Preparation and Characterization of Glucose-Based Self-Blowing Non-Isocyanate Polyurethane (NIPU) Foams with Different Acid Catalysts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Glucose-Based Non-Isocyanate Polyurethane (GNIPU) [25]
2.3. Preparation of GNIPU Foams
2.4. Water Absorption (24 h) Test
2.5. Compression Test
2.6. Ignition Test
2.7. Limiting Oxygen Index (LOI)
2.8. TG Analysis
2.9. Fourier Transform Infrared (FT-IR) Spectrometry
2.10. Scanning Electron Microscopy (SEM)
3. Results and Discussion
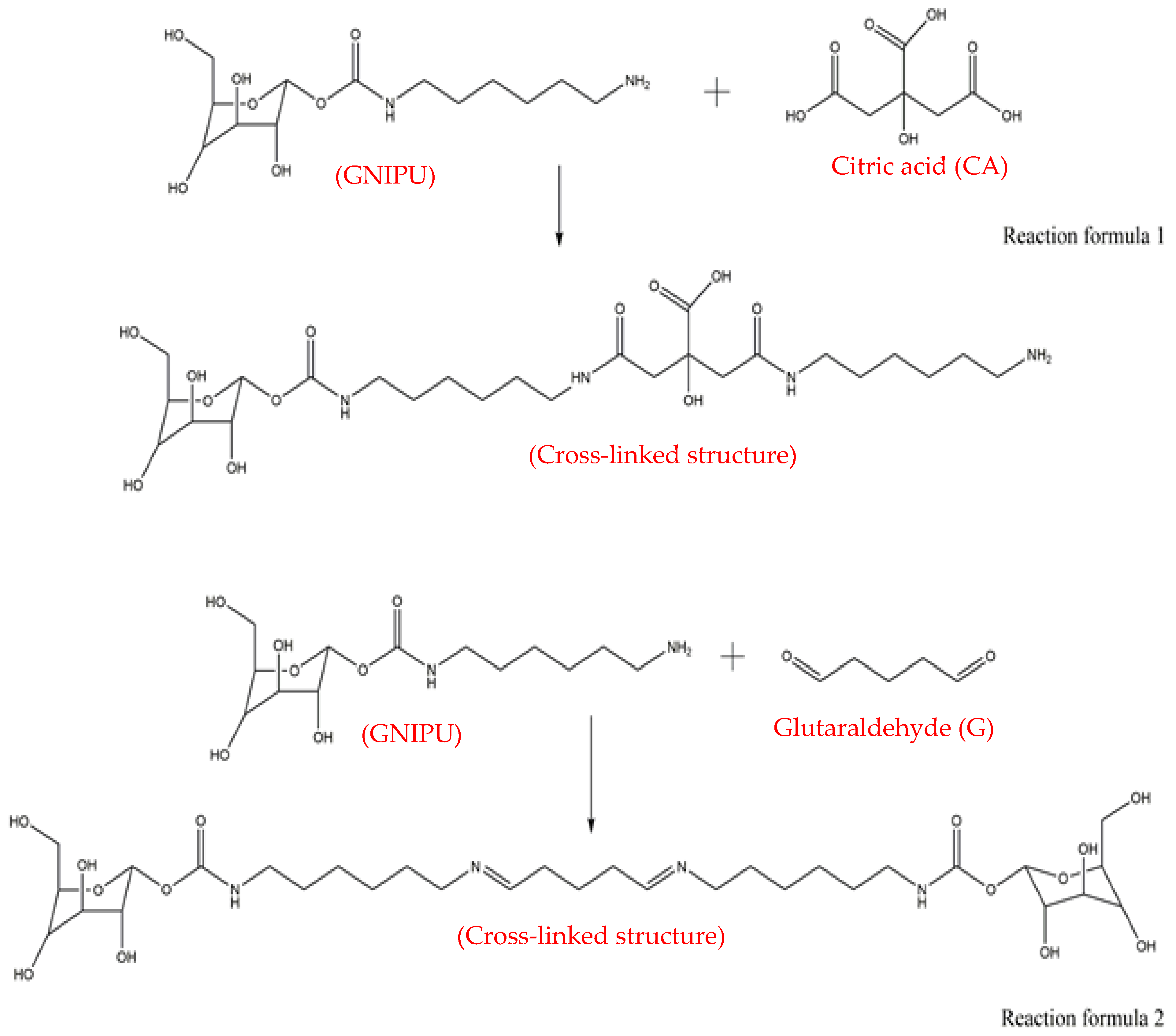
3.1. GNIPU Foam Reaction Mechanism Analysis
3.2. Effects of Different Acid and Glutaraldehyde Additions on Basic Foam Properties
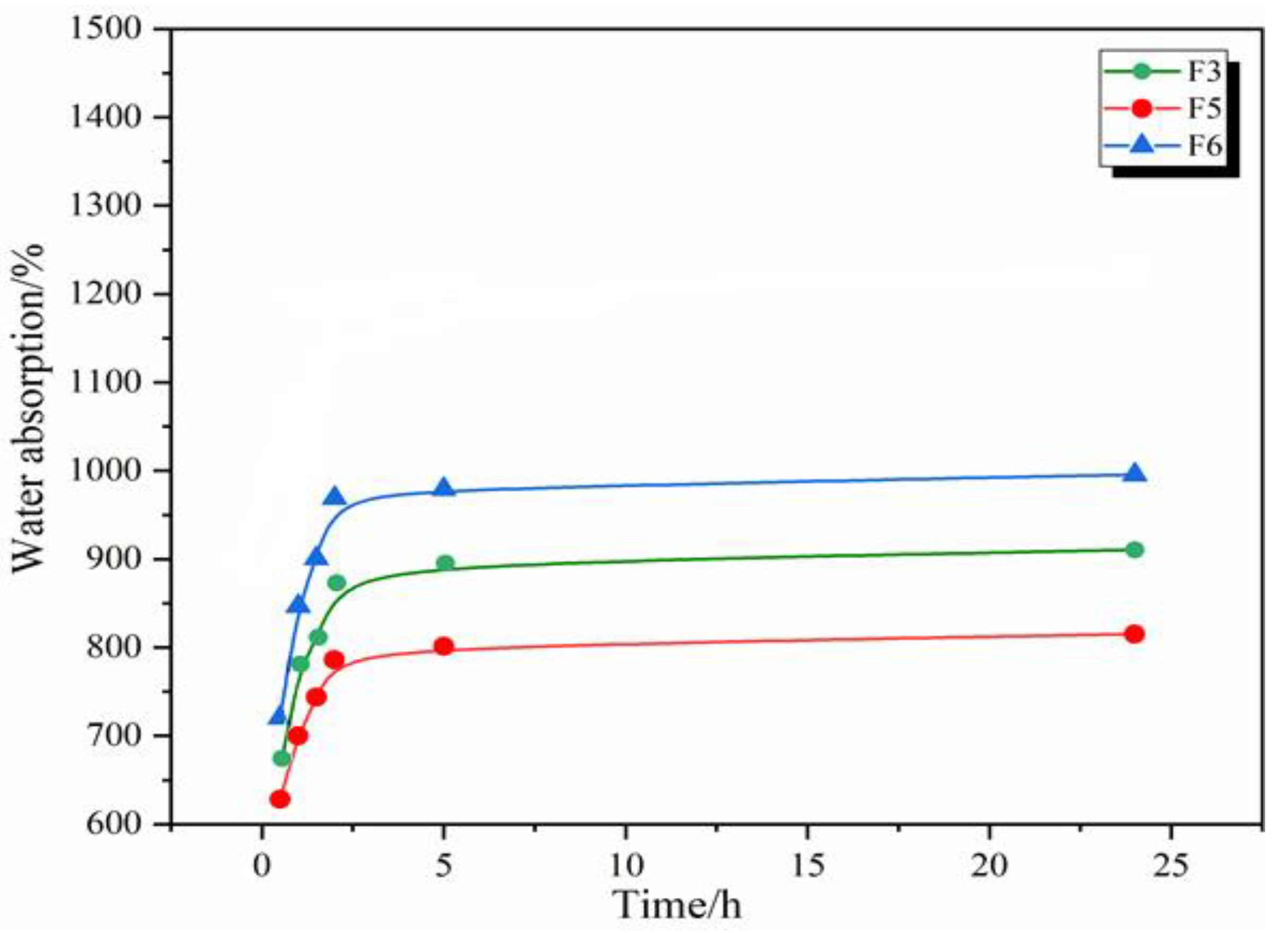
3.3. Water Absorption of GNIPU Foams
3.4. Compression Properties of GNIPU Foams
3.5. Ignition Test Analysis
3.6. Limiting Oxygen Index (LOI) Analysis
3.7. Thermogravimetric Analysis
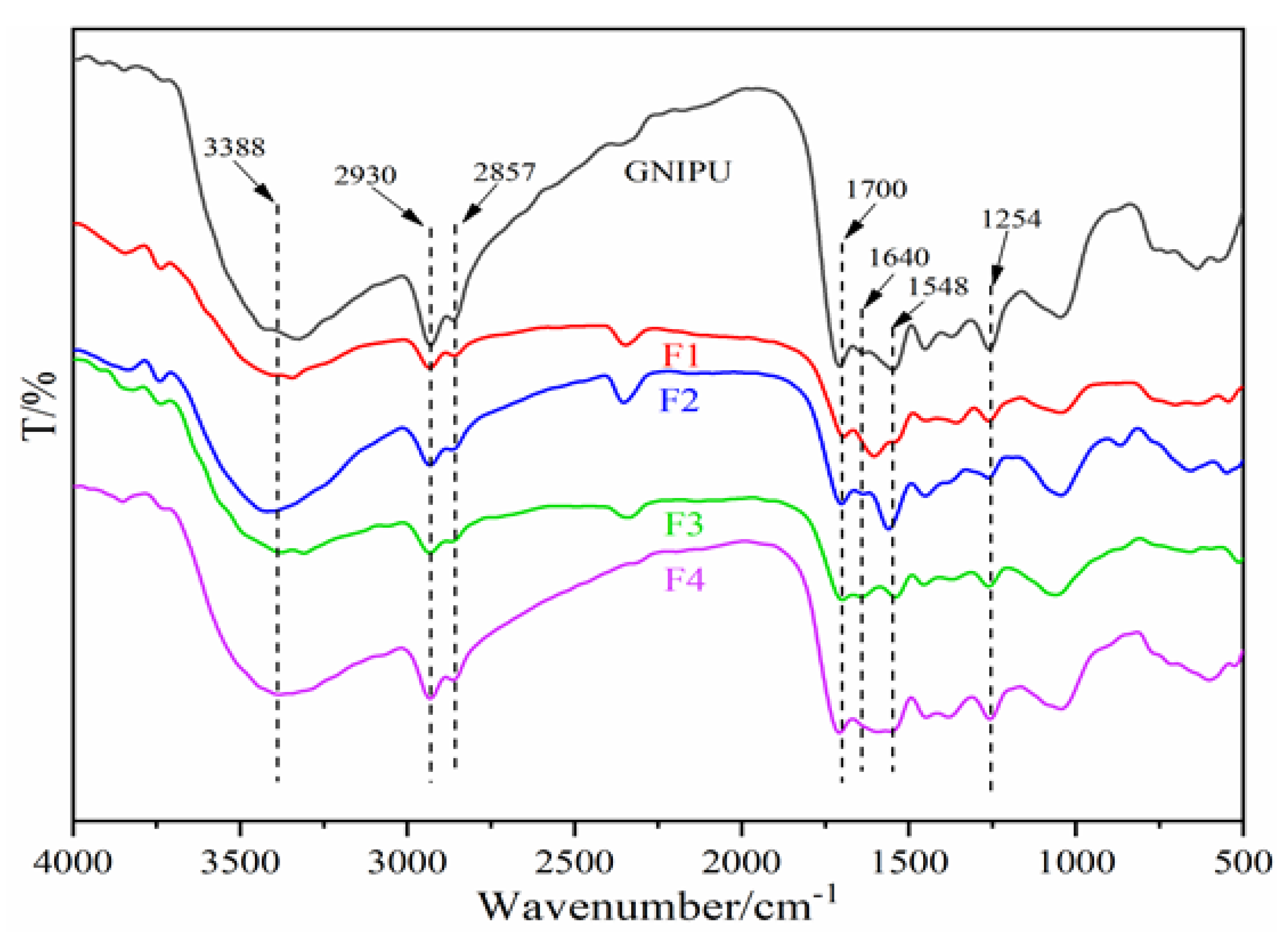
3.8. Fourier Transform Infrared (FT-IR) Spectroscopy
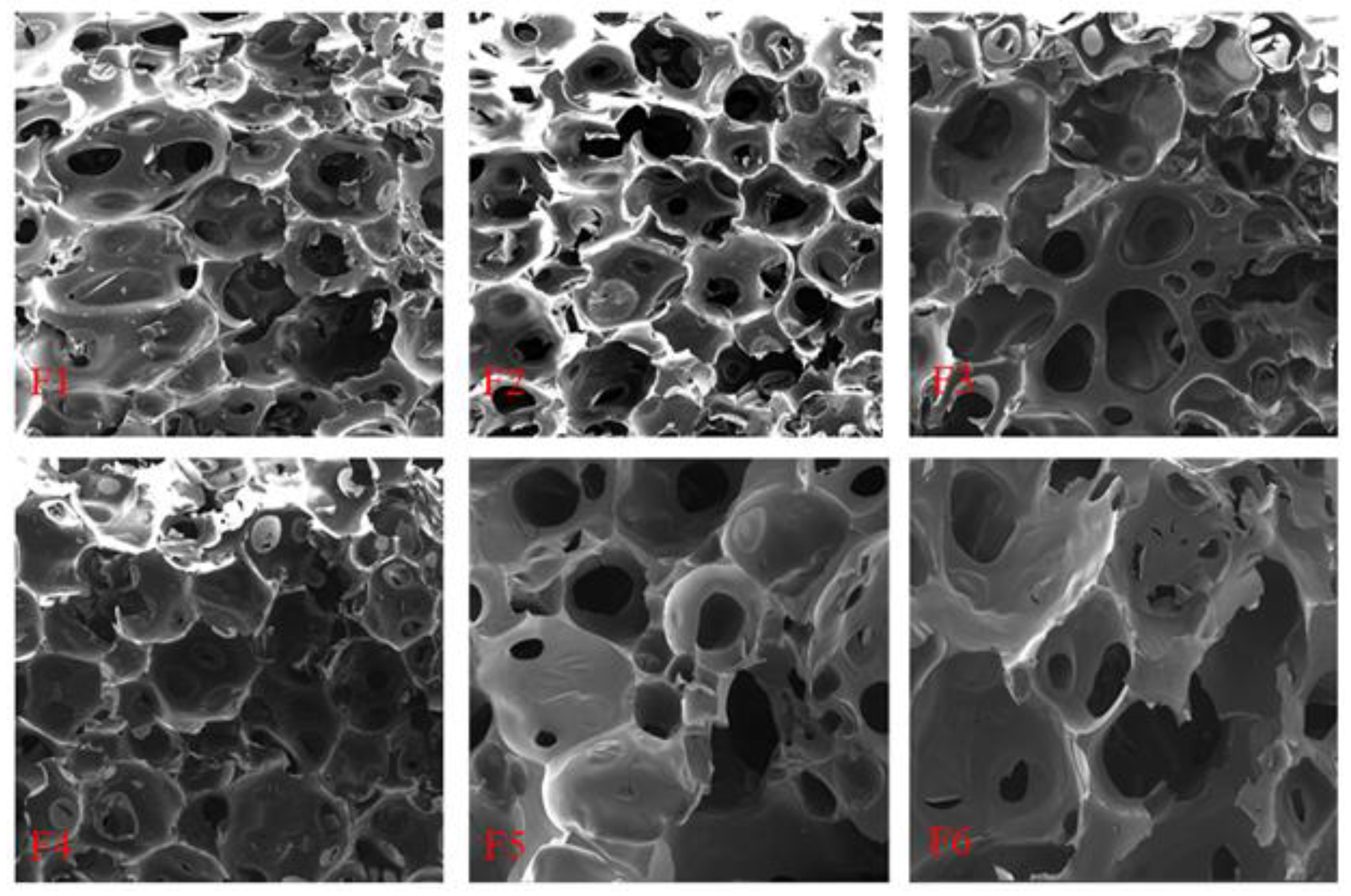
3.9. SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane foams: Past, present, and future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Kirpluks, M.; Kalnbund, D.; Benes, H.; Cabulis, U. Natural oil based highly functional polyols as feedstock for rigid polyurethane foam thermal insulation. Ind. Crops Prod. 2018, 122, 627–636. [Google Scholar] [CrossRef]
- Gama, N.; Costa, L.C.; Amara, V.; Ferreira, A.; Barros-Timmons, A.M.M.V. Insights into the physical properties of biobased polyurethane/expanded graphite composite foams. Comp. Sci. Technol. 2017, 138, 24–31. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, S. Optimization of acoustic performances of a new tung oleic acid-based composite polyurethane foam. J. Appl. Polym. Sci. 2019, 136, 47861. [Google Scholar] [CrossRef]
- Bourguignon, M.; Thomassin, J.M.; Grignard, B.; Jerome, C.; Detrembleur, C. Fast and facile one-pot one-step preparation of nonisocyanate polyurethane hydrogels in water at room temperature. ACS Sustain. Chem. Eng. 2019, 7, 12601–12610. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Xi, X.; Pizzi, A.; Zhou, X.; Fredon, E.; Du, G.; Gerardin, C. Condensed tannin-glucose-based NIPU bio-foams of improved fire retardancy. Polym. Degrad. Stab. 2020, 175, 109121. [Google Scholar] [CrossRef]
- Tondi, G.; Zhao, W.; Pizzi, A.; Fierro, V.; Celzard, A. Tannin-based rigid foams: A survey of chemical and physical properties. Bioresour. Technol. 2009, 100, 5162–5169. [Google Scholar] [CrossRef]
- Li, X.; Pizzi, A.; Cangemi, M.; Navarrete, P.; Segovia, C.; Fierro, V.; Celzard, A. Flexible natural tannin-based and protein-based biosourced foams. Ind. Crops Prod. 2012, 37, 389–393. [Google Scholar] [CrossRef]
- Basso, M.C.; Pizzi, A.; Al-Marzouki, F.; Abdalla, S. Horticultural/hydroponics and floral natural foams from tannins. Ind. Crops Prod. 2016, 87, 177–181. [Google Scholar] [CrossRef]
- Zhang, J.; Hori, N.; Takemura, A. Optimization of agricultural wastes liquefaction process and preparing bio-based polyurethane foams by the obtained polyols. Ind. Crops Prod. 2019, 138, 111455. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.C.; Yuan, Z.; Wei, Q. Synthesis of bio-based polyurethane foams with liquefied wheat straw: Process optimization. Biomass Bioenergy 2018, 111, 134–140. [Google Scholar] [CrossRef]
- Yan, Y.; Pan, H.; Yang, X.; Zhang, R.; Liao, B. Preparation and characterization of water-blown polyurethane foams from liquefied cornstalk polyol. J. Appl. Polym. Sci. 2008, 110, 1099–1111. [Google Scholar] [CrossRef]
- Li, H.; Feng, S.; Yuan, Z.; Wei, Q.; Xu, C.C. Highly efficient liquefaction of wheat straw for the production of bio-polyols and bio-based polyurethane foams. Ind. Crops Prod. 2017, 109, 426–433. [Google Scholar] [CrossRef]
- Hu, S.; Wan, C.; Li, Y. Production and characterization of biopolyols and polyurethane foams from crude glycerol based liquefaction of soybean straw. Bioresour. Technol. 2012, 103, 227–233. [Google Scholar] [CrossRef]
- Gómez-Fernández, S.; Günther, M.; Schartel, B.; Corcuera, M.A.; Eceiza, A. Impact of the combined use of layered double hydroxides, lignin and phosphorous polyol on the fire behavior of flexible polyurethane foams. Ind. Crops Prod. 2018, 125, 346–359. [Google Scholar] [CrossRef]
- Xi, X.; Wu, Z.; Pizzi, A.; Geradin, C.; Lei, H.; Zhang, B.; Du, G. Non-isocyanate polyurethane adhesive from sucrose used for particleboard. Wood Sci. Technol. 2019, 53, 393–405. [Google Scholar] [CrossRef]
- Figovsky, O.; Shapovalov, L.; Buslov, F. Ultraviolet and thermostable non-isocyanate polyurethane coatings. Surf. Coat. Intern. Part B Coat. Trans. 2005, 88, 67–71. [Google Scholar] [CrossRef]
- Asemani, H.R.; Mannari, V. Synthesis and evaluation of non-isocyanate polyurethane polyols for heat-cured thermoset coatings. Prog. Org. Coat. 2019, 131, 247–258. [Google Scholar] [CrossRef]
- Wu, Z.; Tang, L.; Dai, J.; Qu, J. Synthesis and properties of fluorinated non-isocyanate polyurethanes coatings with good hydrophobic and oleophobic properties. J. Coat. Technol. Res. 2019, 16, 1233–1241. [Google Scholar] [CrossRef]
- Saražin, J.; Pizzi, A.; Amirou, S.; Schmidt, D.; Sernek, M. Organosolv lignin for non-isocyanate based polyurethanes (NIPU) as wood adhesive. J. Renew. Mater. 2021, 9, 881–907. [Google Scholar] [CrossRef]
- Poussard, L.; Mariage, J.; Grignard, B.; Detrembleur, C.; Jérôme, C.; Calberg, C.; Heinrichs, B.; De Winter, J.; Gerbaux, P.; Raquez, J.-M.; et al. Non-isocyanate polyurethanes from carbonated soybean oil using monomeric or oligomeric diamines to achieve thermosets or thermoplastics. Macromolecules 2016, 49, 2162–2171. [Google Scholar] [CrossRef]
- Lee, A.; Deng, Y. Green polyurethane from lignin and soybean oil through non-isocyanate reactions. Eur. Polym. J. 2015, 63, 67–73. [Google Scholar] [CrossRef]
- Esmaeili, N.; Vafayan, M.; Salimi, A.; Zohuriaan-Mehr, M.J. Kinetics of curing and thermo-degradation, antioxidizing activity, and cell viability of a tannic acid based epoxy resin: From natural waste to value-added biomaterial. Thermochim. Acta 2017, 655, 21–33. [Google Scholar] [CrossRef]
- Liu, G.; Guomin, W.; Shuping, H.; Can, J.; Kong, Z. Synthesis and Properties of Non-Isoyanate Polyurethane Coatings derived from Cyclic Carbonate-Functionalized Polysiloxanes. Prog. Org. Coat. 2017, 112, 169–175. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Lei, H.; Chen, X.; Amirou, S. Preparation and evaluation of glucose based non-isocyanate polyurethane self-blowing rigid foams. Polymers 2019, 11, 1802. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Gerardin, C.; Du, G. Glucose-biobased non-isocyanate polyurethane rigid foams. J. Renew. Mater. 2019, 7, 301–312. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Fredon, E.; Gerardin, C.; Zhou, X.; Zhang, B.; Du, G. Low curing temperature tannin-based non-isocyanate polyurethane (NIPU) wood adhesives: Preparation and properties evaluation. Int. J. Adhes. Adhes. 2022, 112, 103001. [Google Scholar] [CrossRef]
- Annunziata, L.; Diallo, A.K.; Fouquay, S.; Michaud, G.; Simon, F.; Brusson, J.-M.; Carpentier, J.-F.; Guillaume, S.M. α, ω-Di (Glycerol carbonate) telechelic polyesters and polyolefins as precursors to polyhydroxyurethanes: An isocyanate-free approach. Green Chem. 2014, 16, 1947–1956. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The chemistry of dimethyl carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Delmotte, L. Isocyanate-free polyurethane coatings and adhesives from mono-and di-saccharides. Polymers 2018, 10, 402. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Essawy, H.; Fredon, E.; Gerardin, C.; Guigo, N.; Sbirrazzuoli, N. Non-furanic humins-based non-isocyanate polyurethane (NIPU) thermoset wood adhesives. Polymers 2021, 13, 372. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Dumarçay, S.; Gerardin, P.; Fredon, E.; Delmotte, L. Polyurethanes from hydrolysable tannins obtained without using isocyanates. Ind. Crops Prod. 2014, 59, 329–336. [Google Scholar] [CrossRef]
- Azadeh, E.; Chen, X.; Pizzi, A.; Gerardin, C.; Gerardin, P.; Essawy, H. Self-Blowing Non-Isocyanate Polyurethane Foams Based on Hydrolysable Tannins. J. Renew. Mater. 2022, 10, 3217–3227. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Essawy, H.A.; Baroum, A.; van Assche, G. Isocyanate free condensed tannin-based polyurethanes. Eur. Polym. J. 2015, 67, 513–526. [Google Scholar] [CrossRef]
- Thébault, M.; Pizzi, A.; Santiago-Medina, F.J.; Al-Marzouki, F.M.; Abdalla, S. Isocyanate-free polyurethanes by coreaction of condensed tannins with aminated tannins. J. Renew. Mater. 2017, 5, 21–29. [Google Scholar] [CrossRef]
- Tondi, G.; Pizzi, A. Tannin-based rigid foams: Characterization and modification. Ind. Crops Prod. 2009, 29, 356–363. [Google Scholar] [CrossRef]
- Chen, M.J.; Chen, C.R.; Tan, Y.; Huang, J.-Q.; Wang, X.-L.; Chen, L.; Wang, Y.-Z. Inherently flame-retardant flexible polyurethane foam with low content of phosphorus-containing cross-linking agent. Ind. Eng. Chem. Res. 2014, 53, 1160–1171. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhao, H.B.; Rao, W.H.; Wang, T.; Liao, W.; Wang, Y.-Z. Inherently flame-retardant rigid polyurethane foams with excellent thermal insulation and mechanical properties. Polymer 2018, 153, 616–625. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Y.; Wang, S.; Shaghaleh, H.; Sun, P.; Huang, X.; Xu, X.; Wang, S.; Liu, H. Flame-retarded polyurethane foam conferred by a bio-based nitrogen phosphorus-containing flame retardant. React. Funct. Polym. 2021, 168, 105057. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Z.; Zhang, J.; Chen, S.; Zhou, Y. Effects of a novel phosphorus–nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym. Degrad. Stab. 2015, 120, 427–434. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Larch tannin-based rigid phenolic foam with high compressive strength, low friability, and low thermal conductivity reinforced by cork powder. Compos. Part B Eng. 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Shi, S.Q.; Gao, Q.; Li, J. Preparation of a moderate viscosity, high performance and adequately-stabilized soy protein-based adhesive via recombination of protein molecules. J. Clean. Prod. 2020, 255, 120303. [Google Scholar] [CrossRef]
- Chen, X.; Pizzi, A.; Xi, X.; Zhou, X.; Fredon, E.; Gerardin, C. Soy protein isolate non-isocyanates polyurethanes (NIPU) wood adhesives. J. Renew. Mater. 2021, 9, 1045–1057. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Pizzi, A.; Fredon, E.; Zhou, X.; Li, J.; Gerardin, C.; Du, G. Preparation and characterization of condensed tannin non-isocyanate polyurethane (NIPU) rigid foams by ambient temperature blowing. Polymers 2020, 12, 750. [Google Scholar] [CrossRef]
- Del Menezzi, C.; Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with Wood Carbohydrates and Lignin of Citric Acid as a Bond Promoter of Wood Veneer Panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef]








| Foam Number | Formulations | Density (g/cm3) | Ignition Time (s) |
|---|---|---|---|
| F1 | 10 g GNIPU + 1.0 g FA + 3.0 g G | 0.119 | 75 s |
| F2 | 10 g GNIPU + 1.0 g MA + 3.0 g G | 0.101 | 85 s |
| F3 | 10 g GNIPU + 1.0 g PA + 3.0 g G | 0.105 | 0 s |
| F4 | 10 g GNIPU + 1.0 g CA + 3.0 g G | 0.108 | 80 s |
| F5 | 10 g GNIPU + 1.0 g PA + 2.0 g G | 0.107 | 0 s |
| F6 | 10 g GNIPU + 1.0 g PA + 4.0 g G | 0.112 | 0 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Pizzi, A.; Xi, X.; Zhou, X.; Zhang, Q. Preparation and Characterization of Glucose-Based Self-Blowing Non-Isocyanate Polyurethane (NIPU) Foams with Different Acid Catalysts. Polymers 2024, 16, 2899. https://doi.org/10.3390/polym16202899
Yang T, Pizzi A, Xi X, Zhou X, Zhang Q. Preparation and Characterization of Glucose-Based Self-Blowing Non-Isocyanate Polyurethane (NIPU) Foams with Different Acid Catalysts. Polymers. 2024; 16(20):2899. https://doi.org/10.3390/polym16202899
Chicago/Turabian StyleYang, Tianjiao, Antonio Pizzi, Xuedong Xi, Xiaojian Zhou, and Qianyu Zhang. 2024. "Preparation and Characterization of Glucose-Based Self-Blowing Non-Isocyanate Polyurethane (NIPU) Foams with Different Acid Catalysts" Polymers 16, no. 20: 2899. https://doi.org/10.3390/polym16202899
APA StyleYang, T., Pizzi, A., Xi, X., Zhou, X., & Zhang, Q. (2024). Preparation and Characterization of Glucose-Based Self-Blowing Non-Isocyanate Polyurethane (NIPU) Foams with Different Acid Catalysts. Polymers, 16(20), 2899. https://doi.org/10.3390/polym16202899








