Leveraging Microneedles for Raised Scar Management
Abstract
1. Introduction
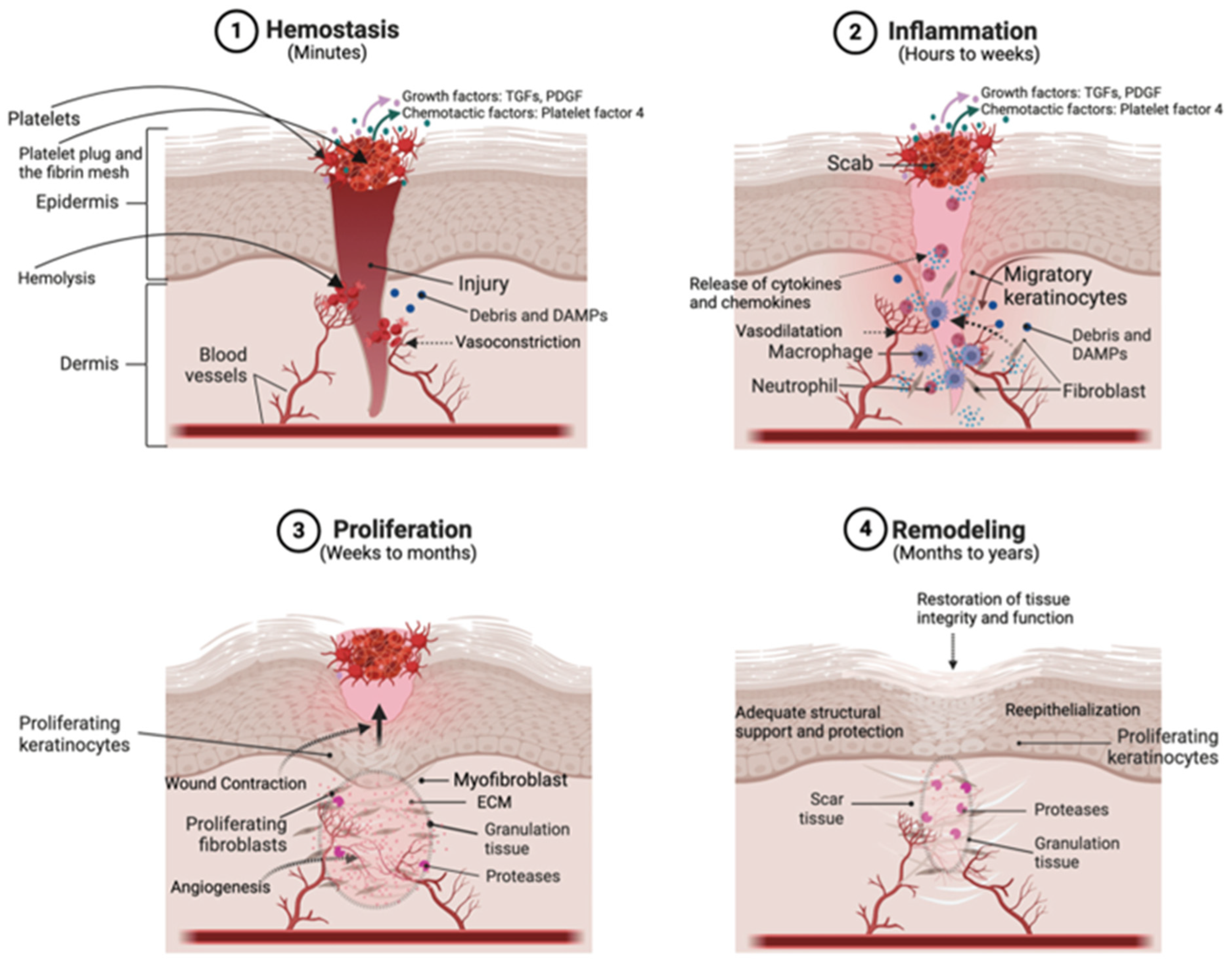
2. Underlying Mechanism of Scar Development
3. MNs for Raised Scare Management
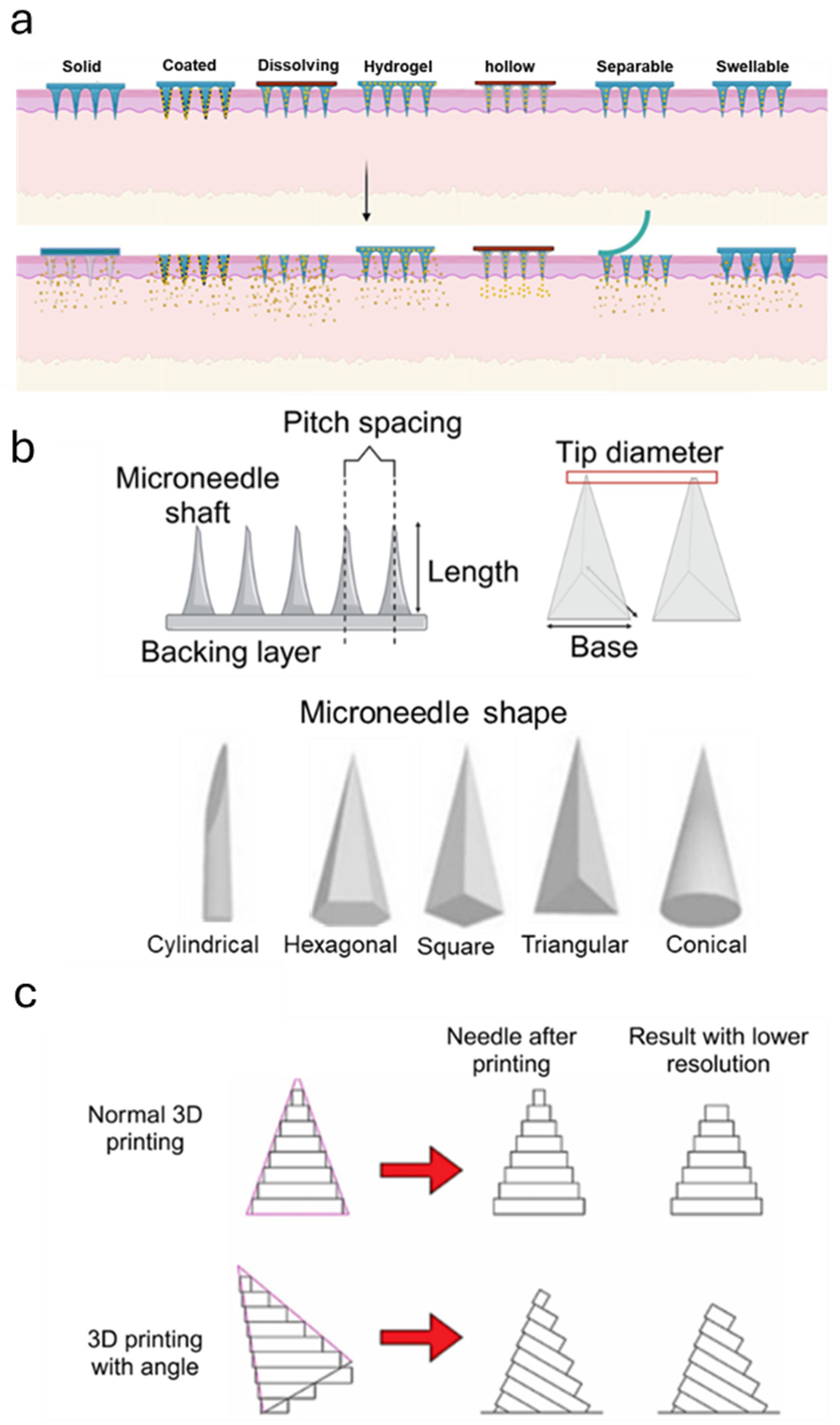
3.1. Design, Material Selection, and Fabrication of MNs for Raised Scar Treatment
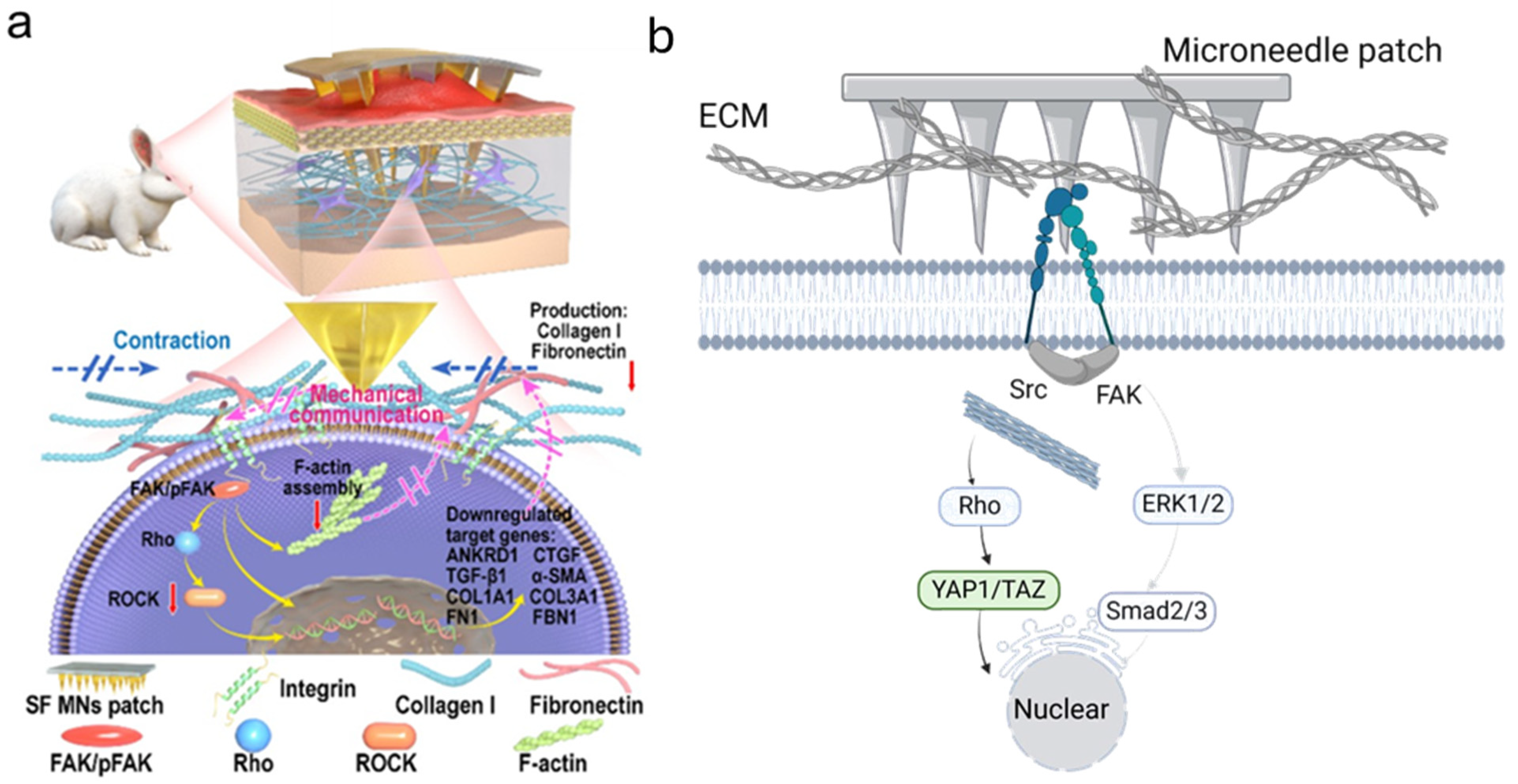
3.2. MNs as a Physical Intervention
3.3. Microneedles as Drug Carrier
3.3.1. Corticosteroids-Loaded MNs

3.3.2. Plant-Extract-Loaded MNs
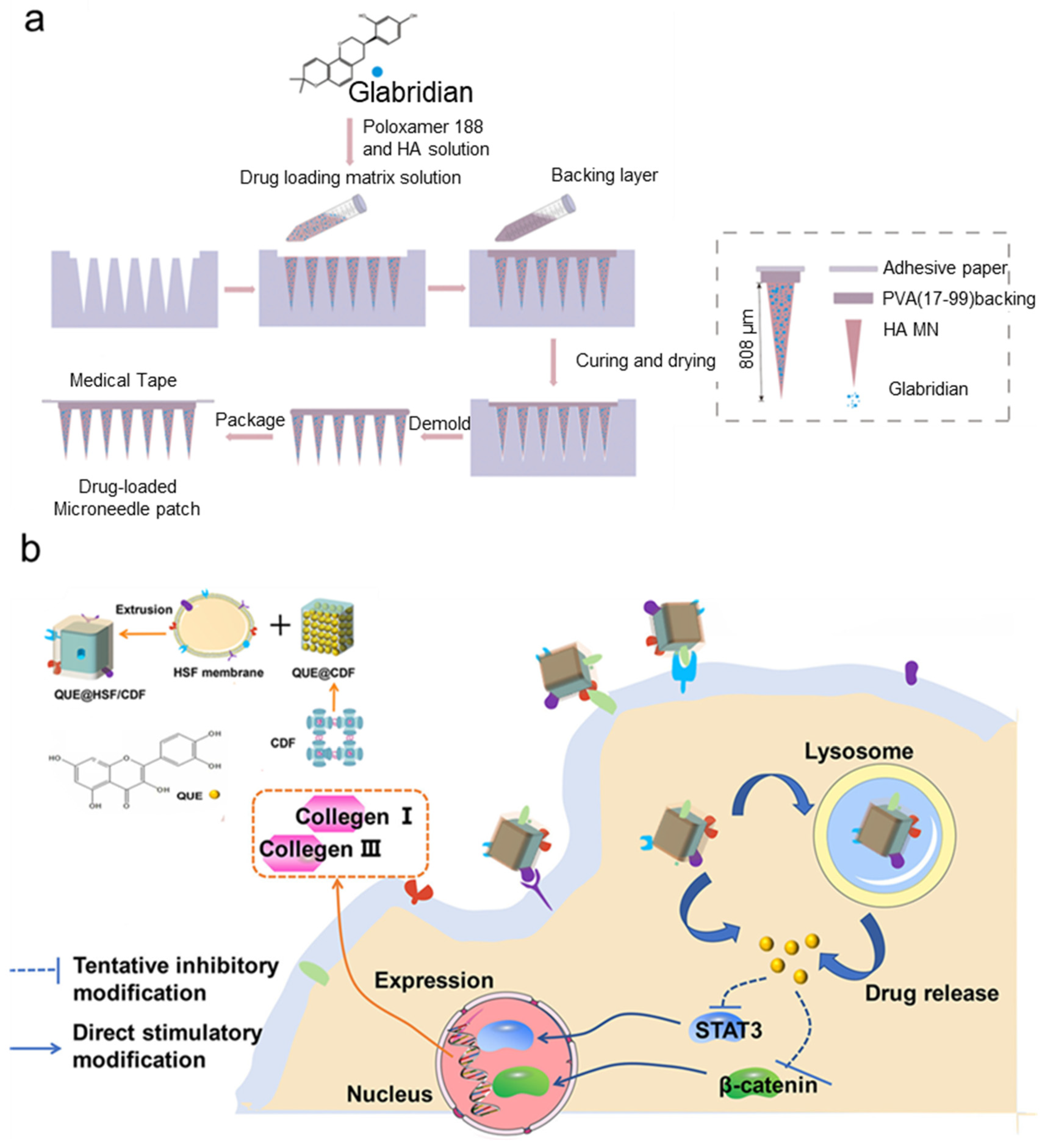
3.3.3. MNs Loaded with Small Molecules
3.4. Combinative Strategies Based on MNs
4. Challenges and Future Perspective
5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Lin, X.; Lai, Y. Scarring skin: Mechanisms and therapies. Int. J. Mol. Sci. 2024, 25, 1458. [Google Scholar] [CrossRef]
- Monstrey, S.; Middelkoop, E.; Vranckx, J.J.; Bassetto, F.; Ziegler, U.E.; Meaume, S.; Téot, L. Updated scar management practical guidelines: Non-invasive and invasive measures. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Pethe, P. Focus: Cell Fate: The Molecular Mechanisms Involved in the Hypertrophic Scars Post-Burn Injury. Yale J. Biol. Med. 2023, 96, 549. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Update on Hypertrophic Scar Management in Burn Patients. Clin. Plast. Surg. 2024, 51, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Kuribayashi, S.; Tosa, M.; Aoki, M.; Akaishi, S.; Ogawa, R. Z-plasty and postoperative radiotherapy for upper-arm keloids: An analysis of 38 patients. Plast. Reconstr. Surg.–Glob. Open 2019, 7, e2496. [Google Scholar] [CrossRef]
- Gajbhiye, S.; Wairkar, S. Collagen fabricated delivery systems for wound healing: A new roadmap. Biomater. Adv. 2022, 142, 213152. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wu, X.; Zhang, Y.; Qian, X.; Sun, W.; Zhao, Y. Emerging biomedical technologies for scarless wound healing. Bioact. Mater. 2024, 42, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Yang, Y.; Zhao, S.; You, J.; Wang, J.; Cai, J.; Wang, H.; Wang, J.; Zhang, W.; et al. Scarless wound healing programmed by core-shell microneedles. Nat. Commun. 2023, 14, 3431. [Google Scholar] [CrossRef]
- Pham, H.P.; Vo, V.T.; Nguyen, T.Q. Optimizing CNC milling parameters for manufacturing of ultra-sharp tip microneedle with various tip angles. Drug Deliv. Transl. Res. 2024, 18, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, O.; Goutos, I. Scar Symptoms: Pruritus and Pain. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer: Cham, Switzerland, 2020; pp. 87–101. [Google Scholar] [CrossRef]
- Peng, K.; Vora, L.K.; Tekko, I.A.; Permana, A.D.; Domínguez-Robles, J.; Ramadon, D.; Chambers, P.; McCarthy, H.O.; Larrañeta, E.; Donnelly, R.F. Dissolving microneedle patches loaded with amphotericin B microparticles for localised and sustained intradermal delivery: Potential for enhanced treatment of cutaneous fungal infections. J. Control Release 2021, 339, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Reish, R.G.; Eriksson, E. Scar treatments: Preclinical and clinical studies. J. Am. Coll. Surg. 2008, 206, 719–730. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Lee, C.; Yu, J.-W. Distinctive role of inflammation in tissue repair and regeneration. Arch. Pharmacal Res. 2023, 46, 78–89. [Google Scholar] [CrossRef]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Chen, K.; Henn, D.; Januszyk, M.; Barrera, J.A.; Noishiki, C.; Bonham, C.A.; Griffin, M.; Tevlin, R.; Carlomagno, T.; Shannon, T. Disrupting mechanotransduction decreases fibrosis and contracture in split-thickness skin grafting. Sci. Transl. Med. 2022, 14, eabj9152. [Google Scholar] [CrossRef]
- Klingberg, F.; Chow, M.L.; Koehler, A.; Boo, S.; Buscemi, L.; Quinn, T.M.; Costell, M.; Alman, B.A.; Genot, E.; Hinz, B. Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J. Cell Biol. 2014, 207, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of acute and chronic wound healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, K.; Irnaten, M.; O’Brien, C. p53 and myofibroblast apoptosis in organ fibrosis. Int. J. Mol. Sci. 2023, 24, 6737. [Google Scholar] [CrossRef]
- Park, S.M.; Lee, J.H.; Ahn, K.S.; Shim, H.W.; Yoon, J.Y.; Hyun, J.; Lee, J.H.; Jang, S.; Yoo, K.H.; Jang, Y.K. Cyclic Stretch Promotes Cellular Reprogramming Process through Cytoskeletal-Nuclear Mechano-Coupling and Epigenetic Modification. Adv. Sci. 2023, 10, 2303395. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, L.; He, H.; Liu, X.; Huang, Y.; Xu, D.; Yao, M.; Zhang, N.; Guo, Y.; Lu, Y. Down-regulating scar formation by microneedles directly via a mechanical communication pathway. ACS Nano 2022, 16, 10163–10178. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Godin, M.; Pelling, A.E. Mechanical stretch sustains myofibroblast phenotype and function in microtissues through latent TGF-β1 activation. Integr. Biol. 2020, 12, 199–210. [Google Scholar] [CrossRef]
- Piersma, B.; de Rond, S.; Werker, P.M.; Boo, S.; Hinz, B.; van Beuge, M.M.; Bank, R.A. YAP1 is a driver of myofibroblast differentiation in normal and diseased fibroblasts. Am. J. Pathol. 2015, 185, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell Biosci. 2019, 9, 98. [Google Scholar] [CrossRef]
- Berry, C.E.; Downer, M., Jr.; Morgan, A.G.; Griffin, M.; Liang, N.E.; Kameni, L.; Laufey Parker, J.B.; Guo, J.; Longaker, M.T.; Wan, D.C. The effects of mechanical force on fibroblast behavior in cutaneous injury. Front. Surg. 2023, 10, 1167067. [Google Scholar] [CrossRef]
- Huth, S.; Blumberg, J.W.; Probst, D.; Lammerding, J.; Schwarz, U.S.; Selhuber-Unkel, C. Quantifying force transmission through fibroblasts: Changes of traction forces under external shearing. Eur. Biophys. J. 2022, 51, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Roca-Cusachs, P.; Iskratsch, T.; Sheetz, M.P. Finding the weakest link–exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 2012, 125, 3025–3038. [Google Scholar] [CrossRef] [PubMed]
- Berdiaki, A.; Neagu, M.; Tzanakakis, P.; Spyridaki, I.; Pérez, S.; Nikitovic, D. Extracellular Matrix Components and Mechanosensing Pathways in Health and Disease. Biomolecules 2024, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef]
- Liu, X.-G.; Zhang, D. Evaluation of efficacy of corticosteroid and corticosteroid combined with botulinum toxin type A in the treatment of keloid and hypertrophic scars: A meta-analysis. Aesthetic Plast. Surg. 2021, 45, 3037–3044. [Google Scholar] [CrossRef]
- Kabel, A.M.; Sabry, H.H.; Sorour, N.E.; Moharm, F.M. Comparative study between intralesional injection of bleomycin and 5-fluorouracil in the treatment of keloids and hypertrophic scars. J. Dermatol. Dermatol. Surg. 2016, 20, 32–38. [Google Scholar] [CrossRef]
- Gragnani, A.; Warde, M.; Furtado, F.; Ferreira, L.M. Topical Tamoxifen Therapy in Hypertrophic Scars or Keloids in Burns; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Murray, J.C.; Pollack, S.V.; Pinnell, S.R. Keloids and hypertrophic scars. Clin. Dermatol. 1984, 2, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Elshahed, A.R.; Elmanzalawy, K.S.; Shehata, H.; ElSaie, M.L. Effect of botulinum toxin type a for treating hypertrophic scars: A split-scar, double-blind randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 2252–2258. [Google Scholar] [CrossRef]
- Kant, S.; Van den Kerckhove, E.; Colla, C.; Tuinder, S.; Van der Hulst, R.; Piatkowski de Grzymala, A. A new treatment of hypertrophic and keloid scars with combined triamcinolone and verapamil: A retrospective study. Eur. J. Plast. Surg. 2018, 41, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Irie, H.; Inoue, M.; Mitani, H.; Sunami, H.; Sano, S. Factors affecting hypertrophic scar development in median sternotomy incisions for congenital cardiac surgery. J. Am. Coll. Surg. 1997, 185, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Hong, J.P.; Choi, J.W.; Seo, D.K.; Lee, E.S.; Lee, H.S. The Efficacy of a Silicone Sheet in Postoperative Scar Management. Adv. Ski. Wound Care 2016, 29, 414–420. [Google Scholar] [CrossRef]
- de Oliveira, G.V.; Gold, M.H. Silicone sheets and new gels to treat hypertrophic scars and keloids: A short review. Dermatol. Ther. 2020, 33, e13705. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.A.; Wu, E.; Pontes, D.; Kwan, K.R.; Poondru, S.; Miller, C.H.; Kundu, R.V. Keloid treatments: An evidence-based systematic review of recent advances. Syst. Rev. 2023, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, R.; da Silva, C.A.; Pinto, A.; Kuczynski, U.; da Silva, E.M. Laser therapy for treating hypertrophic and keloid scars. Cochrane Database Syst. Rev. 2022, 9, Cd011642. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Zhang, S.; Wang, B.; Gao, Y. Layered dissolving microneedle containing a three-drug combination on the treatment of hypertrophic scar. J. Drug Deliv. Sci. Technol. 2024, 95, 105572. [Google Scholar] [CrossRef]
- Disphanurat, W.; Sivapornpan, N.; Srisantithum, B.; Leelawattanachai, J. Efficacy of a triamcinolone acetonide-loaded dissolving microneedle patch for the treatment of hypertrophic scars and keloids: A randomized, double-blinded, placebo-controlled split-scar study. Arch. Dermatol. Res. 2023, 315, 989–997. [Google Scholar] [CrossRef]
- Younas, A.; Asad, M.; Wan, X.; Zhang, Y.; Ma, X.; Wang, L.; Gu, H.; Shang, H.; Zhang, N. Oregano essential oil-infused mucin microneedle patch for the treatment of hypertrophic scar. Int. J. Pharm. 2024, 665, 124748. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dong, Y.; Shen, Y.; Hou, A.; Quan, G.; Pan, X.; Wu, C. Bilayer dissolving microneedle array containing 5-fluorouracil and triamcinolone with biphasic release profile for hypertrophic scar therapy. Bioact. Mater. 2021, 6, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wei, Q.; Chen, S.; Liu, X.; Cui, S.; Huang, Q.; Chu, Z.; Ma, K.; Zhang, W.; Hu, W. MiR-141-3p-Functionalized Exosomes Loaded in Dissolvable Microneedle Arrays for Hypertrophic Scar Treatment. Small 2024, 20, 2305374. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Dong, Z.; Xu, X.; Bei, H.P.; Yuen, H.Y.; James Cheung, C.W.; Wong, M.S.; He, Y.; Zhao, X. Going below and beyond the surface: Microneedle structure, materials, drugs, fabrication, and applications for wound healing and tissue regeneration. Bioact. Mater. 2023, 27, 303–326. [Google Scholar] [CrossRef] [PubMed]
- Tariq, N.; Ashraf, M.W.; Tayyaba, S. A review on solid microneedles for biomedical applications. J. Pharm. Innov. 2022, 17, 1464–1483. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Gade, S.; Glover, K.; Mishra, D.; Sharma, S.; Guy, O.; Donnelly, R.F.; Vora, L.K.; Thakur, R.R.S. Hollow microneedles for ocular drug delivery. J. Control. Release 2024, 371, 43–66. [Google Scholar] [CrossRef]
- Thakur, R.R.S.; Tekko, I.A.; Al-Shammari, F.; Ali, A.A.; McCarthy, H.; Donnelly, R.F. Rapidly dissolving polymeric microneedles for minimally invasive intraocular drug delivery. Drug Deliv. Transl. Res. 2016, 6, 800–815. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.R.; Dobson, L.J.; Pattanayek, S.K.; Das, D.B. Swellable microneedles based transdermal drug delivery: Mathematical model development and numerical experiments. Chem. Eng. Sci. 2022, 247, 117005. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Zeng, Y.; Terry, R.N.; Li, W. Rapidly separable microneedle patches for controlled release of therapeutics for long-acting therapies. Med. Drug Discov. 2022, 13, 100118. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, X.; Liang, L.; Chen, B.Z.; Liu, C.; Wang, Y. A stimulus responsive microneedle-based drug delivery system for cancer therapy. Biomater. Sci. 2024, 12, 6274–6283. [Google Scholar] [CrossRef]
- Luo, X.; Yang, L.; Cui, Y. Microneedles: Materials, fabrication, and biomedical applications. Biomed. Microdevices 2023, 25, 20. [Google Scholar] [CrossRef]
- Le, Z.; Yu, J.; Quek, Y.J.; Bai, B.; Li, X.; Shou, Y.; Myint, B.; Xu, C.; Tay, A. Design principles of microneedles for drug delivery and sampling applications. Mater. Today 2023, 63, 137–169. [Google Scholar] [CrossRef]
- Makvandi, P.; Kirkby, M.; Hutton, A.R.; Shabani, M.; Yiu, C.K.; Baghbantaraghdari, Z.; Jamaledin, R.; Carlotti, M.; Mazzolai, B.; Mattoli, V. Engineering microneedle patches for improved penetration: Analysis, skin models and factors affecting needle insertion. Nano-Micro Lett. 2021, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Ando, D.; Miyatsuji, M.; Sakoda, H.; Yamamoto, E.; Miyazaki, T.; Koide, T.; Sato, Y.; Izutsu, K.-I. Mechanical characterization of dissolving microneedles: Factors affecting physical strength of needles. Pharmaceutics 2024, 16, 200. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, M.; Akbari, M. The effect of 3D printing tilt angle on the penetration of 3D-printed microneedle arrays. Micromachines 2023, 14, 1157. [Google Scholar] [CrossRef]
- Hosseini, M.; Brown, J.; Khosrotehrani, K.; Bayat, A.; Shafiee, A. Skin biomechanics: A potential therapeutic intervention target to reduce scarring. Burn. Trauma 2022, 10, tkac036. [Google Scholar] [CrossRef]
- Huang, C.; Liu, L.; You, Z.; Wang, B.; Du, Y.; Ogawa, R. Keloid progression: A stiffness gap hypothesis. Int. Wound J. 2017, 14, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Liu, M.; Pan, L.; Wu, J.; Wang, C.; Yang, L.; Liu, W.; Xu, W.; Lei, M. Biomechanical regulatory factors and therapeutic targets in keloid fibrosis. Front. Pharmacol. 2022, 13, 906212. [Google Scholar] [CrossRef] [PubMed]
- Hang, J.; Chen, J.; Zhang, W.; Yuan, T.; Xu, Y.; Zhou, B. Correlation between elastic modulus and clinical severity of pathological scars: A cross-sectional study. Sci. Rep. 2021, 11, 23324. [Google Scholar] [CrossRef]
- Juhasz, M.L.W.; Cohen, J.L. Microneedling for the Treatment of Scars: An Update for Clinicians. Clin. Cosmet. Investig. Dermatol. 2020, 13, 997–1003. [Google Scholar] [CrossRef]
- Zhang, X.P.; He, Y.T.; Li, W.X.; Chen, B.Z.; Zhang, C.Y.; Cui, Y.; Guo, X.D. An update on biomaterials as microneedle matrixes for biomedical applications. J. Mater. Chem. B 2022, 10, 6059–6077. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wu, C. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef]
- O’Mahony, C. Structural characterization and in-vivo reliability evaluation of silicon microneedles. Biomed. Microdevices 2014, 16, 333–343. [Google Scholar] [CrossRef]
- Davis, S.P.; Martanto, W.; Allen, M.G.; Prausnitz, M.R. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Costa, M. Elucidating the mechanisms of nickel compound uptake: A review of particulate and nano-nickel endocytosis and toxicity. Toxicol. Appl. Pharmacol. 2012, 260, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Azizi Machekposhti, S.; Khanna, S.; Shukla, S.; Narayan, R. Microneedle fabrication methods and applications. MRS Commun. 2023, 13, 212–224. [Google Scholar] [CrossRef]
- Abrbekoh, F.N.; Salimi, L.; Saghati, S.; Amini, H.; Karkan, S.F.; Moharamzadeh, K.; Sokullu, E.; Rahbarghazi, R. Application of microneedle patches for drug delivery; doorstep to novel therapies. J. Tissue Eng. 2022, 13, 20417314221085390. [Google Scholar] [CrossRef]
- Jeon, E.Y.; Lee, J.; Kim, B.J.; Joo, K.I.; Kim, K.H.; Lim, G.; Cha, H.J. Bio-inspired swellable hydrogel-forming double-layered adhesive microneedle protein patch for regenerative internal/external surgical closure. Biomaterials 2019, 222, 119439. [Google Scholar] [CrossRef]
- Yeo, D.C.; Balmayor, E.R.; Schantz, J.-T.; Xu, C. Microneedle physical contact as a therapeutic for abnormal scars. Eur. J. Med. Res. 2017, 22, 28. [Google Scholar] [CrossRef]
- Liu, F.; Luo, Y.; Chen, H.; Xu, S.; Zhang, D.; Sang, H.; Xu, C.; Zhang, M. Comparison of the efficacy of seven types of microneedles for treating a rabbit hypertrophic scar model. Nanoscale Adv. 2023, 5, 927–933. [Google Scholar] [CrossRef]
- Aarabi, S.; Longaker, M.T.; Gurtner, G.C. Hypertrophic scar formation following burns and trauma: New approaches to treatment. PLoS Med. 2007, 4, e234. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Henn, D.; Sivaraj, D.; Abbas, D.; Trotsyuk, A.; Perrault, D.; Longaker, M.; Gurtner, G.C. Mechano-Immunomodulation of fibrosis and healing. Plast. Reconstr. Surg.–Glob. Open 2022, 10, 139. [Google Scholar] [CrossRef]
- Kambhampati, S.P.; Mishra, M.K.; Mastorakos, P.; Oh, Y.; Lutty, G.A.; Kannan, R.M. Intracellular delivery of dendrimer triamcinolone acetonide conjugates into microglial and human retinal pigment epithelial cells. Eur. J. Pharm. Biopharm. 2015, 95, 239–249. [Google Scholar] [CrossRef]
- Min, M.S.; Mazori, D.R.; Lee, M.S.; Merola, J.F.; Vleugels, R.A.; Cobos, G.; LaChance, A.H. Successful treatment of keloids and hypertrophic scars with systemic and intralesional dupilumab. J. Drugs Dermatol. JDD 2023, 22, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Q.; Zhang, H.; Kou, F.; Li, Q.; Lyu, C.; Wei, H. Traditional Chinese medicine for hypertrophic scars—A review of the therapeutic methods and potential effects. Front. Pharmacol. 2022, 13, 1025602. [Google Scholar] [CrossRef]
- Xu, Y.; Bian, Q.; Zhang, Y.; Zhang, Y.; Li, D.; Ma, X.; Wang, R.; Hu, W.; Hu, J.; Ye, Y. Single-dose of integrated bilayer microneedles for enhanced hypertrophic scar therapy with rapid anti-inflammatory and sustained inhibition of myofibroblasts. Biomaterials 2025, 312, 122742. [Google Scholar] [CrossRef]
- Elsaie, M.L. Update on management of keloid and hypertrophic scars: A systemic review. J. Cosmet. Dermatol. 2021, 20, 2729–2738. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, X.; Lin, Z.; Mao, H.; Qiu, Z.; Xiang, K.; Ke, T.; Li, L.; Lu, L.; Xiao, L. Layered GelMA/PEGDA hydrogel microneedle patch as an intradermal delivery system for hypertrophic scar treatment. ACS Appl. Mater. Interfaces 2023, 15, 43309–43320. [Google Scholar] [CrossRef] [PubMed]
- Traverso, V.; Christian, H.C.; Morris, J.F.; Buckingham, J.C. Lipocortin 1 (annexin 1): A candidate paracrine agent localized in pituitary folliculo-stellate cells. Endocrinology 1999, 140, 4311–4319. [Google Scholar] [CrossRef]
- Prado, C.; de Paz, B.; Gómez, J.; López, P.; Rodríguez-Carrio, J.; Suárez, A. Glucocorticoids enhance Th17/Th1 imbalance and signal transducer and activator of transcription 3 expression in systemic lupus erythematosus patients. Rheumatology 2011, 50, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Faruq, O.; Chien, P.N.; Dönmez, N.; Nam, S.-Y.; Heo, C.-Y. Functionalization of silicone surface with drugs and polymers for regulation of capsular contracture. Polymers 2021, 13, 2731. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yi, B.; Xu, Q.; Ma, J.; Yuan, L.; Liu, Y.; Liu, W.; Zhou, Z.; Ning, X.; Zhang, J. ADSCC-CM-Induced Keratin Hydrogel-Based Bioactive Microneedle Patch Containing Triamcinolone Acetonide for the Treatment of Pathological Scar. Adv. Funct. Mater. 2024, 34, 2400457. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Y.; Hao, Y.; Shan, M.; Liu, H.; Liang, Z.; Kuang, X. Deciphering the single-cell transcriptome network in keloids with intra-lesional injection of triamcinolone acetonide combined with 5-fluorouracil. Front. Immunol. 2023, 14, 1106289. [Google Scholar] [CrossRef]
- Yang, Z.-R.; Suo, H.; Fan, J.-W.; Lv, N.; Du, K.; Ma, T.; Qin, H.; Li, Y.; Yang, L.; Zhou, N. Endogenous stimuli-responsive separating microneedles to inhibit hypertrophic scar through remodeling the pathological microenvironment. Nat. Commun. 2024, 15, 2038. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Tan, W.D.; Srivastava, R.; Yow, A.P.; Wong, D.W.; Tey, H.L. Dissolving triamcinolone-embedded microneedles for the treatment of keloids: A single-blinded intra-individual controlled clinical trial. Dermatol. Ther. 2019, 9, 601–611. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Y.; Yan, D.; Sun, Y.; Li, M.; Cao, H.; Wang, S.; Meng, J. Integrating pharmacokinetics and network analysis to investigate the mechanism of Moutan Cortex in blood-heat and blood stasis syndrome. Chin. Med. 2022, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic acid and diabetes mellitus: Its association with oxidative stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef]
- Huang, H.; Shen, Y.; Yang, X.; Hou, C.; Ke, X.; Yang, R. Dissolvable Microneedles Loaded with Asiaticoside Nanocrystals Stabilized by Panax Notoginseng Saponins for Hypertrophic Scar Treatment. J. Drug Deliv. Sci. Technol. 2024, 98, 105854. [Google Scholar] [CrossRef]
- Zhao, B.; Guo, W.; Zhou, X.; Xue, Y.; Wang, T.; Li, Q.; Tan, L.L.; Shang, L. Ferroptosis-mediated synergistic therapy of hypertrophic scarring based on metal–organic framework microneedle patch. Adv. Funct. Mater. 2023, 33, 2300575. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and biological activity of medicinal plants in wound healing: An overview of current research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, X.; Zhang, Y.; Zhao, X.; Zhao, J.; Wang, X. The potential of functionalized dressing releasing flavonoids facilitates scar-free healing. Front. Med. 2022, 9, 978120. [Google Scholar] [CrossRef]
- Ning, X.; Wiraja, C.; Chew, W.T.S.; Fan, C.; Xu, C. Transdermal delivery of Chinese herbal medicine extract using dissolvable microneedles for hypertrophic scar treatment. Acta Pharm. Sin. B 2021, 11, 2937–2944. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Jang, H.-S.; Shim, J.; Yeo, E.; Kim, M.-H.; Noh, H.; Oh, S.; Park, J.-H.; Lee, D.; Lee, J.H. 3D keloid spheroid model: Development and application for personalized drug response prediction. Commun. Biol. 2024, 7, 1470. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, Z.; Huang, R.; Tang, D.; Wang, Y.; Song, P.; Mei, L.; Hou, S.; Peng, W.; He, L. Development and optimization of the Glabridin-loaded dissolving microneedle for enhanced treatment of keloid. Int. J. Pharm. X 2024, 8, 100267. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Cheng, H.-W.; Yen, W.-Y.; Tsai, J.-H.; Yeh, C.-Y.; Chen, C.-J.; Liu, J.T.; Chen, S.-Y.; Chang, S.-J. The treatment of keloid scars via modulating heterogeneous gelatin-structured composite microneedles to control transdermal dual-drug release. Polymers 2022, 14, 4436. [Google Scholar] [CrossRef]
- Wu, T.; Hou, X.; Li, J.; Ruan, H.; Pei, L.; Guo, T.; Wang, Z.; Ci, T.; Ruan, S.; He, Y. Microneedle-mediated biomimetic cyclodextrin metal organic frameworks for active targeting and treatment of hypertrophic scars. ACS Nano 2021, 15, 20087–20104. [Google Scholar] [CrossRef]
- Morihara, K.; Takai, S.; Takenaka, H.; Sakaguchi, M.; Okamoto, Y.; Morihara, T.; Miyazaki, M.; Kishimoto, S. Cutaneous tissue angiotensin–converting enzyme may participate in pathologic scar formation in human skin. J. Am. Acad. Dermatol. 2006, 54, 251–257. [Google Scholar] [CrossRef]
- Ehanire, T.; Ren, L.; Bond, J.; Medina, M.; Li, G.; Bashirov, L.; Chen, L.; Kokosis, G.; Ibrahim, M.; Selim, A. Angiotensin II stimulates canonical TGF-β signaling pathway through angiotensin type 1 receptor to induce granulation tissue contraction. J. Mol. Med. 2015, 93, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, J.; Wang, Y.; Chen, D.; Huang, J.; Dai, W.; Peng, P.; Guo, L.; Lei, Y. Intradermal delivery of an angiotensin II receptor blocker using a personalized microneedle patch for treatment of hypertrophic scars. Biomater. Sci. 2023, 11, 583–595. [Google Scholar] [CrossRef]
- Hay, J.; Shahzeidi, S.; Laurent, G. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol. 1991, 65, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.I.; Kim, S.; Cho, S.W.; Cho, M.K. The efficacy of bleomycin for treating keloid and hypertrophic scar: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2020, 19, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.B.; Romanelli, C.; Delatti, M.d.A.; Santos, M.A.S.d.; Siqueira, D.M.; Victó, B. Uso de bleomicina em queloides e cicatrizes hipertróficas: Revisão da literatura. Surg. Cosmet. Dermatol. 2016, 8, 97–101. [Google Scholar] [CrossRef]
- Huu, N.D.; Huu, S.N.; Le Thi, X.; Van, T.N.; Minh, P.P.T.; Minh, T.T.; Van, T.H.; Le Huyen, M.; Hau, K.T.; Gandolfi, M. Successful treatment of intralesional bleomycin in keloids of Vietnamese population. Open Access Maced. J. Med. Sci. 2019, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Ritz, R.; Scheidle, C.; Noell, S.; Roser, F.; Schenk, M.; Dietz, K.; Strauss, W.S. In vitro comparison of hypericin and 5-aminolevulinic acid-derived protoporphyrin IX for photodynamic inactivation of medulloblastoma cells. PLoS ONE 2012, 7, e51974. [Google Scholar] [CrossRef]
- Tosa, M.; Ogawa, R. Photodynamic therapy for keloids and hypertrophic scars: A review. Scars Burn. Heal. 2020, 6, 2059513120932059. [Google Scholar] [CrossRef]
- Heckenkamp, J.; Aleksic, M.; Gawenda, M.; Breuer, S.; Brabender, J.; Mahdavi, A.; Aydin, F.; Brunkwall, J. Modulation of human adventitial fibroblast function by photodynamic therapy of collagen matrix. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 651–659. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, D.; Zhang, Y.; Long, W.; Chai, L.; Myint, T.P.; Zhou, W.; Zhou, L.; Wang, M.; Guo, L. Visible light-driven photodynamic therapy for hypertrophic scars with MOF armored microneedles patch. Front. Chem. 2023, 11, 1128255. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, L.; Ning, X.; Hu, T.; Xu, C.; Liu, W. Enhanced drug permeation into human keloid tissues by sonophoresis-assisted microneedling. SLAS Technol. Transl. Life Sci. Innov. 2021, 26, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Petchsangsai, M.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T. The combination of microneedles with electroporation and sonophoresis to enhance hydrophilic macromolecule skin penetration. Biol. Pharm. Bull. 2014, 37, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Heath, B. Iontophoresis and electroporation-assisted microneedles: Advancements and therapeutic potentials in transdermal drug delivery. Drug Deliv. Transl. Res. 2024, 1–23. [Google Scholar] [CrossRef]
- Falk, H.; Vissing, M.; Wooler, G.; Gehl, J. Calcium electroporation for keloids: A first-in-man phase I study. Dermatology 2021, 237, 961–969. [Google Scholar] [CrossRef]
- Karina, K.; Ekaputri, K.; Andrew, H.; Biben, J.A. Microneedle electroporation for intralesional administration of corticosteroid treatment of keloid scar. Acta Derm.-Venereol. 2023, 103, adv13402. [Google Scholar] [CrossRef] [PubMed]
- Tawfic, S.O.; Hassan, A.S.; El-Zahraa SH Aly, F.; Elbendary, A.; Shaker, O.G.; AlOrbani, A.M. Fractional microneedle radiofrequency versus fractional carbon dioxide laser in the treatment of postburn hypertrophic scars. Lasers Surg. Med. 2022, 54, 1089–1098. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.G.; Choi, S.; Lee, J.; Lee, Y.I.; Kim, J.; Lee, J.H. Combination of Fractional Microneedling Radiofrequency and Ablative Fractional Laser versus Ablative Fractional Laser Alone for Acne and Acne Scars. Yonsei Med. J. 2023, 64, 721. [Google Scholar] [CrossRef] [PubMed]
- McClure, C.M.; McClure, K. Keloid Treatment with High Intensity Focused Radiofrequency: A Case Report. Med. Lasers 2018, 7, 79–82. [Google Scholar] [CrossRef]
- Cucu, C.; Butacu, A.I.; Niculae, B.D.; Tiplica, G.S. Benefits of fractional radiofrequency treatment in patients with atrophic acne scars-Literature review. J. Cosmet. Dermatol. 2021, 20, 381–385. [Google Scholar] [CrossRef]
- Tripathi, S.; Soni, K.; Agrawal, P.; Gour, V.; Mondal, R.; Soni, V. Hypertrophic scars and keloids: A review and current treatment modalities. Biomed. Dermatol. 2020, 4, 11. [Google Scholar] [CrossRef]
- Murakami, T.; Shigeki, S. Pharmacotherapy for keloids and hypertrophic scars. Int. J. Mol. Sci. 2024, 25, 4674. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Wang, Y.; Chi, J.; Yu, Y.; Zhao, Y.; Luo, Y.; Wang, Y. Porous MOF microneedle array patch with photothermal responsive nitric oxide delivery for wound healing. Adv. Sci. 2022, 9, 2103449. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-X.; Chen, Y.-L.; Feng, P.-F.; Wang, C.-C.; Li, X.-K.; Liu, L.-L.; Tang, Y. Hierarchically porous MOF-based microneedles for glucose-responsive infected diabetic wound treatment. Mater. Chem. Front. 2022, 6, 680–688. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Ye, R.; Zheng, Y.; Li, X.; Chen, Y.; Xie, X.; Jiang, L. Smartphone-powered iontophoresis-microneedle array patch for controlled transdermal delivery. Microsyst. Nanoeng. 2020, 6, 112. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, R.; Gong, X.; Yang, J.; Liu, B.; Xu, Y.; Nie, G.; Xie, X.; Jiang, L. Iontophoresis-driven microneedle patch for the active transdermal delivery of vaccine macromolecules. Microsyst. Nanoeng. 2023, 9, 35. [Google Scholar] [CrossRef]
- Bok, M.; Zhao, Z.-J.; Jeon, S.; Jeong, J.-H.; Lim, E. Ultrasonically and iontophoretically enhanced drug-delivery system based on dissolving microneedle patches. Sci. Rep. 2020, 10, 2027. [Google Scholar] [CrossRef]
- Chen, B.; Wei, J.; Iliescu, C. Sonophoretic enhanced microneedles array (SEMA)—Improving the efficiency of transdermal drug delivery. Sens. Actuators B Chem. 2010, 145, 54–60. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Yang, F.; Wang, X.; Zhang, J.; Han, X.; Zhang, X.; Wang, Z. Application and progress of new technologies and new materials in the treatment of pathological scar. Front. Chem. 2024, 12, 1389399. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; He, T.; Shen, K.; Wang, Y.; Liu, J. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.Y.; Wang, W.B.; Wu, X.L.; Zhang, W.J.; Zhou, G.D.; Gao, Z.; Liu, W. Nintedanib inhibits keloid fibroblast functions by blocking the phosphorylation of multiple kinases and enhancing receptor internalization. Acta Pharmacol. Sin. 2020, 41, 1234–1245. [Google Scholar] [CrossRef]

| MN Type | Material Composition | Therapeutic Agent | Scar Type | Outcome | Ref. |
|---|---|---|---|---|---|
| Layered dissolving MNs | Sodium carboxymethyl cellulose | Asiaticoside (AS), ginsenoside Rb1, and L-carnosine | HTSs | Enhanced therapeutic effects comparable to triamcinolone acetonide (TAC) injections. Improved drug delivery through skin barrier. | [43] |
| Dissolving MNs (DMNs) | Dextran-Polyethyleneimine (PEI) | TAC | HTSs and Keloid | TAC-DMNs showed significant reduction in HTSs, while pristine DMN and TAC-DMNs showed no impacts on keloids. | [44] |
| DMNs | Mucin | Oregano essential oil | HTSs | Leveraged anti-inflammatory properties of oregano essential oil. Effective delivery through microneedle system. | [45] |
| Bilayer dissolving MNs (BMNs) | Chitosan and Dextran for tips | TAC and 5-Fluorouraci | HTSs | TA-5-Fu-BMN, with its ability to reduce fibroblast proliferation, collagen deposition, and scar elevation while downregulating Collagen I and TGF-β1. | [46] |
| DMNs | Hyaluronic acid incorporated with Hydroxypropyl-β-cyclodextrin for tips | miR-141-3p-functionalized exosomes | HTSs | miR-141OE-Exos@DMNs reduces scar thickness, modulates the TGF-β2/Smad pathway, improves collagen fiber organization, and enhances fibroblast distribution. | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Kim, Y.-S.; Lim, J.Y. Leveraging Microneedles for Raised Scar Management. Polymers 2025, 17, 108. https://doi.org/10.3390/polym17010108
Jin Z, Kim Y-S, Lim JY. Leveraging Microneedles for Raised Scar Management. Polymers. 2025; 17(1):108. https://doi.org/10.3390/polym17010108
Chicago/Turabian StyleJin, Zhengyun, Young-Seong Kim, and Joong Yeon Lim. 2025. "Leveraging Microneedles for Raised Scar Management" Polymers 17, no. 1: 108. https://doi.org/10.3390/polym17010108
APA StyleJin, Z., Kim, Y.-S., & Lim, J. Y. (2025). Leveraging Microneedles for Raised Scar Management. Polymers, 17(1), 108. https://doi.org/10.3390/polym17010108






