Pharmaceutical Applications of Biomass Polymers: Review of Current Research and Perspectives
Abstract
1. Introduction
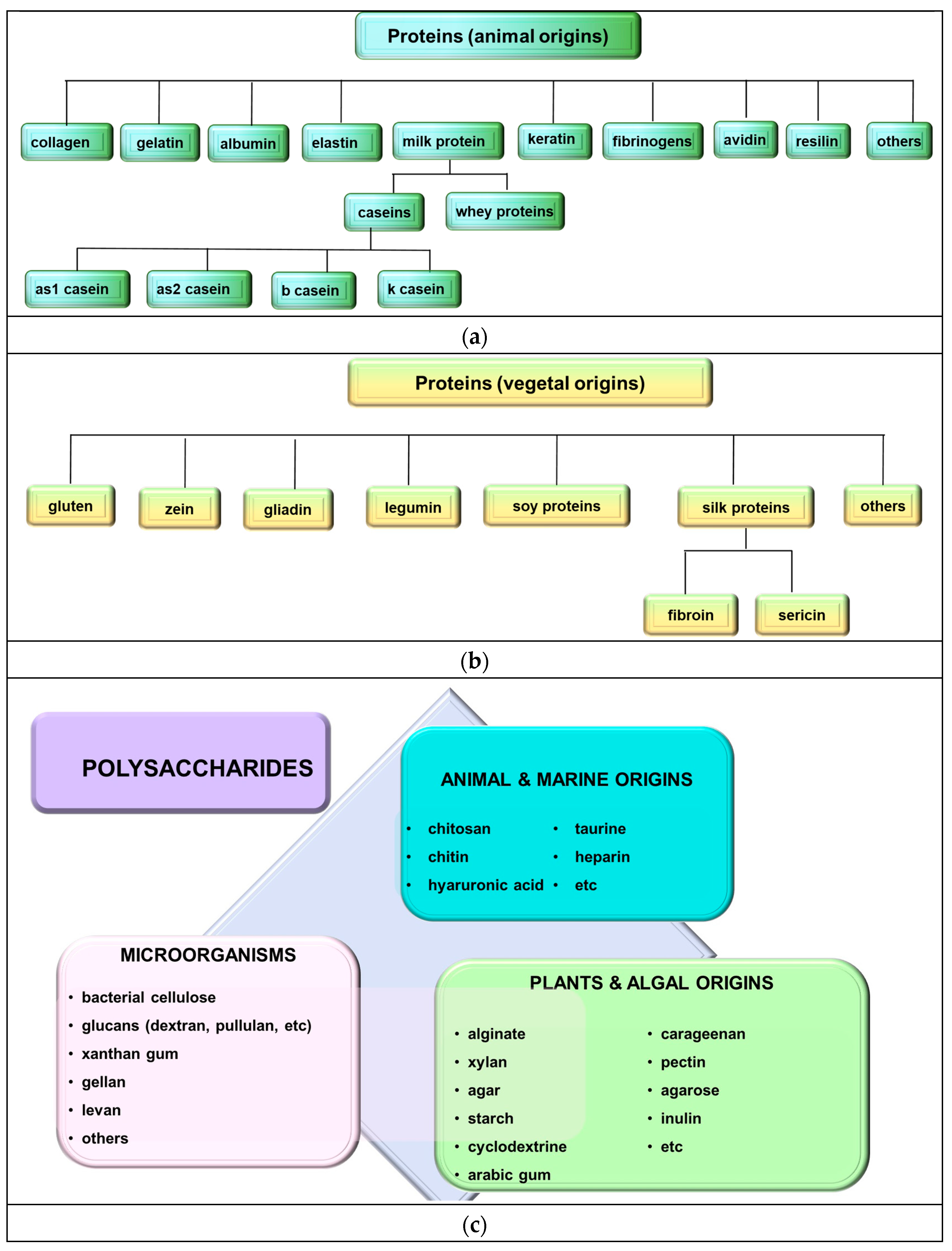
2. Biopolymers
2.1. Polysaccharides
2.1.1. Homoglycans
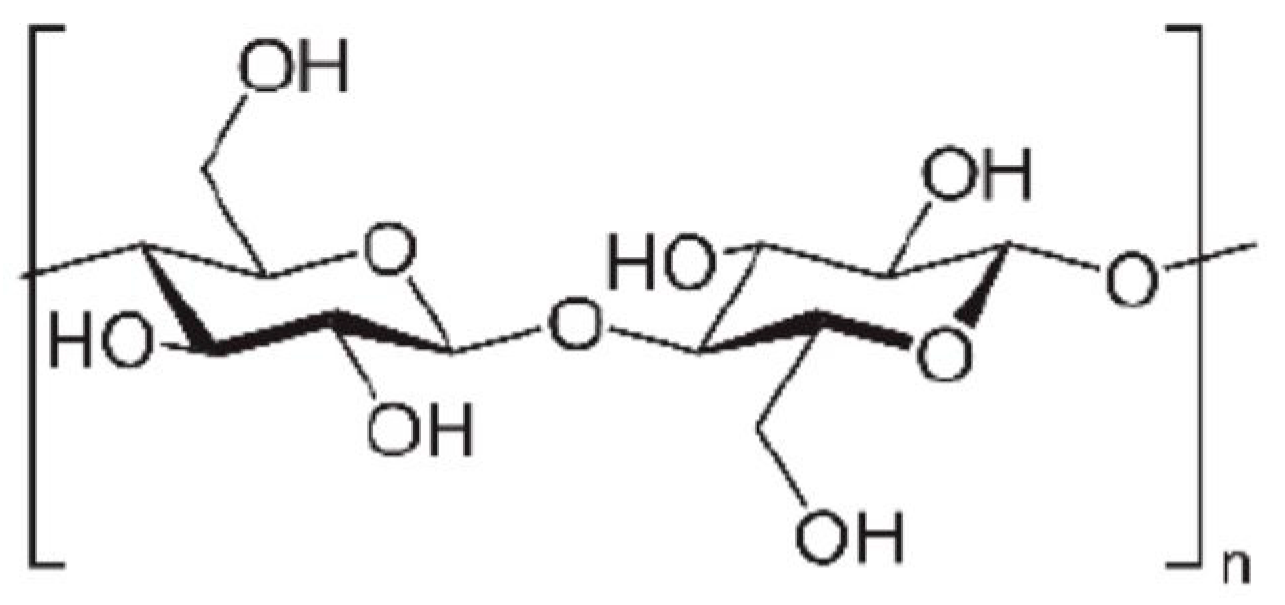
Starch
Dextran
Cyclodextrins
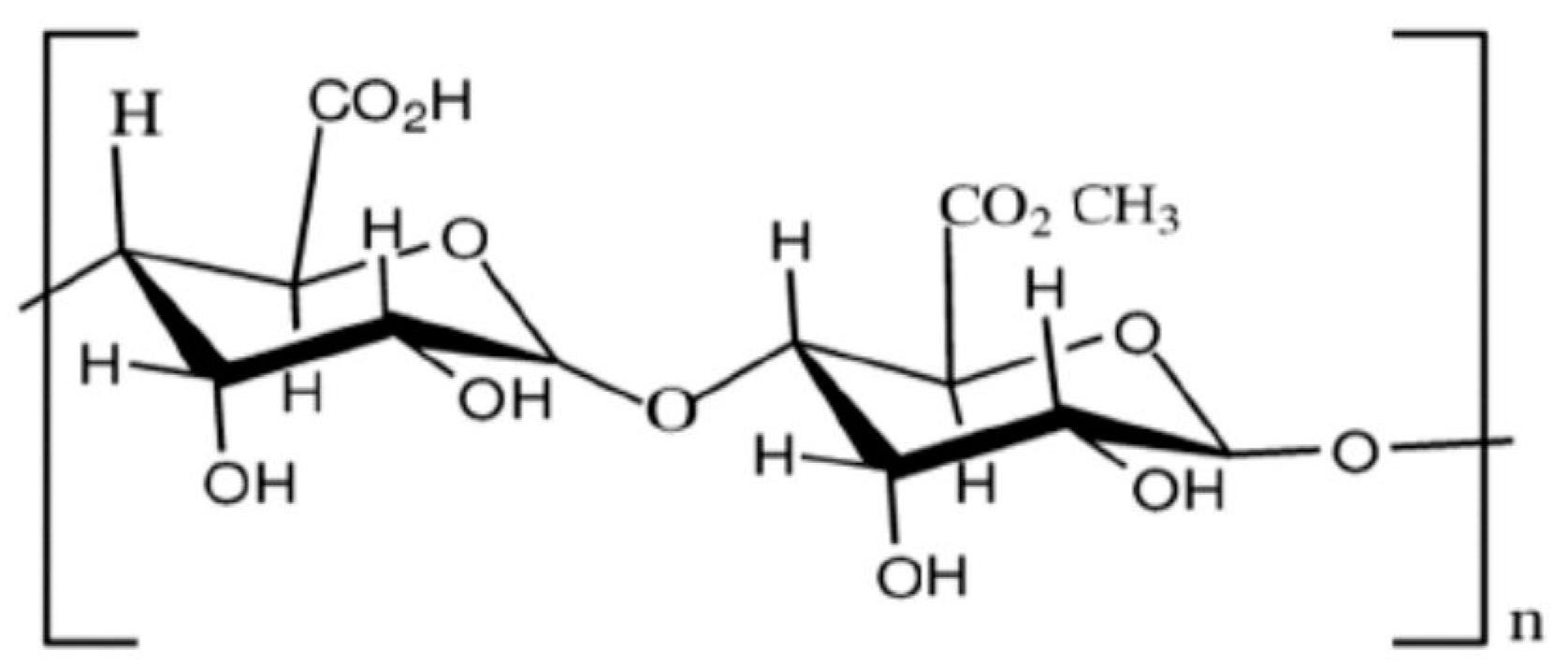
Cellulose
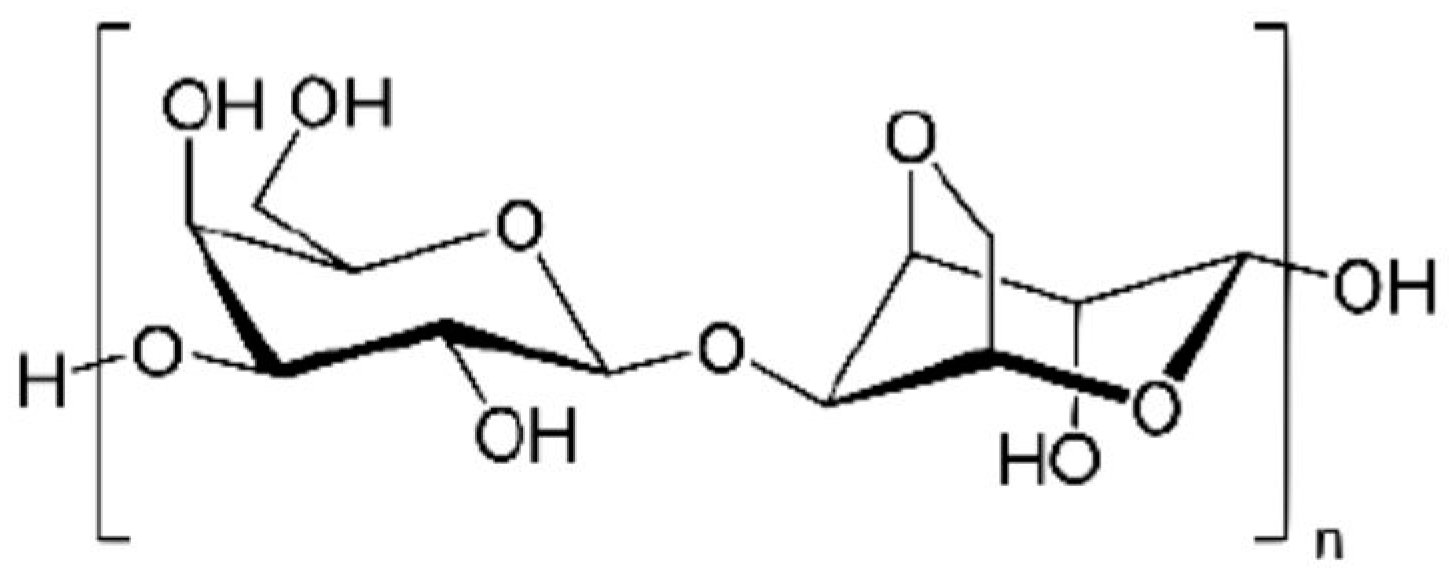
Chitin and Chitosan
2.1.2. Heteroglycans
Alginate
Agarose
Carrageenans
Pectins
Arabic Gum
Guar Gum
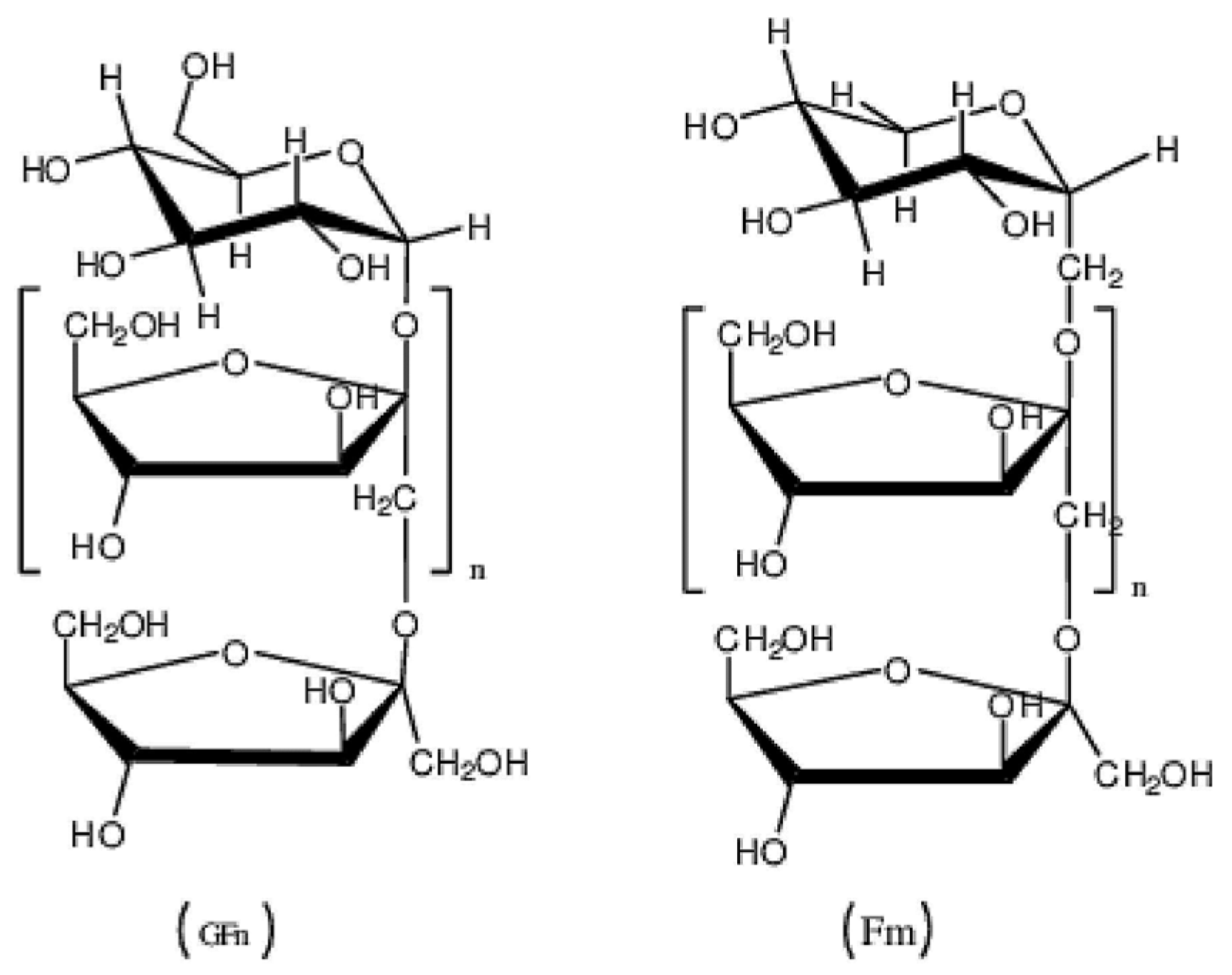
Inulin
Glycosaminoglycans
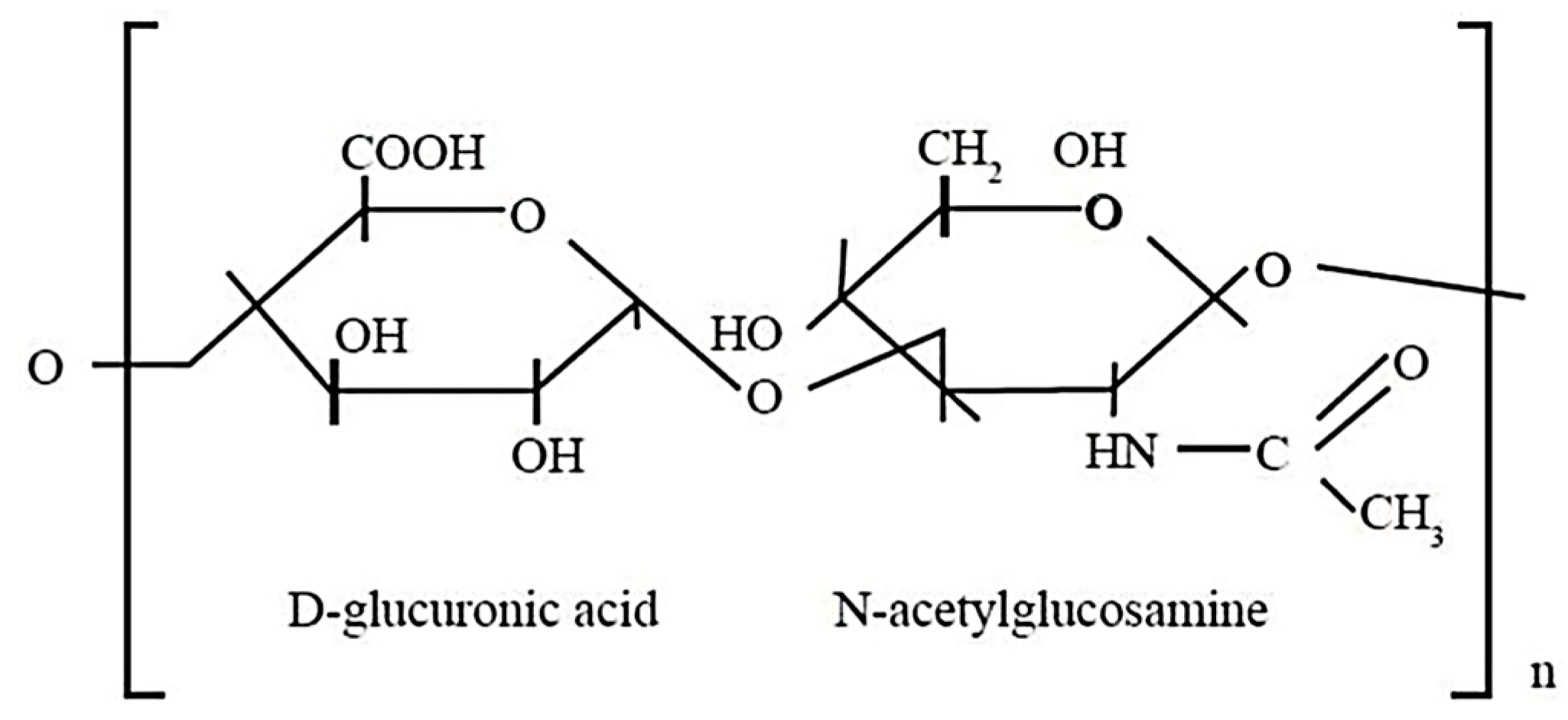
- Hyaluronic Acid
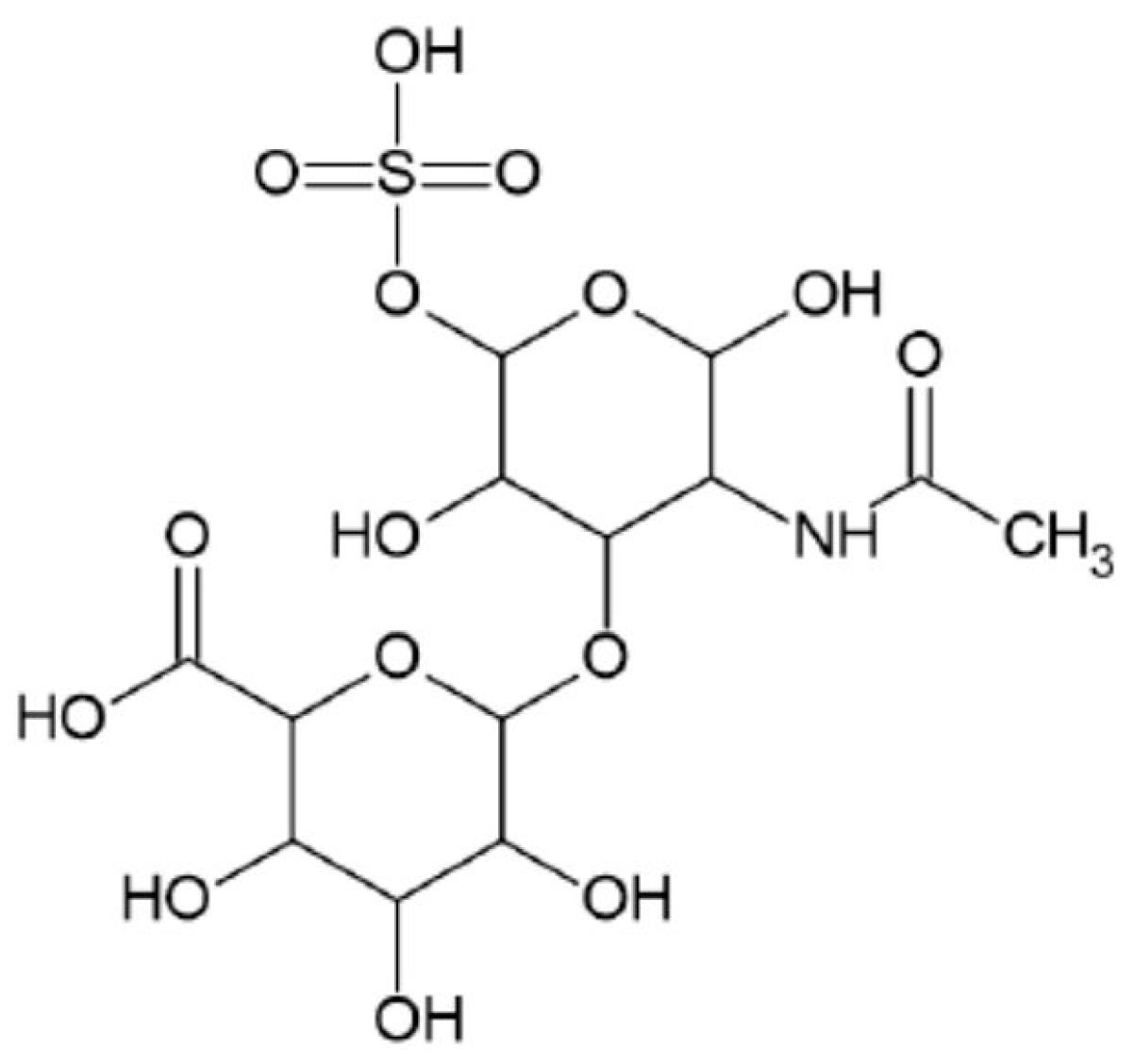
- Chondroitin
2.2. Proteins
2.2.1. Collagen
2.2.2. Gelatin
2.2.3. Silk Protein
2.2.4. Albumin
Bovine Serum Albumin
Other Proteins
- Zein
- Legumin
- Gliadin
- Avidin
2.3. Lignin
2.4. Shellac
3. Chemical Modifications of Biopolymers
3.1. Cross-Linking
3.2. Functionalization and Conjugation
3.3. Interpenetrating Polymer Networks
3.4. Graft Copolymers
3.5. Block Copolymers
3.6. Polyion Complexes
4. Pharmaceutical and Biomedical Applications of Biopolymers
4.1. Drug Delivery Systems
4.2. Gene Delivery
4.3. Lesion Recovery
4.4. Targeted Diagnosis
4.5. Tissue Engineering and Regeneration
4.6. Biosensors
4.7. Medical Implants
4.8. Challenges
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A comprehensive review on chemical properties and applications of biopolymers and their composites. Int. J. Biol. Macromol. 2020, 154, 329–338. [Google Scholar] [CrossRef] [PubMed]
- López, A.S.; Ramos, M.P.; Herrero, R.; Vilariño, J.M.L. Synthesis of magnetic green nanoparticle—Molecular imprinted polymers with emerging contaminants templates. J. Environ. Chem. Eng. 2020, 8, 103889. [Google Scholar] [CrossRef]
- Hassan, M.S.; Bai, J.; Dou, D.-Q. Biopolymers; definition, classification and applications. Egypt. J. Chem. 2019, 62, 1725–1737. [Google Scholar] [CrossRef]
- Rao, M.G.; Bharathi, P.; Akila, R.M. A comprehensive review on biopolymers. Sci. Rev. Chem. Commun. 2014, 4, 61–68. [Google Scholar]
- Merlettini, A. Micro-Nanostructured Polymeric Materials with Specific Functionalities for Advanced Biomedical Applications. Ph.D. Thesis, Università di Bologna, Bologna, Italy, 2019. [Google Scholar]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Veeramachineni, A.K.; Owh, C.; Loh, X.J. Biodegradable polysaccharides for controlled drug delivery. ChemPlusChem 2016, 81, 504–514. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.Y.; Mubarak, N.M.; Tanjung, F.A. Structure–property relationship of cellulose nanowhiskers reinforced chitosan biocomposite films. J. Environ. Chem. Eng. 2017, 5, 6132–6136. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Chapter 4—Natural polymers. In Natural and Synthetic Biomedical Polymers, 1st ed.; Kumbar, S.G., Kaurencin, C.T., Deng, M., Eds.; Elsevier Science: Burlington, MA, USA, 2014; pp. 67–89. [Google Scholar]
- Soltani, R.; Pishnamazi, M.; Pelalak, R.; Rezakazemi, M.; Marjani, A.; Dinari, M.; Sarkar, S.M.; Shirazian, S. Preparation of COOH-KCC-1/polyamide 6 composite by in situ ring-opening polymerization: Synthesis, characterization, and Cd(II) adsorption study. J. Environ. Chem. Eng. 2021, 9, 104683. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sean, T.C.; Kudaiyappan, T.; Bisyarah, U.; Mirarmandi, A.; Faradjeva, E.; Abubakar, A.; Ali, H.H.; Angalene, J.L.A.; Suresh Kumar, S. Production, characterization and application of nanocarriers made of polysaccharides, proteins, bio-polyesters and other biopolymers: A review. Int. J. Biol. Macromol. 2020, 165, 3088–3105. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Peng, F.; André, A.A.M.; Men, Y.; Srinivas, M.; Wilson, D.A. Biodegradable hybrid stomatocyte nanomotors for drug delivery. ACS Nano 2017, 11, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Huang, P.K.; Law, W.C.; Chu, C.H.; Chen, N.T.; Lo, L.W. Biodegradable polymers for gene-delivery applications. Int. J. Nanomed. 2020, 15, 2131–2150. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, R.A.; Sapuan, S.M.; Kadier, A.; Krishnan, S.; Atikah, M.S.N.; Ibrahim, R.; Nazrin, A.; Syafiq, R.; Misri, S.; Huzaifah, M.R.M.; et al. Chapter 7—Mechanical testing of sugar palm fiber reinforced sugar palm biopolymer composites. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers, 1st ed.; Al-Oqla, F.M., Sapuan, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–110. [Google Scholar]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Alam, A.; Bera, R.; Hassan, A.; Goswami, S.; Das, N. Synthesis, characterization and adsorption studies of a novel triptycene based hydroxyl azo-nanoporous polymer for environmental remediation. J. Environ. Chem. Eng. 2020, 8, 103558. [Google Scholar] [CrossRef]
- Eltz, F.Z.; Vebber, M.C.; Aguzzoli, C.; Machado, G.; da Silva Crespo, J.; Giovanela, M. Preparation, characterization and application of polymeric thin films containing silver and copper nanoparticles with bactericidal activity. J. Environ. Chem. Eng. 2020, 8, 103745. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A review on natural fiber reinforced polymer composite and its applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Kirthikaa, G.B.; Muthusamy, S.; Ramakrishnan, B.; Sivarajasekar, N. Comparison and evaluation of electrospun nanofiber membrane for the clarification of grape juice. In Sustainable Development in Energy and Environment. Select Proceedings of ICSDEE 2019; Sivasubramanian, V., Pugazhendhi, A., Moorthy, I.G., Eds.; Springer Proceedings in Energy (SPE) Book Series; Springer Nature: Singapore, 2020; pp. 77–92. [Google Scholar]
- Al Battashi, H.; Al-Kindi, S.; Gupta, V.K.; Sivakumar, N. Polyhydroxyalkanoate (PHA) production using volatile fatty acids derived from the anaerobic digestion of waste paper. J. Polym. Environ. 2020, 29, 250–259. [Google Scholar] [CrossRef]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the polyhydroxyalkanoate biopolymer by Cupriavidus necator using beer brewery wastewater containing maltose as a primary carbon source. J. Environ. Chem. Eng. 2020, 8, 103588. [Google Scholar] [CrossRef]
- Al-Battashi, H.; Annamalai, N.; Al-Kindi, S.; Nair, A.S.; Al-Bahry, S.; Verma, J.P.; Sivakumar, N. Production of bioplastic (poly-3-hydroxybutyrate) using waste paper as a feedstock: Optimization of enzymatic hydrolysis and fermentation employing Burkholderia sacchari. J. Clean. Prod. 2019, 214, 236–247. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.K. Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Francis, R.; Sasikumar, S.; Gopalan, G.P. Chapter 2—Synthesis, structure, and properties of biopolymers (natural and synthetic). In Polymer Composite, 1st ed.; Thomas, S., Joseph, K., Malhotra, S.K., Goda, K., Sreekala, M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; Volume 3, pp. 11–107. [Google Scholar]
- Jha, A.; Kumar, A. Biobased technologies for the efficient extraction of biopolymers from waste biomass. Bioprocess Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A sustainable material for food and medical applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Pothan, L.A.; Oommen, Z.; Thomas, S. Dynamic mechanical analysis of banana fiber reinforced polyester composites. Compos. Sci. Technol. 2003, 63, 283–293. [Google Scholar] [CrossRef]
- Abotbina, W.; Sapuan, S.M.; Ilyas, R.A.; Sultan, M.T.H.; Alkbir, M.F.M.; Sulaiman, S.; Harussani, M.M.; Bayraktar, E. Recent developments in cassava (Manihot esculenta) based biocomposites and their potential industrial applications: A comprehensive review. Materials 2022, 15, 6992. [Google Scholar] [CrossRef] [PubMed]
- Iliou, K.; Kikionis, S.; Ioannou, E.; Roussis, V. Marine biopolymers as bioactive functional ingredients of electrospun nanofibrous scaffolds for biomedical applications. Mar. Drugs 2022, 20, 314. [Google Scholar] [CrossRef] [PubMed]
- Flaris, V.; Singh, G. Recent developments in biopolymers. J. Vinyl Addit. Technol. 2009, 15, 1–11. [Google Scholar] [CrossRef]
- Devadas, V.V.; Khoo, K.S.; Chia, W.Y.; Chew, K.W.; Munawaroh, H.S.H.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Lee, K.T.; Show, P.L. Algae biopolymer towards sustainable circular economy. Bioresour. Technol. 2021, 325, 124702. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Kundu, P.P. Addition polymers from natural oils—A review. Prog. Polym. Sci. 2006, 31, 983–1008. [Google Scholar] [CrossRef]
- Awe, O.W.; Zhao, Y.; Nzihou, A.; Minh, D.P.; Lyczko, N. A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valoriz. 2017, 8, 267–283. [Google Scholar] [CrossRef]
- Lelkes, P.I.; Har-El, Y.H.; Marcinkiewicz, C.; Lazarovici, P.; Baharlou, S.M.; Gerstenhaber, J.A. Soy-Derived Bioactive Peptides for Use in Compositions and Methods for Wound Healing, Tissue Engineering, and Regenerative Medicine. Patent WO 2017/015488 A1, 26 January 2017. [Google Scholar]
- Othman, S.H. Bio-nanocomposite materials for food packaging applications: Types of biopolymer and nano-sized filler. Agric. Agric. Sci. Procedia 2014, 2, 296–303. [Google Scholar] [CrossRef]
- Chaabouni, E.; Gassara, F.; Brar, S.K. Biopolymers synthesis and application. In Biotransformation Waste Biomass into High Value Biochemicals, 1st ed.; Brar, S.K., Dhillon, G.S., Soccol, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 415–443. [Google Scholar]
- Atanase, L.I.; Desbrieres, J.; Riess, G. Micellization of synthetic and polysaccharides-based graft copolymers in aqueous media. Prog. Polym. Sci. 2017, 73, 32–60. [Google Scholar] [CrossRef]
- Atanase, L.I. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Sakeer, K.; Ispas-Szabo, P.; Benyerbah, N.; Mateescu, M.A. Ampholytic starch excipients for high loaded drug formulations: Mechanistic insights. Int. J. Pharm. 2018, 535, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Singh, J.; Liu, Q. Chapter 5—Starch—A potential biomaterial for biomedical applications. In Nanomaterials Nanosystems for Biomedical Applications, 1st ed.; Mozafari, M.R., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 83–98. [Google Scholar]
- Lu, D.R.; Xiao, C.M.; Xu, S.J. Starch-based completely biodegradable polymer materials. Express Polym. Lett. 2009, 3, 366–375. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, T.; Lin, Q.; Liu, G.-Q.; Zhang, L.; Yu, F.; Chen, Y. Acetylated starch nanocrystals: Preparation and antitumor drug delivery study. Int. J. Biol. Macromol. 2016, 89, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G.; Huang, H. Preparation and application of dextran and its derivatives as carriers. Int. J. Biol. Macromol. 2020, 145, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Bandopadhyay, R. Use of dextran nanoparticle: A paradigm shift in bacterial exopolysaccharide based biomedical applications. Int. J. Biol. Macromol. 2016, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiong, S.; You, J.; Guan, W.; Xu, G.; Dou, H. Dextran-based coacervate nanodroplets as potential gene carriers for efficient cancer therapy. Carbohydr. Polym. 2020, 231, 115687. [Google Scholar]
- Huang, S.; Huang, G. Design and application of dextran carrier. J. Drug Deliv. Sci. Technol. 2020, 55, 101392. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B. Continuous production of β-cyclodextrin by cyclodextrin glycosyltransferase immobilized in mixed gel beads: Comparative study in continuous stirred tank reactor and packed bed reactor. Biochem. Eng. J. 2016, 105, 107–113. [Google Scholar] [CrossRef]
- Mejia-Ariza, R.; Graña-Suárez, L.; Verboom, W.; Huskens, J. Cyclodextrin-based supramolecular nanoparticles for biomedical applications. J. Mater. Chem. B 2017, 5, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, R.; Li, Y.; Kang, H.; Shen, D.; Wu, M.; Huang, Y. Self-assembly of ethyl cellulose-graft-polystyrene copolymers in acetone. Polymer 2009, 50, 211–217. [Google Scholar] [CrossRef]
- Wang, D.; Tan, J.; Kang, H.; Ma, L.; Jin, X.; Liu, R.; Huang, Y. Synthesis, self-assembly and drug release behaviors of pH-responsive copolymers ethyl cellulose-graft-PDEAEMA through ATRP. Carbohydr. Polym. 2011, 84, 195–202. [Google Scholar] [CrossRef]
- Kamel, R.; El-Wakil, N.A.; Dufresne, A.; Elkasabgy, N.A. Nanocellulose: From an agricultural waste to a valuable pharmaceutical ingredient. Int. J. Biol. Macromol. 2020, 163, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Merlusca, I.P.; Ibanescu, C.; Tuchilus, C.; Danu, M.; Atanase, L.I.; Popa, I.M. Characterization of neomycin-loaded xanthan-chitosan hydrogels for topical applications. Cellul. Chem. Technol. 2019, 53, 709–719. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Popa, M.; Atanase, L.I.; Daraba, O.M.; Popescu, I.; Romila, L.E.; Ichim, D.L. Biocomposite hydrogels for the treatment of bacterial infections: Physicochemical characterization and in vitro assessment. Pharmaceutics 2021, 13, 2079. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Kolesinska, B.; Sujka, W. Chitosan and its derivatives—Biomaterials with diverse biological activity for manifold applications. Mini Rev. Med. Chem. 2019, 19, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and its derivatives: Structural properties and biomedical applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Nithya, R.; Sudha, P.N.; Kim, S.-K. Role of alginate in bone tissue engineering. Adv. Food Nutr. Res. 2014, 73, 45–57. [Google Scholar] [PubMed]
- Sosnik, A. Alginate particles as platform for drug delivery by the oral route: State-of-the-art. ISRN Pharm. 2014, 2014, 926157. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; Oliveira, D.; Chen, M.; Souto, E.B. Chapter 13—Advanced applications of alginates in biomedical. In Applications of Advanced Green Materials, 1st ed.; Ahmed, S., Ed.; Woodhead Publishing in Materials; Woodhead Publishing–Elsevier: Kidlington, UK, 2021; pp. 321–337. [Google Scholar]
- Mollah, M.Z.I.; Zahid, H.M.; Mahal, Z.; Faruque, M.R.I.; Khandaker, M.U. The usages and potential uses of alginate for healthcare applications. Front. Mol. Biosci. 2021, 8, 719972. [Google Scholar] [CrossRef] [PubMed]
- Pokusaev, B.G.; Karlov, S.P.; Nekrasov, D.A.; Zakharov, N.S.; Khramtsov, D.P.; Reznik, V.V.; Vyazmin, A.V.; Shumova, N.V. Agarose gels with bioresorbable additives: The kinetics of the formation, structure, some properties. Chem. Eng. Trans. 2019, 74, 1171–1176. [Google Scholar]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F.; et al. Agarose-based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- López-Marcial, G.R.; Zeng, A.Y.; Osuna, C.; Dennis, J.; García, J.M.; O’Connell, G.D. Agarose-based hydrogels as suitable bioprinting materials for tissue engineering. ACS Biomater. Sci. Eng. 2018, 4, 3610–3616. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhan, X.; Wan, J.; Wang, Y.; Wang, C. Review for carrageenan-based pharmaceutical biomaterials: Favourable physical features versus adverse biological effects. Carbohydr. Polym. 2015, 121, 27–36. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Kim, D.; Banerjee, I.; Pal, K. Carrageenan: A wonder polymer from marine algae for potential drug delivery applications. Curr. Pharm. Des. 2019, 25, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Rode, M.P.; Batti Angulski, A.B.; Gomes, F.A.; da Silva, M.M.; Jeremias, T.D.S.; de Carvalho, R.G.; Iucif Vieira, D.G.; Oliveira, L.F.C.; Fernandes Maia, L.; Trentin, A.G.; et al. Carrageenan hydrogel as a scaffold for skin-derived multipotent stromal cells delivery. J. Biomater. Appl. 2018, 33, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in biomedical applications of pectin gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Alkarib, S.Y.; MohamedElhassan, D.E.; Nur, A.O. Evaluation of gum Arabic as a film coating former for immediate release oral tablet formulation. World J. Pharm. Pharmaceut. Sci. 2016, 5, 32–41. [Google Scholar]
- Li, M.; Li, H.; Li, X.; Zhu, H.; Xu, Z.; Liu, L.; Ma, J.; Zhang, M. A bioinspired alginate–gum Arabic hydrogel with micro-/nanoscale structures for controlled drug release in chronic wound healing. ACS Appl. Mater. Interfaces 2017, 9, 22160–22175. [Google Scholar] [CrossRef] [PubMed]
- Gamal-Eldeen, A.M.; Moustafa, D.; El-Daly, S.M.; El-Hussieny, E.A.; Saleh, S.; Khoobchandani, M.; Bacon, K.L.; Gupta, S.; Katti, K.; Shukla, R.; et al. Photothermal therapy mediated by gum Arabic-conjugated gold nanoparticles suppresses liver preneoplastic lesions in mice. J. Photochem. Photobiol. B 2016, 163, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Gerola, A.P.; Rubira, A.F.; Muniz, E.C.; Valente, A.J.M. Gum Arabic: A remarkable biopolymer for food and biomedical applications. In Engineering Technology and Industrial Chemistry with Applications, 1st ed.; Haghi, R.K., Torrens, F., Eds.; Apple Academic Press: New York, NY, USA, 2019; pp. 281–312. [Google Scholar]
- Seeli, D.S.; Prabaharan, M. Guar gum oleate-graft-poly(methacrylic acid) hydrogel as a colon-specific controlled drug delivery carrier. Carbohydr. Polym. 2017, 158, 51–57. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Y.; He, J.; Bu, T.; He, X.; He, K.; Xiang, C.; Yu, Z.; Wu, H. The gelation of hydroxypropyl guar gum by nano-ZrO2. Polym. Adv. Technol. 2018, 29, 587–593. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Guar gum: Versatile natural polymer for drug delivery applications. Eur. Polym. J. 2019, 112, 722–735. [Google Scholar] [CrossRef]
- Amjed, N.; Zeshan, M.; Farooq, A.; Naz, S. Applications of guar gum polysaccharide for pharmaceutical drug delivery: A review. Int. J. Biol. Macromol. 2024, 257, 128390. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Hyaluronic acid-based biopharmaceutical delivery and tumor-targeted drug delivery system. J. Control. Release 2018, 278, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cao, Z.; Li, W.; Liu, R.; Chen, Y.; Song, Y.; Liu, G.; Song, Z.; Liu, Z.; Lu, C.; et al. A review on the wide range applications of hyaluronic acid as a promising rejuvenating biomacromolecule in the treatments of bone related diseases. Int. J. Biol. Macromol. 2020, 165, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Peng, X.; Li, B.; Liu, Y.; Sun, H.; Li, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 151, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Yang, X.; Du, X.; Yang, H.; Liu, Y.; Khan, A.Q.; Zhai, G. Chondroitin sulfate derived theranostic and therapeutic nanocarriers for tumor-targeted drug delivery. Carbohydr. Polym. 2020, 233, 115837. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef] [PubMed]
- Varghese, O.P.; Liu, J.; Sundaram, K.; Hilborn, J.; Oommen, O.P. Chondroitin sulfate derived theranostic nanoparticles for targeted drug delivery. Biomater. Sci. 2016, 4, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Han, Y. Chondroitin sulfate-based biomaterials for tissue engineering. Turk. J. Biol. 2016, 40, 290–299. [Google Scholar] [CrossRef]
- Pal, D.; Saha, S. Chondroitin: A natural biomarker with immense biomedical applications. RSC Adv. 2019, 9, 28061–28077. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Rao, S.; Bhatnagar, I.; Kim, S.-K. Sulfated polysaccharides from macroalgae for bone tissue regeneration. Curr. Pharm. Des. 2019, 25, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and supramolecular X-ray characterization of engineered tissues from equine tendon, bovine dermis, and fish skin type-I collagen. Macromol. Biosci. 2020, 20, e2000017. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-based formulations for wound healing: A literature review. Life Sci. 2022, 290, 120096. [Google Scholar] [CrossRef] [PubMed]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine collagen: A promising biomaterial for wound healing, skin anti-aging, and bone regeneration. Mar. Drugs. 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Puig, D.; Costa-Larrión, E.; Rubio-Rodríguez, N.; Gálvez-Martín, P. Collagen supplementation for joint health: The link between composition and scientific knowledge. Nutrients 2023, 15, 1332. [Google Scholar] [CrossRef] [PubMed]
- Davison-Kotler, E.; Marshall, W.S.; García-Gareta, E. Sources of collagen for biomaterials in skin wound healing. Bioengineering 2019, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Bax, D.V.; Smalley, H.E.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Cellular response to collagen–elastin composite materials. Acta Biomater. 2019, 86, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.M.; Ianchis, R.; Leu Alexa, R.; Gifu, I.C.; Kaya, M.G.A.; Savu, D.I.; Popescu, R.C.; Alexandrescu, E.; Ninciuleanu, C.M.; Preda, S.; et al. Development of new collagen/clay composite biomaterials. Int. J. Mol. Sci. 2021, 23, 401. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Polkowska, I.; Małek, M.; Kluczyński, J.; Paździor-Czapula, K.; Wekwejt, M.; Michno, A.; Ronowska, A.; Pałubicka, A.; Nowicka, B.; et al. The characterization of collagen-based scaffolds modified with phenolic acids for tissue engineering application. Sci. Rep. 2023, 13, 9966. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Lei, X.; Li, Y.; Kong, Y.; Huang, D.; Zhang, G. Three-dimensional polyvinyl alcohol scaffolds modified with collagen for HepG2 cell culture. J. Biomater. Appl. 2020, 35, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian Soc. Periodontol. 2013, 17, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Alpaslan, D.; Bitlisli, B.O. Collagen-based hydrogel films as drug-delivery devices with antimicrobial properties. Polym. Bull. 2014, 71, 3017–3033. [Google Scholar] [CrossRef]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N.; Prasad, R.G.S.V. Gelatin based scaffolds for tissue engineering—A review. Polym. Res. J. 2015, 9, 15–32. [Google Scholar]
- Saraogi, G.K.; Gupta, P.; Gupta, U.D.; Jain, N.K.; Agrawal, G.P. Gelatin nanocarriers as potential vectors for effective management of tuberculosis. Int. J. Pharm. 2010, 385, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.B.; Pacelli, S.; El Haj, A.J.; Dua, H.S.; Hopkinson, A.; White, L.J.; Rose, F.R.A.J. Gelatin-based materials in ocular tissue engineering. Materials 2014, 7, 3106–3135. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, R.; Shah, M.; Khan, S.A.; Ali, R. Gelatin nanoparticles: A potential candidate for medical applications. Nanotechnol. Rev. 2017, 6, 191–207. [Google Scholar] [CrossRef]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Felekori, M.; Khakbiz, M.; Nezafati, N.; Mohammadi, J.; Eslaminejad, M.B. Comparative analysis and properties evaluation of gelatin microspheres crosslinked with glutaraldehyde and 3-glycidoxypropyltrimethoxysilane as drug delivery systems for the antibiotic vancomycin. Int. J. Pharm. 2019, 557, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-linking strategies for electrospun gelatin scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- De Colli, M.; Massimi, M.; Barbetta, A.; Di Rosario, B.L.; Nardecchia, S.; Conti Devirgiliis, L.; Dentini, M. A biomimetic porous hydrogel of gelatin and glycosaminoglycans cross-linked with transglutaminase and its application in the culture of hepatocytes. Biomed Mater. 2012, 7, 055005. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lazarovici, P.; Pomerantz, C.; Chen, X.; Wei, Y.; Lelkes, P.I. Co-electrospun blends of PLGA, gelatin, and elastin as potential nonthrombogenic scaffolds for vascular tissue engineering. Biomacromolecules 2011, 12, 399–408. [Google Scholar] [CrossRef]
- Gonçalves, A.S.C.; Rodrigues, C.F.; Fernandes, N.; de Melo-Diogo, D.; Ferreira, P.; Moreira, A.F.; Correia, I.J. IR780 loaded gelatin–PEG coated gold core silica shell nanorods for cancer-targeted photothermal/photodynamic therapy. Biotechnol. Bioeng. 2022, 119, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Nguyen, Q.V.; Nguyen, V.-H.; Le, T.-H.; Huynh, V.Q.N.; Vo, D.-V.N.; Trinh, Q.T.; Kim, S.Y.; Le, Q.V. Silk fibroin-based biomaterials for biomedical applications: A review. Polymers 2019, 11, 1933. [Google Scholar] [CrossRef] [PubMed]
- Gianak, O.; Kyzas, G.Z.; Samanidou, V.F.; Deliyanni, E.A. A review for the synthesis of silk fibroin nanoparticles with different techniques and their ability to be used for drug delivery. Curr. Anal. Chem. 2019, 15, 339–348. [Google Scholar] [CrossRef]
- Pham, D.T.; Saelim, N.; Tiyaboonchai, W. Paclitaxel loaded EDC-crosslinked fibroin nanoparticles: A potential approach for colon cancer treatment. Drug Deliv. Transl. Res. 2020, 10, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Totten, J.D.; Wongpinyochit, T.; Carrola, J.; Duarte, I.F.; Seib, F.P. PEGylation-dependent metabolic rewiring of macrophages with silk fibroin nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 14515–14525. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, B.; Bari, E.; Perteghella, S.; Catenacci, L.; Sorrenti, M.; Mocchi, M.; Faragò, S.; Tripodo, G.; Prina-Mello, A.; Torre, M.L. Silk fibroin nanoparticles for celecoxib and curcumin delivery: ROS-scavenging and anti-inflammatory activities in an in vitro model of osteoarthritis. Eur. J. Pharm. Biopharm. 2019, 137, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Aiyelabegan, H.T.; Zaidi, S.S.Z.; Fanuel, S.; Eatemadi, A.; Ebadi, M.T.K.; Sadroddiny, E. Albumin-based biomaterial for lung tissue engineering applications. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 853–861. [Google Scholar] [CrossRef]
- Tao, C.; Chuah, Y.J.; Xu, C.; Wang, D.-A. Albumin conjugates and assemblies as versatile bio-functional additives and carriers for biomedical applications. J. Mater. Chem. B 2019, 7, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Roufegarinejad, L.; Jahanban-Esfahlan, A.; Sajed-Amin, S.; Panahi-Azar, V.; Tabibiazar, M. Molecular interactions of thymol with bovine serum albumin: Spectroscopic and molecular docking studies. J. Mol. Recognit. 2018, 31, e2704. [Google Scholar] [CrossRef] [PubMed]
- Andishmand, H.; Roufegari-Nejad, L.; Tabibiazar, M. Nanostructure characterization of bovine serum albumin–resveratrol complex. Res. Innov. Food Sci. Technol. 2017, 6, 291–300. [Google Scholar]
- Ghorbani, M.; Hamishehkar, H.; Tabibiazar, M. BSA/chitosan polyelectrolyte complex: A platform for enhancing the loading and cancer cell-uptake of resveratrol. Macromol. Res. 2018, 26, 808–813. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Jahanban-Esfahlan, R.; Roufegarinejad, L.; Tabibiazar, M.; Amarowicz, R. Recent developments in the detection of bovine serum albumin. Int. J. Biol. Macromol. 2019, 138, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Panahi-Azar, V.; Sajedi, S. Spectroscopic and molecular docking studies on the interaction between N-acetyl cysteine and bovine serum albumin. Biopolymers 2015, 103, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Davaran, S.; Moosavi-Movahedi, A.A.; Dastmalchi, S. Investigating the interaction of juglone (5-hydroxy-1,4-naphthoquinone) with serum albumins using spectroscopic and in silico methods. J. Iran. Chem. Soc. 2017, 14, 1527–1540. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Roufegarinejad, L.; Amarowicz, R.; Jahanban-Esfahlan, A. Characterizing the interaction between pyrogallol and human serum albumin by spectroscopic and molecular docking methods. J. Biomol. Struct. Dyn. 2019, 37, 2766–2775. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Chianella, I.; Larcombe, L.; Piletsky, S.A.; Noble, J.; Porter, R.; Horgan, A. The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem. Soc. Rev. 2011, 40, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Roufegarinejad, L.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Amarowicz, R. Latest developments in the detection and separation of bovine serum albumin using molecularly imprinted polymers. Talanta 2020, 207, 120317. [Google Scholar] [CrossRef]
- Gao, R.; Kong, X.; Wang, X.; He, X.; Chen, L.; Zhang, Y. Preparation and characterization of uniformly sized molecularly imprinted polymers functionalized with core–shell magnetic nanoparticles for the recognition and enrichment of protein. J. Mater. Chem. 2011, 21, 17863–17871. [Google Scholar] [CrossRef]
- Ansari, S. Application of magnetic molecularly imprinted polymer as a versatile and highly selective tool in food and environmental analysis: Recent developments and trends. TrAC Trends Anal. Chem. 2017, 90, 89–106. [Google Scholar] [CrossRef]
- Sood, A.; Arora, V.; Shah, J.; Kotnala, R.K.; Jain, T.K. Multifunctional gold-coated iron oxide core-shell nanoparticles stabilized using thiolated sodium alginate for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 274–281. [Google Scholar] [CrossRef]
- Guoning, C.; Pengqi, G.; Yan, W.; Lu, W.; Hua, S.; Yunzhe, L.; Wanghui, J.; Chun, C.; Qiang, F. Preparation of molecularly imprinted polymers and application in a biomimetic biotin–avidin–ELISA for the detection of bovine serum albumin. Talanta 2019, 198, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Muthusankar, E.; Ragupathy, D. Chitosan based nanocomposite biosensors: A recent review. Sens. Lett. 2018, 16, 81–91. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J.; Wang, J.; Zhang, Q.; Zhang, B. Design and preparation of self-driven BSA surface imprinted tubular carbon nanofibers and their specific adsorption performance. Chem. Eng. J. 2019, 373, 923–934. [Google Scholar] [CrossRef]
- Xia, J.; Cao, X.; Wang, Z.; Yang, M.; Zhang, F.; Lu, B.; Li, F.; Xia, L.; Li, Y.; Xia, Y. Molecularly imprinted electrochemical biosensor based on chitosan/ionic liquid–graphene composites modified electrode for determination of bovine serum albumin. Sens. Actuators B Chem. 2016, 225, 305–311. [Google Scholar] [CrossRef]
- Qi, M.; Zhao, K.; Bao, Q.; Pan, P.; Zhao, Y.; Yang, Z.; Wang, H.; Wei, J. Adsorption and electrochemical detection of bovine serum albumin imprinted calcium alginate hydrogel membrane. Polymers 2019, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhao, K.; Qi, M.; Li, S.; Xu, G.; Wei, J.; He, X. Preparation of protein molecular imprinted polysiloxane membrane using calcium alginate film as matrix and its application for cell culture. Polymers 2018, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Ma, Y.; Zhang, B.; Zhang, Q. Surface molecularly imprinted thermo-sensitive polymers based on light-weight hollow magnetic microspheres for specific recognition of BSA. Appl. Surf. Sci. 2019, 486, 265–273. [Google Scholar] [CrossRef]
- Saha, T.; Hoque, M.E.; Mahbub, T. Chapter 13—Biopolymers for sustainable packaging in food, cosmetics, and pharmaceuticals. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers, 1st ed.; Al-Oqla, F.M., Sapuan, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 197–214. [Google Scholar]
- Tortorella, S.; Maturi, M.; Vetri Buratti, V.; Vozzolo, G.; Locatelli, E.; Sambri, L.; Franchini, M.C. Zein as a versatile biopolymer: Different shapes for different biomedical applications. RSC Adv. 2021, 62, 39004–39026. [Google Scholar] [CrossRef]
- Tivano, F.; Chiono, V. Zein as a renewable material for the preparation of green nanoparticles for drug delivery. Front. Biomater. Sci. 2023, 2, 1156403. [Google Scholar] [CrossRef]
- Voci, S.; Gagliardi, A.; Fresta, M.; Cosco, D. Antitumor features of vegetal protein-based nanotherapeutics. Pharmaceutics 2020, 12, 65. [Google Scholar] [CrossRef]
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.U.; Jaber, F.; Dera, A.A.; Awwad, N.S.; Ibrahium, H.A. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 2023, 13, 23087–23121. [Google Scholar] [CrossRef] [PubMed]
- Marjanović-Balaban, Ž.; Gojković Cvjetković, V.; Grujić, R. Gliadin proteins from wheat flour: The optimal determination conditions by ELISA. Foods Raw Mater. 2021, 9, 364–370. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Manish; Bansal, P.; Kumar, A.; Saxena, V.; Kumar, V.; Katiyar, D. Overview and in-silico pharmacological profiling of Gliadin: A potential biomaterial. Mater. Today Proc. 2022, 62, 276–282. [Google Scholar] [CrossRef]
- Jain, A.; Cheng, K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J. Control. Release 2017, 245, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Terzioğlu, P.; Parın, F.N.; Sıcak, Y. Lignin composites for biomedical applications: Status, challenges and perspectives. In Lignin: Biosynthesis and Transformation for Industrial Applications, 1st ed.; Sharma, S., Kumar, A., Eds.; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2020; pp. 253–273. [Google Scholar]
- Iravani, S. Biomedical applications of lignin-based nanoparticles. In Nanoparticles and their Biomedical Applications, 1st ed.; Shukla, A.K., Ed.; Springer Nature: Singapore, 2020; pp. 217–224. [Google Scholar]
- Mohammad, S.H.; Bhukya, B. Biotransformation of toxic lignin and aromatic compounds of lignocellulosic feedstock into eco-friendly biopolymers by Pseudomonas putida KT2440. Bioresour. Technol. 2022, 363, 128001. [Google Scholar] [CrossRef] [PubMed]
- Macarie, C.A.; Segneanu, A.E.; Balcu, I.; Pop, R.; Burtica, G.; Ungurean, M.; Grozescu, I. Use of alkaline lyophilization process for lignocellulosic biomass pretreatment. Dig. J. Nanomater. Biostruct. 2012, 7, 1577–1586. [Google Scholar]
- Balcu, I.; Segneanu, A.E.; Mirica, M.C.; Iorga, M.I.; Macarie, C.; Martagiu, R. Microwaves effect over biomass hydrolysis. Environ. Eng. Manag. J. 2009, 8, 741–746. [Google Scholar]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How far is Lignin from being a biomedical material? Bioact. Mater. 2021, 8, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Thombare, N.; Kumar, S.; Kumari, U.; Sakare, P.; Yogi, R.K.; Prasad, N.; Sharma, K.K. Shellac as a multifunctional biopolymer: A review on properties, applications and future potential. Int. J. Biol. Macromol. 2022, 215, 203–223. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Dai, Y.; Xia, F.; Zhang, X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111626. [Google Scholar] [CrossRef] [PubMed]
- Tincu Iurciuc, C.E.; Bouhadiba, B.; Atanase, L.I.; Stan, C.S.; Popa, M.; Ochiuz, L. An accessible method to improve the stability and reusability of porcine pancreatic α-amylase via immobilization in gellan-based hydrogel particles obtained by ionic cross-linking with Mg2+ ions. Molecules 2023, 28, 4695. [Google Scholar] [CrossRef] [PubMed]
- Iurciuc-Tincu, C.E.; Atanase, L.I.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I—Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Suflet, D.M.; Popescu, I.; Pelin, I.M.; Ichim, D.L.; Daraba, O.M.; Constantin, M.; Fundueanu, G. Dual cross-linked chitosan/PVA hydrogels containing silver nanoparticles with antimicrobial properties. Pharmaceutics 2021, 13, 1461. [Google Scholar] [CrossRef] [PubMed]
- Dellali, K.Z.; Dellali, M.; Raţă, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Spataru, M.C.; Solcan, C. Assessment of physicochemical and in vivo biological properties of polymeric nanocapsules based on chitosan and poly(N-vinyl pyrrolidone-alt-itaconic anhydride). Polymers 2022, 14, 1811. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Rossi, F.; van Griensven, M. Polymer functionalization as a powerful tool to improve scaffold performances. Tissue Eng. Part A 2014, 20, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chinoy, Z.S.; Pecastaings, G.; Bathany, K.; Garanger, E.; Lecommandoux, S. Design of polysaccharide–b–elastin-like polypeptide bioconjugates and their thermoresponsive self-assembly. Biomacromolecules 2020, 21, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Thakor, P.; Bhavana, V.; Sharma, R.; Srivastava, S.; Singh, S.B.; Mehra, N.K. Polymer–drug conjugates: Recent advances and future perspectives. Drug Discov. Today 2020, 25, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhu, Y.; Li, X.; Liu, X.; Yeung, K.W.K.; Wu, S.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Surface functionalization of biomaterials by radical polymerization. Prog. Mater. Sci. 2016, 83, 191–235. [Google Scholar] [CrossRef]
- Schatz, C.; Lecommandoux, S. Polysaccharide-containing block copolymers: Synthesis, properties and applications of an emerging family of glycoconjugates. Macromol. Rapid Commun. 2010, 31, 1664–1684. [Google Scholar] [CrossRef] [PubMed]
- Lohani, A.; Singh, G.; Bhattacharya, S.S.; Verma, A. Interpenetrating polymer networks as innovative drug delivery systems. J. Drug Deliv. 2014, 2014, 583612. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Teuwen, J.; Dransfeld, C. Toughening of epoxy systems with interpenetrating polymer network (IPN): A review. Polymers 2020, 12, 1908. [Google Scholar] [CrossRef]
- Zoratto, N.; Matricardi, P. Chapter 4—Semi-IPNs and IPN-based hydrogels. In Polymeric Gels: Characterization, Properties and Biomedical Applications, 1st ed.; Pal, K., Banerjee, I., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing–Elsevier: Kidlington, UK, 2018; pp. 91–124. [Google Scholar]
- Ganguly, S.; Maity, P.P.; Mondal, S.; Das, P.; Bhawal, P.; Dhara, S.; Das, N.C. Polysaccharide and poly(methacrylic acid) based biodegradable elastomeric biocompatible semi-IPN hydrogel for controlled drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhang, B.; Nie, X.; Cheng, Y.; Hu, Z.; Liao, M.; Li, S. A sodium alginate-based sustained-release IPN hydrogel and its applications. RSC Adv. 2020, 10, 39722–39730. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K. Biopolymer Grafting: Synthesis and Properties, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–580. [Google Scholar]
- Nishimura, T.; Shishi, S.; Sasaki, Y.; Akiyoshi, K. Thermoresponsive polysaccharide graft polymer vesicles with tunable size and structural memory. J. Am. Chem. Soc. 2020, 142, 11784–11790. [Google Scholar] [CrossRef] [PubMed]
- Noreen, A.; Zia, K.M.; Tabasum, S.; Khalid, S.; Shareef, R. A review on grafting of hydroxyethylcellulose for versatile applications. Int. J. Biol. Macromol. 2020, 150, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.E.; Macarie, C.; Ungureanu, M.; Balcu, I.; Gherman, V.; Grozescu, I. Comparative study on enzymatic hydrolysis of cellulose. Digest J. Nanomater. Biostruct. 2013, 8, 1061–1068. [Google Scholar]
- Carvalho, L.T.; Moraes, R.M.; Alves, G.M.; Lacerda, T.M.; Santos, J.C.; Santos, A.M.; Medeiros, S.F. Synthesis of amphiphilic pullulan-graft-poly(ε-caprolactone) via click chemistry. Int. J. Biol. Macromol. 2020, 145, 701–711. [Google Scholar] [CrossRef]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.; Liu, Y.; Bai, R. Recent advances in the preparation, structural characteristics, biological properties and applications of gallic acid grafted polysaccharides. Int. J. Biol. Macromol. 2020, 156, 1539–1555. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Z. Polyion complexes via electrostatic interaction of oppositely charged block copolymers. Macromolecules 2020, 53, 8737–8740. [Google Scholar] [CrossRef]
- Zhang, Q.; Ko, N.R.; Oh, J.K. Recent advances in stimuli-responsive degradable block copolymer micelles: Synthesis and controlled drug delivery applications. Chem. Commun. 2012, 48, 7542–7552. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Pandey, V.; Asati, S.; Gour, V.; Tekade, R.K. Chapter 11—Biodegradable block copolymers and their applications for drug delivery. In Basic Fundamentals of Drug Delivery, 1st ed.; Tekade, R.K., Ed.; Academic Press–Elsevier: Cambridge, MA, USA, 2019; pp. 401–447. [Google Scholar]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte complexes of natural polymers and their biomedical applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-based colloidal polyelectrolyte complexes for drug delivery: A review. Carbohydr. Polym. 2020, 238, 116126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-based platforms for the delivery of peptides and proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Kuperkar, K.; Atanase, L.I.; Bahadur, A.; Crivei, I.C.; Bahadur, P. Degradable polymeric bio(nano)materials and their biomedical applications: A comprehensive overview and recent updates. Polymers 2024, 16, 206. [Google Scholar] [CrossRef]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Park, S.B.; Lih, E.; Park, K.S.; Joung, Y.K.; Han, D.K. Biopolymer-based functional composites for medical applications. Prog. Polym. Sci. 2017, 68, 77–105. [Google Scholar] [CrossRef]
- Jacob, J.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Singh, A.V. Biopolymers in drug delivery: A review. Pharmacol. Online 2011, 1, 666–674. [Google Scholar]
- Gopi, S.; Amalraj, A.; Sukumaran, N.P.; Haponiuk, J.T.; Thomas, S. Biopolymers and their composites for drug delivery: A brief review. Macromol. Symp. 2018, 380, 1800114. [Google Scholar] [CrossRef]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The biopolymer bacterial nanocellulose as drug delivery system: Investigation of drug loading and release using the model protein albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Pal, S. Modified biopolymer-dextrin based crosslinked hydrogels: Application in controlled drug delivery. RSC Adv. 2015, 5, 25014–25050. [Google Scholar] [CrossRef]
- Revia, R.A.; Stephen, Z.R.; Zhang, M. Theranostic nanoparticles for RNA-based cancer treatment. Acc. Chem. Res. 2019, 52, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Chandakavathe, B.N.; Kulkarni, R.G.; Dhadde, S.B. Grafting of natural polymers and gums for drug delivery applications: A perspective review. Crit. Rev. Ther. Drug Carrier Syst. 2022, 39, 45–83. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical applications of quaternized chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.J.; Anchordoquy, T.J. Questioning the use of PEGylation for drug delivery. Drug Deliv. Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, A.; Abu Samah, N.H. A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Ren, X.; Long, H.; Qian, H.; Ma, K.; Guo, Y. Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 2016, 4, e2497. [Google Scholar] [CrossRef]
- Dozier, J.K.; Distefano, M.D. Site-specific PEGylation of therapeutic proteins. Int. J. Mol. Sci. 2015, 16, 25831–25864. [Google Scholar] [CrossRef]
- Zong, Y.; Wu, J.; Shen, K. Nanoparticle albumin-bound paclitaxel as neoadjuvant chemotherapy of breast cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 17360–17372. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, A.; Miyata, K.; Kataoka, K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv. Drug Deliv. Rev. 2016, 104, 61–77. [Google Scholar] [CrossRef]
- Kim, K.; Chen, W.C.W.; Heo, Y.; Wang, Y. Polycations and their biomedical applications. Prog. Polym. Sci. 2016, 60, 18–50. [Google Scholar] [CrossRef]
- Debele, T.A.; Su, W.P. Polysaccharide and protein-based functional wound dressing materials and applications. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 87–108. [Google Scholar] [CrossRef]
- Xu, R.; Fang, Y.; Zhang, Z.; Cao, Y.; Yan, Y.; Gan, L.; Xu, J.; Zhou, G. Recent advances in biodegradable and biocompatible synthetic polymers used in skin wound healing. Materials 2023, 16, 5459. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, X.; Cao, X.; Wang, Y.; Wang, J.; Zhao, Y. Developing natural polymers for skin wound healing. Bioact. Mater. 2024, 33, 355–376. [Google Scholar] [CrossRef]
- Pandian, M.; Reshma, G.; Arthi, C.; Másson, M.; Rangasamy, J. Biodegradable polymeric scaffolds and hydrogels in the treatment of chronic and infectious wound healing. Eur. Polym. J. 2023, 198, 112390. [Google Scholar] [CrossRef]
- Prete, S.; Dattilo, M.; Patitucci, F.; Pezzi, G.; Parisi, O.I.; Puoci, F. Natural and synthetic polymeric biomaterials for application in wound management. J. Funct. Biomater. 2023, 14, 455. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, A.T.; Le, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509–5528. [Google Scholar] [CrossRef]
- Alven, S.; Peter, S.; Mbese, Z.; Aderibigbe, B.A. Polymer-based wound dressing materials loaded with bioactive agents: Potential materials for the treatment of diabetic wounds. Polymers 2022, 14, 724. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Zhang, Z.; Zou, X.; Song, H.; Zheng, W. Preparation and evaluation of luteolin-loaded PLA-based shape memory gastroretentive drug delivery systems. Int. J. Pharm. 2024, 650, 123670. [Google Scholar] [CrossRef]
- Bajracharya, R.; Song, J.G.; Patil, B.R.; Lee, S.H.; Noh, H.-M.; Kim, D.-H.; Kim, G.L.; Seo, S.H.; Park, J.W.; Jeong, S.H.; et al. Functional ligands for improving anticancer drug therapy: Current status and applications to drug delivery systems. Drug Deliv. 2022, 29, 1959–1970. [Google Scholar] [CrossRef]
- Raţă, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Bacaita, S.E.; Mihalache, C.; Daraba, O.-M.; Gherghel, D.; Popa, M. “In vitro” behaviour of aptamer-functionalized polymeric nanocapsules loaded with 5-fluorouracil for targeted therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109828. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.-T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111591. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Biopolymer based three-dimensional biomimetic micro/nanofibers scaffolds with porous structures via tailored charge repulsions for skin tissue regeneration. Polym. Adv. Technol. 2021, 32, 3535–3548. [Google Scholar] [CrossRef]
- Samiei, M.; Fathi, M.; Barar, J.; Fathi, N.; Amiryaghoubi, N.; Omidi, Y. Bioactive hydrogel-based scaffolds for the regeneration of dental pulp tissue. J. Drug Deliv. Sci. Technol. 2021, 64, 102600. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 111, 110862. [Google Scholar] [CrossRef]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658. [Google Scholar] [CrossRef]
- Rahmani, F.; Larbi Bouamrane, O.; Ben Bouabdallah, A.; Atanase, L.I.; Hellal, A.; Apintiliesei, A.N. Biomimetic hydroxyapatite crystals growth on phosphorylated chitosan films by in vitro mineralization used as dental substitute materials. Polymers 2023, 15, 2470. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 16, 108293. [Google Scholar] [CrossRef]
- Gamboa, J.; Paulo-Mirasol, S.; Estrany, F.; Torras, J. Recent progress in biomedical sensors based on conducting polymer hydrogels. ACS Appl. Bio Mater. 2023, 6, 1720–1741. [Google Scholar] [CrossRef]
- Jadoun, S.; Rathore, D.S. Polymer-based biosensors for medical applications. In Handbook of Polymers in Medicine, 1st ed.; Mozafari, M., Chauhan, N.P.S., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing–Elsevier: Kidlington, UK, 2023; pp. 635–652. [Google Scholar]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951–967. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled drug delivery systems: Current status and future directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Mansour, A.; Romani, M.; Acharya, A.B.; Rahman, B.; Verron, E.; Badran, Z. Drug delivery systems in regenerative medicine: An updated review. Pharmaceutics 2023, 15, 695. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef]
- Liu, G.; Yang, L.; Chen, G.; Xu, F.; Yang, F.; Yu, H.; Li, L.; Dong, X.; Han, J.; Cao, C.; et al. A review on drug delivery system for tumor therapy. Front. Pharmacol. 2021, 12, 735446. [Google Scholar] [CrossRef]
- Ahmad, W.; Khan, T.; Basit, I.; Imran, J. A comprehensive review on targeted drug delivery system. Asian J. Pharm. Res. 2022, 12, 335–340. [Google Scholar] [CrossRef]
- Prakash, S. Nano-based drug delivery system for therapeutics: A comprehensive review. Biomed. Phys. Eng. Express 2023, 9, 052002. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Demina, N.B. Implants as targeted drug delivery systems (Review). Pharm. Chem. J. 2022, 56, 396–402. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Fortuni, B.; Inose, T.; Ricci, M.; Fujita, Y.; Van Zundert, I.; Masuhara, A.; Fron, E.; Mizuno, H.; Latterini, L.; Rocha, S.; et al. Polymeric engineering of nanoparticles for highly efficient multifunctional drug delivery systems. Sci. Rep. 2019, 9, 2666. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic block copolymers: Their structures, and self-assembly to polymeric micelles and polymersomes as drug delivery vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef]
- De, A.; Nayak, A.K.; Kundu, A.; Das, B.; Samanta, A. Chapter 7—Gum Arabic-based nanomaterials in drug delivery and biomedical applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications, 1st ed.; Bera, H., Mobaswar, C.M., Saha, S., Eds.; Academic Press–Elsevier: Cambridge, MA, USA, 2021; pp. 165–182. [Google Scholar]
- Bratek-Skicki, A. Towards a new class of stimuli-responsive polymer-based materials—Recent advances and challenges. Appl. Surf. Sci. Adv. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-L. Review of stimuli-responsive polymers in drug delivery and textile application. Molecules 2019, 24, 2547. [Google Scholar] [CrossRef]
- Zhu, M.; Whittaker, A.K.; Han, F.Y.; Smith, M.T. Journey to the market: The evolution of biodegradable drug delivery systems. Appl. Sci. 2022, 12, 935. [Google Scholar] [CrossRef]
- Warrington, K.J.; Nair, U.; Carbone, L.D.; Kang, A.H.; Postlethwaite, A.E. Characterisation of the immune response to type I collagen in scleroderma. Arthritis Res. Ther. 2006, 8, R136. [Google Scholar] [CrossRef]
- Dingman, R.; Balu-Iyer, S.V. Immunogenicity of protein pharmaceuticals. J. Pharm. Sci. 2019, 108, 1637–1654. [Google Scholar] [CrossRef]
- Kuten Pella, O.; Hornyák, I.; Horváthy, D.; Fodor, E.; Nehrer, S.; Lacza, Z. Albumin as a biomaterial and therapeutic agent in regenerative medicine. Int. J. Mol. Sci. 2022, 23, 10557. [Google Scholar] [CrossRef]
- Lee, C.H. A simple outline of methods for protein isolation and purification. Endocrinol. Metab. 2017, 32, 18–22. [Google Scholar] [CrossRef]
- Amaecha, B.T.; Higham, S.M.; Edgar, W.M. Effect of sterilisation methods on the structural integrity of artificial enamel caries for intra-oral cariogenicity tests. J. Dent. 1999, 27, 313–316. [Google Scholar] [CrossRef]
- Meyer, M.; Prade, I.; Leppchen-Fröhlich, K.; Felix, A.; Herdegen, V.; Haseneder, R.; Repke, J.U. Sterilisation of collagen materials using hydrogen peroxide doted supercritical carbon dioxide and its effects on the materials properties. J. Supercrit. Fluids 2015, 102, 32–39. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 1–13. [Google Scholar] [CrossRef]
- Prakash, A.; Soni, H.; Mishra, A.; Sarma, P. Are your capsules vegetarian or nonvegetarian: An ethical and scientific justification. Indian J. Pharmacol. 2017, 49, 401–404. [Google Scholar]
- Feier, A.M.; Portan, D.; Manu, D.R.; Kostopoulos, V.; Kotrotsos, A.; Strnad, G.; Dobreanu, M.; Salcudean, A.; Bataga, T. Primary MSCs for personalized medicine: Ethical challenges, isolation and biocompatibility evaluation of 3D electrospun and printed scaffolds. Biomedicines 2022, 10, 1563. [Google Scholar] [CrossRef]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of biopolymers for drugs and probiotics delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef]
- Opriş, O.; Mormile, C.; Lung, I.; Stegarescu, A.; Soran, M.L.; Soran, A. An overview of biopolymers for drug delivery applications. Appl. Sci. 2024, 14, 1383. [Google Scholar] [CrossRef]
- Mandal, D.D.; Singh, G.; Majumdar, S.; Chanda, P. Challenges in developing strategies for the valorization of lignin—A major pollutant of the paper mill industry. Environ. Sci. Pollut. Res. Int. 2023, 30, 11119–11140. [Google Scholar] [CrossRef]










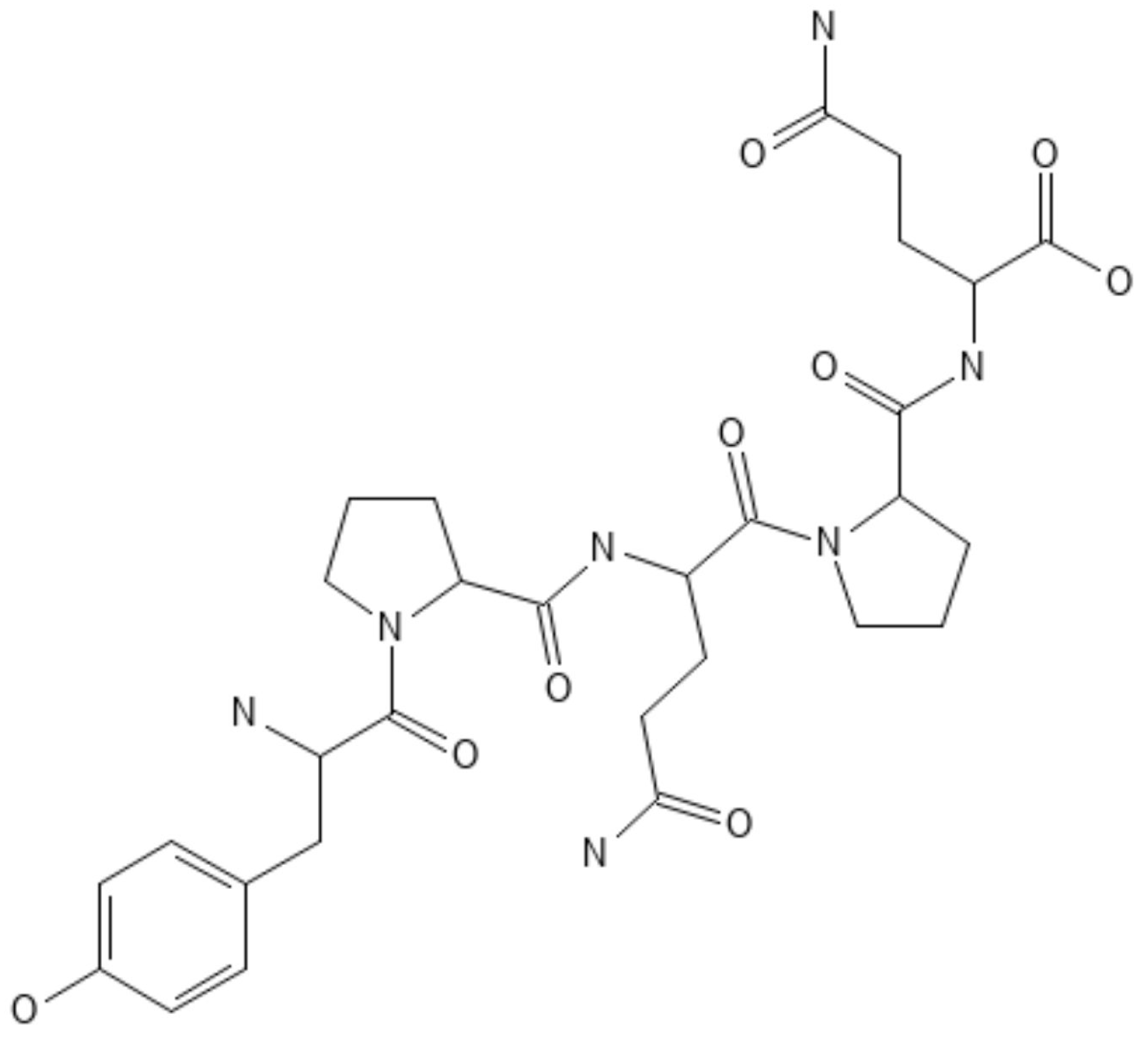
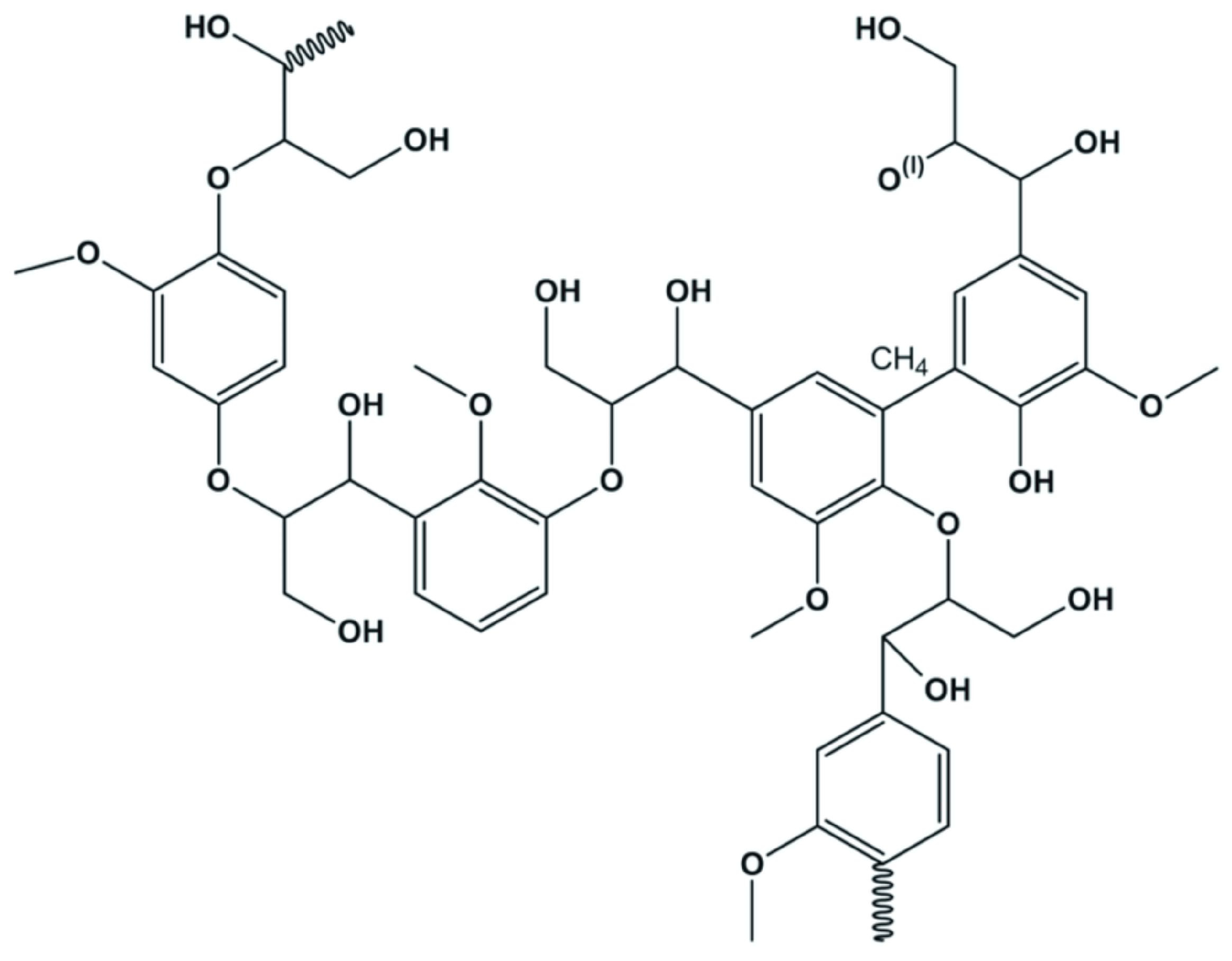

| Biopolymers | Chemical Modifications | Enhanced Properties | |
|---|---|---|---|
| Polysaccharides | Chitin and chitosan | IPNs [169], grafting [170], cross-linking quaternization [171], hydroxypropylation carboxymethylation, sulfation, esterification | Improved mechanical strength and stability; prolonged release time; enhanced interaction with other molecules, water absorption capacity, and resistance to enzymatic degradation; and increased surface activity |
| Cellulose | |||
| Hyaluronic acid | |||
| Alginate | |||
| Pectins | |||
| Proteins | Collagen | Glutaraldehyde cross-linking, carbodiimide cross-linking, glycosylation, hydroxylation, PEGylation [172], acetylation | Increased stability and resistance to enzymatic degradation, improved mechanical properties, mimics the native extracellular matrix, enhanced solubility |
| Gelatin | PEGylation, hydroxylation, glycosylation, acetylation [173], cross-linking (glutaraldehyde [113] or transglutaminase [174]) | Resistance to enzymatic degradation; increased bioactivity; and enhanced solubility, stability, and mechanical properties | |
| Albumin | Site-specific PEGylation [175], drug conjugation [176] | Minimized interference with albumin’s binding and transport functions, enhanced drug pharmacokinetic properties, improved biodistribution, and reduced toxicity | |
| Biopolymers | Applications | References |
|---|---|---|
| Chitosan Fibroin Starch Gelatin Cellulose Bacterial nanocellulose Collagen Biopolymer composites Elastin-like polypeptides Albumin microspheres | Drug delivery systems | [197,198,199,200,201,202,203,204,205,206,207,208,209] |
| Polyethylene imine Poly(L-lysine) Albumin Gelatin Chitosan | Gene delivery | [202,210,211] |
| Hyaluronic acid Cellulose Chitosan Alginate | Lesion recovery | [212,213,214,215,216,217,218,219,220] |
| Chitosan nanoparticles | Targeted diagnosis | [221,222,223,224,225,226,227] |
| Silk Gelatin Collagen Chitosan Hyaluronic acid Alginate Polyurethanes Polyphosphazenes Polyanhydrides Polyesters Polyhydroxyalkanoates Acrylate polymers polyblends | Tissue engineering and regeneration | [228,229,230,231,232,233] |
| Chitosan-based films | Biosensors | [234,235,236,237,238] |
| Chitosan Polylactic acid Gelatin Collagen Polyhydroxyalkanoates Polyhydroxybutyrate | Medical implants | [203,239,240,241] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejenaru, C.; Radu, A.; Segneanu, A.-E.; Biţă, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bradu, I.A.; Vlase, T.; Vlase, G.; Bejenaru, L.E. Pharmaceutical Applications of Biomass Polymers: Review of Current Research and Perspectives. Polymers 2024, 16, 1182. https://doi.org/10.3390/polym16091182
Bejenaru C, Radu A, Segneanu A-E, Biţă A, Ciocîlteu MV, Mogoşanu GD, Bradu IA, Vlase T, Vlase G, Bejenaru LE. Pharmaceutical Applications of Biomass Polymers: Review of Current Research and Perspectives. Polymers. 2024; 16(9):1182. https://doi.org/10.3390/polym16091182
Chicago/Turabian StyleBejenaru, Cornelia, Antonia Radu, Adina-Elena Segneanu, Andrei Biţă, Maria Viorica Ciocîlteu, George Dan Mogoşanu, Ionela Amalia Bradu, Titus Vlase, Gabriela Vlase, and Ludovic Everard Bejenaru. 2024. "Pharmaceutical Applications of Biomass Polymers: Review of Current Research and Perspectives" Polymers 16, no. 9: 1182. https://doi.org/10.3390/polym16091182
APA StyleBejenaru, C., Radu, A., Segneanu, A.-E., Biţă, A., Ciocîlteu, M. V., Mogoşanu, G. D., Bradu, I. A., Vlase, T., Vlase, G., & Bejenaru, L. E. (2024). Pharmaceutical Applications of Biomass Polymers: Review of Current Research and Perspectives. Polymers, 16(9), 1182. https://doi.org/10.3390/polym16091182