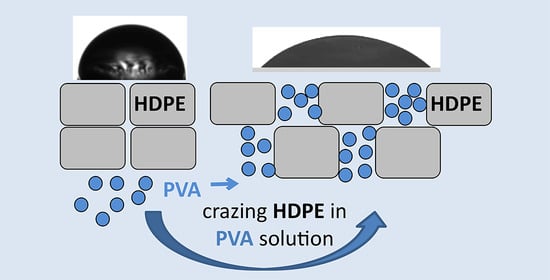
Modification of High-Density Polyethylene with a Fibrillar–Porous Structure by Biocompatible Polyvinyl Alcohol via Environmental Crazing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HDPE/PVA Nanocomposite
2.3. Methods
2.3.1. Volume Porosity
2.3.2. Gravimetric Measurements
2.3.3. Tensile Tests
2.3.4. Strain Recovery
2.3.5. Differential Scanning Calorimetry (DSC)
2.3.6. Atomic Force Microscopy (AFM)
2.3.7. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (EDX SEM)
2.3.8. Wettability
2.3.9. Degree of Swelling
2.3.10. Water Vapor Permeability
2.3.11. Wide Angle X-ray Scattering (WAXS)
2.3.12. Statistical Analysis
3. Results
3.1. Preparation of the HDPE/PVA Nanocomposites by Environmental Crazing
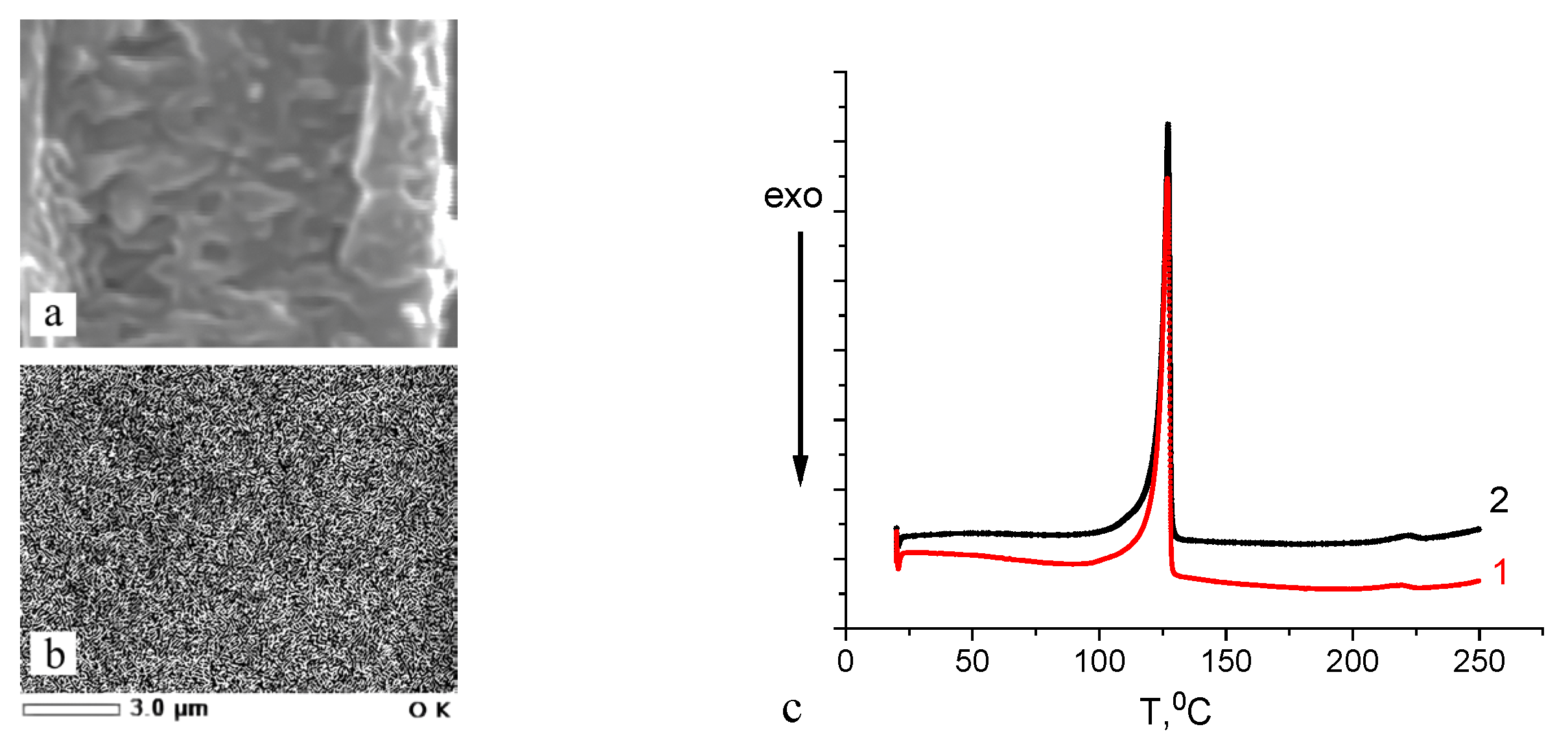
3.2. Structure of the HDPE/PVA Nanocomposites
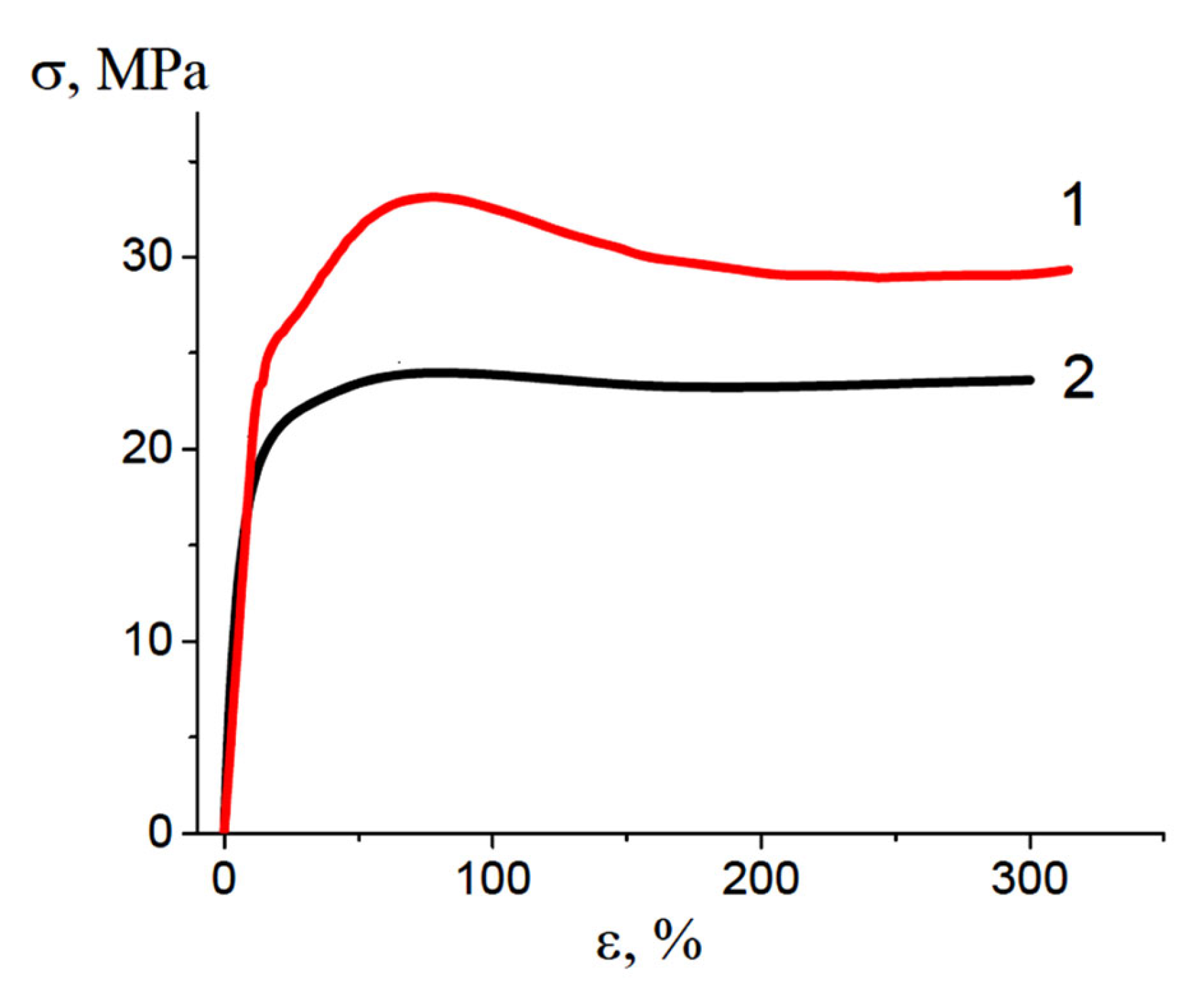
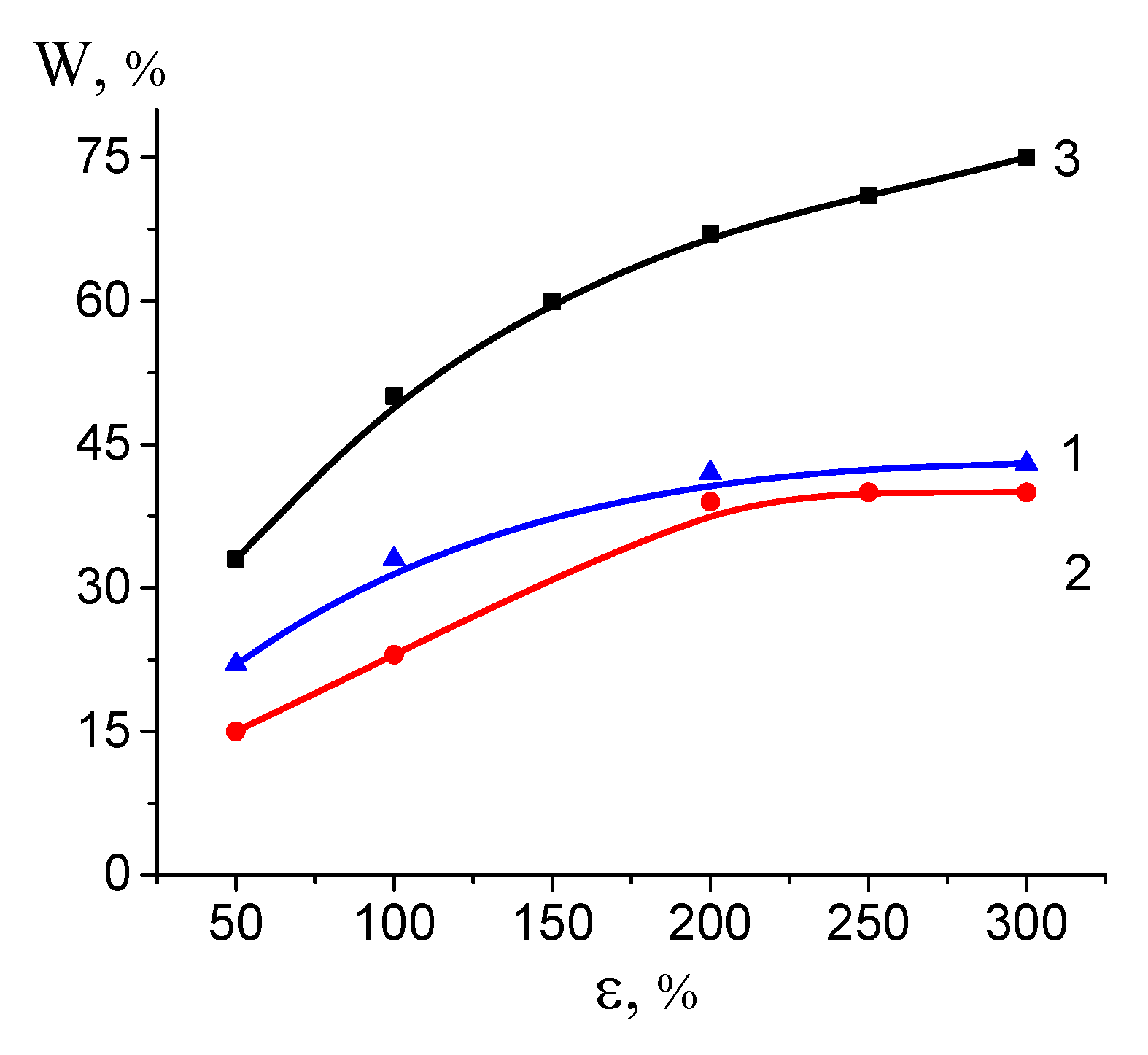
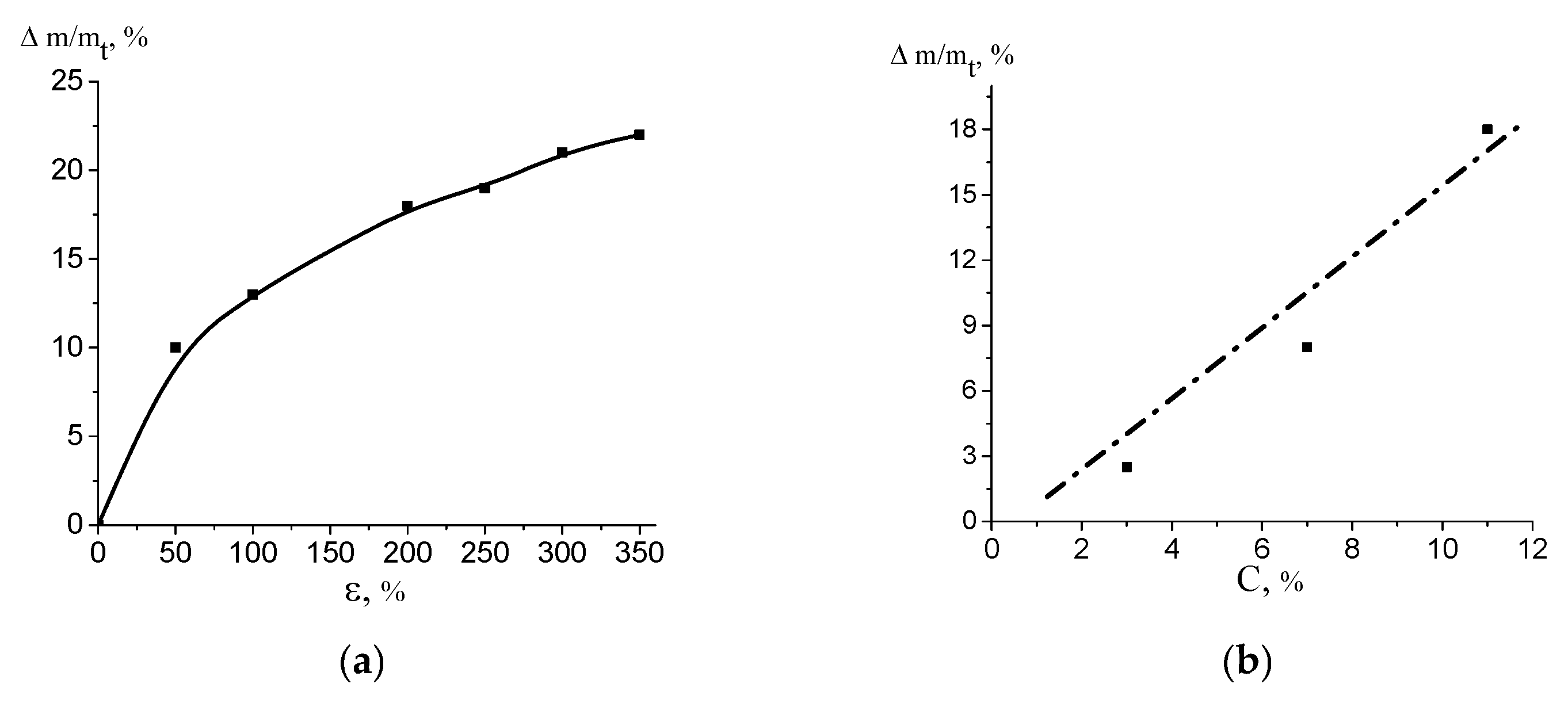
3.3. Properties (Performance) of the HDPE/PVA Nanocomposites
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.-N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly(vinyl alcohol)/poly(acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6, 8105–8114. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M.; Ludwiczak, S.; Kawinska, M. Kinetics of water sorption by chitosan and its blends with poly(vinyl alcohol). Carbohydr. Polym. 2005, 62, 42–49. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.K. Oxygen barrier properties of biaxially oriented polypropylene/polyvinyl alcohol blend films. Polymer 2004, 45, 1599–1607. [Google Scholar] [CrossRef]
- Tang, X.; Alavi, S. Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability (Review). Carbohydr. Polym. 2011, 85, 7–16. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000, 153, 37–65. [Google Scholar] [CrossRef]
- Oviedoe, I.R.; Mendez, N.A.N.; Gómez, M.P.G.; Rodriguez, H.C.; Martinez, A.R. Design of a Physical and Nontoxic Crosslinked Poly(Vinyl Alcohol) Hydrogel. Int. J. Polym. Mater. 2008, 57, 1095–1103. [Google Scholar] [CrossRef]
- Armstrong, J.D.S.R.; Baer, E. Co-extruded multilayer shape memory materials: Comparing layered and blend architectures. Polymer 2013, 54, 5399–5407. [Google Scholar] [CrossRef]
- Wang, J.; Langhe, D.; Ponting, M.; Wnek, G.E.; Korley, L.T.J.; Baer, E. Manufacturing of polymer continuous nanofibers using a novel co-extrusion and multiplication technique. Polymer 2014, 55, 673–685. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer. Multicomponent Polymeric Materials; Wiley: Hoboken, NJ, USA, 2006; pp. 687–756. [Google Scholar]
- Lipatov, Y.S. Polymer blends and interpenetrating polymer networks at the interface with solids. Prog. Polym. Sci. 2002, 27, 1721–1801. [Google Scholar] [CrossRef]
- Stribeck, N.; Zeinolebadi, A.; Fakirov, S.; Bhattacharyya, D.; Botta, S. Extruded blend films of poly(vinyl alcohol) and polyolefins: Common and hard-elastic nanostructure evolution in the polyolefin during straining as monitored by SAXS. Sci. Technol. Adv. Mater. 2013, 14, 035006. [Google Scholar] [CrossRef]
- Kim, D.; Jung, J.; Park, S.; Seo, J. Preparation and characterization of LDPE/PVA blend films filled with glycerin-plasticized polyvinyl alcohol. J. Appl. Polym. Sci. 2015, 132, 41985. [Google Scholar] [CrossRef]
- Yan, X.; Cayla, A.; Devaux, E.; Salaün, F. Microstructure Evolution of Immiscible PP-PVA Blends Tuned by Polymer Ratio and Silica Nanoparticles. Polymers 2018, 10, 1031. [Google Scholar] [CrossRef] [PubMed]
- Ameer, Z.J.A.; Al-Mutairi, N.H. Miscibility Improvement of LDPE/PVA Blends with Maleic Acid Additions. IOP Conf. Ser. Mater. Sci. Eng. 2018, 433, 012074. [Google Scholar] [CrossRef]
- Argon, A. The Physics of Deformation and Fracture of Polymers; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Kramer, E. Microscopic and molecular fundamentals of crazing. In Crazing in Polymers; Kausch, H.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 1–56. [Google Scholar]
- Volynskii, A.L.; Bakeev, N.F. Surface Phenomena in the Structural and Mechanical Behaviour of Solid Polymers; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Yarysheva, A.; Bagrov, D.; Bakirov, A.; Tarasevich, B.; Grohovskaya, T.; Yarysheva, L.; Chvalun, S.; Volynskii, A. Polyethylene–poly(ethylene oxide) hybrid films obtained by crazing and their structural peculiarities. Macromolecules 2017, 50, 2881–2888. [Google Scholar] [CrossRef]
- Yarysheva, A.Y.; Yarysheva, L.M.; Drozdov, F.V.; Arzhakova, O.V.; Muzafarov, A.M. Controlled wettability of polymers via environmental crazing. Chin. J. Polym. Sci. (Engl. Ed.) 2022, 40, 1307–1314. [Google Scholar] [CrossRef]
- Yarysheva, A.; Rukhlya, E.; Yarysheva, L.; Bagrov, D.; Volynskii, A.; Bakeev, N. The structural evolution of high-density polyethylene during crazing in liquid medium. Eur. Polym. J. 2015, 66, 458–469. [Google Scholar] [CrossRef]
- ASTM E96; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2017.
- Andrade, E.N.d.C.; Randall, R.F.Y.; Makin, M.J. The Rehbinder Effect. Proc. Phys. Soc. Sect. B 1950, 63, 990. [Google Scholar] [CrossRef]
- Keller, A.; Machin, M.J. Oriented crystallization in polymers. J. Macromol. Sci. Part B Phys. 1967, 1, 41–91. [Google Scholar] [CrossRef]
- Everett, D.H. IUPAC Manual of Symbols and Terminology. Pure Appl. Chem. 1972, 31, 577. [Google Scholar] [CrossRef]
- Yarysheva, A.Y.; Bagrov, D.V.; Kechekyan, P.A.; Rukhlya, E.G.; Bakirov, A.V.; Yarysheva, L.M.; Chvalun, S.N.; Volynskii, A.L. Which way do fibrils disappear? Polymer 2019, 169, 234–242. [Google Scholar] [CrossRef]
- De Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Doi, M.; Edwards, S.F. The Theory of Polymer Dynamics; Clarendon Press: Oxford, UK, 1986. [Google Scholar]
- Strobl, G.R. The Physics of Polymers; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Dunne, L.J.; Manos, G. Adsorption and Phase Behaviour in Nanochannels and Nanotubes; Springer Science+Business Media B.V.: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Michell, R.M.; Lorenzo, A.T.; Müller, A.J.; Lin, M.-C.; Chen, H.-L.; Blaszczyk-Lezak, I.; Martín, J.; Mijangos, C. The Crystallization of Confined Polymers and Block Copolymers Infiltrated Within Alumina Nanotube Templates. Macromolecules 2012, 45, 1517–1528. [Google Scholar] [CrossRef]
- Woo, A.M.; Huh, J.; Jeong, Y.G.; Shin, K. From Homogeneous to Heterogeneous Nucleation of Chain Molecules under Nanoscopic Cylindrical Confinement. Phys. Rev. Lett. 2007, 98, 136103. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarysheva, A.; Arzhakova, O. Modification of High-Density Polyethylene with a Fibrillar–Porous Structure by Biocompatible Polyvinyl Alcohol via Environmental Crazing. Polymers 2024, 16, 1184. https://doi.org/10.3390/polym16091184
Yarysheva A, Arzhakova O. Modification of High-Density Polyethylene with a Fibrillar–Porous Structure by Biocompatible Polyvinyl Alcohol via Environmental Crazing. Polymers. 2024; 16(9):1184. https://doi.org/10.3390/polym16091184
Chicago/Turabian StyleYarysheva, Alena, and Olga Arzhakova. 2024. "Modification of High-Density Polyethylene with a Fibrillar–Porous Structure by Biocompatible Polyvinyl Alcohol via Environmental Crazing" Polymers 16, no. 9: 1184. https://doi.org/10.3390/polym16091184
APA StyleYarysheva, A., & Arzhakova, O. (2024). Modification of High-Density Polyethylene with a Fibrillar–Porous Structure by Biocompatible Polyvinyl Alcohol via Environmental Crazing. Polymers, 16(9), 1184. https://doi.org/10.3390/polym16091184