Gamma Irradiation Effect on Polymeric Chains of Epoxy Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Gamma Irradiation Test
2.3. FTIR Spectroscopy
2.4. EPR Spectroscopy
2.5. THz Time-Domain Spectroscopy
3. Results and Discussion
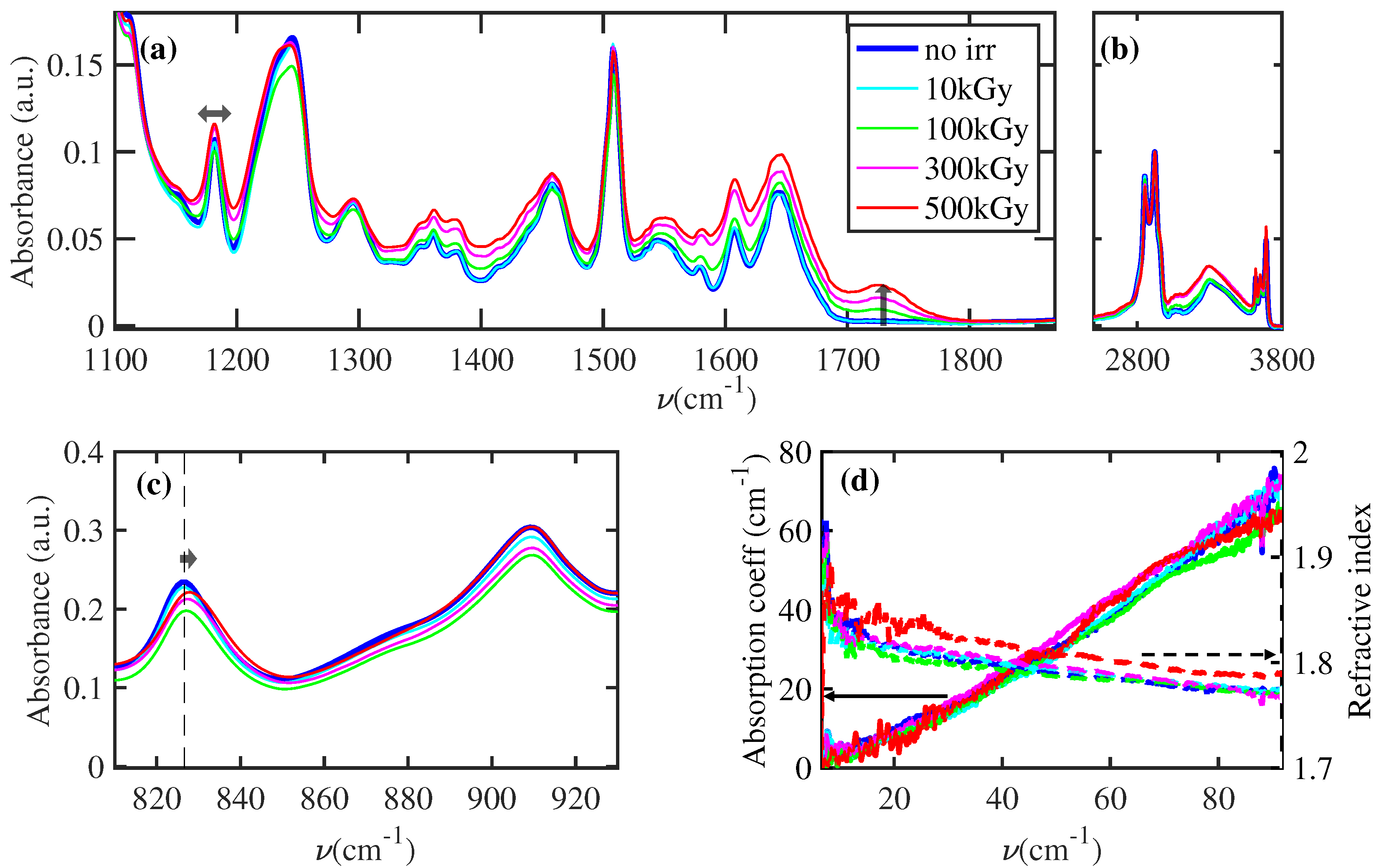
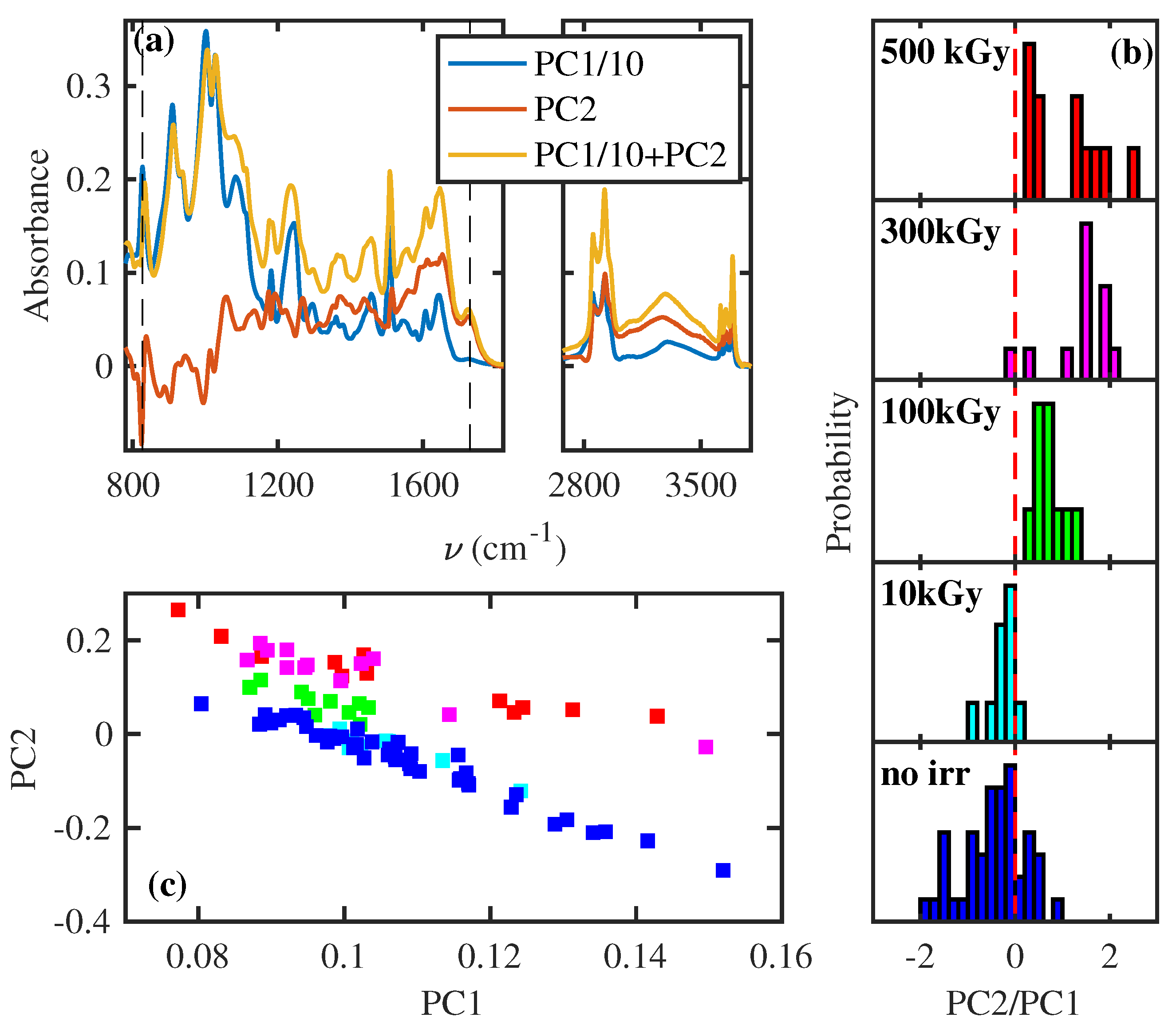
3.1. Structural Change of Polymeric Chains
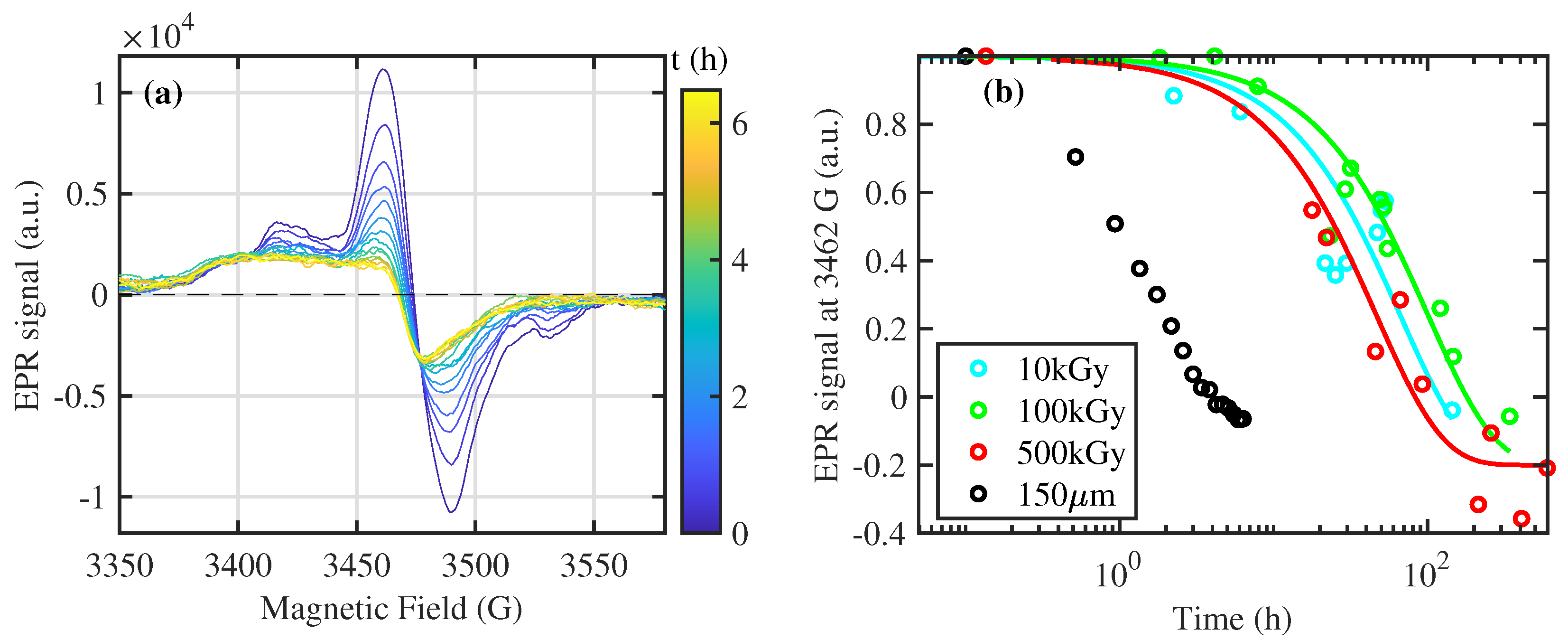
3.2. Dynamic of Free Radicals
4. Conclusions
- The rays exposure induces several free radicals at low exposure (10 kGy). The dependence on sample thickness demonstrates a recombination mediated by the exposure to the gaseous environment.
- At higher exposure (>100 kGy), the FTIR spectra show a dose-dependent structural modification ascribable to the scission of polymeric chains and the increase in crosslinking between polymeric chains. To explain this effect, we should consider that such structural modification can be realized by the generation of free radicals in the same spatial region [5]. This specific condition can be obtained only by increasing the rays exposure.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EA | Epoxy adhesive |
| FTIR | Fourier transform infrared spectroscopy |
| TDS | Time-domain spectroscopy |
| EPR | Electron paramagnetic resonance |
| HEP | High-energy physics |
| rays | Gamma rays |
| PTFE | Polytetrafluoroethylene |
| PA | Photoconductive antenna |
| PCA | Principal component analysis |
| PC1 | First principal component |
| PC2 | Second principal component |
| Parallel components of the anisotropic g tensor | |
| Perpendicular components of the anisotropic g tensor | |
| h | hour |
References
- Kajal, R.; Kataria, B.; Asokan, K.; Mohan, D. Effects of gamma radiation on structural, optical, and electrical properties of SnO2 thin films. Appl. Surf. Sci. Adv. 2023, 15, 100406. [Google Scholar] [CrossRef]
- Chen, J.; Ding, N.; Li, Z.; Wang, W. Organic polymer materials in the space environment. Prog. Aerosp. Sci. 2016, 83, 37–56. [Google Scholar] [CrossRef]
- Jenkins, A.; Kratochvíl, P.; Stepto, R.; Suter, U. Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Jeong, J.O.; Park, J.S.; Kim, Y.A.; Yang, S.J.; Jeong, S.I.; Lee, J.Y.; Lim, Y.M. Gamma Ray-Induced Polymerization and Cross-Linking for Optimization of PPy/PVP Hydrogel as Biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef]
- Ashfaq, A.; Clochard, M.C.; Coqueret, X.; Dispenza, C.; Driscoll, M.S.; Ulański, P.; Al-Sheikhly, M. Polymerization Reactions and Modifications of Polymers by Ionizing Radiation. Polymers 2020, 12, 2877. [Google Scholar] [CrossRef]
- Lapique, F.; Redford, K. Curing effects on viscosity and mechanical properties of a commercial epoxy resin adhesive. Int. J. Adhes. Adhes. 2002, 22, 337–346. [Google Scholar] [CrossRef]
- Rahatekar, S.; Koziol, K.; Butler, S.; Elliott, J.; Shaffer, M.; Mackley, M.; Windle, A. Optical microstructure and viscosity enhancement for an epoxy resin matrix containing multiwall carbon nanotubes. J. Rheol. 2006, 50, 599–610. [Google Scholar] [CrossRef]
- Mohan, P. A critical review: The modification, properties, and applications of epoxy resins. Polym. Plast. Technol. Eng. 2013, 52, 107–125. [Google Scholar] [CrossRef]
- Ramsdale-Capper, R.; Foreman, J.P. Internal antiplasticisation in highly crosslinked amine cured multifunctional epoxy resins. Polymer 2018, 146, 321–330. [Google Scholar] [CrossRef]
- Kumar, A.; Sundararaghavan, V.; Browning, A.R. Study of temperature dependence of thermal conductivity in cross-linked epoxies using molecular dynamics simulations with long range interactions. Model. Simul. Mater. Sci. Eng. 2014, 22, 025013. [Google Scholar] [CrossRef]
- Cleland, M.; Parks, L.; Cheng, S. Applications for radiation processing of materials. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 66–73. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, X.; Zhang, F.; Wu, X.; Huang, W.; Liu, W.; Wang, X. Influence of gamma irradiation on the molecular dynamics and mechanical properties of epoxy resin. Polym. Degrad. Stab. 2019, 168, 108940. [Google Scholar] [CrossRef]
- Diao, F.; Zhang, Y.; Liu, Y.; Fang, J.; Luan, W. γ-Ray irradiation stability and damage mechanism of glycidyl amine epoxy resin. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2016, 383, 227–233. [Google Scholar] [CrossRef]
- Zimmermann, J.; Sadeghi, M.; Schroeder, K. The effect of γ-radiation on the mechanical properties of structural adhesive. Int. J. Adhes. Adhes. 2019, 93, 102334. [Google Scholar] [CrossRef]
- Kacem, I.; Daoudi, M.; Dridi, W.; Sellemi, H.; Harzli, K.; De Izzara, G.; Geslot, B.; Guermazi, H.; Blaise, P.; Hosni, F.; et al. Effects of neutron–gamma radiation on the free radical contents in epoxy resin: Upconversion luminescence and structural stabilization. Appl. Phys. A 2019, 125, 758. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Y.; Zhang, C.; Liu, L.; Zhang, Y.; Wang, L. Effect of γ-ray irradiation grafting on the carbon fibers and interfacial adhesion of epoxy composites. Compos. Sci. Technol. 2007, 67, 3261–3270. [Google Scholar] [CrossRef]
- Przybytniak, G.; Nowicki, A.; Mirkowski, K.; Stobiński, L. Gamma-rays initiated cationic polymerization of epoxy resins and their carbon nanotubes composites. Radiat. Phys. Chem. 2016, 121, 16–22. [Google Scholar] [CrossRef]
- Nhamoinesu, S.; Overend, M. The mechanical performance of adhesives for a steel-glass composite façade system. Chall. Glass 2012, 3, 293–306. [Google Scholar]
- Côté, P.; Desnoyers, N. Thermal stress failure criteria for a structural epoxy. In Proceedings of the Optomechanics 2011: Innovations and Solutions, International Society for Optics and Photonics, San Diego, CA, USA, 21–25 August 2011; Volume 8125, pp. 173–184. [Google Scholar]
- Lucchesi, L.; Beghini, M.; Monelli, B.D.; Raffaelli, F.; Cemmi, A.; Di Sarcina, I. Post-cure and γ-irradiation effects on structural epoxy adhesive bulk specimens. Int. J. Adhes. Adhes. 2022, 118, 103225. [Google Scholar] [CrossRef]
- Lucchesi, L.; Beghini, M.; Monelli, B.D.; Raffaelli, F.; Cemmi, A.; Di Sarcina, I.; Ferrante, C. γ-irradiation effect on the mechanical properties of in situ specimens made of structural epoxy adhesive and comparison with adhesive bulk behavior. Int. J. Adhes. Adhes. 2023, 124, 103387. [Google Scholar] [CrossRef]
- Baccaro, S.; Cemmi, A.; Di Sarcina, I.; Ferrara, G. Gamma Irradiation Calliope Facility at ENEA-Casaccia Research Centre; ENEA: Rome, Italy, 2019. [Google Scholar]
- Taschin, A.; Bartolini, P.; Tasseva, J.; Torre, R. THz time-domain spectroscopic investigations of thin films. Measurement 2018, 118, 282–288. [Google Scholar] [CrossRef]
- Peccianti, M.; Fastampa, R.; Mosca Conte, A.; Pulci, O.; Violante, C.; Łojewska, J.; Clerici, M.; Morandotti, R.; Missori, M. Terahertz Absorption by Cellulose: Application to Ancient Paper Artifacts. Phys. Rev. Appl. 2017, 7, 064019. [Google Scholar] [CrossRef]
- Tatsuoka, S.; Sato, H. Stress-induced crystal transition of poly(butylene succinate) studied by terahertz and low-frequency Raman spectroscopy and quantum chemical calculation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 197, 95–102. [Google Scholar] [CrossRef]
- Nakajima, S.; Horiuchi, S.; Ikehata, A.; Ogawa, Y. Determination of starch crystallinity with the Fourier-transform terahertz spectrometer. Carbohydr. Polym. 2021, 262, 117928. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Abbate, M.; Martuscelli, E.; Musto, P.; Ragosta, G. Thermosetting bismaleimide/reactive rubber blends: Curing kinetics and mechanical behavior. J. Appl. Polym. Sci. 1997, 65, 979–990. [Google Scholar] [CrossRef]
- Musto, P.; Martuscelli, E.; Ragosta, G.; Russo, P.; Scarinzi, G. An interpenetrated system based on a tetrafunctional epoxy resin and a thermosetting bismaleimide: Structure–properties correlation. J. Appl. Polym. Sci. 1998, 69, 1029–1042. [Google Scholar] [CrossRef]
- Parker, S.F.; Mason, S.M.; Williams, K.P. Fourier Transform Raman and infrared spectroscopy of N-phenylmaleimide and methylene dianiline bismaleimide. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1990, 46, 315–321. [Google Scholar] [CrossRef]
- Smith, R.E.; Larsen, F.N.; Long, C.L. Epoxy resin cure. II. FTIR analysis. J. Appl. Polym. Sci. 1984, 29, 3713–3726. [Google Scholar] [CrossRef]
- Brunton, S.L.; Kutz, J.N. Data-Driven Science and Engineering: Machine Learning, Dynamical Systems, and Control; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Ranby, B.; Rabek, J.F. ESR Spectroscopy in Polymer Research; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 1. [Google Scholar]
- Moriuchi, S.; Nakamura, M.; Shimada, S.; Kashiwabara, H.; Sohma, J. ESR study on molecular motion of peroxy radicals of polytetrafluoroethylene. Polymer 1970, 11, 630–639. [Google Scholar] [CrossRef]
- Zanni-Deffarges, M.; Shanahan, M. Diffusion of water into an epoxy adhesive: Comparison between bulk behaviour and adhesive joints. Int. J. Adhes. Adhes. 1995, 15, 137–142. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrante, C.; Lucchesi, L.; Cemmi, A.; Di Sarcina, I.; Scifo, J.; Verna, A.; Taschin, A.; Senni, L.; Beghini, M.; Monelli, B.D.; et al. Gamma Irradiation Effect on Polymeric Chains of Epoxy Adhesive. Polymers 2024, 16, 1202. https://doi.org/10.3390/polym16091202
Ferrante C, Lucchesi L, Cemmi A, Di Sarcina I, Scifo J, Verna A, Taschin A, Senni L, Beghini M, Monelli BD, et al. Gamma Irradiation Effect on Polymeric Chains of Epoxy Adhesive. Polymers. 2024; 16(9):1202. https://doi.org/10.3390/polym16091202
Chicago/Turabian StyleFerrante, Carino, Leonardo Lucchesi, Alessia Cemmi, Ilaria Di Sarcina, Jessica Scifo, Adriano Verna, Andrea Taschin, Luca Senni, Marco Beghini, Bernardo Disma Monelli, and et al. 2024. "Gamma Irradiation Effect on Polymeric Chains of Epoxy Adhesive" Polymers 16, no. 9: 1202. https://doi.org/10.3390/polym16091202
APA StyleFerrante, C., Lucchesi, L., Cemmi, A., Di Sarcina, I., Scifo, J., Verna, A., Taschin, A., Senni, L., Beghini, M., Monelli, B. D., & Raffaelli, F. (2024). Gamma Irradiation Effect on Polymeric Chains of Epoxy Adhesive. Polymers, 16(9), 1202. https://doi.org/10.3390/polym16091202





