Preparation and Structural Analysis of a Water-Soluble Aminated Lignin
Abstract
1. Introductions
2. Methods and Materials
2.1. Experimental Materials
2.2. Preparation Method of Fine Lignin
2.3. Synthetic Method of Aminated Lignin
2.4. Product Morphology Analysis
2.5. Method for Determining the Molecular Weight of the Products
2.6. Methods for Element Analysis of the Product
2.7. Methods for Structural Analysis
2.8. Methods for the Thermal Stability Analysis of the Product
2.9. Methods for the Water Solubility Analysis of the Product
3. Results and Discussions
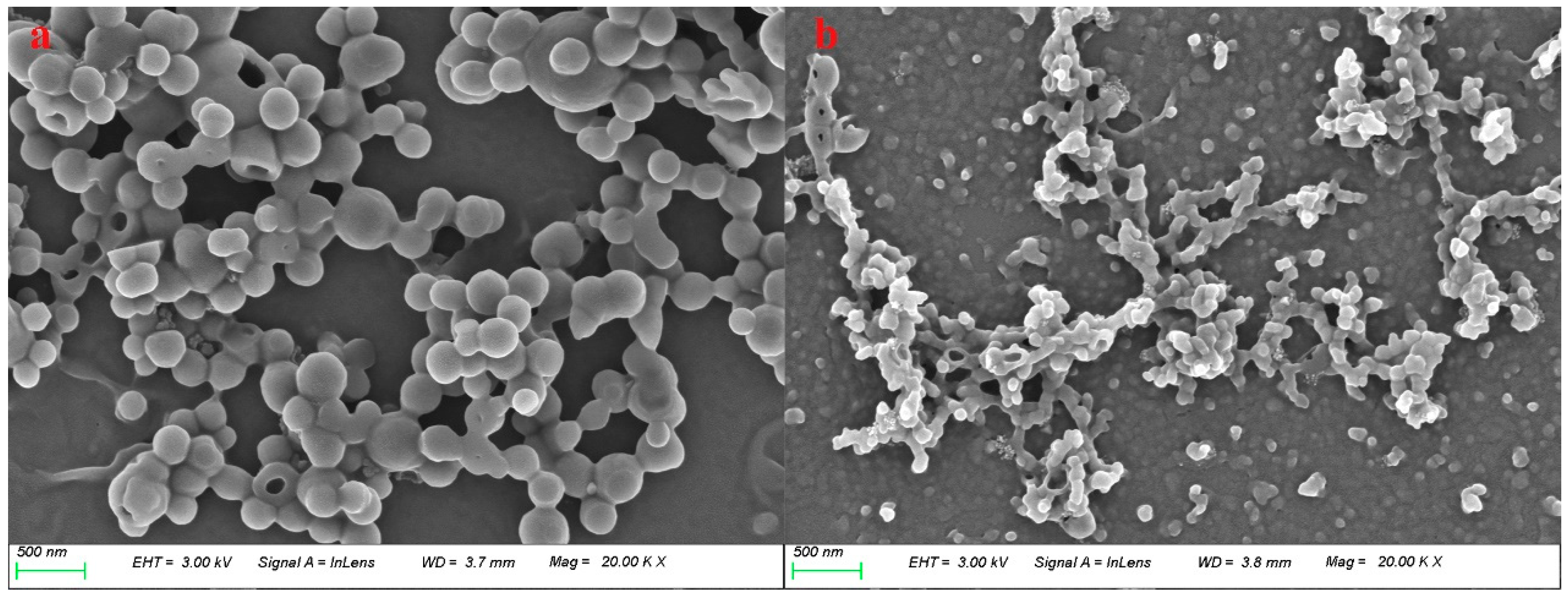
3.1. Product Morphology
3.2. The Molecular Weight of the Generated Product
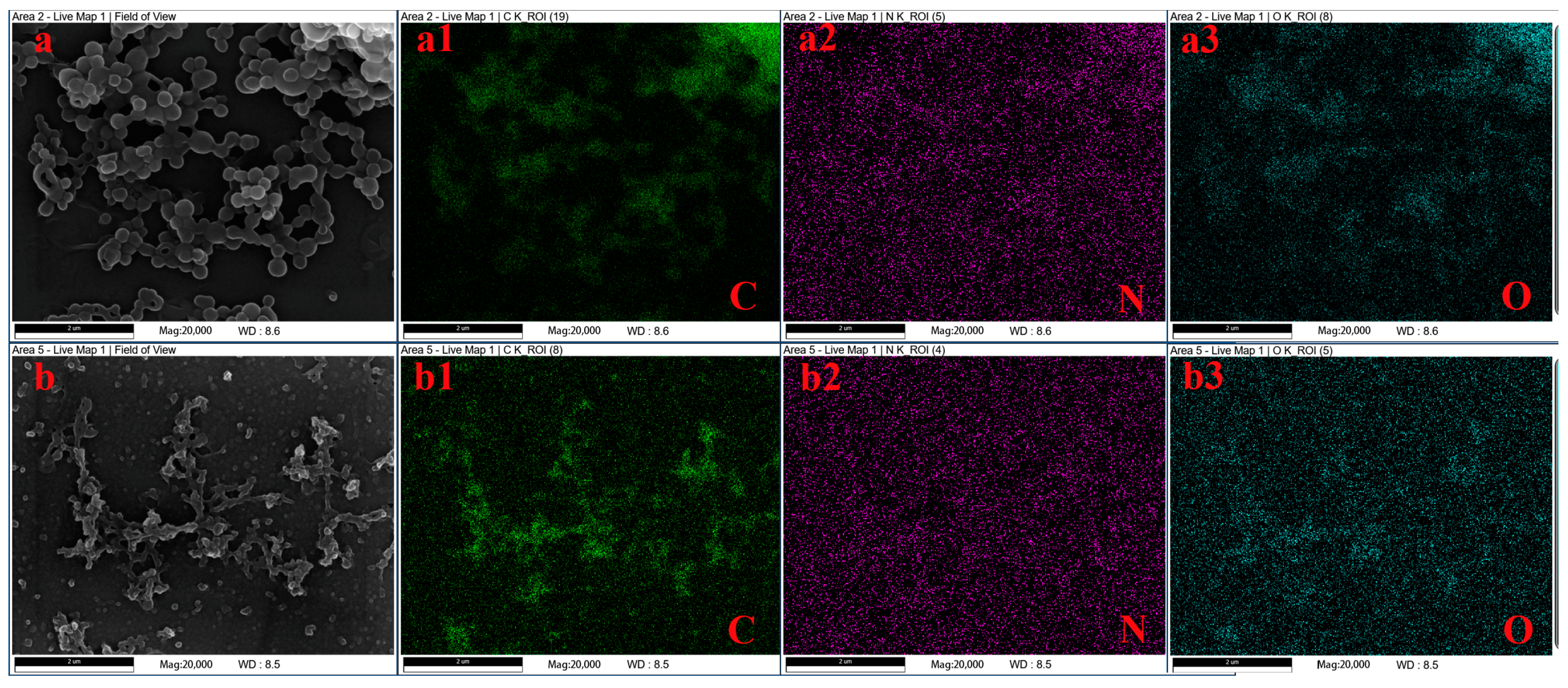
3.3. Element Compositions
- (1)
- Results of analysis using an energy-dispersive spectrometer (EDS).
- (2)
- Results of elemental analysis.
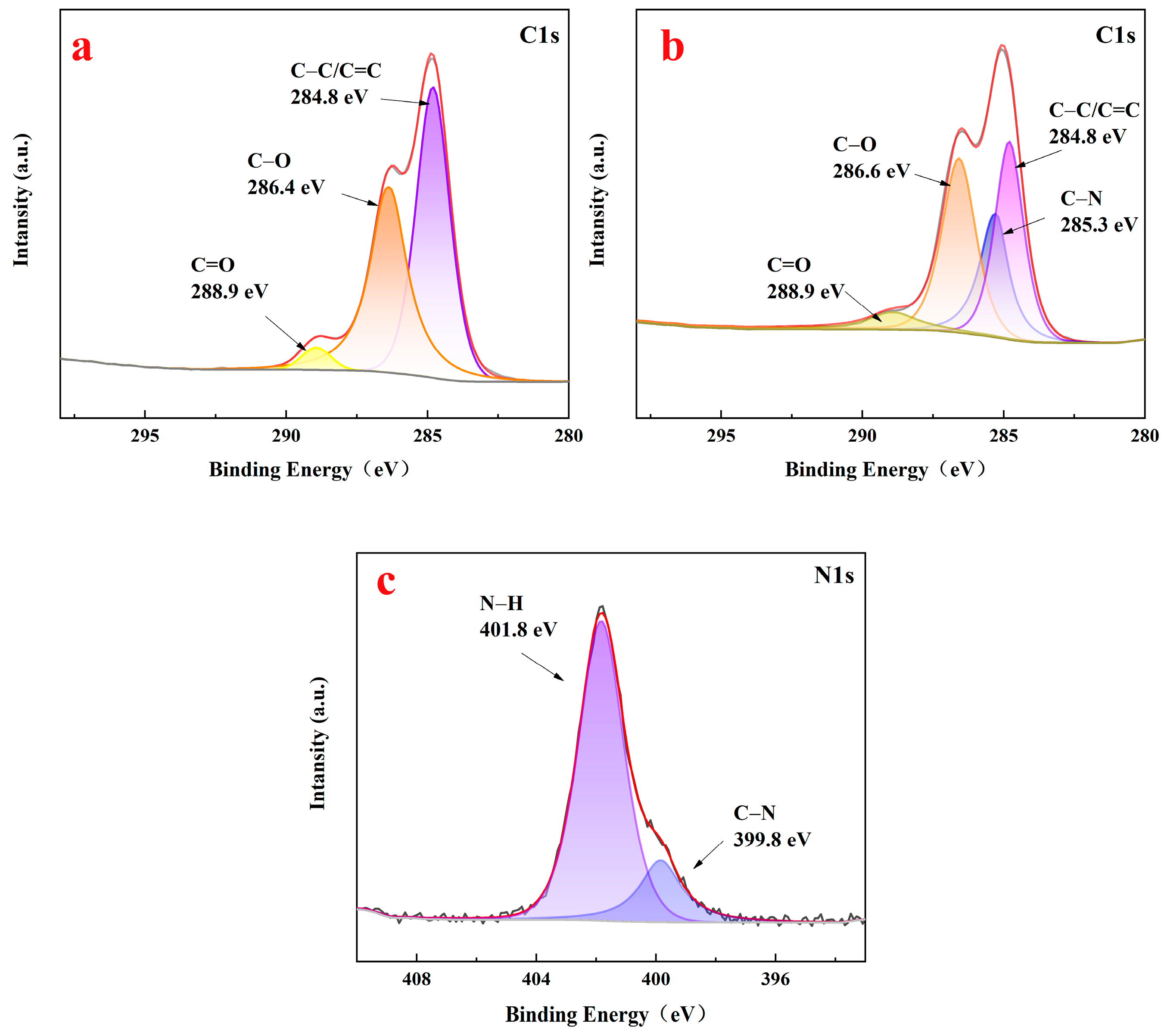
3.4. Results of the XPS Analysis
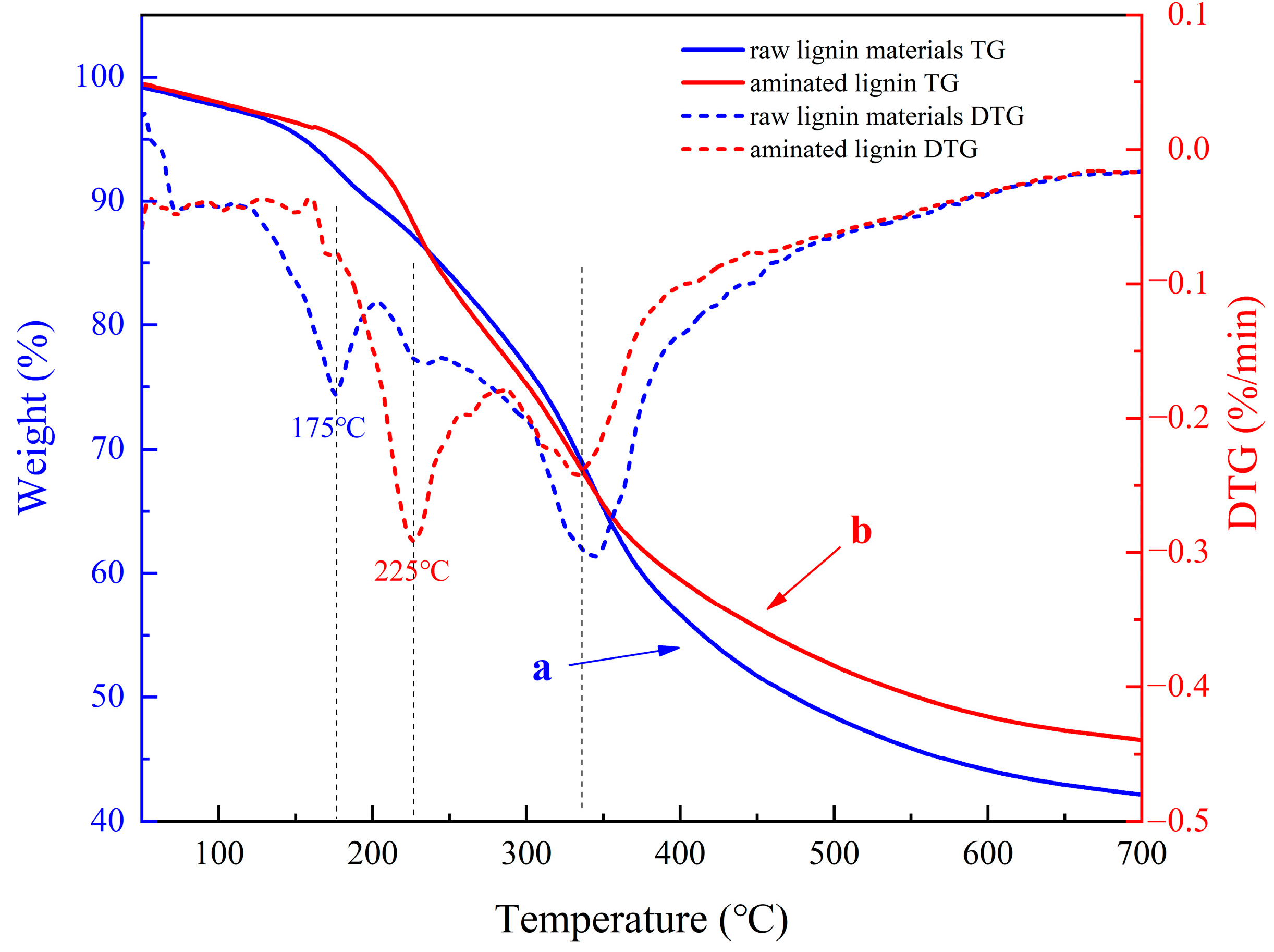
3.5. Test Results of Thermal Stability
3.6. Test Results of Water Solubility
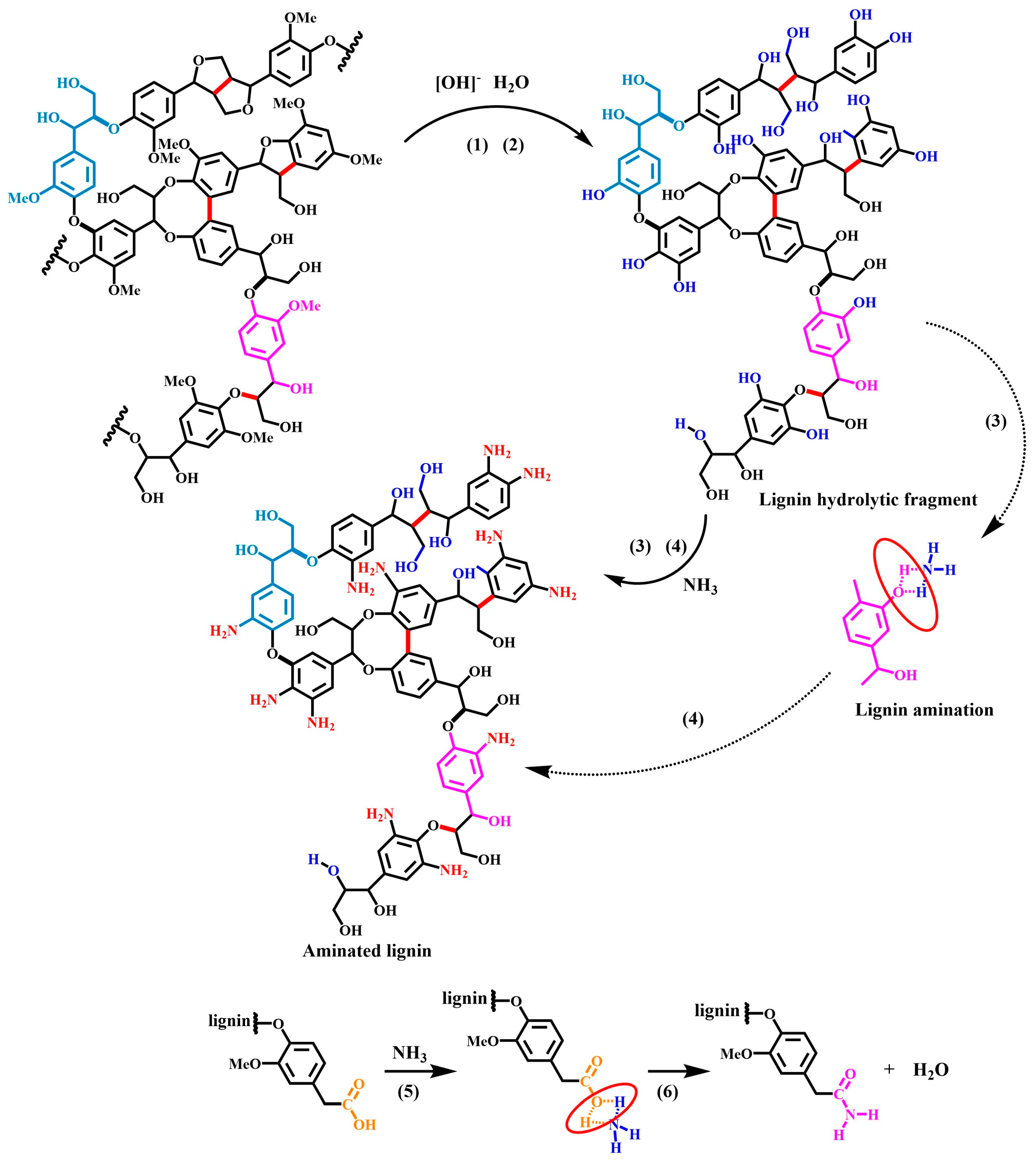
3.7. Discussion of the Mechanisms of the Lignin Ammonification Reaction
- (1)
- (2)
- Subsequently, under alkaline and high-temperature conditions, the methoxyether bonds on the side chains of the lignin molecules undergo hydrolysis, producing methanol and hydroxylated lignin, which contain a large amount of phenolic and fatty hydroxyl groups [36]. (The principle of alkaline-catalyzed ether bond hydrolysis).
- (3)
- (4)
- (5)
- In addition, lignin also contains small amounts of carboxylic acids, and these carboxyl groups combine with ammonia molecules to produce carboxylic acid–ammonia transition-state intermediates [41]. (The principle of acid–base combination).
- (6)
3.8. Discussion of the Innovations and Limitations to This Research
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, E.; Mercado, M.I.V.; Segato, F.; Wilkins, M.R. A green pathway for lignin valorization: Enzymatic lignin depolymerization in biocompatible ionic liquids and deep eutectic solvents. Enzyme. Microb. Technol. 2024, 174, 110392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Hou, Y.C.; Wu, W.Z.; Li, H.; Ren, S.H.; Li, J.W. Polycyclic Aromatics Observed in Enzymatic Lignin by Spectral Characterization and Ruthenium Ion-Catalyzed Oxidation. J. Agric. Food Chem. 2021, 69, 12148–12155. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.X.; Gong, Z.G.; Luo, X.L.; Chen, L.H.; Shuai, L. Bonding wood with uncondensed lignins as adhesives. Nature 2023, 621, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Peng, Z.W.; Li, J.J.; Li, X.N.; Jiang, X.; Dong, Y.M. Unlocking the role of lignin for preparing the lignin-based wood adhesive: A review. Ind. Crops Prod. 2022, 187, 12. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New Opportunities in the Valorization of Technical Lignins. ChemSusChem 2021, 14, 1016–1036. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.A.E.; Casimiro, F.M.; Vega-Aguilar, C.; Rodrigues, A.E. Lignin Valorization for Added-Value Chemicals: Kraft Lignin versus Lignin Fractions. Chemengineering 2023, 7, 42. [Google Scholar] [CrossRef]
- Ghavidel, N.; Konduri, M.K.R.; Fatehi, P. Chemical reactivity and sulfo-functionalization response of enzymatically produced lignin. Ind. Crops Prod. 2021, 172, 10. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.L.; Hult, E.L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef]
- Ghosh, T.; Elo, T.; Parihar, V.S.; Maiti, P.; Layek, R. Poly (itaconic acid) functionalized lignin/polyvinyl acetate composite resin with improved sustainability and wood adhesion strength. Ind. Crops Prod. 2022, 187, 12. [Google Scholar] [CrossRef]
- Vishtal, A.; Kraslawski, A. Challenges in Industrial Applications of Technical Lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Chatel, G.; Rogers, R.D. Review: Oxidation of Lignin Using Ionic Liquids-An Innovative Strategy To Produce Renewable Chemicals. ACS Sustain. Chem. Eng. 2014, 2, 322–339. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.Y.; Zong, Q.J.; Wang, L.; Xu, T.; Gong, J.B.; Liu, Z.H.; Li, B.Z.; Yuan, Y.J. High-solid ethylenediamine pretreatment to fractionate new lignin streams from lignocellulosic biomass. Chem. Eng. J. 2022, 427, 12. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.J.; Zhong, C.; Li, B.Z.; Yuan, Y.J. Alkali-Based Pretreatment-Facilitated Lignin Valorization: A Review. Ind. Eng. Chem. Res. 2020, 59, 16923–16938. [Google Scholar] [CrossRef]
- Zhao, W.W.; Xiao, L.P.; Song, G.Y.; Sun, R.C.; He, L.L.; Singh, S.; Simmons, B.A.; Cheng, G. From lignin subunits to aggregates: Insights into lignin solubilization. Green Chem. 2017, 19, 3272–3281. [Google Scholar] [CrossRef]
- Konduri, M.K.R.; Fatehi, P. Production of Water-Soluble Hardwood Kraft Lignin via Sulfomethylation Using Formaldehyde and Sodium Sulfite. ACS Sustain. Chem. Eng. 2015, 3, 1172–1182. [Google Scholar] [CrossRef]
- Prinsen, P.; Narani, A.; Hartog, A.F.; Wever, R.; Rothenberg, G. Dissolving Lignin in Water through Enzymatic Sulfation with Aryl Sulfotransferase. Chemsuschem 2017, 10, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Tahir, P.M.; Halis, R. Lignin-based copolymer adhesives for composite wood panels—A review. Int. J. Adhes. Adhes. 2019, 95, 12. [Google Scholar] [CrossRef]
- Peng, Z.W.; Jiang, X.; Si, C.L.; Cárdenas-Oscanoa, A.J.; Huang, C.X. Advances of Modified Lignin as Substitute to Develop Lignin-Based Phenol-Formaldehyde Resin Adhesives. Chemsuschem 2023, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, S.; Zhuang, J.; Liu, Y.; Wei, W.; Zhang, F. Preparation of high-performance lignin-phenolic resin adhesive by oxidative pretreatment of lignin. Appl. Chem. Ind. 2022, 51, 907–911. [Google Scholar]
- Li, M.; Wilkins, M. Lignin bioconversion into valuable products: Fractionation, depolymerization, aromatic compound conversion, and bioproduct formation. Syst. Microbiol. Biomanuf. 2021, 1, 166–185. [Google Scholar] [CrossRef]
- Limarta, S.O.; Kim, H.; Ha, J.M.; Park, Y.K.; Jae, J. High-quality and phenolic monomer-rich bio-oil production from lignin in supercritical ethanol over synergistic Ru and Mg-Zr-oxide catalysts. Chem. Eng. J. 2020, 396, 125175. [Google Scholar] [CrossRef]
- Chen, J.S.; An, L.L.; Bae, J.H.; Heo, J.W.; Han, S.Y.; Kim, Y.S. Green and facile synthesis of aminated lignin-silver complex and its antibacterial activity. Ind. Crops Prod. 2021, 173, 8. [Google Scholar] [CrossRef]
- Xue, Z.M.; Zhao, X.H.; Sun, R.C.; Mu, T.C. Biomass-Derived γ-Valerolactone-Based Solvent Systems for Highly Efficient Dissolution of Various Lignins: Dissolution Behavior and Mechanism Study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870. [Google Scholar] [CrossRef]
- Wu, W.J.; Jiang, B.; Yang, L.F.; Jin, Y.C. Isolation of Lignin from Masson Pine by Liquid-Liquid Extraction Based on Complete Dissolution in NaOH Aqueous Solution. BioResources 2018, 13, 231–240. [Google Scholar] [CrossRef]
- Chen, R.; Hu, K.X.; Tang, H.; Wang, J.J.; Zhu, F.S.; Zhou, H. A novel flame retardant derived from DOPO and piperazine and its application in epoxy resin: Flame retardance, thermal stability and pyrolysis behavior. Polym. Degrad. Stabil. 2019, 166, 334–343. [Google Scholar] [CrossRef]
- Shang, Y.P.; Zhu, G.Z.; Yan, D.X.; Liu, Q.Z.; Gao, T.T.; Zhou, G.W. Tannin cross-linked polyethyleneimine for highly ef fi cient removal of hexavalent chromium. J. Taiwan Inst. Chem. Eng. 2021, 119, 52–59. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Ma, W.; Liu, H.; Zhu, J.; Wang, L.; Pei, H.; Liu, Q.; Yao, J. Insight into the synergistic adsorption-reduction character of chromium(VI) onto poly(pyrogallol-tetraethylene pentamine) microsphere in synthetic wastewater. J. Colloid Interface Sci. 2022, 609, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Seydibeyoglu, M.; Mohanty, A.K.; Misra, M. Characterization of industrial lignins for their utilization in future value added applications. Biomass Bioenerg. 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, D.Y.; Wang, S.S.; Feng, X.; Zhu, J.H.; Lu, X.H.; Mu, L.W. Valorization of industrial lignin as lubricating additives by C-C Bond Cleavage and doping heteroelement-rich groups. Biomass Bioenerg. 2022, 161, 10. [Google Scholar] [CrossRef]
- Majeke, B.M.; Collard, F.X.; Tyhoda, L.; Görgens, J.F. The synergistic application of quinone reductase and lignin peroxidase for the deconstruction of industrial (technical) lignins and analysis of the degraded lignin products. Bioresour. Technol. 2021, 319, 9. [Google Scholar] [CrossRef] [PubMed]
- Naron, D.R.; Collard, F.X.; Tyhoda, L.; Görgens, J.F. Production of phenols from pyrolysis of sugarcane bagasse lignin: Catalyst screening using thermogravimetric analysis—Thermal desorption—Gas chromatography—Mass spectroscopy. J. Anal. Appl. Pyrolysis 2019, 138, 120–131. [Google Scholar] [CrossRef]
- Wang, C.; Feng, X.Z.; Shang, S.B.; Liu, H.; Song, Z.Q.; Zhang, H.B. Lignin/sodium alginate hydrogel for efficient removal of methylene blue. Int. J. Biol. Macromol. 2023, 237, 11. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.B.; He, C.; Liu, C.; Tong, H.; Wu, L.Q.; Wu, S.B. A computational study on thermal decomposition mechanism of β-1 linkage lignin dimer. Comput. Theor. Chem. 2015, 1054, 80–87. [Google Scholar] [CrossRef]
- Roberts, V.M.; Stein, V.; Reiner, T.; Lemonidou, A.; Li, X.B.; Lercher, J.A. Towards Quantitative Catalytic Lignin Depolymerization. Chem.–A Eur. J. 2011, 17, 5939–5948. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, F.; Fierro, V.; Pizzi, A.; Rode, K.; Radke, W.; Delmotte, L.; Parmentier, J.; Celzard, A. Reaction of condensed tannins with ammonia. Ind. Crops Prod. 2013, 44, 330–335. [Google Scholar] [CrossRef]
- Hashida, K.; Makino, R.; Ohara, S. Amination of pyrogallol nucleus of condensed tannins and related polyphenols by ammonia water treatment. Holzforschung 2009, 63, 319–326. [Google Scholar] [CrossRef]
- Dai, L.L.; Wang, Y.P.; Liu, Y.H.; He, C.; Ruan, R.; Yu, Z.T.; Jiang, L.; Zeng, Z.H.; Wu, Q.H. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass. Sci. Total Environ. 2020, 749, 20. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Yao, O.; Zhang, Y.; Fu, Y. Integrated Production of Aromatic Amines and N-Doped Carbon from Lignin via Catalytic Fast Pyrolysis in the Presence of Ammonia over Zeolites. ACS Sustain. Chem. Eng. 2017, 5, 2960–2969. [Google Scholar] [CrossRef]
- Jursic, B.S.; Zdravkovski, Z. A Simple Preparation of Amides from Acids and Amines by Heating of Their Mixture. Synth. Commun. 1993, 23, 2761–2770. [Google Scholar] [CrossRef]
- D’Amaral, M.C.; Jamkhou, N.; Adler, M.J. Efficient and accessible silane-mediated direct amide coupling of carboxylic acids and amines. Green Chem. 2021, 23, 288–295. [Google Scholar] [CrossRef]
- Stoll, E.L.; Tongue, T.; Andrews, K.G.; Valette, D.; Hirst, D.J.; Denton, R.M. A practical catalytic reductive amination of carboxylic acids. Chem. Sci. 2020, 11, 9494–9500. [Google Scholar] [CrossRef] [PubMed]



| Element | Raw Lignin Material | Aminated Lignin | ||
|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | |
| C | 84.90 | 88.23 | 82.20 | 85.65 |
| N | 0.03 | 0.02 | 3.84 | 3.43 |
| O | 15.06 | 11.75 | 13.94 | 10.90 |
| S | 0.01 | 0 | 0.02 | 0.01 |
| N( %) | C (%) | H (%) | S (%) | O (%) | Residual | Unsaturation | |
|---|---|---|---|---|---|---|---|
| Lignin | 0.40 | 52.01 | 5.19 | 0.14 | 36.46 | 5.80 | 4.61 |
| Aminated lignin | 4.25 | 48.67 | 6.35 | 0 | 34.32 | 6.41 | 2.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Q.; Nong, G.; Li, N. Preparation and Structural Analysis of a Water-Soluble Aminated Lignin. Polymers 2024, 16, 1237. https://doi.org/10.3390/polym16091237
Zheng Q, Nong G, Li N. Preparation and Structural Analysis of a Water-Soluble Aminated Lignin. Polymers. 2024; 16(9):1237. https://doi.org/10.3390/polym16091237
Chicago/Turabian StyleZheng, Qi, Guangzai Nong, and Ning Li. 2024. "Preparation and Structural Analysis of a Water-Soluble Aminated Lignin" Polymers 16, no. 9: 1237. https://doi.org/10.3390/polym16091237
APA StyleZheng, Q., Nong, G., & Li, N. (2024). Preparation and Structural Analysis of a Water-Soluble Aminated Lignin. Polymers, 16(9), 1237. https://doi.org/10.3390/polym16091237






