Characterization of Chitosan Hydrogels Obtained through Phenol and Tripolyphosphate Anionic Crosslinking
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
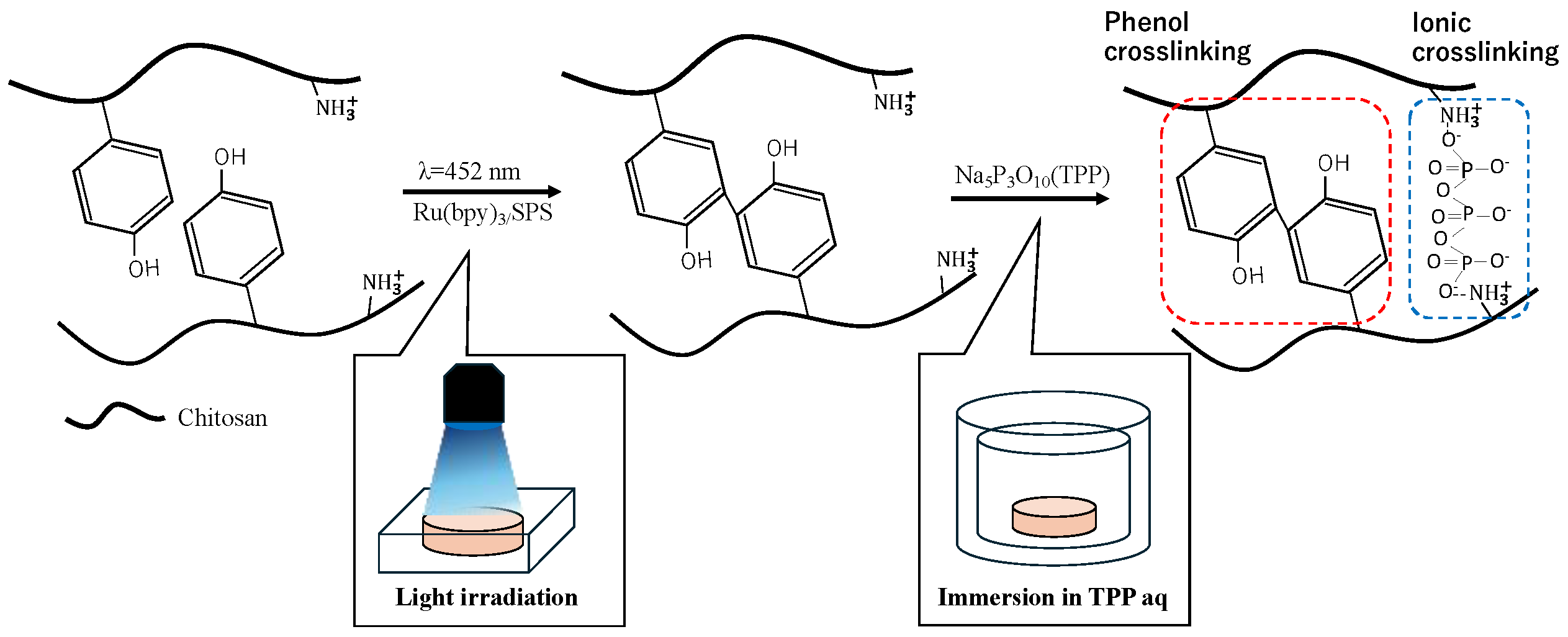
2.2. ChPh Synthesis
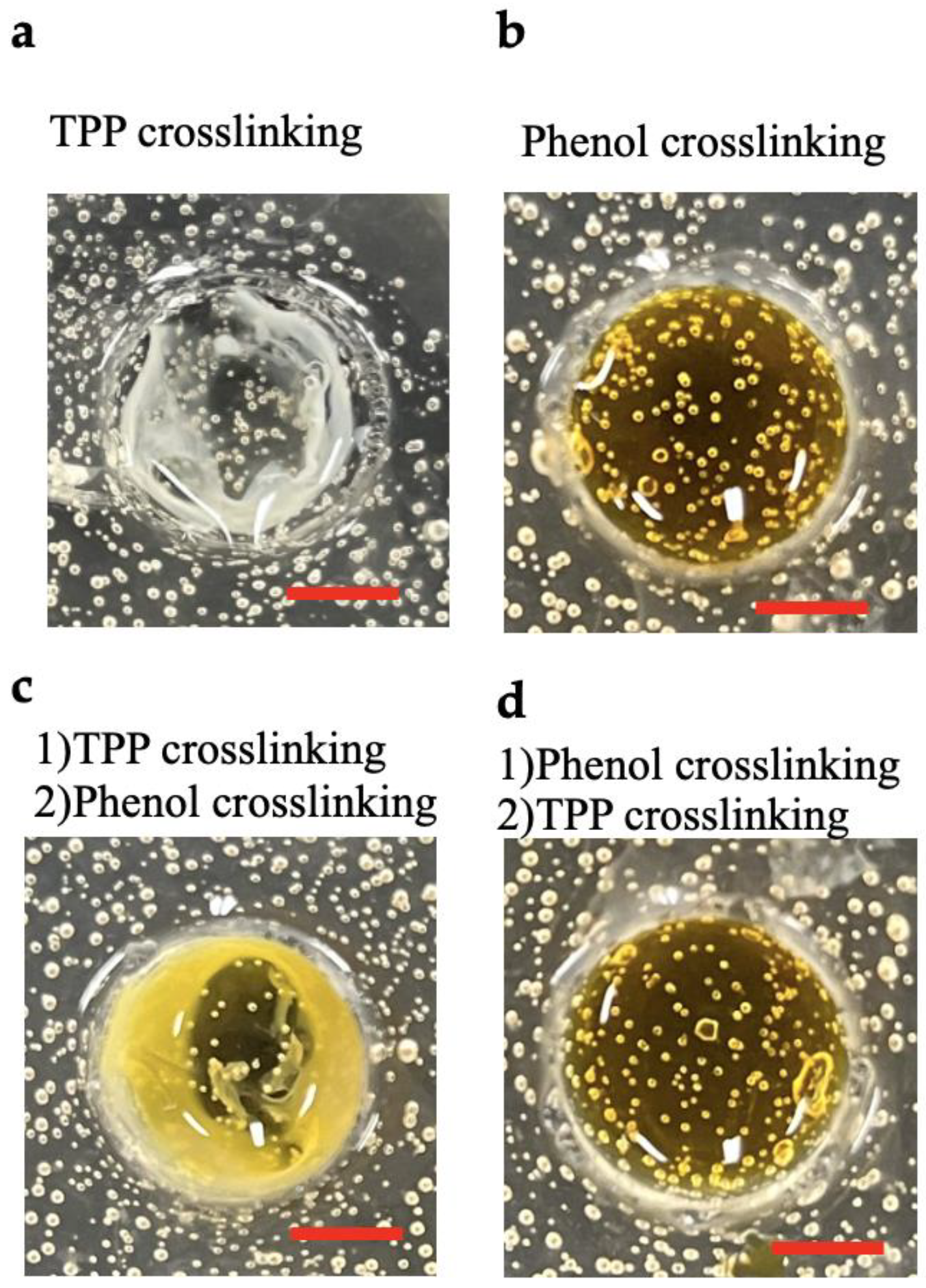
2.3. Comparison of Chitosan Hydrogels Obtained Using Different Crosslinking Methods
2.4. TPP Phenol-Crosslinked Hydrogel Preparation
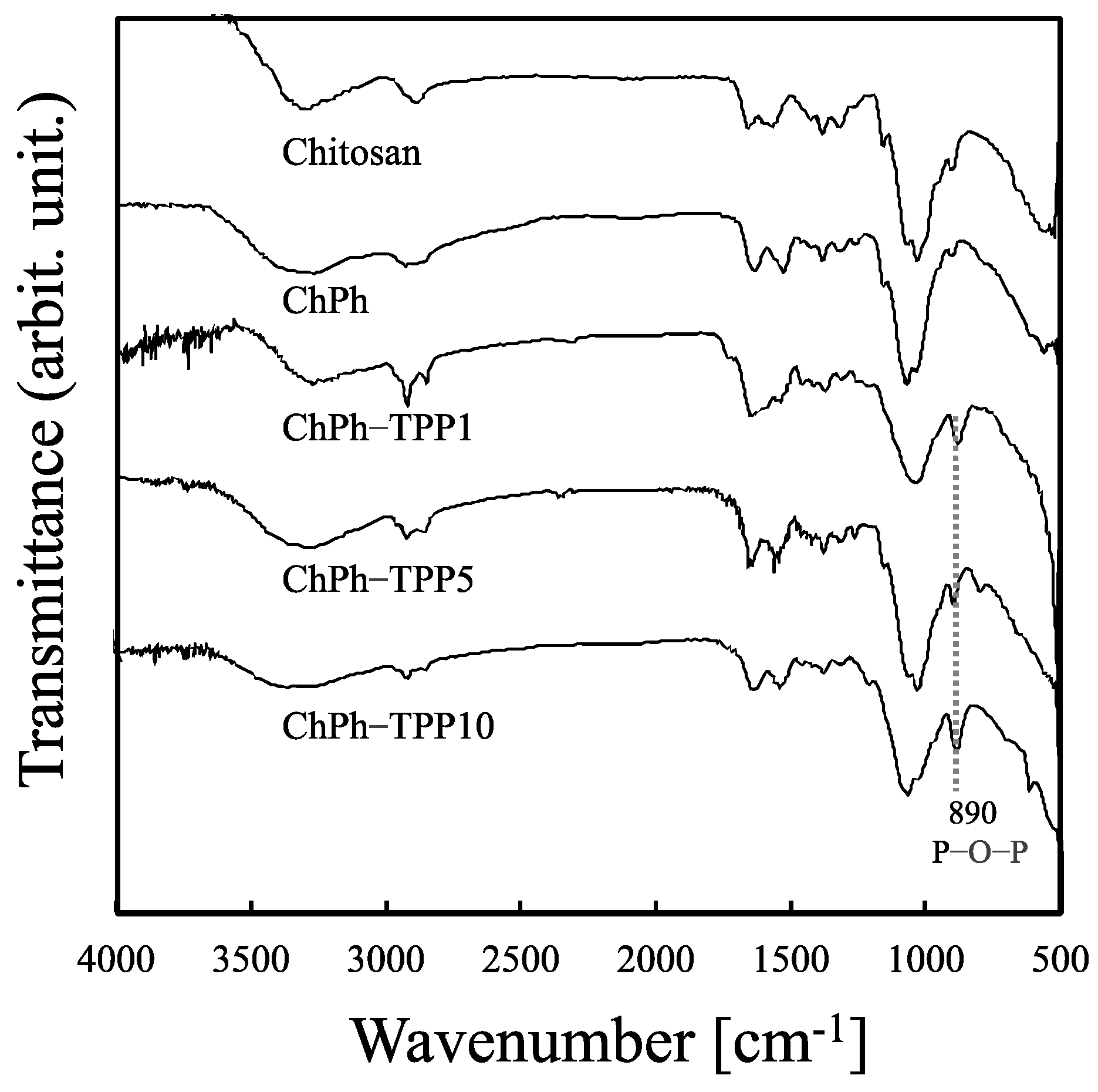
2.5. Fourier-Transform Infrared Spectroscopy
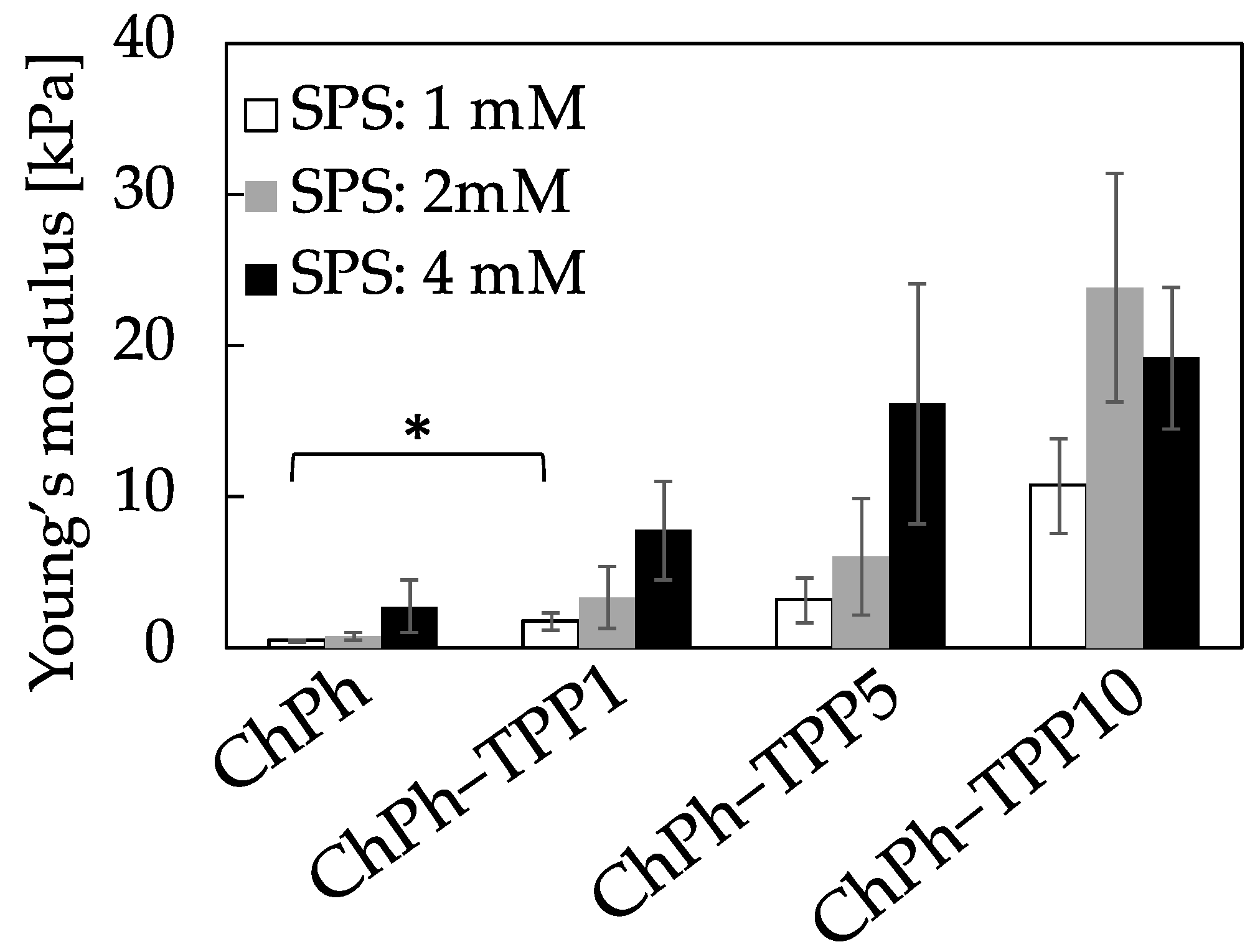
2.6. Young’s Modulus
2.7. Degree of Swelling
2.8. Water Retention
2.9. Antimicrobial Activity
2.10. Removal of Ionic Crosslinking
2.11. Statistical Analysis
3. Results and Discussion
3.1. Comparison of Chitosan Hydrogels Obtained Using Different Crosslinking Methods
3.2. FTIR Spectroscopy
3.3. Young’s Modulus
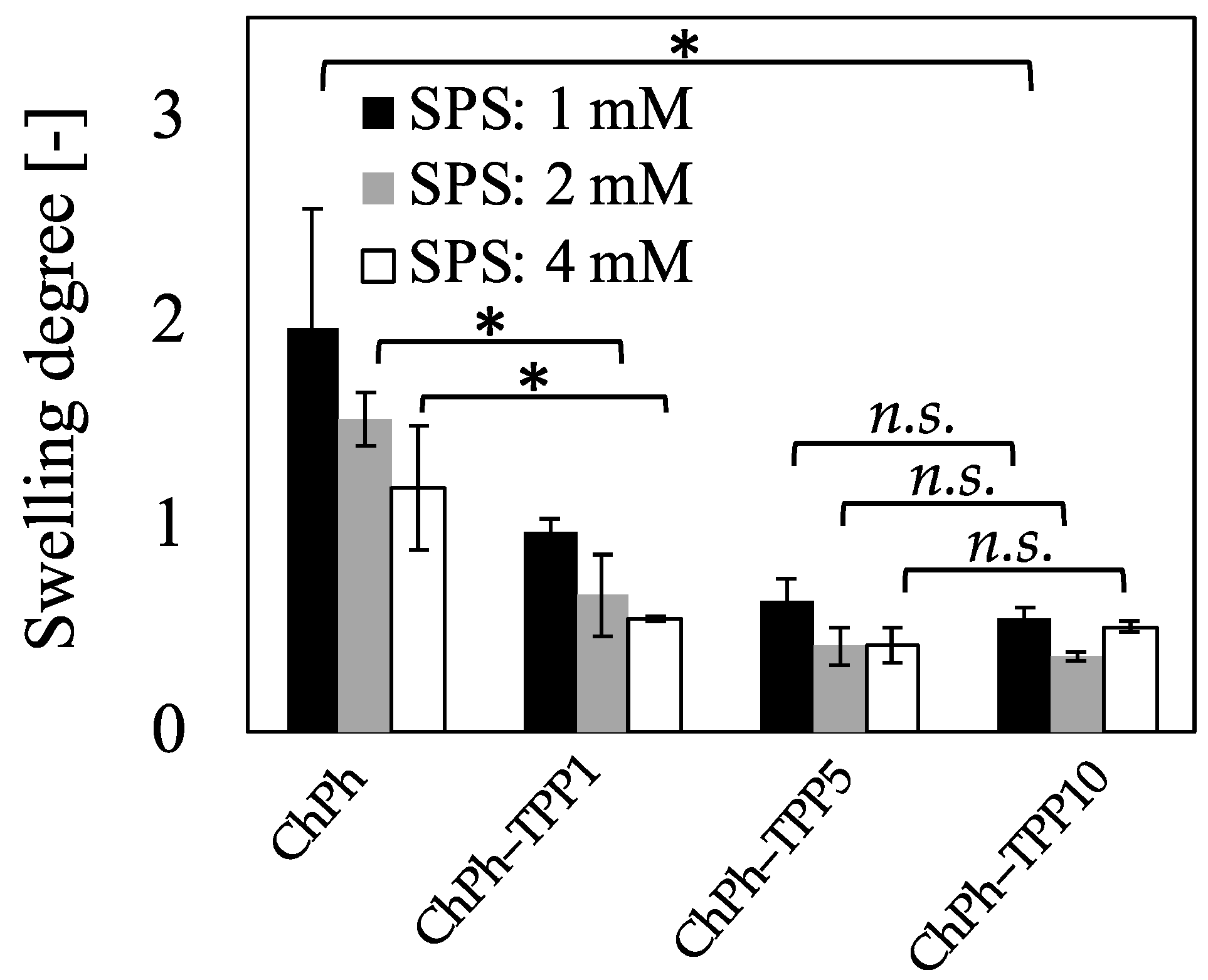
3.4. Degree of Swelling
3.5. Water Retention
3.6. Antimicrobial Activity
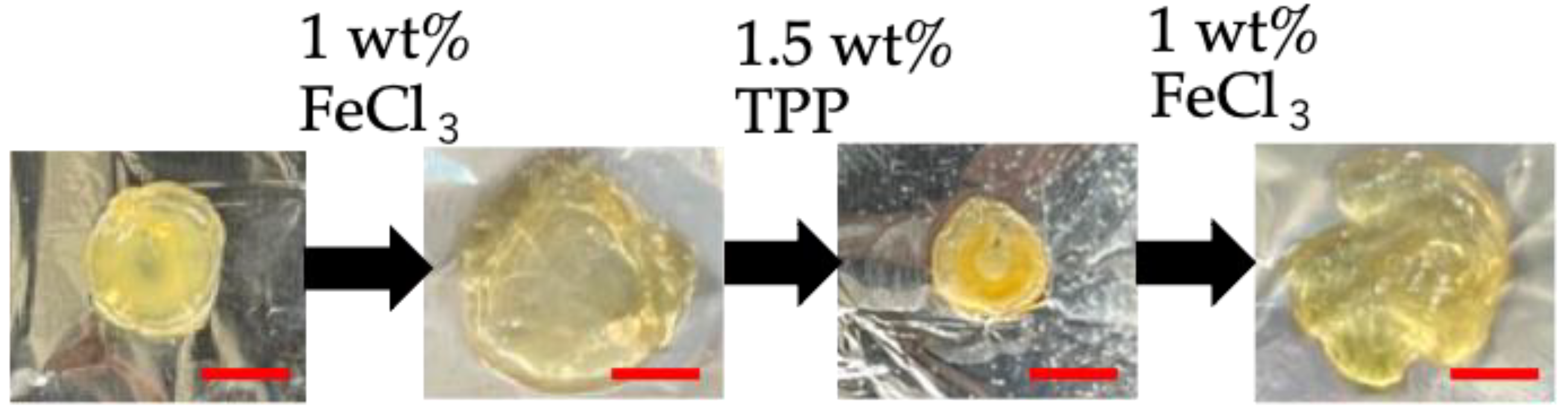
3.7. Removal of Ionic Crosslinking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spicer, C.D. Hydrogel Scaffolds for Tissue Engineering: The Importance of Polymer Choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Won, P.; Ko, S.H.; Majidi, C.; Feinberg, A.W.; Webster-Wood, V.A. Biohybrid Actuators for Soft Robotics: Challenges in Scaling up. Actuators 2020, 9, 96. [Google Scholar] [CrossRef]
- Nie, M.; Takeuchi, S. 3D Biofabrication Using Living Cells for Applications in Biohybrid Sensors and Actuators. ACS Appl. Bio Mater. 2020, 3, 8121–8126. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Peng, J.; Xiao, H.; Xu, X.; Qian, Z. Polysaccharide Hydrogels: Functionalization, Construction and Served as Scaffold for Tissue Engineering. Carbohydr. Polym. 2022, 278, 118952. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Niu, Y.; Xing, P.; Wang, C. Bioactive Polysaccharides from Natural Resources Including Chinese Medicinal Herbs on Tissue Repair. Chin. Med. 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Song, H.Q.; Fan, Y.; Hu, Y.; Cheng, G.; Xu, F.J. Polysaccharide–Peptide Conjugates: A Versatile Material Platform for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2005978. [Google Scholar] [CrossRef]
- McCarthy, R.R.; Ullah, M.W.; Pei, E.; Yang, G. Antimicrobial Inks: The Anti-Infective Applications of Bioprinted Bacterial Polysaccharides. Trends Biotechnol. 2019, 37, 1155–1159. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y. Chitosan-Based Biomaterials: From Discovery to Food Application. Polym. Adv. Technol. 2020, 31, 2408–2421. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the Antimicrobial Activity of Chitosan. Polimeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- el Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, Chemical Modification and Characterization of Chitin and Chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biot. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Singh, R.P.; Kumari, P.; Reddy, C.R.K. Antimicrobial Compounds from Seaweeds-Associated Bacteria and Fungi. Appl. Microbiol. Biotechnol. 2015, 99, 1571–1586. [Google Scholar] [CrossRef]
- Cabañas-Romero, L.V.; Valls, C.; Valenzuela, S.V.; Roncero, M.B.; Pastor, F.I.J.; Diaz, P.; Martínez, J. Bacterial Cellulose-Chitosan Paper with Antimicrobial and Antioxidant Activities. Biomacromolecules 2020, 21, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-Acorn Starch-Eugenol Edible Film: Physico-Chemical, Barrier, Antimicrobial, Antioxidant and Structural Properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Han, C.; Liu, S.; Hao, X.; Rao, Y.; Gong, Z.; Sun, Z. Sodium Alginate-Chitosan Hydrogel-Based Soft Ionic Artificial Muscle with Different Moisture Content. Ionics 2020, 26, 6371–6378. [Google Scholar] [CrossRef]
- Godeau, X.Y.; Andrianandrasana, F.J.; Volkova, O.; Szczepanski, C.R.; Zenerino, A.; Montreuil, O.; Godeau, R.P.; Kuzhir, P.; Godeau, G. Investigation on Dung Beetle’s (Heliocopris Hope, 1838) Chitosan Valorisation for Hydrogel 3D Printing. Int. J. Biol. Macromol. 2022, 199, 172–180. [Google Scholar] [CrossRef]
- Ramirez Caballero, S.S.; Saiz, E.; Montembault, A.; Tadier, S.; Maire, E.; David, L.; Delair, T.; Grémillard, L. 3-D Printing of Chitosan-Calcium Phosphate Inks: Rheology, Interactions and Characterization. J. Mater. Sci. Mater. Med. 2019, 30, 6. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fu, H.; Wang, Z.; Meng, Q.; Liu, S.; Wang, H.; Zheng, X.; Dai, J.; Zhang, Z. BMSCs-Laden Gelatin/Sodium Alginate/Carboxymethyl Chitosan Hydrogel for 3D Bioprinting. RSC Adv. 2016, 6, 108423–108430. [Google Scholar] [CrossRef]
- Lu, Z.; Zou, L.; Zhou, X.; Huang, D.; Zhang, Y. High Strength Chitosan Hydrogels Prepared from NaOH/Urea Aqueous Solutions: The Role of Thermal Gelling. Carbohydr. Polym. 2022, 297, 120054. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; et al. Effects of Genipin Cross-Linking of Chitosan Hydrogels on Cellular Adhesion and Viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Srivastava, P.; Singh, A.; Singh, D.; Malviya, T. Polysaccharide-Silica Hybrids: Design and Applications. Polym. Rev. 2016, 56, 113–136. [Google Scholar] [CrossRef]
- Condi Mainardi, J.; Rezwan, K.; Maas, M. Genipin-Crosslinked Chitosan/Alginate/Alumina Nanocomposite Gels for 3D Bioprinting. Bioprocess Biosyst. Eng. 2022, 45, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Ramaswamy, Y.; Roberts, J.J.; Alves, M.H.; Poole-Warren, L.A.; Martens, P.J. Promoting Cell Survival and Proliferation in Degradable Poly(Vinyl Alcohol)-Tyramine Hydrogels. Macromol. Biosci. 2015, 15, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Kamei, H.; Mori, T.; Hotta, T.; Ohi, H.; Nakahata, M.; Taya, M. Visible Light-Induced Hydrogelation of an Alginate Derivative and Application to Stereolithographic Bioprinting Using a Visible Light Projector and Acid Red. Biomacromolecules 2018, 19, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, M.; Kojima, M.; Nakahata, M.; Sakai, S. Visible Light-Curable Chitosan Ink for Extrusion-Based and Vat Polymerization-Based 3d Bioprintings. Polymers 2021, 13, 1382. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, C.; Chang, S.; Hsu, S. An Injectable, Self-Healing Phenol-Functionalized Chitosan Hydrogel with Fast Gelling Property and Visible Light-Crosslinking Capability for 3D Printing. Acta Biomater. 2020, 122, 211–219. [Google Scholar] [CrossRef]
- Sakai, S.; Komatani, K.; Taya, M. Glucose-Triggered Co-Enzymatic Hydrogelation of Aqueous Polymer Solutions. RSC Adv. 2012, 2, 1502–1507. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of Surface Charge, Particle Size and Morphological Properties of Chitosan-TPP Nanoparticles Intended for Gene Delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Farrugia, B.L.; Dargaville, T.R.; Dhara, S. Physico-Chemical/Biological Properties of Tripolyphosphate Cross-Linked Chitosan Based Nanofibers. Mater. Sci. Eng. C 2013, 33, 1446–1454. [Google Scholar] [CrossRef]
- Sakai, S.; Yamada, Y.; Zenke, T.; Kawakami, K. Novel Chitosan Derivative Soluble at Neutral PH and In-Situ Gellable via Peroxidase-Catalyzed Enzymatic Reaction. J. Mater. Chem. 2009, 19, 230–235. [Google Scholar] [CrossRef]
- Sakai, S.; Khanmohammadi, M.; Khoshfetrat, A.B.; Taya, M. Horseradish Peroxidase-Catalyzed Formation of Hydrogels from Chitosan and Poly(Vinyl Alcohol) Derivatives Both Possessing Phenolic Hydroxyl Groups. Carbohydr. Polym. 2014, 111, 404–409. [Google Scholar] [CrossRef]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An Injectable, in Situ Enzymatically Gellable, Gelatin Derivative for Drug Delivery and Tissue Engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef]
- Sakai, S.; Ohi, H.; Taya, M. Gelatin/Hyaluronic Acid Content in Hydrogels Obtained through Blue Light-Induced Gelation Affects Hydrogel Properties and Adipose Stem Cell Behaviors. Biomolecules 2019, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Lohrasbi, S.; Mirzaei, E.; Karimizade, A.; Takallu, S.; Rezaei, A. Collagen/Cellulose Nanofiber Hydrogel Scaffold: Physical, Mechanical and Cell Biocompatibility Properties. Cellulose 2020, 27, 927–940. [Google Scholar] [CrossRef]
- Wahid, M.H.; Eroglu, E.; LaVars, S.M.; Newton, K.; Gibson, C.T.; Stroeher, U.H.; Chen, X.; Boulos, R.A.; Raston, C.L.; Harmer, S.L. Microencapsulation of Bacterial Strains in Graphene Oxide Nano-Sheets Using Vortex Fluidics. RSC Adv. 2015, 5, 37424–37430. [Google Scholar] [CrossRef]
- Lee, S.-T.; Mi, F.-L.; Shen, Y.-J.; Shyu, S.-S. Equilibrium and Kinetic Studies of Copper(II) Ion Uptake by Chitosan-Tripolyphosphate Chelating Resin. Polymer 2001, 42, 1879–1892. [Google Scholar] [CrossRef]
- Fakhreddin Hosseini, S.; Soleimani, M.R.; Nikkhah, M.; Hosseini, S.F. Chitosan/Sodium Tripolyphosphate Nanoparticles as Efficient Vehicles for Antioxidant Peptidic Fraction from Common Kilka. Int. J. Biol. Macromol. 2018, 111, 730–737. [Google Scholar] [CrossRef]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, e1902026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X. Multi-Scale Multi-Mechanism Design of Tough Hydrogels: Building Dissipation into Stretchy Networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.F.; de Oliveira, D.M.; Pereira, A.G.B.; Rubira, A.F.; Muniz, E.C. Chitosan/TPP Microparticles Obtained by Microemulsion Method Applied in Controlled Release of Heparin. Int. J. Biol. Macromol. 2012, 51, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef]
- Vasconcellos, F.C.; Goulart, G.A.S.; Beppu, M.M. Production and Characterization of Chitosan Microparticles Containing Papain for Controlled Release Applications. Powder Technol. 2011, 205, 65–70. [Google Scholar] [CrossRef]
- Martins, A.F.; Piai, J.F.; Schuquel, I.T.A.; Rubira, A.F.; Muniz, E.C. Polyelectrolyte complexes of chitosan/heparin and N,N,N-trimethyl chitosan/heparin obtained at different pH: I. Preparation, characterization, and controlled release of heparin. Colloid Polym. Sci. 2011, 289, 1133–1144. [Google Scholar] [CrossRef]
- Khoshfetrat, A.B.; Khanmohammadi, M.; Sakai, S.; Taya, M. Enzymatically-Gellable Galactosylated Chitosan: Hydrogel Characteristics and Hepatic Cell Behavior. Int. J. Biol. Macromol. 2016, 92, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Kotani, T.; Harada, R.; Goto, R.; Morita, T.; Bouissil, S.; Dubessay, P.; Pierre, G.; Michaud, P.; El Boutachfaiti, R.; et al. Development of Phenol-Grafted Polyglucuronic Acid and Its Application to Extrusion-Based Bioprinting Inks. Carbohydr. Polym. 2022, 277, 118820. [Google Scholar] [CrossRef]
- Ishihara, S.; Kurosawa, H.; Haga, H. Stiffness-Modulation of Collagen Gels by Genipin-Crosslinking for Cell Culture. Gels 2023, 9, 148. [Google Scholar] [CrossRef]
- Lavrentieva, A.; Fleischhammer, T.; Enders, A.; Pirmahboub, H.; Bahnemann, J.; Pepelanova, I. Fabrication of Stiffness Gradients of GelMA Hydrogels Using a 3D Printed Micromixer. Macromol. Biosci. 2020, 20, e2000107. [Google Scholar] [CrossRef]
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Structure-Activity Relationships in the Hydrophobic Interactions of Polyphenols with Cellulose and Collagen. Biopolymers 2003, 70, 403–413. [Google Scholar] [CrossRef]
- Stefani, R. Computational Study of Natural Phenolic Acid Solubility and Their Interactions with Chitosan. Available online: https://sciforum.net/manuscripts/3862/slides.pdf (accessed on 10 March 2024).
- Ahmad Shariff, S.H.; Daik, R.; Haris, M.S.; Ismail, M.W. Hydrophobic Drug Carrier from Polycaprolactone-b-Poly(Ethylene Glycol) Star-Shaped Polymers Hydrogel Blend as Potential for Wound Healing Application. Polymers 2023, 15, 2072. [Google Scholar] [CrossRef]
- Cui, P.F.; Zhuang, W.R.; Hu, X.; Xing, L.; Yu, R.Y.; Qiao, J.B.; He, Y.J.; Li, F.; Ling, D.; Jiang, H.L. A New Strategy for Hydrophobic Drug Delivery Using a Hydrophilic Polymer Equipped with Stacking Units. Chem. Commun. 2018, 54, 8218–8221. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liu, Y.; Yang, G.; Zhang, A. Water- and Fertilizer-Integrated Hydrogel Derived from the Polymerization of Acrylic Acid and Urea as a Slow-Release N Fertilizer and Water Retention in Agriculture. J. Agric. Food Chem. 2018, 66, 5762–5769. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chen, S.Y.; Lin, Y.L.; Liu, D.M. Synthesis and Characterization of Amphiphatic Carboxymethyl-Hexanoyl Chitosan Hydrogel: Water-Retention Ability and Drug Encapsulation. Langmuir 2006, 22, 9740–9745. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation. Mar. Drugs 2020, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Minamisawa, H.; Iwanami, H.; Arai, N.; Okutani, T. Adsorption behavior of cobalt(II) on chitosan and its determination by tungsten metal furnace atomic absorption spectrometry. Anal. Chim. Acta 1999, 378, 279–285. [Google Scholar] [CrossRef]
- Xu, H.; Matysiak, S. Effect of PH on Chitosan Hydrogel Polymer Network Structure. Chem. Commun. 2017, 53, 7373–7376. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Sapner, V.S.; Chavan, P.P.; Digraskar, R.V.; Narwade, S.S.; Mulik, B.B.; Mali, S.M.; Sathe, B.R. Tyramine Functionalized Graphene: Metal-Free Electrochemical Non-Enzymatic Biosensing of Hydrogen Peroxide. ChemElectroChem 2018, 5, 3191–3197. [Google Scholar] [CrossRef]
- Chang, C.C.; Hou, S.S. Intercalation of Poly(Methyl Methacrylate) into Tyramine-Modified Layered Silicates through Hydrogen-Bonding Interaction. Eur. Polym. J. 2008, 44, 1337–1345. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s), not of the MDPI and/or editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidaka, M.; Kojima, M.; Sakai, S.; Delattre, C. Characterization of Chitosan Hydrogels Obtained through Phenol and Tripolyphosphate Anionic Crosslinking. Polymers 2024, 16, 1274. https://doi.org/10.3390/polym16091274
Hidaka M, Kojima M, Sakai S, Delattre C. Characterization of Chitosan Hydrogels Obtained through Phenol and Tripolyphosphate Anionic Crosslinking. Polymers. 2024; 16(9):1274. https://doi.org/10.3390/polym16091274
Chicago/Turabian StyleHidaka, Mitsuyuki, Masaru Kojima, Shinji Sakai, and Cédric Delattre. 2024. "Characterization of Chitosan Hydrogels Obtained through Phenol and Tripolyphosphate Anionic Crosslinking" Polymers 16, no. 9: 1274. https://doi.org/10.3390/polym16091274
APA StyleHidaka, M., Kojima, M., Sakai, S., & Delattre, C. (2024). Characterization of Chitosan Hydrogels Obtained through Phenol and Tripolyphosphate Anionic Crosslinking. Polymers, 16(9), 1274. https://doi.org/10.3390/polym16091274







