Evaluation of Chemical and Biological Properties of Biodegradable Composites Based on Poly(3-hydroxybutyrate) and Chitosan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
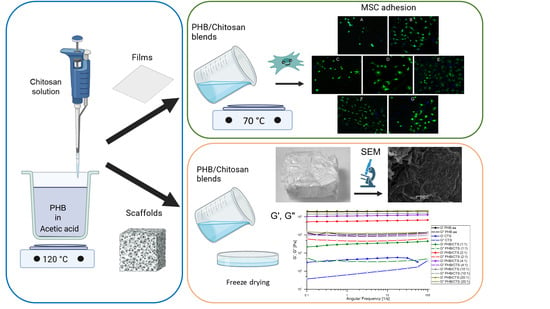
2.2. Preparation of PHB/CTS Films
2.3. In Vitro Enzymatic Degradation
2.4. Cytocompatibility Assay
2.5. Fabrication of PHB/CTS Scaffolds
2.6. Scanning Electron Microscopy (SEM)
2.7. Rheological Measurements
2.8. Moisture Absorption and Porosity of the Scaffolds
2.9. Statistical Analysis
3. Results and Discussion
3.1. Mass Changes during Biodegradation Process
3.2. In Vitro Biocompatibility Assay
3.3. Scanning Electron Microscopy (SEM)
3.4. Rheological Behavior of the Scaffolds
3.5. Moisture Absorption and Porosity of Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayalath, S.; Herath, M.; Epaarachchi, J.; Trifoni, E.; Gdoutos, E.E.; Fang, L. Durability and long-term behaviour of shape memory polymers and composites for the space industry—A review of current status and future perspectives. Polym. Degrad. Stab. 2023, 211, 110297. [Google Scholar] [CrossRef]
- Navaratnam, S.; Selvaranjan, K.; Jayasooriya, D.; Rajeev, P.; Sanjayan, J. Applications of natural and synthetic fiber reinforced polymer in infrastructure: A suitability assessment. J. Build. Eng. 2023, 66, 105835. [Google Scholar] [CrossRef]
- Namazi, H. Polymers in our daily life. BioImpacts 2017, 7, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Parlak, M.E.; Sahin, O.I.; Dundar, A.N.; Saricaoglu, F.T.; Smaoui, S.; Goksen, G.; Koirala, P.; Al-Asmari, F.; Nirmal, N.P. Natural colorant incorporated biopolymers-based pH-sensing films for indicating the food product quality and safety. Food Chem. 2024, 439, 138160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tarahomi, M.; Sheibani, R.; Xia, C.; Wang, W. Progresses in lignin, cellulose, starch, chitosan, chitin, alginate, and gum/carbon nanotube (nano)composites for environmental applications: A review. Int. J. Biol. Macromol. 2023, 241, 124472. [Google Scholar] [CrossRef] [PubMed]
- Swarupa, S.; Thareja, P. Techniques, applications and prospects of polysaccharide and protein based biopolymer coatings: A review. Int. J. Biol. Macromol. 2024, 266, 131104. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q. Plastics from Bacteria; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Jeyachandran, D.; Cerruti, M. Glass, Ceramic, Polymeric, and Composite Scaffolds with Multiscale Porosity for Bone Tissue Engineering. Adv. Eng. Mater. 2023, 25, 2201743. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, X.; Shen, D.; Li, W.; Cheng, Y.; Yang, M.; Kou, Y.; Jiang, B. Perceiving the connection between the bone healing process and biodegradation of biodegradable metal implants through precise bioadaptability principle. J. Mater. Sci. Technol. 2023, 147, 132–144. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Varghese, S.A.; Pulikkalparambil, H.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Novel biodegradable polymer films based on poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and Ceiba pentandra natural fibers for packaging applications. Food Packag. Shelf Life 2020, 25, 100538. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Zhao, S.; Liu, P.; Xie, F.; Zhang, B. Biofunctional chitosan–biopolymer composites for biomedical applications. Mater. Sci. Eng. R Rep. 2024, 159, 100775. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Shi, Z.; Wang, Y.; Song, X.; Wang, L.; Han, M.; Du, H.; He, C.; Zhao, W.; et al. Anticoagulant chitosan-kappa-carrageenan composite hydrogel sorbent for simultaneous endotoxin and bacteria cleansing in septic blood. Carbohydr. Polym. 2020, 243, 116470. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Xing, Y.; Liu, Y.; Zhang, Y.; Shen, X.; Li, X.; Miao, X.; Feng, Z.; Peng, X.; Qin, S. Fungicidal effect of chitosan via inducing membrane disturbance against Ceratocystis fimbriata. Carbohydr. Polym. 2018, 192, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lei, X.; Lv, Y.; Wang, L.; Wang, L.-N. Recent advances of chitosan as a hemostatic material: Hemostatic mechanism, material design and prospective application. Carbohydr. Polym. 2024, 327, 121673. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Rogina, A.; Pušić, M.; Štefan, L.; Ivković, A.; Urlić, I.; Ivanković, M.; Ivanković, H. Characterization of Chitosan-Based Scaffolds Seeded with Sheep Nasal Chondrocytes for Cartilage Tissue Engineering. Ann. Biomed. Eng. 2021, 49, 1572–1586. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, Y.; Li, H.; Liu, X.; Fu, Y.; Ding, F. Recent advances in chitosan-based layer-by-layer biomaterials and their biomedical applications. Carbohydr. Polym. 2021, 271, 118427. [Google Scholar] [CrossRef] [PubMed]
- Bisla, V.; Yoshitake, H. Control of mechanical and hydrophobic properties of silylated chitosan-starch films by cross-linking using carboxylic acids. Carbohydr. Polym. Technol. Appl. 2024, 7, 100462. [Google Scholar] [CrossRef]
- Reddy, C.S.; Ghai, R.; Rashmi; Kalia, V. Polyhydroxyalkanoates: An overview. Bioresour. Technol. 2003, 87, 137–146. [Google Scholar] [CrossRef]
- Iordanskii, A.; Bonartseva, G.; Makhina, T.; Sklyanchuk, E.; Zaikov, G. Bacterial Poly(3-Hydroxybutyrate) as a Biodegradable Polymer for Biomedicine. In Physical Chemistry Research for Engineering and Applied Sciences, Volume One; Apple Academic Press: New York, NY, USA, 2015; pp. 1–44. [Google Scholar] [CrossRef]
- Volova, T.; Shishatskaya, E.; Sevastianov, V.; Efremov, S.; Mogilnaya, O. Results of biomedical investigations of PHB and PHB/PHV fibers. Biochem. Eng. J. 2003, 16, 125–133. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Voinova, V.V.; Volkov, A.V.; Muraev, A.A.; Boyko, E.M.; Venediktov, A.A.; Didenko, N.N.; Dolgalev, A.A. Scaffolds Based on Poly(3-Hydroxybutyrate) and Its Copolymers for Bone Tissue Engineering (Review). Sovrem. Technol. Med. 2022, 14, 78. [Google Scholar] [CrossRef]
- Movahedi, M.; Karbasi, S. Electrospun halloysite nanotube loaded polyhydroxybutyrate-starch fibers for cartilage tissue engineering. Int. J. Biol. Macromol. 2022, 214, 301–311. [Google Scholar] [CrossRef]
- Hosseini, F.S.; Soleimanifar, F.; Aidun, A.; Enderami, S.E.; Saburi, E.; Marzouni, H.Z.; Khani, M.; Khojasteh, A.; Ardeshirylajimi, A. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) improved osteogenic differentiation of the human induced pluripotent stem cells while considered as an artificial extracellular matrix. J. Cell. Physiol. 2019, 234, 11537–11544. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, D.; Wacławek, S.; Sobel, B.; Torres-Mendieta, R.; Novotný, V.; Nguyen, N.H.A.; Ševců, A.; Padil, V.V.T.; Müllerová, J.; Stuchlík, M.; et al. A poly(3-hydroxybutyrate)–chitosan polymer conjugate for the synthesis of safer gold nanoparticles and their applications. Green Chem. 2018, 20, 4975–4982. [Google Scholar] [CrossRef]
- Jacquel, N.; Lo, C.; Wu, H.; Wei, Y.; Wang, S.S. Solubility of polyhydroxyalkanoates by experiment and thermodynamic correlations. AIChE J. 2007, 53, 2704–2714. [Google Scholar] [CrossRef]
- Zhuikova, Y.; Zhuikov, V.; Varlamov, V. Biocomposite Materials Based on Poly(3-hydroxybutyrate) and Chitosan: A Review. Polymers 2022, 14, 5549. [Google Scholar] [CrossRef]
- Hardy, A.; Seguin, C.; Brion, A.; Lavalle, P.; Schaaf, P.; Fournel, S.; Bourel-Bonnet, L.; Frisch, B.; De Giorgi, M. β-Cyclodextrin-Functionalized Chitosan/Alginate Compact Polyelectrolyte Complexes (CoPECs) as Functional Biomaterials with Anti-Inflammatory Properties. ACS Appl. Mater. Interfaces 2018, 10, 29347–29356. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.; Saha, S.; Biswas, A. Preparation and Characterization of HAp Coated Chitosan-Alginate PEC Porous Scaffold for Bone Tissue Engineering. Macromol. Symp. 2017, 376, 1600205. [Google Scholar] [CrossRef]
- de Souza, F.C.B.; de Souza, R.F.B.; Drouin, B.; Mantovani, D.; Moraes, Â.M. Comparative study on complexes formed by chitosan and different polyanions: Potential of chitosan-pectin biomaterials as scaffolds in tissue engineering. Int. J. Biol. Macromol. 2019, 132, 178–189. [Google Scholar] [CrossRef]
- Zayed, H.S.; Saleh, S.; Omar, A.E.; Saleh, A.K.; Salama, A.; Tolba, E. Development of collagen–chitosan dressing gel functionalized with propolis–zinc oxide nanoarchitectonics to accelerate wound healing. Int. J. Biol. Macromol. 2024, 261, 129665. [Google Scholar] [CrossRef]
- Anbardan, M.A.; Alipour, S.; Mahdavinia, G.R.; Rezaei, P.F. Synthesis of magnetic chitosan/hyaluronic acid/κ-carrageenan nanocarriers for drug delivery. Int. J. Biol. Macromol. 2023, 253, 126805. [Google Scholar] [CrossRef]
- Zhuikova, Y.V.; Zhuikov, V.A.; Zubareva, A.A.; Akhmedova, S.A.; Sviridova, I.K.; Sergeeva, N.S.; Varlamov, V.P. Physicochemical and biological characteristics of chitosan/κ-carrageenan thin layer-by-layer films for surface modification of nitinol. Micron 2020, 138, 102922. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Alahmadi, T.E.; Jayasuriya, A.C. Chitosan microparticles based polyelectrolyte complex scaffolds for bone tissue engineering in vitro and effect of calcium phosphate. Carbohydr. Polym. 2018, 199, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, K.L.; Ma, T. Perfusion conditioning of hydroxyapatite-chitosan-gelatin scaffolds for bone tissue regeneration from human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2012, 6, 49–59. [Google Scholar] [CrossRef]
- Ke, Y.; Zhang, X.Y.; Ramakrishna, S.; He, L.M.; Wu, G. Reactive blends based on polyhydroxyalkanoates: Preparation and biomedical application. Mater. Sci. Eng. C 2017, 70, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Saika, A.; Watanabe, Y.; Sudesh, K.; Tsuge, T. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxy-4-methylvalerate) by recombinant Escherichia coli expressing leucine metabolism-related enzymes derived from Clostridium difficile. J. Biosci. Bioeng. 2014, 117, 670–675. [Google Scholar] [CrossRef]
- Mousavioun, P.; George, G.A.; Doherty, W.O.S. Environmental degradation of lignin/poly(hydroxybutyrate) blends. Polym. Degrad. Stab. 2012, 97, 1114–1122. [Google Scholar] [CrossRef]
- dos Santos, A.J.; Valentina, L.V.O.D.; Schulz, A.A.H.; Duarte, M.A.T. From Obtaining to Degradation of PHB:Material Properties. Part I. Ing. Cienc. 2017, 13, 269–298. [Google Scholar] [CrossRef]
- Kervran, M.; Shabanian, M.; Vagner, C.; Ponçot, M.; Meier-Haack, J.; Laoutid, F.; Gaan, S.; Vahabi, H. Flame retardancy of sustainable polylactic acid and polyhydroxybutyrate (PLA/PHB) blends. Int. J. Biol. Macromol. 2023, 251, 126208. [Google Scholar] [CrossRef]
- Kausar, A.; Ijaz, S.; Rafaqat, M.; Dahshan, A.; Latif, A.A.; Bibi, S.; Al-Kadhi, N.S.; Alissa, S.A.; Nazir, A.; Iqbal, M. Chitosan-cellulose composite for the adsorptive removal of anionic dyes: Experimental and theoretically approach. J. Mol. Liq. 2023, 391, 123347. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Gong, Y.; Guo, Y.; Quan, F.; Shi, Q. Study on polysaccharide polyelectrolyte complex and fabrication of alginate/chitosan derivative composite fibers. Int. J. Biol. Macromol. 2021, 184, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhuikova, Y.V.; Zhuikov, V.A.; Makhina, T.K.; Efremov, Y.M.; Aksenova, N.A.; Timashev, P.S.; Bonartseva, G.A.; Varlamov, V.P. Preparation and characterization of poly(3-hydroxybutyrate)/chitosan composite films using acetic acid as a solvent. Int. J. Biol. Macromol. 2023, 248, 125970. [Google Scholar] [CrossRef]
- Carrière, F.; Renou, C.; Lopez, V.; De Caro, J.; Ferrato, F.; Lengsfeld, H.; De Caro, A.; Laugier, R.; Verger, R. The specific activities of human digestive lipases measured from the in vivo and in vitro lipolysis of test meals. Gastroenterology 2000, 119, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Zhuikov, V.A.; Zhuikova, Y.V.; Makhina, T.K.; Myshkina, V.L.; Rusakov, A.; Useinov, A.; Voinova, V.V.; Bonartseva, G.A.; Berlin, A.A.; Bonartsev, A.P.; et al. Comparative Structure-Property Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)s Films under Hydrolytic and Enzymatic Degradation: Finding a Transition Point in 3-Hydroxyvalerate Content. Polymers 2020, 12, 728. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Martins, A.M.; Castelhano-Carlos, M.J.; Correlo, V.M.; Sol, P.C.; Longatto-Filho, A.; Battacharya, M.; Reis, R.L.; Neves, N.M. In vitro degradation and in vivo biocompatibility of chitosan–poly(butylene succinate) fiber mesh scaffolds. J. Bioact. Compat. Polym. 2014, 29, 137–151. [Google Scholar] [CrossRef]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-Induced Degradation of Chitosan: The Characterisation of Degraded Chitosan Scaffolds. J. Tissue Repair Regen. 2017, 1, 12–22. [Google Scholar] [CrossRef]
- Valente, B.F.A.; Silvestre, A.J.D.; Neto, C.P.; Vilela, C.; Freire, C.S.R. Improving the Processability and Performance of Micronized Fiber-Reinforced Green Composites through the Use of Biobased Additives. Polymers 2022, 14, 3451. [Google Scholar] [CrossRef] [PubMed]
- Ufere, S.K.J.; Sultana, N. Fabrication and Characterization of PCL/HA/PPY Composite Scaffold Using Freeze-Drying Technique. J. Teknol. 2016, 78, 89–94. [Google Scholar] [CrossRef]
- Knowles, J.C. Development of a Natural Degradable Polymer for Orthopaedic Use. J. Med. Eng. Technol. 1993, 17, 129–137. [Google Scholar] [CrossRef]
- Koyama, N.; Doi, Y. Morphology and biodegradability of a binary blend of poly((R)-3-hydroxybutyric acid) and poly((R,S)-lactic acid). Can. J. Microbiol. 1995, 41, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Benbow, N.L.; Sebben, D.A.; Karpiniec, S.; Stringer, D.; Krasowska, M.; Beattie, D.A. Lysozyme uptake into pharmaceutical grade fucoidan/chitosan polyelectrolyte multilayers under physiological conditions. J. Colloid Interface Sci. 2020, 565, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Kołakowska, A.; Gadomska-Gajadhur, A.; Ruśkowski, P. Biomimetic scaffolds based on chitosan in bone regeneration. A review. Chem. Process. Eng. 2023, 43, 305–330. [Google Scholar] [CrossRef]
- Zhu, X.; Chian, K.S.; Chan-Park, M.B.E.; Lee, S.T. Effect of argon-plasma treatment on proliferation of human-skin–derived fibroblast on chitosan membrane in vitro. J. Biomed. Mater. Res. Part A 2005, 73A, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef] [PubMed]
- Sert, A.B.Ö.; Bittrich, E.; Uhlmann, P.; Kok, F.N.; Kılıç, A. Monitoring Cell Adhesion on Polycaprolactone–Chitosan Films with Varying Blend Ratios by Quartz Crystal Microbalance with Dissipation. ACS Omega 2023, 8, 17017–17027. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Schickle, K.; Bergmann, C.; Fischer, H. Ensuring defined porosity and pore size using ammonium hydrogen carbonate as porosification agent for calcium phosphate scaffolds. BioNanoMaterials 2013, 14, 101–108. [Google Scholar] [CrossRef]
- Nam, Y.S.; Yoon, J.J.; Park, T.G. A novel fabrication method of macroporous biodegradable polymer scaffolds using gas foaming salt as a porogen additive. J. Biomed. Mater. Res. 2000, 53, 1–7. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Zharkova, I.I.; Voinova, V.V.; Kuznetsova, E.S.; Zhuikov, V.A.; Makhina, T.K.; Myshkina, V.L.; Potashnikova, D.M.; Chesnokova, D.V.; Khaydapova, D.D.; et al. Poly(3-hydroxybutyrate)/poly(ethylene glycol) scaffolds with different microstructure: The effect on growth of mesenchymal stem cells. 3 Biotech 2018, 8, 328. [Google Scholar] [CrossRef]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Yadav, P.; Beniwal, G.; Saxena, K.K. A review on pore and porosity in tissue engineering. Mater. Today Proc. 2021, 44, 2623–2628. [Google Scholar] [CrossRef]
- Zharkova, I.I.; Volkov, A.V.; Muraev, A.A.; Makhina, T.K.; Voinova, V.V.; Ryabova, V.M.; Gazhva, Y.V.; Kashirina, A.S.; Kashina, A.V.; Bonartseva, G.A.; et al. Poly(3-hydroxybutyrate) 3D-Scaffold–Conduit for Guided Tissue Sprouting. Int. J. Mol. Sci. 2023, 24, 6965. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, J.; Boccaccini, A.R.; Covarrubias, C.; Palza, H. Effect of Cu- and Zn-Doped Bioactive Glasses on the In Vitro Bioactivity, Mechanical and Degradation Behavior of Biodegradable PDLLA Scaffolds. Materials 2020, 13, 2908. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Kanungo, B.P.; Gibson, L.J. Density–property relationships in mineralized collagen–glycosaminoglycan scaffolds. Acta Biomater. 2009, 5, 1006–1018. [Google Scholar] [CrossRef]





| Sample | Quantity of PHB | Quantity of CTS | ||
|---|---|---|---|---|
| % | Mg per 1 mL of blend | % | Mg per 1 mL of blend | |
| PHB | 100 | 20 | 0 | 0 |
| PHB/CTS (20:1) | 95.23 | 19.047 | 4.76 | 0.953 |
| PHB/CTS (10:1) | 90.90 | 18.18 | 9.1 | 1.82 |
| PHB/CTS (4:1) | 80 | 16 | 20 | 4 |
| PHB/CTS (2:1) | 66.67 | 13.33 | 33.33 | 6.67 |
| PHB/CTS (1:1) | 50 | 10 | 50 | 10 |
| CTS | 0 | 0 | 100 | 20 |
| Sample | Moisture Absorption (%) | Density, g/cm3 | Porosity (%) |
|---|---|---|---|
| PHB aa | 162 ± 20 | 0.04 | 97 ± 1 |
| PHB:CTS 20:1 | 175 ± 26 | 0.05 | 96 ± 0.5 |
| PHB:CTS 10:1 | 240 ± 20 | 0.04 | 97 ± 1 |
| PHB:CTS 4:1 | 295 ± 26 | 0.05 | 96 ± 1 |
| PHB:CTS 2:1 | 2800 ± 150 | - | - |
| PHB:CTS 1:1 | 5000 ± 278 | - | - |
| CTS | 6976 ± 305 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuikova, Y.V.; Zhuikov, V.A.; Khaydapova, D.D.; Lunkov, A.P.; Bonartseva, G.A.; Varlamov, V.P. Evaluation of Chemical and Biological Properties of Biodegradable Composites Based on Poly(3-hydroxybutyrate) and Chitosan. Polymers 2024, 16, 1124. https://doi.org/10.3390/polym16081124
Zhuikova YV, Zhuikov VA, Khaydapova DD, Lunkov AP, Bonartseva GA, Varlamov VP. Evaluation of Chemical and Biological Properties of Biodegradable Composites Based on Poly(3-hydroxybutyrate) and Chitosan. Polymers. 2024; 16(8):1124. https://doi.org/10.3390/polym16081124
Chicago/Turabian StyleZhuikova, Yulia V., Vsevolod A. Zhuikov, Dolgor D. Khaydapova, Alexey P. Lunkov, Garina A. Bonartseva, and Valery P. Varlamov. 2024. "Evaluation of Chemical and Biological Properties of Biodegradable Composites Based on Poly(3-hydroxybutyrate) and Chitosan" Polymers 16, no. 8: 1124. https://doi.org/10.3390/polym16081124
APA StyleZhuikova, Y. V., Zhuikov, V. A., Khaydapova, D. D., Lunkov, A. P., Bonartseva, G. A., & Varlamov, V. P. (2024). Evaluation of Chemical and Biological Properties of Biodegradable Composites Based on Poly(3-hydroxybutyrate) and Chitosan. Polymers, 16(8), 1124. https://doi.org/10.3390/polym16081124