Recycling Polyethylene/Polyamide Multilayer Films with Poly(isoprene-g-Maleic Anhydride) Compatibilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extruders
2.3. Production of the Films
2.4. Preparation of the Samples for Characterization
2.5. Characterization Techniques
- Thermogravimetric analysis (TGA) and Differential scanning calorimetry (DSC)
- Fourier-transform infrared spectroscopy (FTIR)
- Scanning electron microscopy
- Tensile testing
- Dart Impact test and Tear test
3. Results and Discussion
3.1. Film Preparation
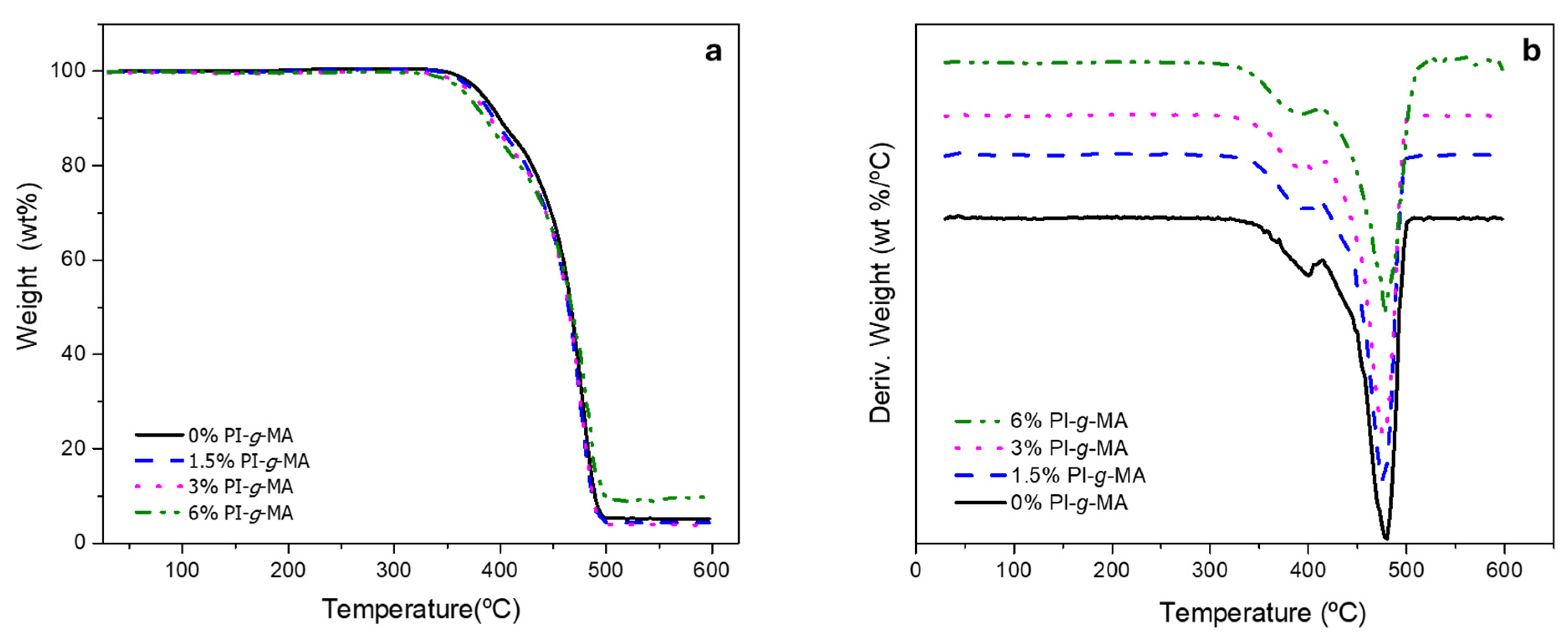
3.2. Thermogravimetric Analysis
3.3. Differential Scanning Calorimetry
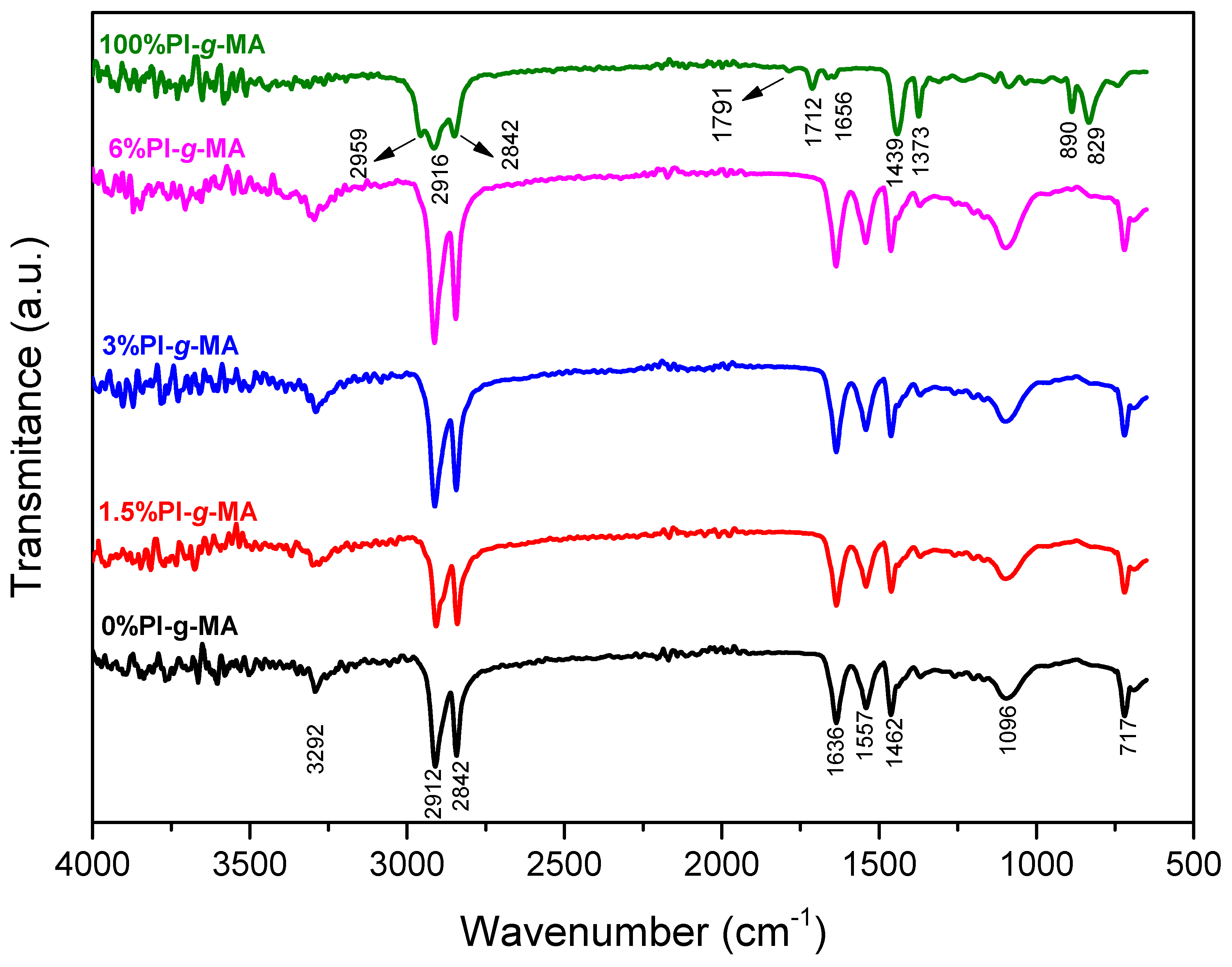
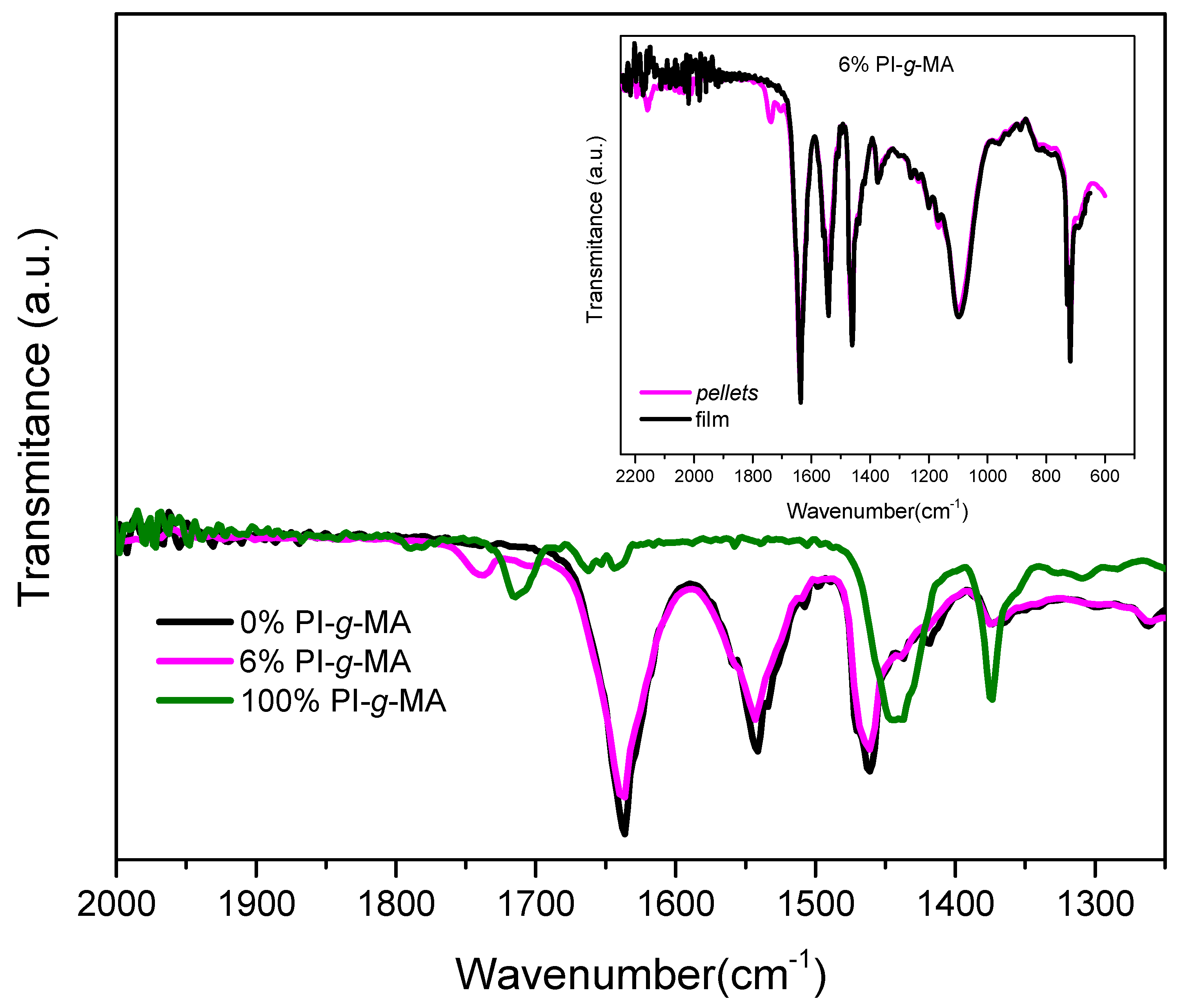
3.4. Infrared Spectroscopy (FTIR)
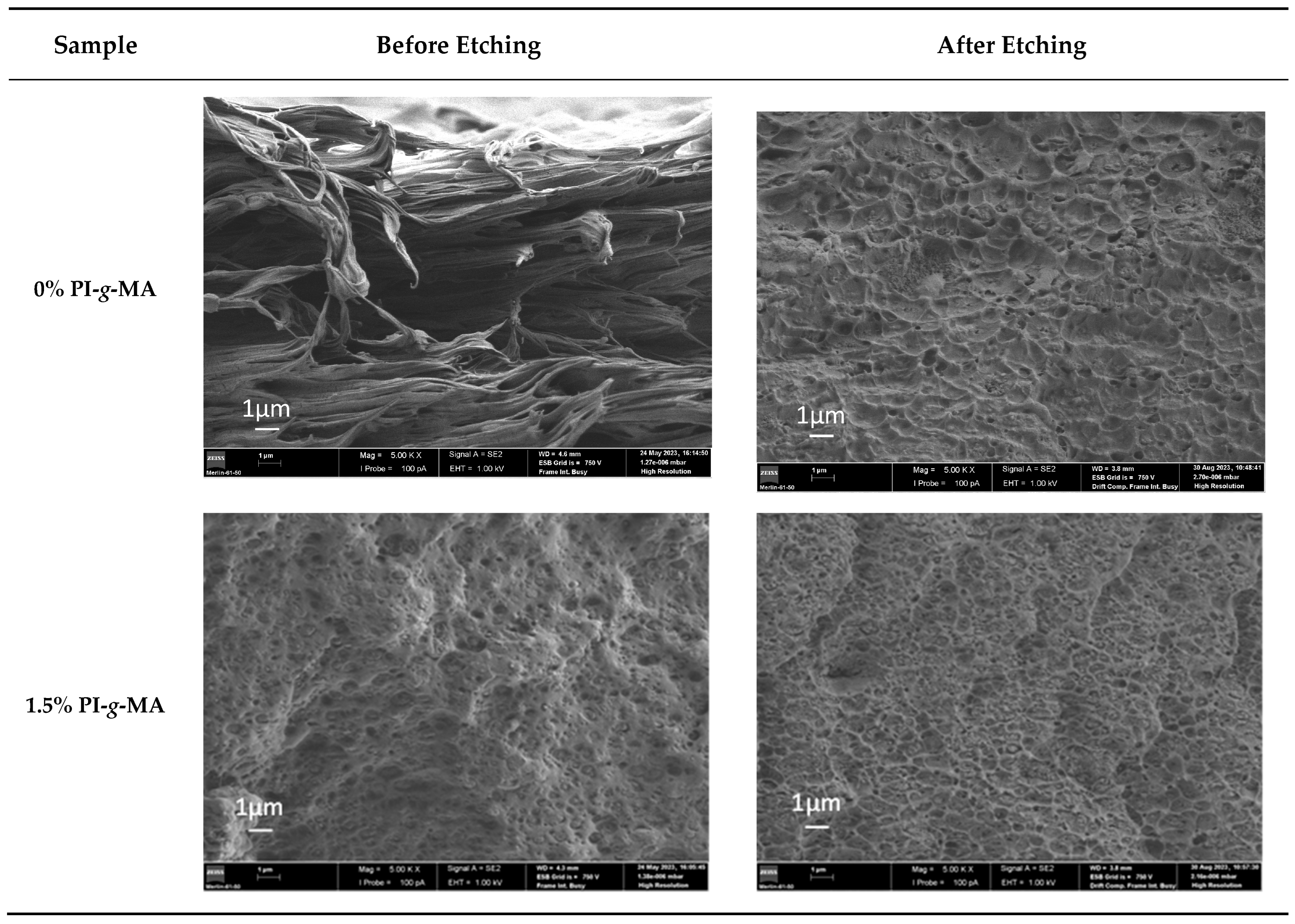
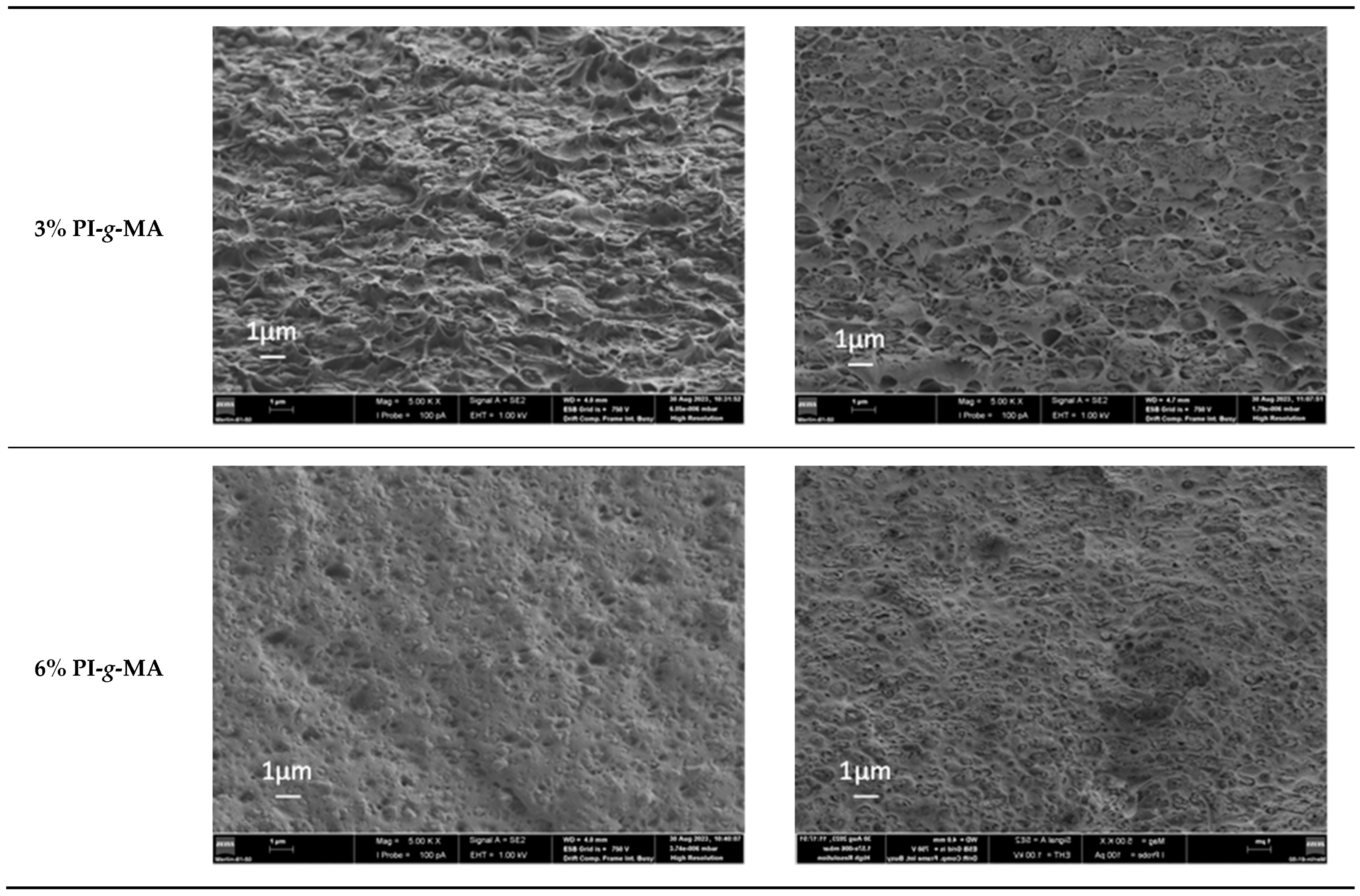
3.5. SEM
3.6. Mechanical Performance
3.7. Tear Resistance, Perforation, and Impact Fall Dart
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Moreno, D.D.P.; Saron, C. Influence of compatibilizer on the properties of low-density polyethylene/polyamide 6 blends obtained by mechanical recycling of multilayer film waste. Waste Manag. Res. 2018, 36, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Alias, A.R.; Wan, M.K.; Sarbon, N.M. Emerging materials and technologies of multi-layer film for food packaging application: A review. Food Control 2022, 136, 108875. [Google Scholar] [CrossRef]
- Koning, C.; Van Duin, M.; Pagnoulle, C.; Jerome, R. Strategies for compatibilization of polymer blends. Prog. Polym. Sci. 1998, 23, 707–757. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- Maris, J.; Bourdon, S.; Brossard, J.-M.; Cauret, L.; Fontaine, L.; Montembault, V. Mechanical recycling: Compatibilization of mixed thermoplastic wastes. Polym. Degrad. Stab. 2018, 147, 245–266. [Google Scholar] [CrossRef]
- Fortelný, I.; Jůza, J. The effects of copolymer compatibilizers on the phase structure evolution in polymer blends—A review. Materials 2021, 14, 7786. [Google Scholar] [CrossRef] [PubMed]
- Ararat, C.A.; Percino, M.J.; Murillo, E.A. Compatibilization of LDPE/PA6 by Using a LDPE Functionalized with a Maleinized Hyperbranched Polyester Polyol. Macromol. Res. 2020, 28, 203–210. [Google Scholar] [CrossRef]
- Filippi, S.; Yordanov, H.; Minkova, L.; Polacco, G.; Talarico, M. Reactive Compatibilizer Precursors for LDPE/PA6 Blends, 4. Macromol. Mater. Eng. 2004, 289, 512–523. [Google Scholar] [CrossRef]
- Scaffaro, R.; La Mantia, F.P.; Canfora, L.; Polacco, G.; Filippi, S.; Magagnini, P. Reactive compatibilization of PA6/LDPE blends with an ethylene–acrylic acid copolymer and a low molar mass bis-oxazoline. Polymer 2003, 44, 6951–6957. [Google Scholar] [CrossRef]
- Minkova, L.; Yordanov, H.; Filippi, S. Characterization of blends of LDPE and PA6 with functionalized polyethylenes. Polymer 2002, 43, 6195–6204. [Google Scholar] [CrossRef]
- Psarski, M.; Pracella, M.; Galeski, A. Crystal phase and crystallinity of polyamide 6/functionalized polyolefin blends. Polymer 2000, 41, 4923–4932. [Google Scholar] [CrossRef]
- Chiono, V.; Filippi, S.; Yordanov, H.; Minkova, L.; Magagnini, P. Reactive compatibilizer precursors for LDPE/PA6 blends. III: Ethylene–glycidylmethacrylate copolymer. Polymer 2003, 44, 2423–2432. [Google Scholar] [CrossRef]
- Taşdemïr, M.; Yildirim, H. Achieving compatibility in blends of low-density polyethylene/polyamide-6 with addition of ethylene vinyl acetate. J. Appl. Polym. Sci. 2001, 82, 1748–1754. [Google Scholar] [CrossRef]
- Lahor, A.; Nithitanakul, M.; Grady, B.P. Blends of low-density polyethylene with nylon compatibilized with a sodium-neutralized carboxylate ionomer. Eur. Polym. J. 2004, 40, 2409–2420. [Google Scholar] [CrossRef]
- Kelar, K.; Jurkowski, B. Preparation of functionalised low-density polyethylene by reactive extrusion and its blend with polyamide 6. Polymer 2000, 41, 1055–1062. [Google Scholar] [CrossRef]
- Jurkowski, B.; Olkhov, Y.A.; Kelar, K.; Olkhova, O.M. Thermomechanical study of low-density polyethylene, polyamide 6 and its blends. Eur. Polym. J. 2002, 38, 1229–1236. [Google Scholar] [CrossRef]
- Filippi, S.; Minkova, L.; Dintcheva, N.; Narducci, P.; Magagnini, P. Comparative study of different maleic anhydride grafted compatibilizer precursors towards LDPE/PA6 blends: Morphology and mechanical properties. Polymer 2005, 46, 8054–8061. [Google Scholar] [CrossRef]
- Lei, F.; Du, Q.; Li, T.; Li, J.; Guo, S. Effect of phase morphology and interfacial strength on barrier properties of high density polyethylene/polyamide 6 membranes. Polym. Eng. Sci. 2013, 53, 1996–2003. [Google Scholar] [CrossRef]
- Mistretta, M.C.; Fontana, P.; Ceraulo, M.; Morreale, M.; La Mantia, F.P. Effect of compatibilization on the photo-oxidation behaviour of polyethylene/polyamide 6 blends and their nanocomposites. Polym. Degrad. Stab. 2015, 112, 192–197. [Google Scholar] [CrossRef]
- Dos Anjos, E.G.R.; Backes, E.H.; Marini, J.; Pessan, L.A.; Montagna, L.S.; Passador, F.R. Effect of LLDPE-g-MA on the rheological, thermal, mechanical properties and morphological characteristic of PA6/LLDPE blends. J. Polym. Res. 2019, 26, 134. [Google Scholar] [CrossRef]
- Moreno, D.D.P.; Saron, C. Low-density polyethylene/polyamide 6 blends from multilayer films waste. J. Appl. Polym. Sci. 2019, 136, 47456. [Google Scholar] [CrossRef]
- Lozay, Q.; Beuguel, Q.; Follain, N.; Lebrun, L.; Guinault, A.; Miquelard-Garnier, G.; Tencé-Girault, S.; Sollogoub, C.; Dargent, E.; Marais, S. Structural and barrier properties of compatibilized PE/PA6 multinanolayer films. Membranes 2021, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Komorowska, D.; Nowak-Grzebyta, J.; Gawdzinska, K.; Mysiukiewicz, O.; Tomasik, M. Polyethylene/Polyamide Blends Made of Waste with Compatibilizer: Processing, Morphology, Rheological and Thermo-Mechanical Behavior. Polymers 2021, 13, 2385. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.d.A.S.d.; d’Almeida, J.R.M. Mechanical properties and morphology of HDPE/PA12 blends compatibilized with HDPE-alt-MAH. Polym. Polym. Compos. 2022, 30. [Google Scholar] [CrossRef]
- Shen, L.; Gorbea, G.D.; Danielsona, E.; Cuib, S.; Ellisona, C.J.; Bates, F.S. Threading-the Needle: Compatibilization of HDPE/iPP blends with butadiene-derived polyolefin block copolymers. Proc. Natl. Acad. Sci. 2023, 120, e2301352120. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K. Performance evaluation of chlorinated polyethylene compatibilized-industrial waste-filled acrylonitrile butadiene rubber/low-density polyethylene blends. J. Vinyl Addit. Technol. 2016, 22, 460–469. [Google Scholar] [CrossRef]
- Hu, T.; Tang, X.; Zhang, L.; Wang, W.; Huang, Z. Preparation of TPI-g-MAH and the application in toughened PA6. Plast. Rubber Compos. 2016, 45, 300–303. [Google Scholar] [CrossRef]
- Ahmed, K. An investigation on chloroprene-compatibilized acrylonitrile butadiene rubber/high density polyethylene blends. J. Adv. Res. 2015, 6, 811–817. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmed, K. Eco-thermoplastic Elastomer Blends Developed by Compatibilizing Chlorinated Polyethylene into Industrial-Waste-Filled Polypropylene/Acrylonitrile Butadiene Rubber System. Arab. J. Sci. Eng. 2015, 40, 2929–2936. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Della Maggiore, I.; Savi, S.; Aglietto, M.; Ciardelli, F. Modified styrene–butadiene–styrene block copolymer as compatibiliser precursor in polyethylene/poly(ethylene terephthalate) blends. Polym. Degrad. Stab. 2005, 90, 211–223. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The thermal degradation of poly(vinyl alcohol). Polymer 2001, 42, 6775–6783. [Google Scholar] [CrossRef]
- Araújo, J.R.; Vallim, M.R.; Spinacé, M.A.S.; De Paoli, M.A. Use of postconsumer polyethylene in blends with polyamide 6: Effects of the extrusion method and the compatibilizer. J. Appl. Polym. Sci. 2008, 110, 1310–1317. [Google Scholar] [CrossRef]
- Mohammadi, R.S.; Zolali, A.M.; Kim, J.-H.; Jalali, A.; Park, C.B. 3D fibrillated network of compatibilized linear low density polyethylene/polyamide with high melt strength and superior foamability. Polymer 2021, 228, 123911. [Google Scholar] [CrossRef]
- Arnal, M.L.; Balsamo, V.; Ronca, G.; Sánchez, A.; Müller, A.J.; Cañizales, E.; Urbina De Navarro, C. Applications of successive self-nucleation and annealing (SSA) to polymer characterization. J. Therm. Anal. Calorim. 2000, 59, 451–470. [Google Scholar] [CrossRef]
- Tarasova, E.; Poltimäe, T.; Krumme, A.; Lehtinen, A.; Viikna, A. Triple crystallization behavior of fractionated ethylene/α-olefin copolymers of different catalyst type. J. Polym. Res. 2011, 18, 207–216. [Google Scholar] [CrossRef]
- Silva, E.F.; Soares, B.G. Polyethylene/Polyamide-6 Blends Containing Mercapto-Modified EVA. J. Appl. Polym. Sci. 1996, 60, 1687–1694. [Google Scholar] [CrossRef]
- Jiang, G.; Filippi, S.; Magagnini, P. Reactive compatibilizer precursors for LDPE/PA6 blends. II: Maleic anhydride grafted polyethylenes. Polymer 2003, 44, 2411–2422. [Google Scholar] [CrossRef]
- Souza, G.P.M.; Anjos, E.G.R.; Montagna, L.S.; Ferro, O.; Passador, F.R. A New Strategy for the Use of Post-Processing Vacuum Bags from Aerospace Supplies: Nucleating Agent to LLDPE Phase in PA6/LLDPE Blends. Recycling 2019, 4, 18. [Google Scholar] [CrossRef]
- Filippi, S.; Chiono, V.; Polacco, G.; Paci, M.; Minkova, L.I.; Magagnini, P. Reactive compatibilizer precursors for LDPE/PA6 blends, 1. Ethylene/acrylic acid copolymers. Macromol. Chem. Phys. 2002, 203, 1512–1525. [Google Scholar] [CrossRef]
- Guilmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Rtter, G.; Ishida, H. FTIR separation of nylon-6 chain conformations: Clarification of the mesomorphous and γ-crystalline phases. J. Polym. Sci. Part B Polym. Phys. 1992, 30, 489–495. [Google Scholar] [CrossRef]
- Vasanthan, N.; Salem, D.R. FTIR spectroscopic characterization of structural changes in polyamide-6 fibers during annealing and drawing. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 536–547. [Google Scholar] [CrossRef]
- Porubská, M.; Szöllős, O.; Kóňová, A.; Janigová, I.; Jašková, M.; Jomová, K.; Chodák, I. FTIR spectroscopy study of polyamide-6 irradiated by electron and proton beams. Polym. Degrad. Stab. 2012, 97, 523–531. [Google Scholar] [CrossRef]
- Bhattacharyya, A.R.; Ghosh, A.K.; Misra, A.; Eichhorn, K.-J. Reactively compatibilised polyamide6/ethylene-co-vinyl acetate blends: Mechanical properties and morphology. Polymer 2005, 46, 1661–1674. [Google Scholar] [CrossRef]
- Bolloju, S.; Chang, Y.L.; Sharma, S.U.; Hsu, M.F.; Lee, J.T. Vulcanized polyisoprene-graft-maleic anhydride as an efficient binder for silicon anodes in lithium-ion batteries. Electrochim. Acta 2022, 419, 140390. [Google Scholar] [CrossRef]
- Armat, R.; Moet, A. Morphological origin of toughness in polyethylene-nylon-6 blends. Polymer 1993, 34, 977–985. [Google Scholar] [CrossRef]
- Hamid, F.; Akhbar, S.; Halim, K.H.K. Mechanical and Thermal Properties of Polyamide 6/HDPE-g- MAH/High Density Polyethylene. Procedia Eng. 2013, 68, 418–424. [Google Scholar] [CrossRef]
- Darie, R.N.; Vasile, C.; Kozłowski, M. The effect of compatibilization in reactive processing low density polyethylene/polyamide 6/EPDM blends. Polimery 2006, 51, 656–661. [Google Scholar] [CrossRef][Green Version]



| Temperature Profile (°C) | |||||||
|---|---|---|---|---|---|---|---|
| Zones | Zone1 | Zone 2 | Zone 3 | Zone 4 | Zone 5 | Zone 6 | Head |
| Set-Point | 100 | 180 | 195 | 220 | 220 | 220 | 220 |
| Spindle Speed (rpm) | Feeder (%) | Cutting Speed (%) |
|---|---|---|
| 290 | 5 | 8 |
| Temperature Profile (°C) | Extrusion Parameters | ||||||
|---|---|---|---|---|---|---|---|
| Zones | Zone 1 | Zone 2 | Zone 3 | Zone A | Zone B | Pull | Spindle Speed (%) |
| Set-Point | 190 | 220 | 220 | 220 | 220 | 2.7 | 27 |
| Sample | 1st Loss | 2nd Loss | Residue (%) | ||
|---|---|---|---|---|---|
| Tonset (°C) | % | Tonset (°C) | % | ||
| 0% PI-g-MA | 372.2 | 15.0 | 447.3 | 80.0 | 5.0 |
| 1.5% PI-g-MA | 343.7 | 16.0 | 453.1 | 80.0 | 4.0 |
| 3% PI-g-MA | 369.3 | 17.7 | 454.8 | 78.6 | 3.7 |
| 6% PI-g-MA | 365.5 | 17.8 | 469.1 | 72.5 | 9.7 |
| Sample | Polyethylene | Polyamide | ||||||
|---|---|---|---|---|---|---|---|---|
| Tm (°C) * | ∆Hm (J/g) | Tc (°C) | ∆Hc (J/g) | Tm (°C) | ∆Hm (J/g) | Tc (°C) | ∆Hc (J/g) | |
| 0% PI-g-MA | 120 (113) | 85.6 | 108 | 55.2 | 211 | 62.8 | 175 | 48.0 |
| 1.5% PI-g-MA | 120 (112) | 85.6 | 107 | 71.1 | 211 | 59.8 | 172 | 41.0 |
| 3% PI-g-MA | 120 (112) | 83.3 | 107 | 61.7 | 210 | 57.7 | 168 | 35.1 |
| 6% PI-g-MA | 120 (112) | 88.3 | 107 | 64.7 | 211 | 61.1 | 168 | 32.2 |
| Sample | Tear Resistance (g) | Puncture Resistance | Impact of the Fall Dart (g) | ||
|---|---|---|---|---|---|
| Longitudinal | Transverse | Energy (J) | Load (N) | ||
| 0% PI-g-MA | 576 ± 42.1 | 828 ± 18.8 | 0.037 ± 0.0 | 15.40 ± 1.07 | 440 |
| 1.5% PI-g-MA | 608 ± 46.2 | 719 ± 12.5 | 0.036 ± 0.0 | 15.27 ± 2.0 | 770 |
| 6% PI-g-MA | 748 ± 38.8 | 816 ± 10.3 | 0.039 ± 0.0 | 14.61 ± 0.87 | 680 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeiro, A.; Teixeira, C.; Costa, H.; Coelho, J.F.J.; Serra, A.C. Recycling Polyethylene/Polyamide Multilayer Films with Poly(isoprene-g-Maleic Anhydride) Compatibilizer. Polymers 2024, 16, 1079. https://doi.org/10.3390/polym16081079
Romeiro A, Teixeira C, Costa H, Coelho JFJ, Serra AC. Recycling Polyethylene/Polyamide Multilayer Films with Poly(isoprene-g-Maleic Anhydride) Compatibilizer. Polymers. 2024; 16(8):1079. https://doi.org/10.3390/polym16081079
Chicago/Turabian StyleRomeiro, Andreia, Cidália Teixeira, Henrique Costa, Jorge F. J. Coelho, and Arménio C. Serra. 2024. "Recycling Polyethylene/Polyamide Multilayer Films with Poly(isoprene-g-Maleic Anhydride) Compatibilizer" Polymers 16, no. 8: 1079. https://doi.org/10.3390/polym16081079
APA StyleRomeiro, A., Teixeira, C., Costa, H., Coelho, J. F. J., & Serra, A. C. (2024). Recycling Polyethylene/Polyamide Multilayer Films with Poly(isoprene-g-Maleic Anhydride) Compatibilizer. Polymers, 16(8), 1079. https://doi.org/10.3390/polym16081079







