A.; Medina, J.; García, L.; Jessop, I.A.; González-Henríquez, C.M.; et al. New Light-Green Thermally Activated Delayed Fluorescence Polymer Based on Dimethylacridine-Triphenyltriazine Light-Emitting Unit and Tetraphenylsilane Moiety as Non-Conjugated Backbone. Polymers 2023, 15, 67. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Zhan, W.; Dong, J.; Ma, Y.; Li, J.; Li, W.; Zhang, C. Dual Polymer Electrochromic Adaptive Camouflage Devices for Multiple Environments Based on Quaterthiophene Polymer and Optional Ion Storage Layers. Chem. Eng. J. 2024, 483, 149078. [Google Scholar] [CrossRef]
- Bruma, M.; Schulz, B. Silicon-Containing Aromatic Polymers. J. Macromol. Sci. Rev. Macromol. Chem. Phys. 2001, 41, 1–40. [Google Scholar] [CrossRef]
- Yao, C.; Peng, C.; Yang, Y.; Li, L.; Bo, M.; Wang, J. Larger VH (Hole Distribution Volume)/VM (Molecular Volume) Induced Higher Charge Mobility of Group IVA Element-Based Host Materials for Potentially Highly Efficient Blue OLEDs. J. Phys. Chem. C 2018, 122, 22273–22279. [Google Scholar] [CrossRef]
- Cohen, S.; Oesper, R.E. The Preparation of Naphthidine. Ind. Eng. Chem. Anal. Ed. 1936, 8, 306–307. [Google Scholar] [CrossRef]
- Tundidor-Camba, A.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Tagle, L.H.; Hauyón, R.A.; Sobarzo, P.A.; González, A.; Ortiz, P.A.; Maya, E.M.; Terraza, C.A. Silylated Oligomeric Poly(Ether-Azomethine)s from Monomers Containing Biphenyl Moieties: Synthesis and Characterization. RSC Adv. 2018, 8, 1296–1312. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Du, M.; Chen, D.; Ye, K.; Zhang, Z.; Liu, Y.; Wang, Y. A Novel Tetraphenylsilane-Phenanthroimidazole Hybrid Host Material for Highly Efficient Blue Fluorescent, Green and Red Phosphorescent OLEDs. J. Mater. Chem. C Mater. 2015, 3, 4394–4401. [Google Scholar] [CrossRef]
- Sobarzo, P.A.; Jessop, I.A.; Mariman, A.P.; González, A.F.; Saldías, C.; Schott, E.; Zarate, X.; Hauyon, R.A.; Recabarren-Gajardo, G.; González-Henríquez, C.M.; et al. New Thiophene-Based Poly(Azomethine)s Bearing Tetraphenylsilane Moieties along Their Backbone. Optical, Electronic, Thermal Properties and Theoretical Calculations. Eur. Polym. J. 2020, 130, 109658. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Gill, P.M.W.; Johnson, B.G.; Cheeseman, J.R.; Keith, T.A.; Petersson, G.A.; Montgomery, J.A.; Raghavachari, K.; et al. Gaussian 03, Revision c. 02; Gaussian: Wallingford, CT, USA, 2004; Volume 4. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Cossi, M.; Scalmani, G.; Rega, N.; Barone, V. New Developments in the Polarizable Continuum Model for Quantum Mechanical and Classical Calculations on Molecules in Solution. J. Chem. Phys. 2002, 117, 43–54. [Google Scholar] [CrossRef]
- Sobarzo, P.A.; González, A.; Jessop, I.A.; Hauyon, R.A.; Medina, J.; Garcia, L.E.; Zarate, X.; González-Henríquez, C.; Schott, E.; Tundidor-Camba, A.; et al. Tetraphenylsilane-Based Oligo(Azomethine)s Containing 3,4-Ethylenedioxythiophene Units along Their Backbone: Optical, Electronic, Thermal Properties and Computational Simulations. Eur. Polym. J. 2022, 181, 111712. [Google Scholar] [CrossRef]
- Sobarzo, P.A.; Mariman, A.P.; Sánchez, C.O.; Hauyon, R.A.; Rodríguez-González, F.E.; Medina, J.; Jessop, I.A.; Recabarren-Gajardo, G.; Tundidor-Camba, A.; Terraza, C.A. Comparison between Poly(Azomethine)s and Poly(p-Phenylvinylene)s Containing a Di-R-Diphenylsilane (R = Methyl or Phenyl) Moiety. Optical, Electronic and Thermal Properties. Eur. Polym. J. 2021, 159, 110714. [Google Scholar] [CrossRef]
- Sobarzo, P.A.; González, A.F.; Schott, E.; Tagle, L.H.; Tundidor-Camba, A.; González-Henríquez, C.; Jessop, I.A.; Terraza, C.A. New Triphenylamine-Based Oligomeric Schiff Bases Containing Tetraphenylsilane Moieties in the Backbone. Polymers 2019, 11, 216. [Google Scholar] [CrossRef]
- Fei, T.; Cheng, G.; Hu, D.; Lu, P.; Ma, Y. A Wide Band Gap Polymer Derived from 3,6-Carbazole and Tetraphenylsilane as Host for Green and Blue Phosphorescent Complexes. J. Polym. Sci. A Polym. Chem. 2009, 47, 4784–4792. [Google Scholar] [CrossRef]
- Iwan, A.; Sek, D. Processible Polyazomethines and Polyketanils: From Aerospace to Light-Emitting Diodes and Other Advanced Applications. Prog. Polym. Sci. 2008, 33, 289–345. [Google Scholar] [CrossRef]
- Tundidor-Camba, A.; González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Tagle, L.H.; Sobarzo, P.A.; González, A.; Hauyón, R.A.; Mariman, A.P.; Terraza, C.A. Diphenylsilane-Containing Linear and Rigid Whole Aromatic Poly(Azomethine)s. Structural and Physical Characterization. Polymer 2018, 150, 232–243. [Google Scholar] [CrossRef]
- Kovalchuk, A.I.; Kobzar, Y.L.; Tkachenko, I.M.; Kurioz, Y.I.; Tereshchenko, O.G.; Shekera, O.V.; Nazarenko, V.G.; Shevchenko, V.V. Photoactive Fluorinated Poly(Azomethine)s with Azo Groups in the Main Chain for Optical Storage Applications and Controlling Liquid Crystal Orientation. ACS Appl. Polym. Mater. 2020, 2, 455–463. [Google Scholar] [CrossRef]
- Liu, F.H.; Bai, J.; Yu, G.; Ma, F.H.; Hou, Y.J.; Niu, H.J. Synthesis, Electrochromic Properties and Flash Memory Behaviors of Novel D-A-D Polyazomethines Containing EDOT and Thiophene Units. Org. Electron. 2020, 77, 105538. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Yu, L.; Hou, F.; Zhu, T.; Bao, X.; Li, F.; Sun, M.; Yang, R. The Regulation of π-Bridge of Indacenodithiophene-Based Donor-π-Acceptor Conjugated Polymers toward Efficient Polymer Solar Cells. Dyes Pigments 2019, 162, 43–51. [Google Scholar] [CrossRef]
- Ma, X.; Wu, Y.; Wen, H.; Niu, H.; Wang, C.; Qin, C.; Bai, X.; Lei, L.; Wang, W. Immobilized Polyazomethines Containing Triphenylamine Groups on ITO: Synthesis and Acidochromic, Electrochemical, Electrochromic and Photoelectronic Properties. RSC Adv. 2016, 6, 4564–4575. [Google Scholar] [CrossRef]
- Barik, S.; Bletzacker, T.; Skene, W.G. π-Conjugated Fluorescent Azomethine Copolymers: Opto-Electronic, Halochromic, and Doping Properties. Macromolecules 2012, 45, 1165–1173. [Google Scholar] [CrossRef]
- Çulhaoğlu, S.; Kolcu, F.; Kaya, İ. Synthesis of Phosphate and Silane-Based Conjugated Polymers Derived from Bis-Azomethine: Photophysical and Thermal Characterization. React. Funct. Polym. 2021, 166, 104978. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Nakamura, T.; Araki, Y.; Ito, O.; Takimiya, K.; Otsubo, T. Fluorescence Up-Conversion Study of Excitation Energy Transport Dynamics in Oligothiophene—Fullerene Linked Dyads. J. Phys. Chem. A 2008, 112, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Gan, X.; Leonhardt, C.; Zhang, Z.; Seibert, J.; Busch, J.M.; Bräse, S. A Brief History of OLEDs—Emitter Development and Industry Milestones. Adv. Mater. 2021, 33, 2005630. [Google Scholar] [CrossRef] [PubMed]
- González-Juárez, E.; Güizado-Rodríguez, M.; Barba, V.; Melgoza-Ramírez, M.; Rodríguez, M.; Ramos-Ortíz, G.; Maldonado, J.L. Polythiophenes Based on Pyrene as Pendant Group: Synthesis, Structural Characterization and Luminescent Properties. J. Mol. Struct. 2016, 1103, 25–34. [Google Scholar] [CrossRef]
- Jebnouni, A.; Leclerc, N.; Teka, S.; Mansour, D.; Jaballah, N.S. Vinylene-versus Azomethine-Bridged Carbazole-Based Polymers for Light Emission and Sensor Applications. J. Mol. Struct. 2021, 1244, 130994. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, Y.; Uddin, M.A.; Jang, B.; Zhao, W.; Liu, D.; Woo, H.Y.; Hou, J. A Fluorinated Polythiophene Derivative with Stabilized Backbone Conformation for Highly Efficient Fullerene and Non-Fullerene Polymer Solar Cells. Macromolecules 2016, 49, 2993–3000. [Google Scholar] [CrossRef]
- Sánchez, C.O.; Bèrnede, J.C.; Cattin, L.; Makha, M.; Gatica, N. Schiff Base Polymer Based on Triphenylamine Moieties in the Main Chain. Characterization and Studies in Solar Cells. Thin Solid Films 2014, 562, 495–500. [Google Scholar] [CrossRef]
- Garbay, G.; Giraud, L.; Gali, S.M.; Hadziioannou, G.; Grau, E.; Grelier, S.; Cloutet, E.; Cramail, H.; Brochon, C. Divanillin-Based Polyazomethines: Toward Biobased and Metal-Free π-Conjugated Polymers. ACS Omega 2020, 5, 5176–5181. [Google Scholar] [CrossRef]
- Guo, X.; Gao, B.; Cui, X.; Wang, J.; Dong, W.; Duan, Q.; Fei, T.; Su, Z. PL Sensor for Sensitive and Selective Detection of 2,4,6-Trinitrophenol Based on Carbazole and Tetraphenylsilane Polymer. Dyes Pigments 2021, 191, 109379. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Parafiniuk, K.; Tazbir, I.; Gorecki, L.; Sikora, A.; Filapek, M.; Schab-Balcerzak, E. New Air-Stable Aromatic Polyazomethines with Triphenylamine or Phenylenevinylene Moieties towards Photovoltaic Application. Synth. Met. 2014, 195, 341–349. [Google Scholar] [CrossRef]
- Gamage, P.L.; Gunawardhana, R.; Bulumulla, C.; Kularatne, R.N.; Gedara, C.M.U.; Ma, Z.; Biewer, M.C.; Stefan, M.C. An Ester Functionalized Wide Bandgap Polythiophene for Organic Field-Effect Transistors. Synth. Met. 2021, 277, 116767. [Google Scholar] [CrossRef]
- Iwan, A. An Overview of LC Polyazomethines with Aliphatic-Aromatic Moieties: Thermal, Optical, Electrical and Photovoltaic Properties. Renew. Sustain. Energy Rev. 2015, 52, 65–79. [Google Scholar] [CrossRef]
- Sánchez, C.O.; Sobarzo, P.; Gatica, N. Electronic and Structural Properties of Polymers Based on Phenylene Vinylene and Thiophene Units. Control of the Gap by Gradual Increases of Thiophene Moieties. New J. Chem. 2015, 39, 7979–7987. [Google Scholar] [CrossRef]
- Cheng, G.; Fei, T.; Zhao, Y.; Ma, Y.; Liu, S. White Phosphorescent Polymer Light-Emitting Devices Based on a Wide Band-Gap Polymer Derived from 3,6-Carbazole and Tetraphenylsilane. Org. Electron. 2010, 11, 498–502. [Google Scholar] [CrossRef]
- Kim, O.Y.; Lee, J.Y. High Efficiency Deep Blue Phosphorescent Organic Light-Emitting Diodes Using a Tetraphenylsilane Based Phosphine Oxide Host Material. J. Ind. Eng. Chem. 2012, 18, 1029–1032. [Google Scholar] [CrossRef]
- Kalinichenko, N.K.; Balakirev, D.O.; Savchenko, P.S.; Mannanov, A.L.; Peregudova, S.M.; Paraschuk, D.Y.; Ponomarenko, S.A.; Luponosov, Y.N. Effects of Electron-Withdrawing Group and π-Conjugation Length in Donor-Acceptor Oligothiophenes on Their Properties and Performance in Non-Fullerene Organic Solar Cells. Dyes Pigments 2021, 194, 109592. [Google Scholar] [CrossRef]
- Tong, J.; Huang, Y.; An, L.; Liang, Z.; Li, J.; Yang, C.; Xia, Y. Fluorination Effect of Benzo[c][1,2,5]Thiadiazole-Alt-Oligothiophene-Based Copolymers Involving All Straight Flexible Side Chain in Photovoltaic Application. Opt. Mater. 2020, 108, 110321. [Google Scholar] [CrossRef]

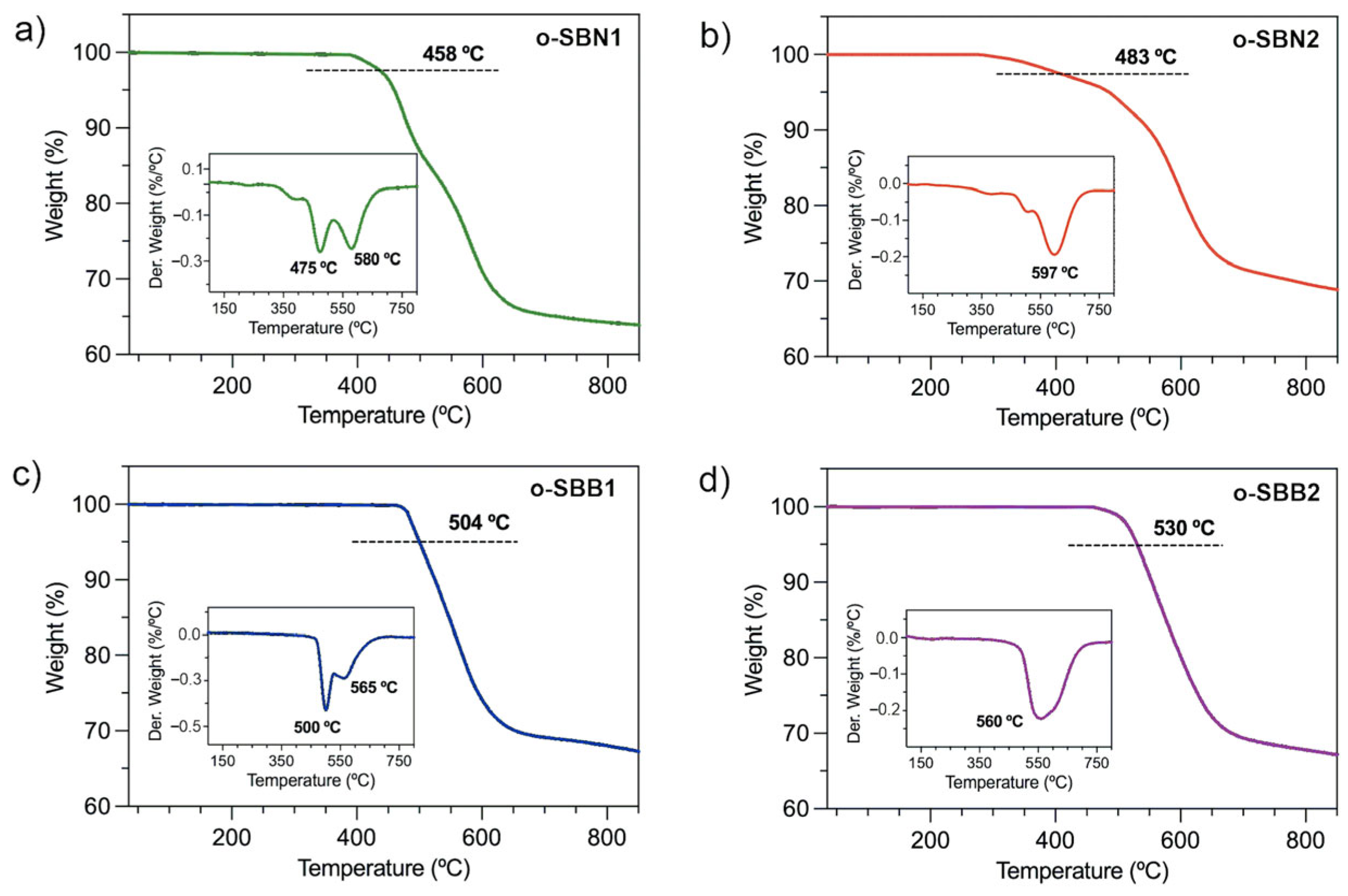
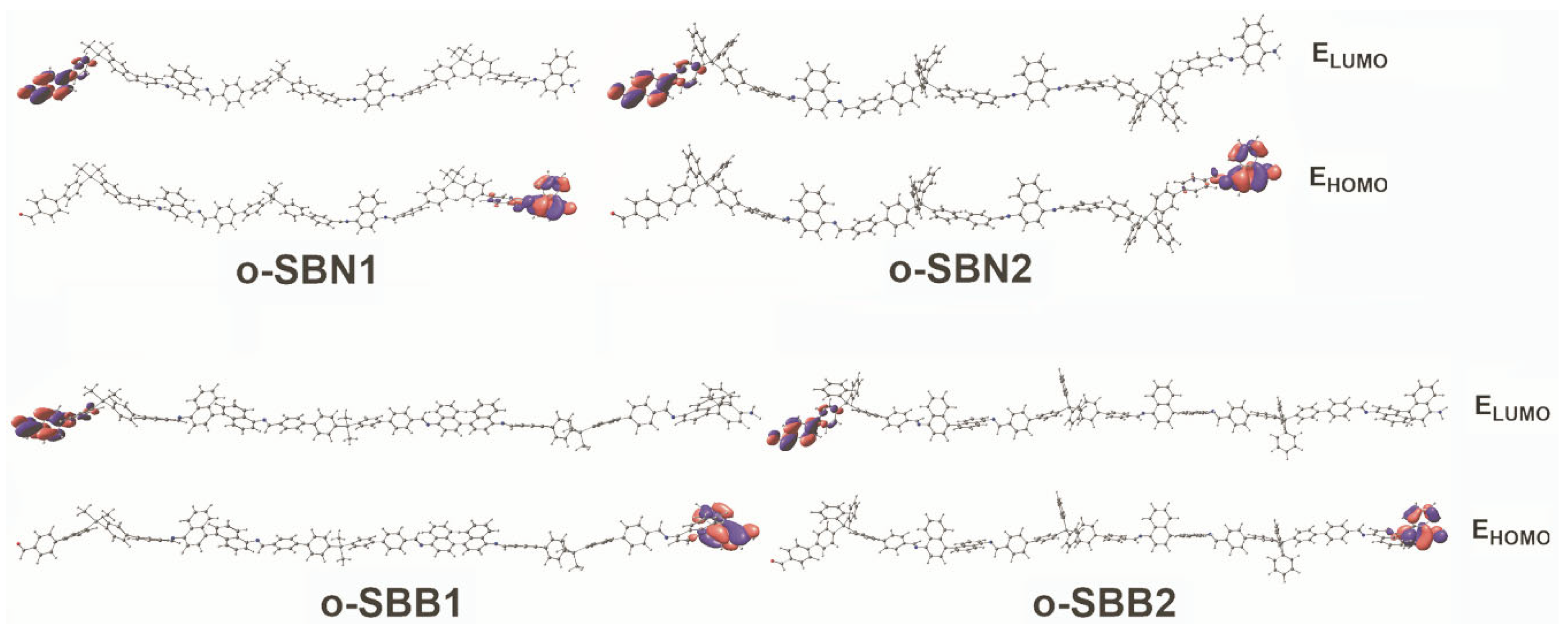
| Oligomer | Mw (kDa) | PDI a | DP b | T5% (°C) c | T10% (°C) c | R (%) d | Tg (°C) e |
|---|---|---|---|---|---|---|---|
| o-SBN1 | 5.10 | 1.78 | 5 | 458 | 481 | 63 | 136 |
| o-SBN2 | 11.53 | 1.75 | 10 | 483 | 548 | 68 | 175 |
| o-SBB1 | n.d. | n.d. | - | 504 | 526 | 66 | 347 |
| o-SBB2 | 10.20 | 2.21 | 6 | 530 | 553 | 67 | 387 |
| Oligomer | λmax (nm) | λmaxem (nm) | Stokes Shift (nm) a | Egopt (eV) b |
|---|---|---|---|---|
| o-SBN1 | 298 | 431 | 133 | 3.67 |
| o-SBN2 | 300, 387 c | 488 | 101 | 2.60 |
| o-SBB1 | 302 | 497 | 195 | 2.82 |
| o-SBB2 | 300, 369 c | 495 | 106 | 2.80 |
| Oligomer | λTh (nm) | ƒ | Composition | p (%) | EHOMO (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|---|---|---|---|
| o-SBN1 | 298 | 0.8952 | HOMO-4 → LUMO+3 | 22 | −4.99 | −2.1 | 2.89 |
| HOMO-1 → LUMO+6 | 11 | ||||||
| HOMO-5 → LUMO+2 | 9 | ||||||
| HOMO-4 → LUMO+2 | 9 | ||||||
| HOMO-1 → LUMO+13 | 7 | ||||||
| HOMO-3 → LUMO+5 | 6 | ||||||
| HOMO-1 → LUMO+16 | 5 | ||||||
| o-SBN2 | 327 | 0.6502 | HOMO-1 → LUMO+7 | 52 | −4.87 | −1.96 | 2.91 |
| HOMO-1 → LUMO+6 | 17 | ||||||
| HOMO-1 → LUMO+17 | 8 | ||||||
| HOMO-4 → LUMO+2 | 5 | ||||||
| 303 | 0.7602 | HOMO-2 → LUMO+6 | 23 | ||||
| HOMO-6 → LUMO+1 | 21 | ||||||
| HOMO-5 → LUMO+1 | 10 | ||||||
| HOMO-11 → LUMO+1 | 9 | ||||||
| HOMO-2 → LUMO+7 | 6 | ||||||
| HOMO-3 → LUMO+2 | 5 | ||||||
| o-SBB1 | 326 | 0.5738 | HOMO-2 → LUMO+6 | 81 | −5.13 | −1.97 | 3.17 |
| HOMO-2 → LUMO+8 | 8 | ||||||
| HOMO-4 → LUMO+8 | 70 | ||||||
| HOMO-6 → LUMO+1 | 15 | ||||||
| HOMO-4 → LUMO+6 | 9 | ||||||
| 310 | 1.2735 | HOMO-4 → LUMO+8 | 70 | ||||
| HOMO-6 → LUMO+1 | 15 | ||||||
| HOMO-4 → LUMO+6 | 9 | ||||||
| o-SBB2 | 397 | 0.1698 | HOMO-5 → LUMO+4 | 58 | −5.11 | −1.97 | 3.14 |
| HOMO-2 → LUMO+4 | 28 | ||||||
| HOMO-2 → LUMO+2 | 6 | ||||||
| HOMO-4 → LUMO+9 | 34 | ||||||
| HOMO-3 → LUMO+9 | 23 | ||||||
| HOMO-4 → LUMO+10 | 7 | ||||||
| HOMO-6 → LUMO+1 | 6 | ||||||
| HOMO-3 → LUMO+10 | 5 | ||||||
| 313 | 0.8675 | HOMO-4 → LUMO+9 | 34 | ||||
| HOMO-3 → LUMO+9 | 23 | ||||||
| HOMO-4 → LUMO+10 | 7 | ||||||
| HOMO-6 → LUMO+1 | 6 | ||||||
| HOMO-3 → LUMO+10 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, E.; González, A.F.; Mariman, A.P.; Jara, C.I.; Velázquez, J.D.; Saldías, C.; Schott, E.; Zarate, X.; Tundidor-Camba, A.; Sobarzo, P.A.; et al. Silicon-Containing π-Conjugated Schiff Base Oligomers with Naphthalene or Binaphthalene Moieties in the Backbone: Synthesis and Study of Properties. Polymers 2025, 17, 1316. https://doi.org/10.3390/polym17101316
González E, González AF, Mariman AP, Jara CI, Velázquez JD, Saldías C, Schott E, Zarate X, Tundidor-Camba A, Sobarzo PA, et al. Silicon-Containing π-Conjugated Schiff Base Oligomers with Naphthalene or Binaphthalene Moieties in the Backbone: Synthesis and Study of Properties. Polymers. 2025; 17(10):1316. https://doi.org/10.3390/polym17101316
Chicago/Turabian StyleGonzález, Enzo, Alexis F. González, Andrea P. Mariman, Camilo I. Jara, Joel D. Velázquez, César Saldías, Eduardo Schott, Ximena Zarate, Alain Tundidor-Camba, Patricio A. Sobarzo, and et al. 2025. "Silicon-Containing π-Conjugated Schiff Base Oligomers with Naphthalene or Binaphthalene Moieties in the Backbone: Synthesis and Study of Properties" Polymers 17, no. 10: 1316. https://doi.org/10.3390/polym17101316
APA StyleGonzález, E., González, A. F., Mariman, A. P., Jara, C. I., Velázquez, J. D., Saldías, C., Schott, E., Zarate, X., Tundidor-Camba, A., Sobarzo, P. A., & Terraza, C. A. (2025). Silicon-Containing π-Conjugated Schiff Base Oligomers with Naphthalene or Binaphthalene Moieties in the Backbone: Synthesis and Study of Properties. Polymers, 17(10), 1316. https://doi.org/10.3390/polym17101316






