Covalent Grafting of Quaternary Ammonium Salt-Containing Polyurethane onto Silicone Substrates to Enhance Bacterial Contact-Killing Ability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
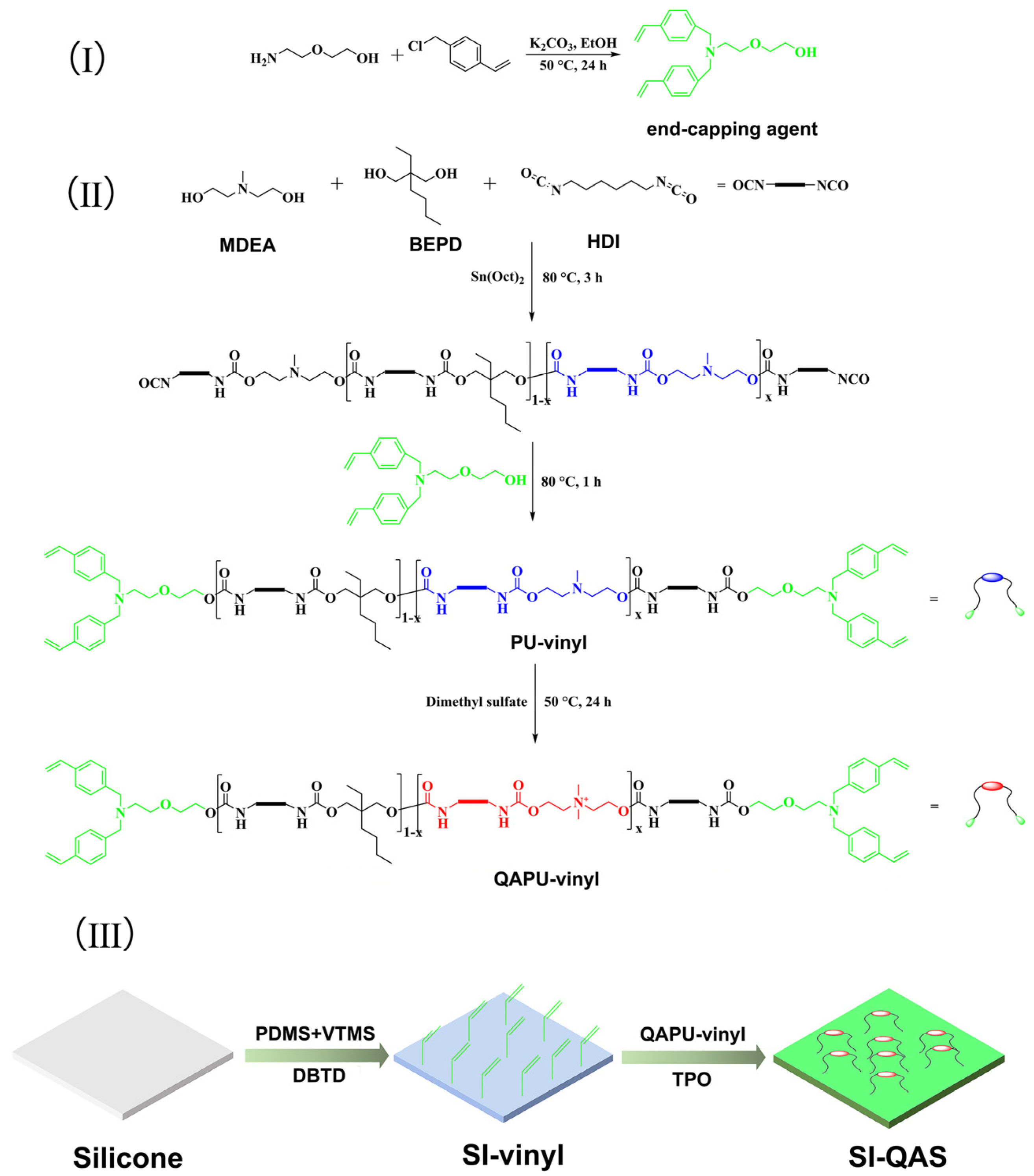
2.2. Synthesis of End-Capping Agent N-Bis(vinylbenzyl) Diglycolamine
2.3. Synthesis of QAPU-Vinyl
2.4. Preparation of SI-Vinyl
2.5. Surface Grafting of QAPU-Vinyl
2.6. Determination of Surface QAS Content on SI-QAS
2.7. Contact-Killing Abilities of SI-QAS
2.8. Bacterial Morphologies on SI-QAS-2
2.9. Long-Term Bactericidal Activity of SI-QAS-2
2.10. Characterization
3. Results
3.1. Synthesis of QAPU-Vinyl
3.1.1. Synthesis of End-Capping Agent N-Bis(vinylbenzyl) Diglycolamine
3.1.2. Characterization of QAPU-Vinyl
3.2. Preparation and Optimum Seeking of SI-QAS Coatings
3.3. The Assessment of the Bactericidal Abilities of the SI-QAS Coatings
3.4. Long-Term Bactericidal Activity of SI-QAS-2 Coating
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Sato, T.; Kitani, I. A novel Foley catheter made of high-intensity near-infrared fluorescent silicone rubber for image-guided surgery of lower rectal cancer. Photodiagnosis Photodyn. Ther. 2024, 45, 103976. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-h.; Zhou, X.; Lei, Z.-Y.; Tian, Y.; Chen, Y.; Zhang, Y.-M.; Mao, T.-C.; Fan, D.-L.; Zhou, S.-W. Novel silicone rubber with carboxyl grafted polyhedral oligomeric silsesquioxane (POSS-COOH) as a potential scaffold for soft tissue filling. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 162–179. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, X.; Mao, T.-C.; Li, X.; Shi, X.-H.; Fan, D.-L.; Zhang, Y.-M. Carbon Ion Implantation: A Good Method to Enhance the Biocompatibility of Silicone Rubber. Plast. Reconstr. Surg. 2016, 137, 690e–699e. [Google Scholar] [CrossRef]
- Dasgupta, M.K.; Ward, K.; Noble, P.A.; Larabie, M.; Costerton, J.W. Development of Bacterial Biofilms on Silastic Catheter Materials in Peritoneal Dialysis Fluid. Am. J. Kidney Dis. 1994, 23, 709–716. [Google Scholar] [CrossRef]
- Gomes, R.N.; Borges, I.; Pereira, A.T.; Maia, A.F.; Pestana, M.; Magalhães, F.D.; Pinto, A.M.; Gonçalves, I.C. Antimicrobial graphene nanoplatelets coatings for silicone catheters. Carbon 2018, 139, 635–647. [Google Scholar] [CrossRef]
- Yelin, I.; Kishony, R. Antibiotic Resistance. Cell 2018, 172, 1136.e1. [Google Scholar] [CrossRef]
- MacLean, R.C.; San Millan, A. The evolution of antibiotic resistance. Science 2019, 365, 1082–1083. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID∗ guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, W.; Liu, X.; Liang, C.; Zheng, Y.; Li, Z.; Liang, Y.; Zheng, D.; Zhu, S.; Cui, Z.; et al. Self-activating anti-infection implant. Nat. Commun. 2021, 12, 6907. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Xi, Y.; Yu, S.; Yang, K.; Zhang, F.; Yang, Y.; Wang, T.; He, S.; Zhu, Y.; Fan, Z.; et al. A polypeptide coating for preventing biofilm on implants by inhibiting antibiotic resistance genes. Biomaterials 2023, 293, 121957. [Google Scholar] [CrossRef] [PubMed]
- Punjataewakupt, A.; Napavichayanun, S.; Aramwit, P. The downside of antimicrobial agents for wound healing. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 39–54. [Google Scholar] [CrossRef]
- Keum, H.; Kim, J.Y.; Yu, B.; Yu, S.J.; Kim, J.; Jeon, H.; Lee, D.Y.; Im, S.G.; Jon, S. Prevention of Bacterial Colonization on Catheters by a One-Step Coating Process Involving an Antibiofouling Polymer in Water. ACS Appl. Mater. Interfaces 2017, 9, 19736–19745. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Ma, Z.; Sun, S.; Zhang, H.; Zhang, Y.; Zuo, L.; Yang, Y.; Xie, J.; Ding, C.; Li, J. Supramolecular Self-Healing Antifouling Coating for Dental Materials. ACS Appl. Mater. Interfaces 2023, 15, 41403–41416. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Nazifi, S.; Cheng, K.; Karim, A.; Ghasemi, H. Scalable inter-diffused zwitterionic polyurethanes for durable antibacterial coatings. Chem. Eng. J. 2021, 422, 130085. [Google Scholar] [CrossRef]
- Wei, M.; Wang, H.; Wu, J.; Yang, D.; Li, K.; Liu, X.; Wang, M.; Lin, B.; Wang, Z. Multihydrogen Bond Modulated Polyzwitterionic Removable Adhesive Hydrogel with Antibacterial and Hemostatic Function for Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 21472–21485. [Google Scholar] [CrossRef]
- Yang, J.; Qian, H.; Wang, J.; Ju, P.; Lou, Y.; Li, G.; Zhang, D. Mechanically durable antibacterial nanocoatings based on zwitterionic copolymers containing dopamine segments. J. Mater. Sci. Technol. 2021, 89, 233–241. [Google Scholar] [CrossRef]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. A sol–gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. Appl. Surf. Sci. 2021, 535, 147675. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, G.B.; Hu, C.; Liu, Z.X.; Liu, H.S.; Wang, Y.L.; Wang, L.; Feng, X.Q. Perfluoropolyether-incorporated polyurethane with enhanced antibacterial and anti-adhesive activities for combating catheter-induced infection. RSC Adv. 2024, 14, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.R.; Liang, J.; Liu, X.H.; Gao, K.X.; Zhang, Y.; Li, A.; Chen, C.; Hou, L.A.; Yang, Y. Graphene oxide/methyl anthranilate modified anti-biofouling membrane possesses dual functions of anti-adhesion and quorum quenching. J. Membr. Sci. 2023, 668, 121265. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, W.; Weng, Y.; Chen, X.; Cheng, Y.; Zhou, P. Fabrication of PDMS surfaces with micro patterns and the effect of pattern sizes on bacteria adhesion. Food Control 2016, 68, 344–351. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, G.; Jiang, N.; Ying, G.; Li, Y.; Cai, X.; Meng, J.; Mai, L.; Guo, M.; Zhang, Y.S.; et al. Nature-inspired micropatterns. Nat. Rev. Methods Primers 2023, 3, 68. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Wei, D.; Zheng, Z.; Han, Z.; Liu, Y. Fluorine-free, robust and self-healing superhydrophobic surfaces with anticorrosion and antibacterial performances. J. Mater. Sci. Technol. 2024, 186, 231–243. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, F.; Chen, H.; Wang, J.; Lopez, A.I.; Chen, Q.; Li, S.; Long, Y.; Darouiche, R.O.; Hull, R.A.; et al. Coating of silicone with mannoside-PAMAM dendrimers to enhance formation of non-pathogenic Escherichia coli biofilms against colonization of uropathogens. Acta Biomater. 2017, 64, 200–210. [Google Scholar] [CrossRef]
- Zhu, M.-M.; Fang, Y.; Chen, Y.-C.; Lei, Y.-Q.; Fang, L.-F.; Zhu, B.-K.; Matsuyama, H. Antifouling and antibacterial behavior of membranes containing quaternary ammonium and zwitterionic polymers. J. Colloid Interface Sci. 2021, 584, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, D.M.; Darouiche, R.O. New strategies to prevent catheter-associated urinary tract infections. Nat. Rev. Urol. 2012, 9, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shang, Z.; Li, C.; Guo, J.; Chen, Z.; Zhao, N.; Liu, G.; Zhou, F.; Liu, W. Excellent lubricating hydrogels with rapid photothermal sterilization for medical catheters coating. Friction 2024, 12, 2679–2691. [Google Scholar] [CrossRef]
- Hu, Y.; Qiao, Y.; Lei, P.; Gu, Y.; Sun, L.; Qiu, Y.; Li, S.; Xu, H.; Wang, R. Dual network hydrogel coatings based on recombinant mussel protein with enhanced antibacterial and super-lubrication properties for urinary catheter applications. Chem. Eng. J. 2023, 474, 145502. [Google Scholar] [CrossRef]
- Cometta, S.; Donose, B.C.; Juárez-Saldivar, A.; Ravichandran, A.; Xu, Y.; Bock, N.; Dargaville, T.R.; Rakić, A.D.; Hutmacher, D.W. Unravelling the physicochemical and antimicrobial mechanisms of human serum albumin/tannic acid coatings for medical-grade polycaprolactone scaffolds. Bioact. Mater. 2024, 42, 68–84. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F.; Catalano, M.; Taurino, A.; Licciulli, A.; Maffezzoli, A.; Sannino, A. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. J. Mater. Sci. Mater. Med. 2011, 22, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-C.; Yu, Y.; Qian, H.-L.; Chen, Y.-F.; Zou, L.-Y.; Zhang, C.-M.; Ren, K.-F.; Yang, Z.-H.; Ji, J. Antibacterial endotracheal tube with silver-containing double-network hydrogel coating. Colloid Interface Sci. Commun. 2023, 55, 100724. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Brzychczy-Włoch, M.; Gosiewski, T.; Jany, B.; Lishchynskyi, O.; Shymborska, Y.; Nastyshyn, S.; Bernasik, A.; et al. “Command” surfaces with thermo-switchable antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109806. [Google Scholar] [CrossRef]
- Dong, J.J.; Muszanska, A.; Xiang, F.; Falkenberg, R.; van de Belt-Gritter, B.; Loontjens, T. Contact Killing of Gram-Positive and Gram-Negative Bacteria on PDMS Provided with Immobilized Hyperbranched Antibacterial Coatings. Langmuir 2019, 35, 14108–14116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jia, Z.; Xiong, P.; Yan, J.; Li, M.; Cheng, Y.; Zheng, Y. Novel pH-responsive tobramycin-embedded micelles in nanostructured multilayer-coatings of chitosan/heparin with efficient and sustained antibacterial properties. Mater. Sci. Eng. C 2018, 90, 693–705. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Li, J.; Gao, Y.; Tan, H.; Wang, K.; Li, J.; Fu, Q. Synthesis and antibacterial characterization of waterborne polyurethanes with gemini quaternary ammonium salt. Sci. Bull. 2015, 60, 1114–1121. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, B.; Shi, J.; Wang, M. Quaternary ammonium antimicrobial agents and their application in antifouling coatings: A review. J. Coat. Technol. Res. 2024, 21, 87–103. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, S.; Zhang, X.; Zeng, S.; Xu, Y.; Nie, W.; Zhou, Y.; Xu, T.; Chen, P. Quaternary Ammonium Salts: Insights into Synthesis and New Directions in Antibacterial Applications. Bioconjugate Chem. 2023, 34, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Zander, Z.K.; Chen, P.; Hsu, Y.-H.; Dreger, N.Z.; Savariau, L.; McRoy, W.C.; Cerchiari, A.E.; Chambers, S.D.; Barton, H.A.; Becker, M.L. Post-fabrication QAC-functionalized thermoplastic polyurethane for contact-killing catheter applications. Biomaterials 2018, 178, 339–350. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, T.; Li, Y.; Lu, J.; Du, H.; Yan, L.; Tan, H.; Li, J.; Yin, Y. Anti-Fouling and Anti-Biofilm Performance of Self-Polishing Waterborne Polyurethane with Gemini Quaternary Ammonium Salts. Polymers 2023, 15, 317. [Google Scholar] [CrossRef]
- Bai, Y.; Li, K.; Ma, L.; Wu, D.; Xiang, J.; Hu, Q.; Du, Z.; Liu, G. Mussel-inspired surface modification of urinary catheters with both zwitterionic and bactericidal properties for effectively preventing catheter-associated infection. Chem. Eng. J. 2023, 455, 140766. [Google Scholar] [CrossRef]
- ISO 22196; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ISO: Geneva, Switzerland, 2024.
- Duan, X.; Xu, Y.; Zhang, Z.; Ma, X.; Wang, C.; Ma, W.; Jia, F.; Pan, X.; Liu, Y.; Zhao, Y.; et al. Piezoelectrically-activated antibacterial catheter for prevention of urinary tract infections in an on-demand manner. Mater. Today Bio 2024, 26, 101089. [Google Scholar] [CrossRef]
- Lundin, J.G.; Coneski, P.N.; Fulmer, P.A.; Wynne, J.H. Relationship between surface concentration of amphiphilic quaternary ammonium biocides in electrospun polymer fibers and biocidal activity. React. Funct. Polym. 2014, 77, 39–46. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Al Lawati, H.; Blair, B.M.; Larnard, J. Urinary Tract Infections: Core Curriculum 2024. Am. J. Kidney Dis. 2024, 83, 90–100. [Google Scholar] [CrossRef]







| PU-Vinyl Sample | Molar Ratio of Reactants in Feed | MDEA in PU a (mol%) | Molecular Weight b | |||||
|---|---|---|---|---|---|---|---|---|
| MDEA | BEPD | HDI | DC | Mn | Mw | PDI | ||
| 1 | 0.1 | 0.9 | 1.1 | 0.11 | 12.2 | 6920 | 11,070 | 1.6 |
| 2 | 0.2 | 0.8 | 1.1 | 0.11 | 21.8 | 6500 | 9750 | 1.5 |
| 3 | 0.3 | 0.7 | 1.1 | 0.11 | 30.5 | 6150 | 9220 | 1.5 |
| 4 | 0.4 | 0.6 | 1.1 | 0.11 | 41.5 | 5980 | 8380 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Z.; Liu, Z.; Yang, S.; Shen, Z.; Wu, Y.; Liu, Y.; Li, J.; Wang, L. Covalent Grafting of Quaternary Ammonium Salt-Containing Polyurethane onto Silicone Substrates to Enhance Bacterial Contact-Killing Ability. Polymers 2025, 17, 17. https://doi.org/10.3390/polym17010017
Pan Z, Liu Z, Yang S, Shen Z, Wu Y, Liu Y, Li J, Wang L. Covalent Grafting of Quaternary Ammonium Salt-Containing Polyurethane onto Silicone Substrates to Enhance Bacterial Contact-Killing Ability. Polymers. 2025; 17(1):17. https://doi.org/10.3390/polym17010017
Chicago/Turabian StylePan, Zihong, Zixu Liu, Sijia Yang, Zhanyu Shen, Yuchen Wu, Yanyu Liu, Jingfan Li, and Liang Wang. 2025. "Covalent Grafting of Quaternary Ammonium Salt-Containing Polyurethane onto Silicone Substrates to Enhance Bacterial Contact-Killing Ability" Polymers 17, no. 1: 17. https://doi.org/10.3390/polym17010017
APA StylePan, Z., Liu, Z., Yang, S., Shen, Z., Wu, Y., Liu, Y., Li, J., & Wang, L. (2025). Covalent Grafting of Quaternary Ammonium Salt-Containing Polyurethane onto Silicone Substrates to Enhance Bacterial Contact-Killing Ability. Polymers, 17(1), 17. https://doi.org/10.3390/polym17010017







