Preparation of a Polymeric Phosphoramide Flame-Retardant and Its Effect on the Flame-Retardant Properties of Epoxy Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
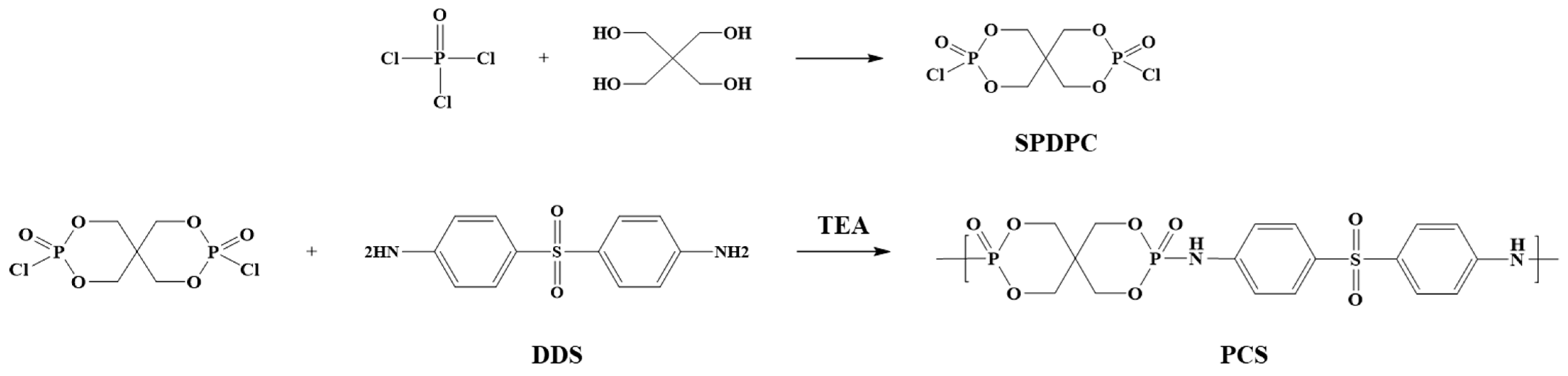
2.2. Synthesis of PCS
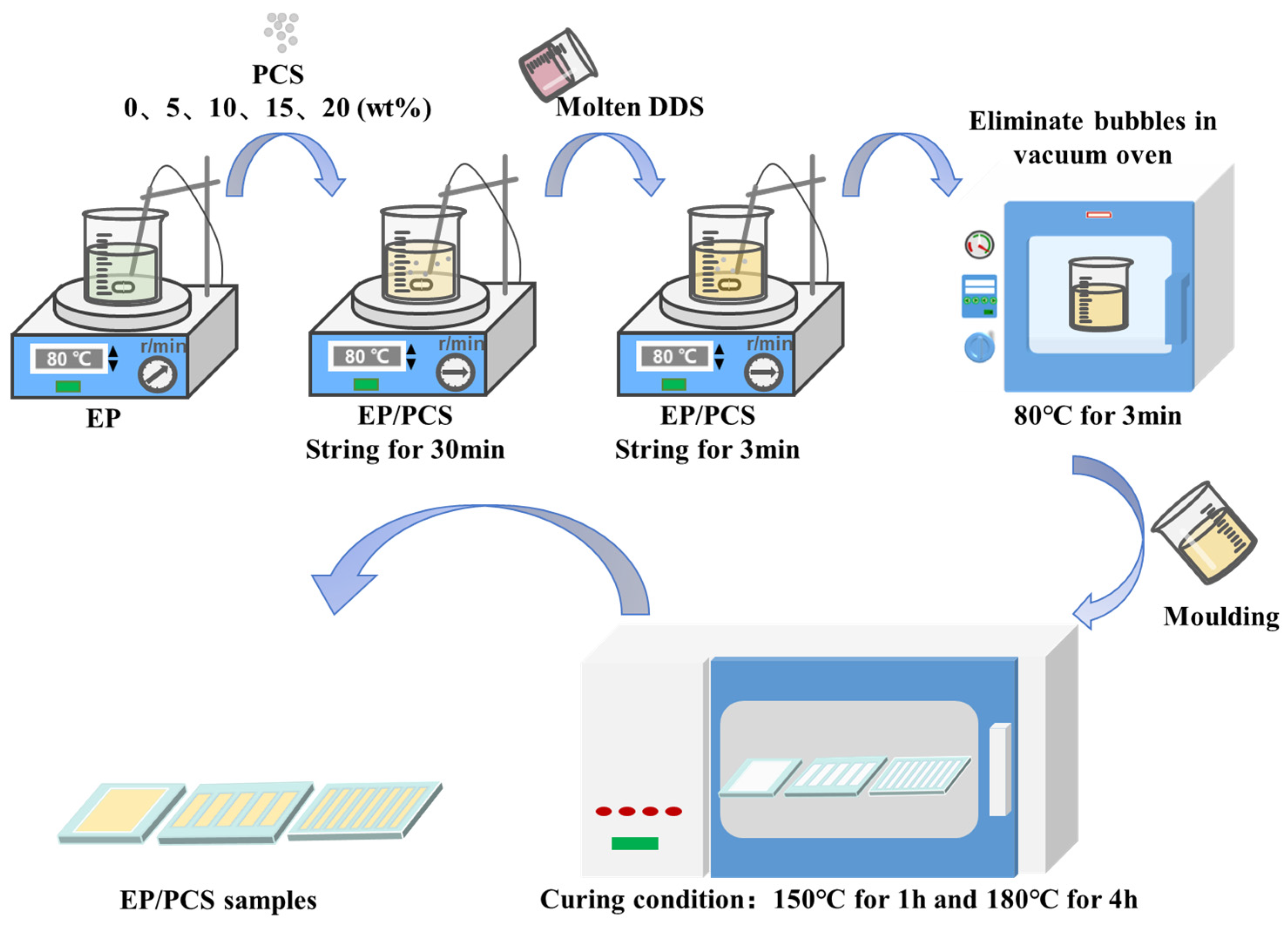
2.3. Preparation of Composites EP/PCS
2.4. Characterizations
3. Results
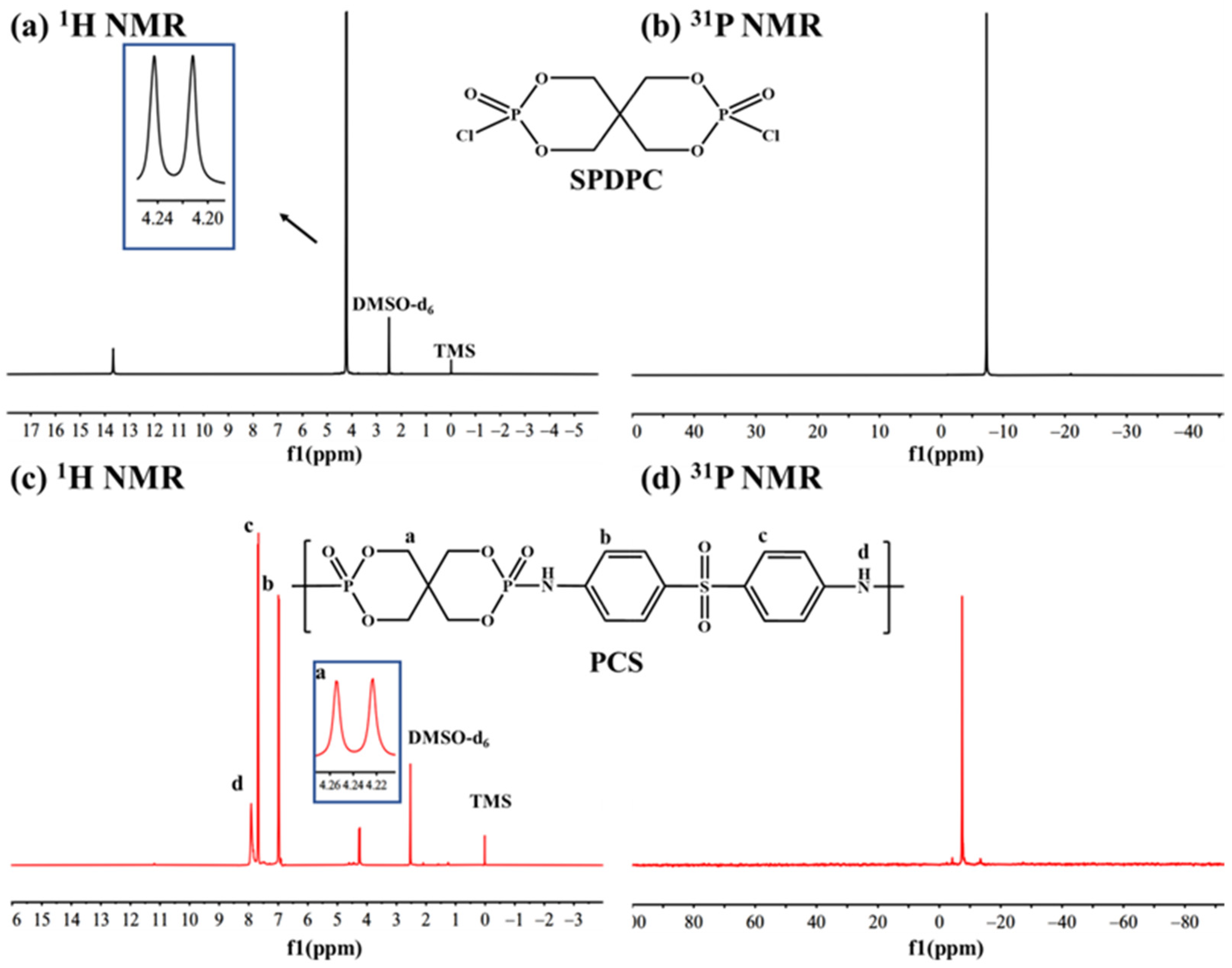
3.1. Characterization of PCS
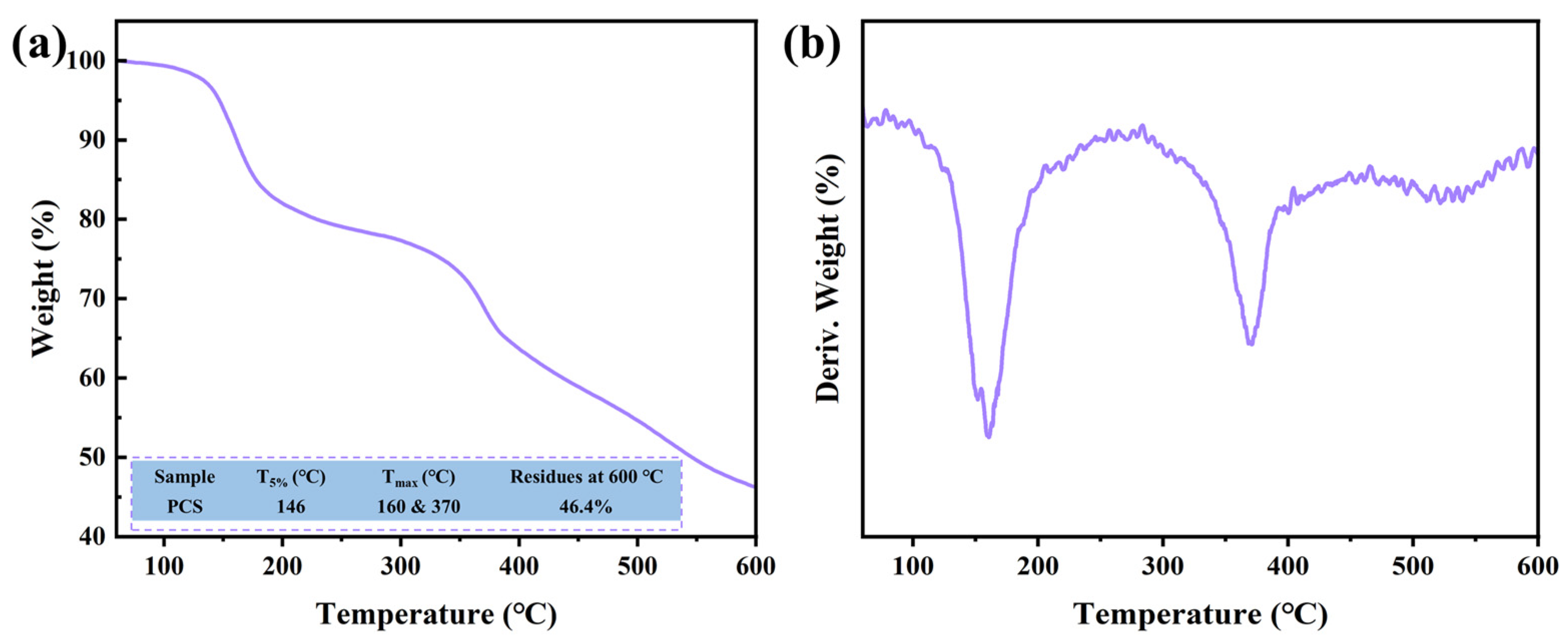
3.2. Thermal Properties and Fire Safety of EP/PCS Composites
3.3. Analysis of EP/PCS Composites in Condensed and Gaseous Phases
3.4. The Mechanism That Makes EP/PCS Composites Resistant to Flame
4. Conclusions
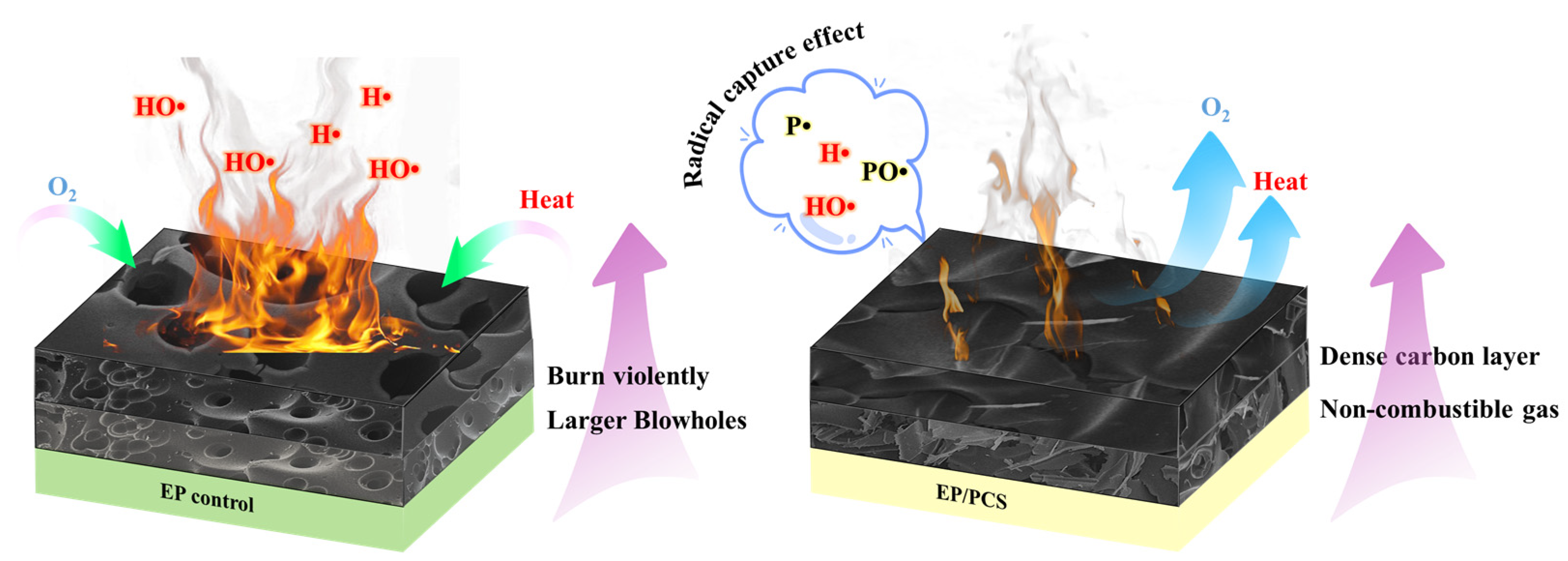
- In the epoxy resin with only PCS flame-retardant, curing at 150 °C for one hour and curing at 180 °C for 4 h can achieve the effect of curing epoxy resin with the traditional curing agent DDS. The existence of PCS can be decomposed before EP, which has a good effect and greatly prevents the further severe combustion of EP. By combining three sources, the carbon chain structure of PCS can serve as a carbon source for carbon formation, the P group as an acid source, and the N group as a gas source. This integration enables the achievement of flame retardancy in both the condensed and gas phases. PCS with both flame-retardant and curing provides a new reference for the development of flame retardants due to its numerous functionalities.
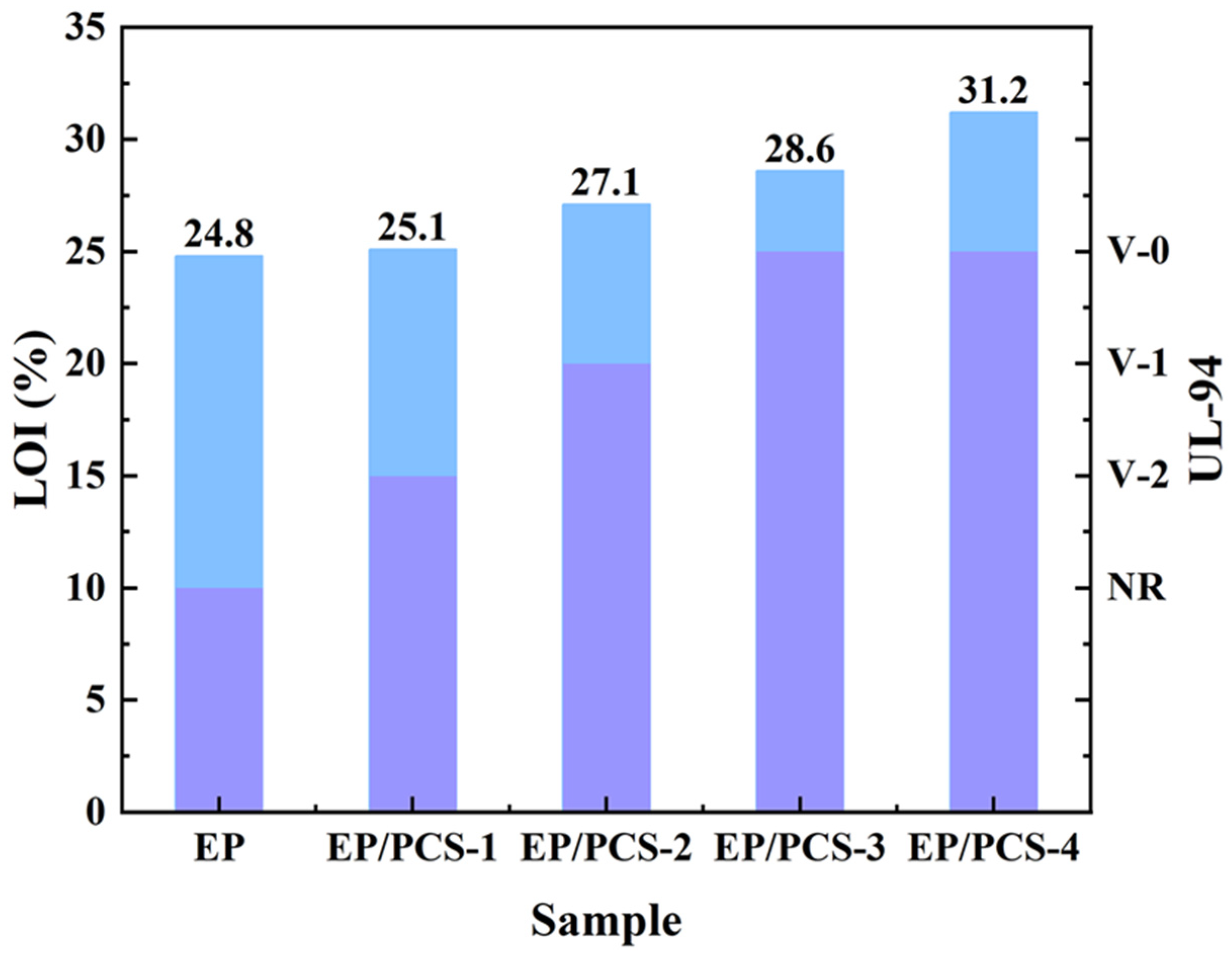
- The EP composites, containing 20 weight percent PCS, exhibited an LOI of 31.2% and successfully cleared the UL-94 examination, receiving a V-0 level. Moreover, with the TTI being advanced, the pHRR, THR, and TSP values for EP/PCS were obviously reduced. With the addition of flame-retardant PCS, we achieved the pHRR reduction of 59.7%, the THR reduction of 63.7%, and the TSP reduction of 42.3%, which is extremely important for flame-retardant polymer materials and suppression of smoke emissions.
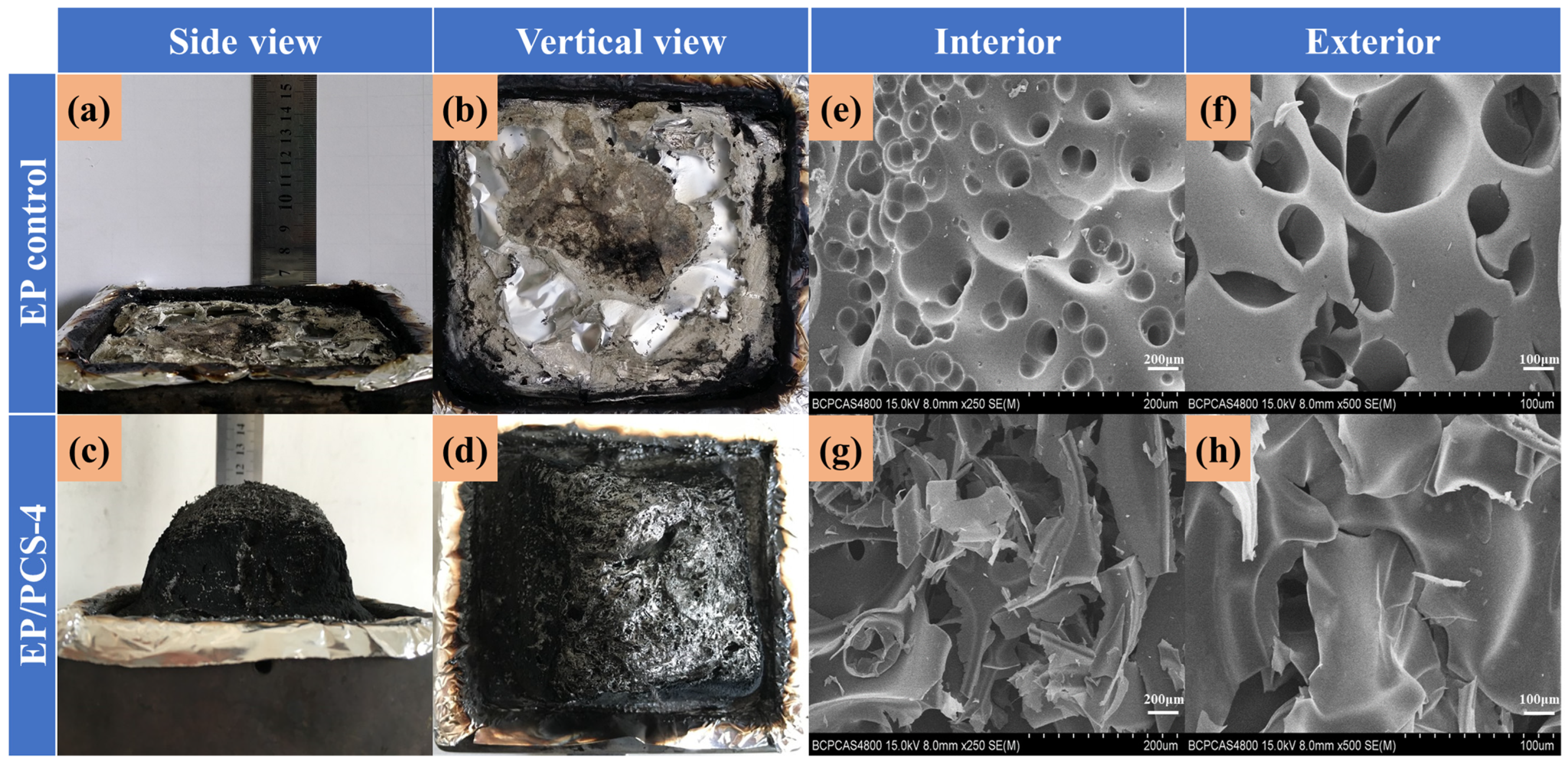
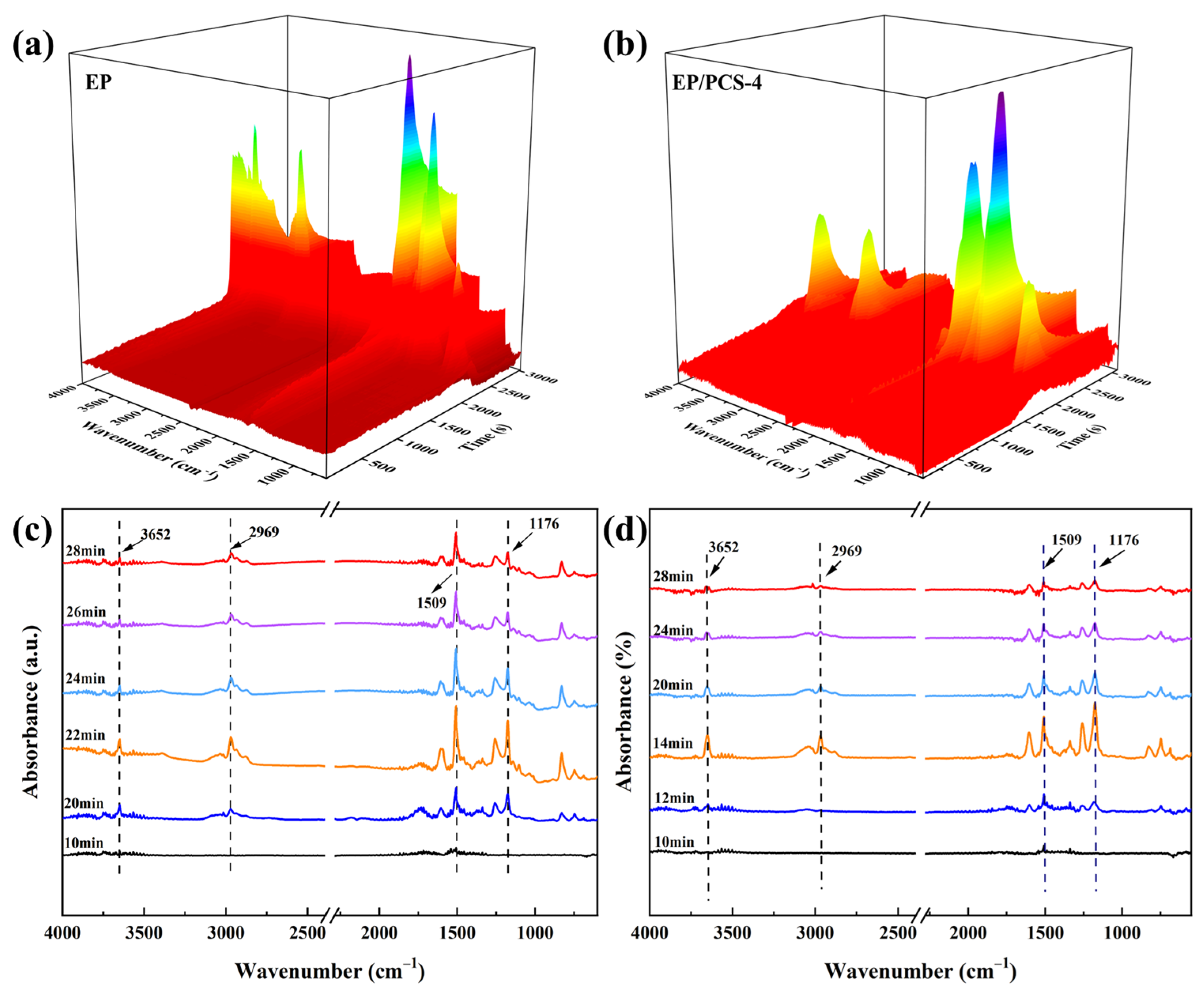
- We found that the addition of PCS increased the thickness of the carbon layer in the combustion process of EP by nearly 5 cm, indicating that this flame-retardant can give full play to the effect of the IFRs. TG-FTIR and SEM results further confirmed that PCS can generate a dense and continuously expanded carbon layer with a stable structure after heating, isolating the transfer of oxygen and heat, and generating a non-combustible gas to dilute oxygen, with excellent flame-retardant performance.
- Although this work has prepared a new type of flame-retardant with both flame-retardant and curing effects, this paper only elaborates on the improvement of flame-retardant performance. The preparation of multifunctional polymer materials should be the focus of our attention. In addition, there are still many possibilities for the preparation of new flame retardants. We should explore the synthesis of more efficient and environmentally friendly flame retardants to achieve our goals while ensuring economic costs.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hao, Y.; Zhong, L.; Li, T.; Zhang, J.; Zhang, D. High-Performance Recyclable and Malleable Epoxy Resin with Vanillin-Based Hyperbranched Epoxy Resin Containing Dual Dynamic Bonds. ACS Sustain. Chem. Eng. 2023, 11, 11077–11087. [Google Scholar] [CrossRef]
- Mi, X.; Liang, N.; Xu, H.; Wu, J.; Jiang, Y.; Nie, B.; Zhang, D. Toughness and its mechanisms in epoxy resins. Prog. Mater. Sci. 2022, 130, 100977. [Google Scholar] [CrossRef]
- Raj, M.; Maheta, J.; Raj, L. Synthesis characterization and application of hexafunctional epoxy resin and comparison against commercial epoxy resin. Polym. Polym. Compos. 2022, 30. [Google Scholar] [CrossRef]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in Epoxy Resins. Polym. Rev. 2019, 60, 1–41. [Google Scholar] [CrossRef]
- Sprenger, S. Nanosilica-Toughened Epoxy Resins. Polymers 2020, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.-G.; Song, C.; Ren, J.; Guo, S.-Y.; Nie, R.; Zhang, L. Mechanical and durability-related performance of graphene/epoxy resin and epoxy resin enhanced OPC mortar. Constr. Build. Mater. 2021, 282, 122644. [Google Scholar] [CrossRef]
- Liu, X.-F.; Xiao, Y.-F.; Luo, X.; Liu, B.-W.; Guo, D.-M.; Chen, L.; Wang, Y.-Z. Flame-Retardant multifunctional epoxy resin with high performances. Chem. Eng. J. 2022, 427, 132031. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, F.; Li, S.; Cheng, Y. Smoke suppression properties of epoxy crosslinked structure and intumescent fire retardant in epoxy-based intumescent fire-retardant coating. J. Appl. Polym. Sci. 2016, 133, 43912. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Mao, Y.; Shi, S.; Lei, L.; Wang, C.; Wang, D.; Hu, J.; Fu, S. A self-healable and highly flame retardant TiO2@MXene/P, N-containing polyimine nanocomposite for dual-mode fire sensing. Chem. Eng. J. 2024, 479, 147545. [Google Scholar] [CrossRef]
- Sun, S.; Yu, Q.; Yu, B.; Zhou, F. New Progress in the Application of Flame-Retardant Modified Epoxy Resins and Fire-Retardant Coatings. Coatings 2023, 13, 1663. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.-Y.; Dong, Y.; Wang, X.; Chen, X.; Ma, X.; Qian, P.-C. Facile Strategy to Design a Cellulose Nanocrystal-Based Nanocomposite Fire Retardant with Strong Smoke Suppression Efficiency. ACS Sustain. Chem. Eng. 2023, 11, 12983–12991. [Google Scholar] [CrossRef]
- Zhou, S.; Tao, R.; Dai, P.; Luo, Z.; He, M. Two-step fabrication of lignin-based flame retardant for enhancing the thermal and fire retardancy properties of epoxy resin composites. Polym. Compos. 2020, 41, 2025–2035. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y. Preparation of High-Transparency Phosphenanthrene-Based Flame Retardants and Studies of Their Flame-Retardant Properties. Polymers 2023, 15, 4665. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Shcherbakov, A.; Zhumabekova, A.; Serikbayeva, G.; Vikulova, M.; Svitkina, V. Effect of Phosphorus and Chlorine Containing Plasticizers on the Physicochemical and Mechanical Properties of Epoxy Composites. J. Compos. Sci. 2023, 7, 178. [Google Scholar] [CrossRef]
- Yan, L.; Xu, Z.; Deng, N.; Chu, Z. Synergistic effects of mono-component intumescent flame retardant grafted with carbon black on flame retardancy and smoke suppression properties of epoxy resins. J. Therm. Anal. Calorim. 2019, 138, 915–927. [Google Scholar] [CrossRef]
- Yu, H.; Xu, X.; Xia, Y.; Pan, M.; Zarshad, N.; Pang, B.; Rahman, A.U.; Wu, M.; Ni, H. Synthesis of a novel modified chitosan as an intumescent flame retardant for epoxy resin. e-Polymers 2020, 20, 303–316. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, L.; Chen, L.; Wang, Y. Synthesis of novel calixarene-based intumescent flame retardant and application of flame retardant epoxy resin. J. Appl. Polym. Sci. 2021, 139, 51986. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Song, L.; Hu, Y. Intrinsically flame retardant bio-based epoxy thermosets: A review. Compos. Part B Eng. 2019, 179, 107487. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Qin, Z.; Zhang, W.; Yang, R. Inorganic/organic phosphorus-based flame retardants synergistic flame retardant rigid polyurethane foam. Polym. Eng. Sci. 2023, 63, 1041–1049. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, D.; Ding, H.; Xu, P.; Zhang, C.; Ren, Y.; Yang, W.; Ma, P. Synthesis of eugenol-based phosphorus-containing epoxy for enhancing the flame-retardancy and mechanical performance of DGEBA epoxy resin. React. Funct. Polym. 2022, 180, 105383. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Zhao, P. Durable Flame Retardancy of Cotton Fabrics with a Novel P-N Intumescent Flame Retardant. Fibers Polym. 2023, 25, 111–119. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, L.; Long, J.; Xing, L.; Huang, Y.; Wu, Z.; Yan, X.; Guo, Z. Reinforced unsaturated polyester composites by chemically grafting amino-POSS onto carbon fibers with active double spiral structural spiralphosphodicholor. Compos. Sci. Technol. 2014, 100, 158–165. [Google Scholar] [CrossRef]
- Jiang, D.; Sun, C.; Zhou, Y.; Wang, H.; Yan, X.; He, Q.; Guo, J.; Guo, Z. Enhanced flame retardancy of cotton fabrics with a novel intumescent flame-retardant finishing system. Fibers Polym. 2015, 16, 388–396. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Cao, Y.; Peng, Y. Synthesis of a Novel Spiro Phosphorus–Nitrogen Concerted Reactive Flame-Retardant Curing Agent and Its Application in Epoxy Resin. Front. Mater. 2020, 7, 293. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H. Preparation, mechanical properties, and thermal degradation of flame retarded epoxy resins with an organophosphorus oligomer. Polym. Bull. 2011, 67, 859–873. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Zhao, W.; Wang, X.; Wang, D. Synthesis of a novel self-intumescent flame retardant with spiro and triazine structure and its performance for polypropylene. J. Fire Sci. 2015, 34, 104–119. [Google Scholar] [CrossRef]
- Luo, X.; Hu, H.; Ma, J.; Huang, Y.; Yang, J. Synthesis and characterization of dicyclic silicon-/phosphorus-grafted alumina and its application in improving flame retardancy of epoxy resin. J. Appl. Polym. Sci. 2020, 138, 49854. [Google Scholar] [CrossRef]
- Liu, J.; Dong, C.; Zhang, Z.; Sun, H.; Kong, D.; Lu, Z. Durable flame retardant cotton fabrics modified with a novel silicon–phosphorus–nitrogen synergistic flame retardant. Cellulose 2020, 27, 9027–9043. [Google Scholar] [CrossRef]
- Ma, H.Y.; Tong, L.F.; Xu, Z.B.; Fang, Z.P. Functionalizing Carbon Nanotubes by Grafting on Intumescent Flame Retardant: Nanocomposite Synthesis, Morphology, Rheology, and Flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Zhang, P.; He, Y.; Tian, S.; Fan, H.; Chen, Y.; Yan, J. Flame retardancy, mechanical, and thermal properties of waterborne polyurethane conjugated with a novel phosphorous-nitrogen intumescent flame retardant. Polym. Compos. 2017, 38, 452–462. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Ruan, F.; Yang, W.; Liu, J. Synthesis of a Novel Spirocyclic Inflatable Flame Retardant and Its Application in Epoxy Composites. Polymers 2020, 12, 2534. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Ruan, F.; Liu, J.; Li, N.; Li, X. Effects of a Macromolecule Spirocyclic Inflatable Flame Retardant on the Thermal and Flame Retardant Properties of Epoxy Resin. Polymers 2020, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Kregl, L.; Wallner, G.M.; Lang, R.W.; Mayrhofer, G. Effect of resin modifiers on the structural properties of epoxy resins. J. Appl. Polym. Sci. 2017, 134, 45348. [Google Scholar] [CrossRef]
- Pu, Z.; Zou, L.; Wu, F.; Wang, X.; Peng, Q.; Zhong, J.; Liu, X.; Wang, Y.; Pan, Y.; Jiang, D.; et al. Preparation of phosphorus-nitrogen flame retardant DOPO-DBAPh and its flame retarded epoxy-based composites. J. Vinyl Addit. Technol. 2023, 30, 398–409. [Google Scholar] [CrossRef]
- Kim, H.-H.; Sim, M.-J.; Lee, J.-C.; Cha, S.-H. The effects of chemical structure for phosphorus-nitrogen flame retardants on flame retardant mechanisms. J. Mater. Sci. 2023, 58, 6850–6864. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Sun, Z. Synthesis of a novel macromolecular carbon-nitrogen-phosphorous intumescent flame retardant. Adv. Powder Technol. 2021, 32, 1341–1349. [Google Scholar] [CrossRef]
- Li, J.; Jiang, W.; Liu, M. Durable phosphorus/nitrogen flame retardant for cotton fabric. Cellulose 2022, 29, 4725–4751. [Google Scholar] [CrossRef]
- Song, K.; Hou, B.; Ur Rehman, Z.; Pan, Y.-T.; He, J.; Wang, D.-Y.; Yang, R. “Sloughing” of metal-organic framework retaining nanodots via step-by-step carving and its flame-retardant effect in epoxy resin. Chem. Eng. J. 2022, 448, 137666. [Google Scholar] [CrossRef]
- Song, K.; Zhang, H.; Pan, Y.-T.; Ur Rehman, Z.; He, J.; Wang, D.-Y.; Yang, R. Metal-organic framework-derived bird’s nest-like capsules for phosphorous small molecules towards flame retardant polyurea composites. J. Colloid Interface Sci. 2023, 643, 489–501. [Google Scholar] [CrossRef]
- Aljamal, A.; Szolnoki, B.; Marosi, G. Improving thermal and flame retardant properties of sorbitol-based bioepoxy systems by phosphorus-based flame retardants. Fire Mater. 2021, 46, 605–614. [Google Scholar] [CrossRef]




| Samples | EP (wt.%) | PCS (wt.%) | DDS (wt.%) |
|---|---|---|---|
| EP-0 | 100 | 0 | 20 |
| EP/PCS-1 | 95 | 5 | 15 |
| EP/PCS-2 | 90 | 10 | 10 |
| EP/PCS-3 | 85 | 15 | 5 |
| EP/PCS-4 | 80 | 20 | 0 |
| Samples | Tonset (°C) | Tmax (°C) | Residual Char (500 °C, wt.%) | Residual Char (700 °C, wt.%) | ||||
|---|---|---|---|---|---|---|---|---|
| N2 | Air | N2 | Air | N2 | Air | N2 | Air | |
| EP-0 | 388 | 367 | 440 | 430 | 15 | 28 | 10 | 12 |
| EP/PCS-2 | 317 | 307 | 366 | 365 | 32 | 36 | 28 | 25 |
| EP/PCS-4 | 296 | 290 | 351 | 349 | 33 | 37 | 29 | 26 |
| Samples | LOI (%) | UL-94 | t1 (s) | t2 (s) | Dripping |
|---|---|---|---|---|---|
| EP-0 | 24.8 | NR | - | - | Yes |
| EP/PCS-1 | 25.1 | V-2 | 23.1 | 8.9 | No |
| EP/PCS-2 | 27.1 | V-1 | 15.2 | 7.3 | No |
| EP/PCS-3 | 28.6 | V-0 | 4.2 | 3.5 | No |
| EP/PCS-4 | 31.2 | V-0 | 3.1 | 1.9 | No |
| Samples | TTI 1 (s) | pHRR (kW/m2) | THR (MJ/m2) | TSP (m2) | Mean COY (g/s) | Mean CO2P (g/s) |
|---|---|---|---|---|---|---|
| EP-0 | 45 | 959 | 66.3 | 15.78 | 0.116 | 2.17 |
| EP/PCS-2 | 38 | 506 | 53.4 | 11.72 | 0.108 | 1.71 |
| EP/PCS-4 | 31 | 386 | 24.1 | 7.01 | 0.104 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, Y.; Su, Y.; Yu, C.; Han, J.; Liu, J. Preparation of a Polymeric Phosphoramide Flame-Retardant and Its Effect on the Flame-Retardant Properties of Epoxy Resin. Polymers 2024, 16, 1224. https://doi.org/10.3390/polym16091224
Wang H, Wang Y, Su Y, Yu C, Han J, Liu J. Preparation of a Polymeric Phosphoramide Flame-Retardant and Its Effect on the Flame-Retardant Properties of Epoxy Resin. Polymers. 2024; 16(9):1224. https://doi.org/10.3390/polym16091224
Chicago/Turabian StyleWang, Hao, Yinjie Wang, Yan Su, Chuang Yu, Jia Han, and Jiping Liu. 2024. "Preparation of a Polymeric Phosphoramide Flame-Retardant and Its Effect on the Flame-Retardant Properties of Epoxy Resin" Polymers 16, no. 9: 1224. https://doi.org/10.3390/polym16091224
APA StyleWang, H., Wang, Y., Su, Y., Yu, C., Han, J., & Liu, J. (2024). Preparation of a Polymeric Phosphoramide Flame-Retardant and Its Effect on the Flame-Retardant Properties of Epoxy Resin. Polymers, 16(9), 1224. https://doi.org/10.3390/polym16091224




